Micro-CT and Microscopy Study of Internal and Marginal Gap to Tooth Surface of Crenelated versus Conventional Dental Indirect Veneers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tooth Surface Preparation
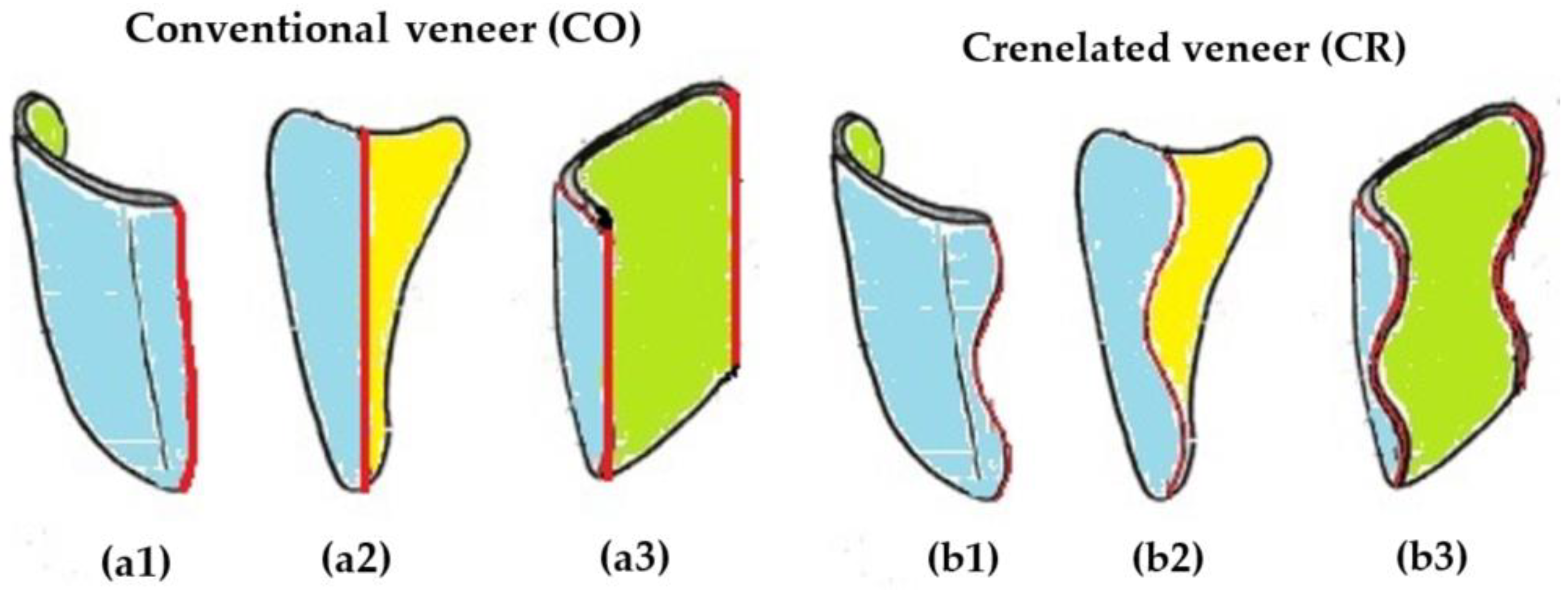
2.2. Manufacturing of the Dental Veneers
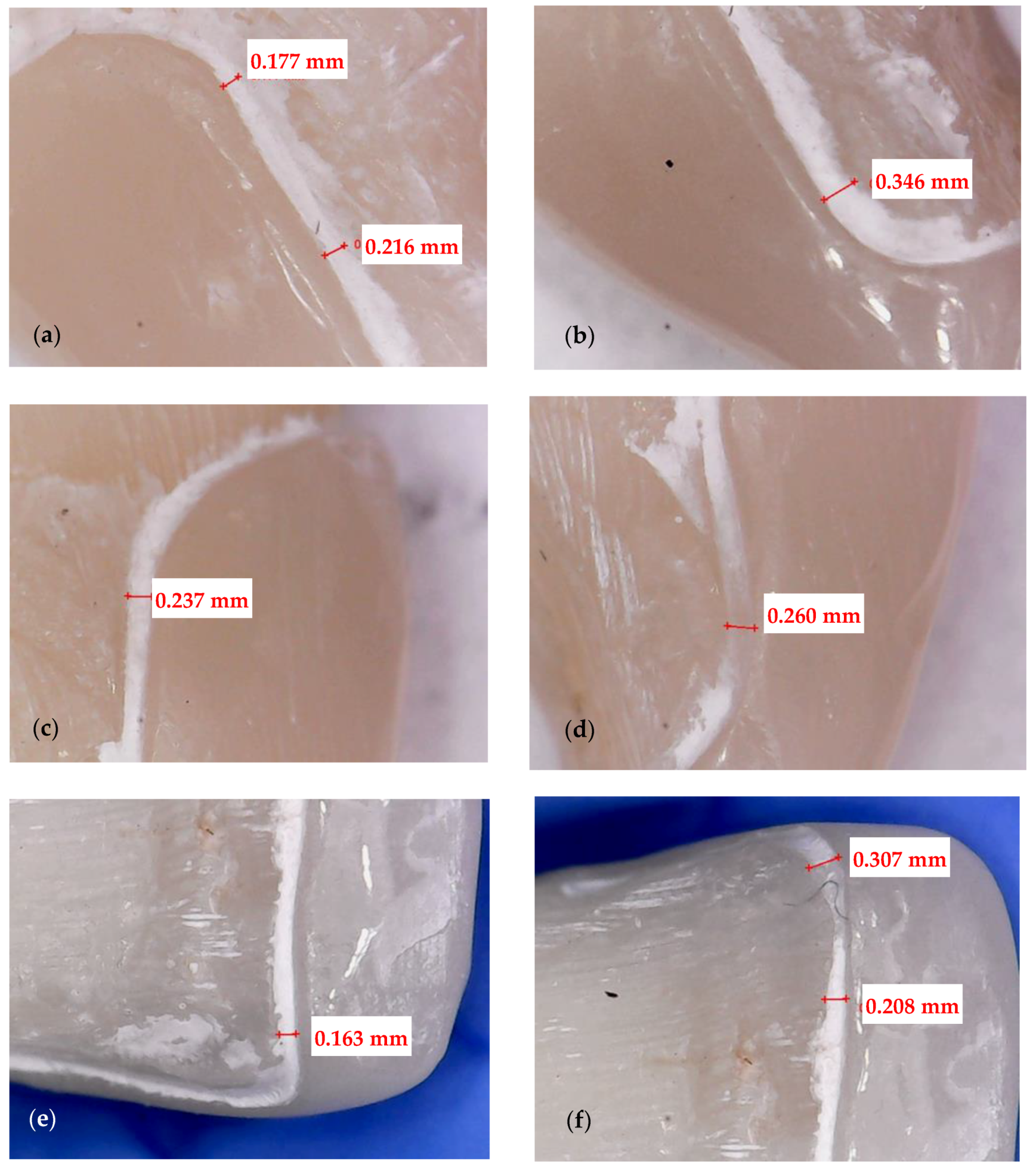
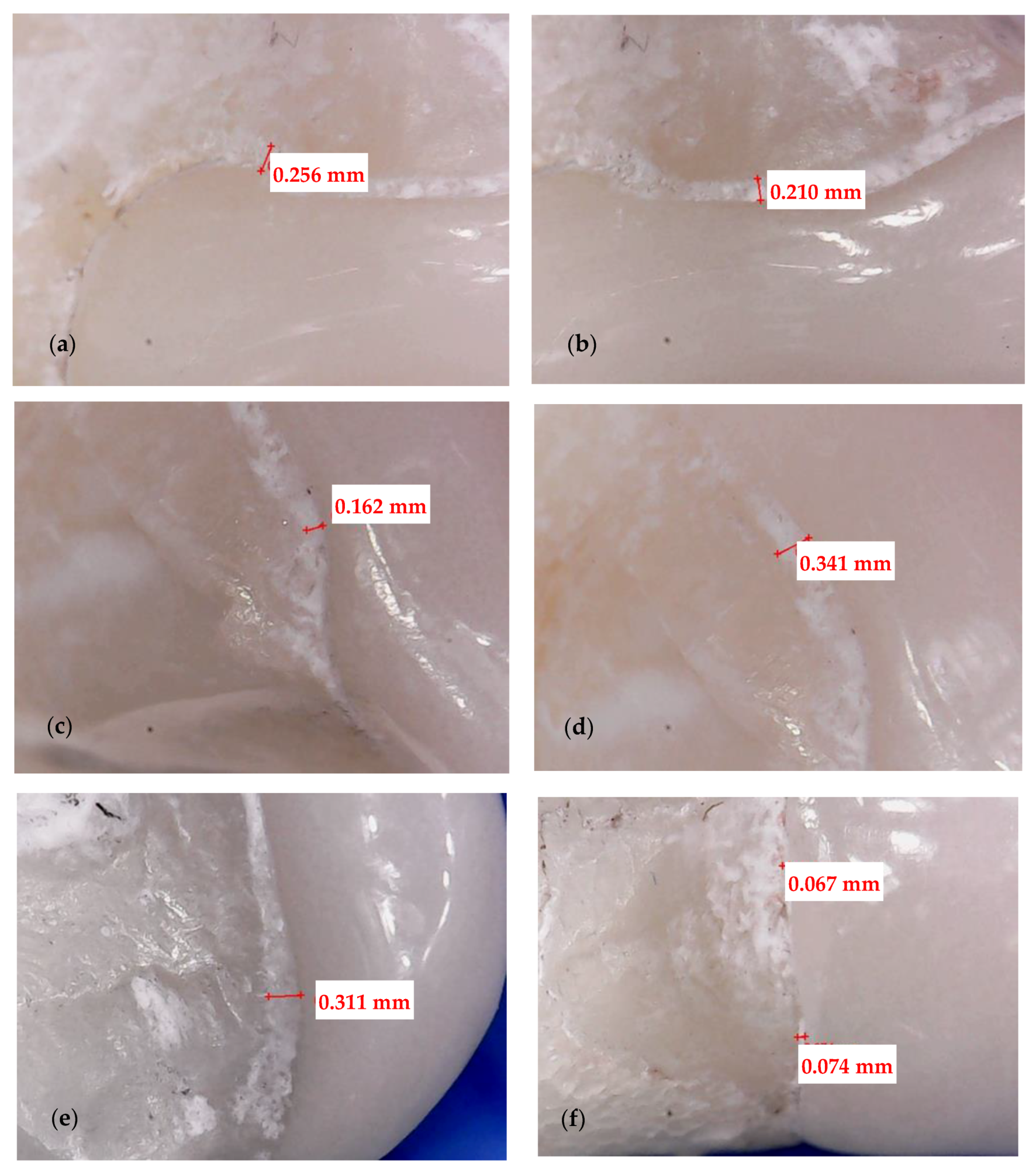
2.3. Optical Microscopy
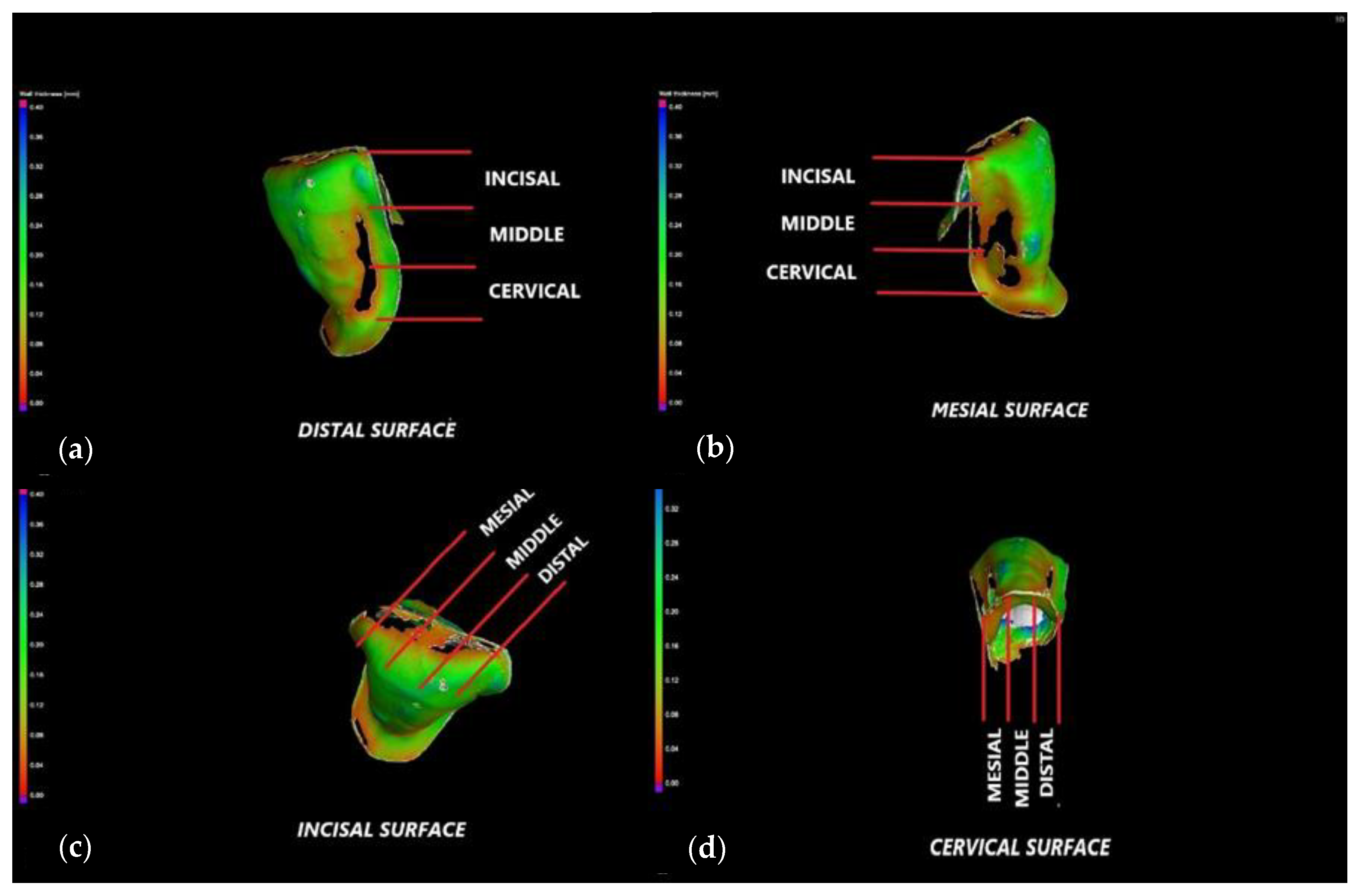
2.4. Micro-CT Investigations
2.5. Porosity Analysis
3. Results
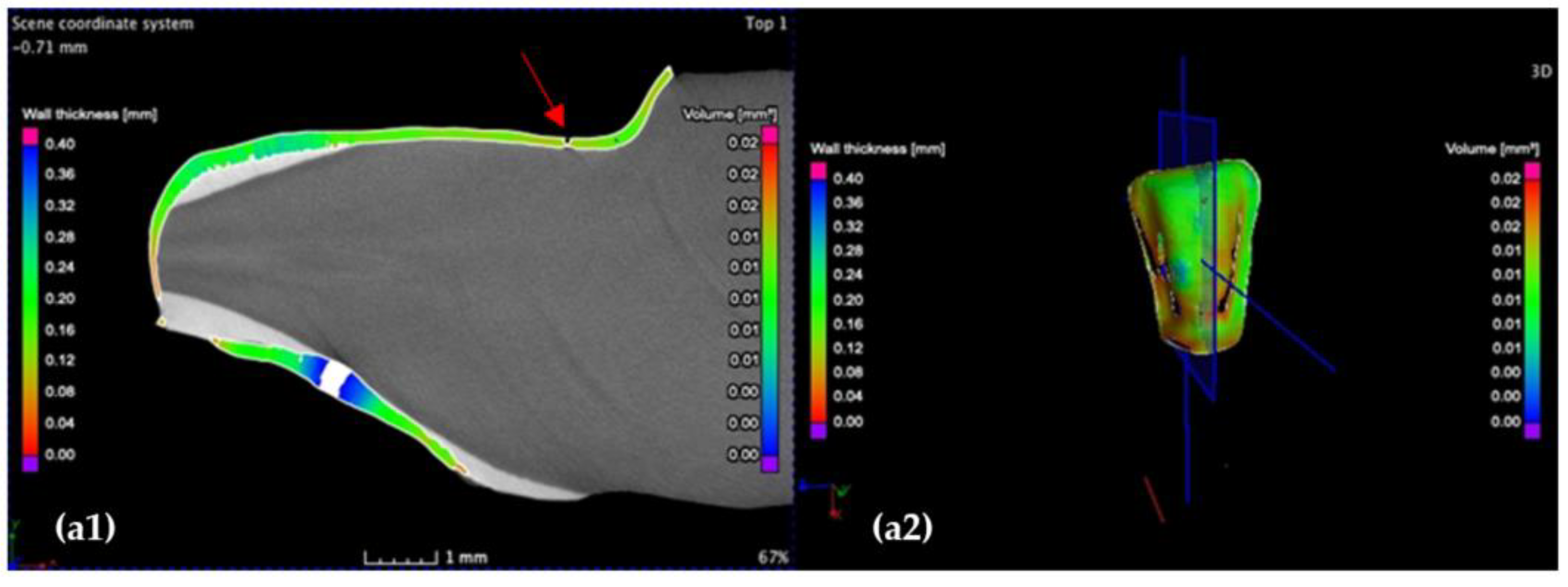
3.1. Optical Microscopy
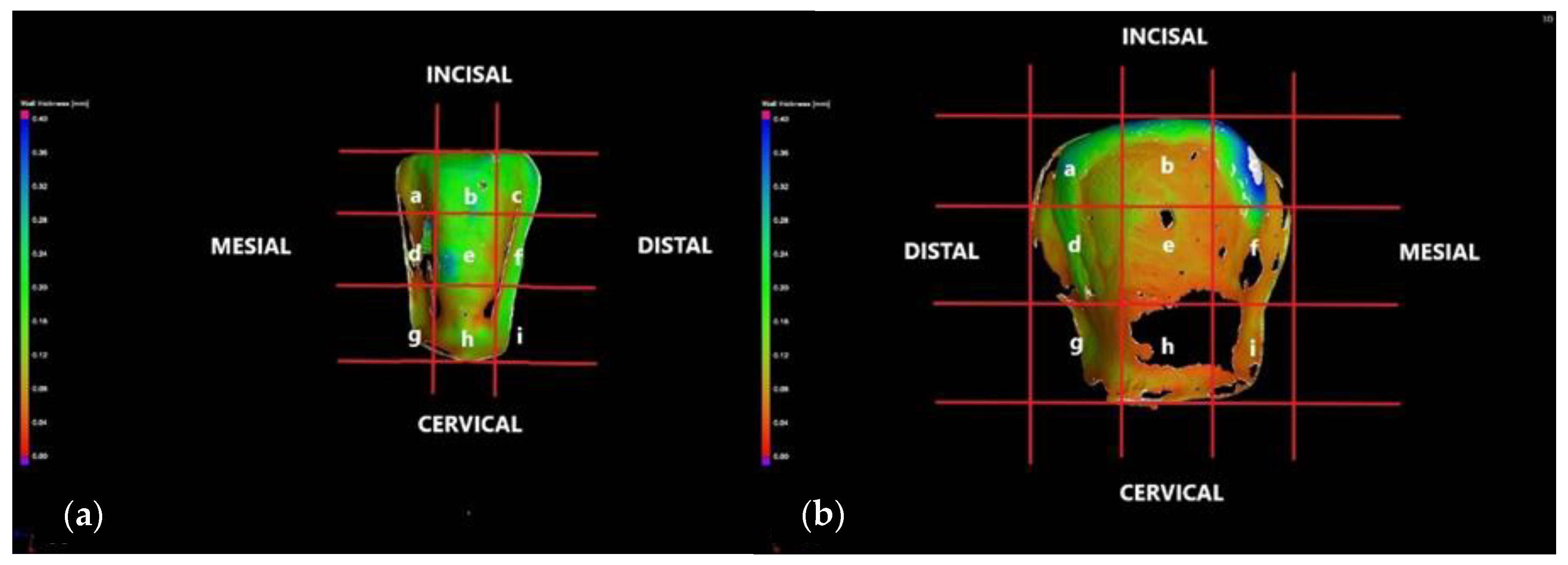
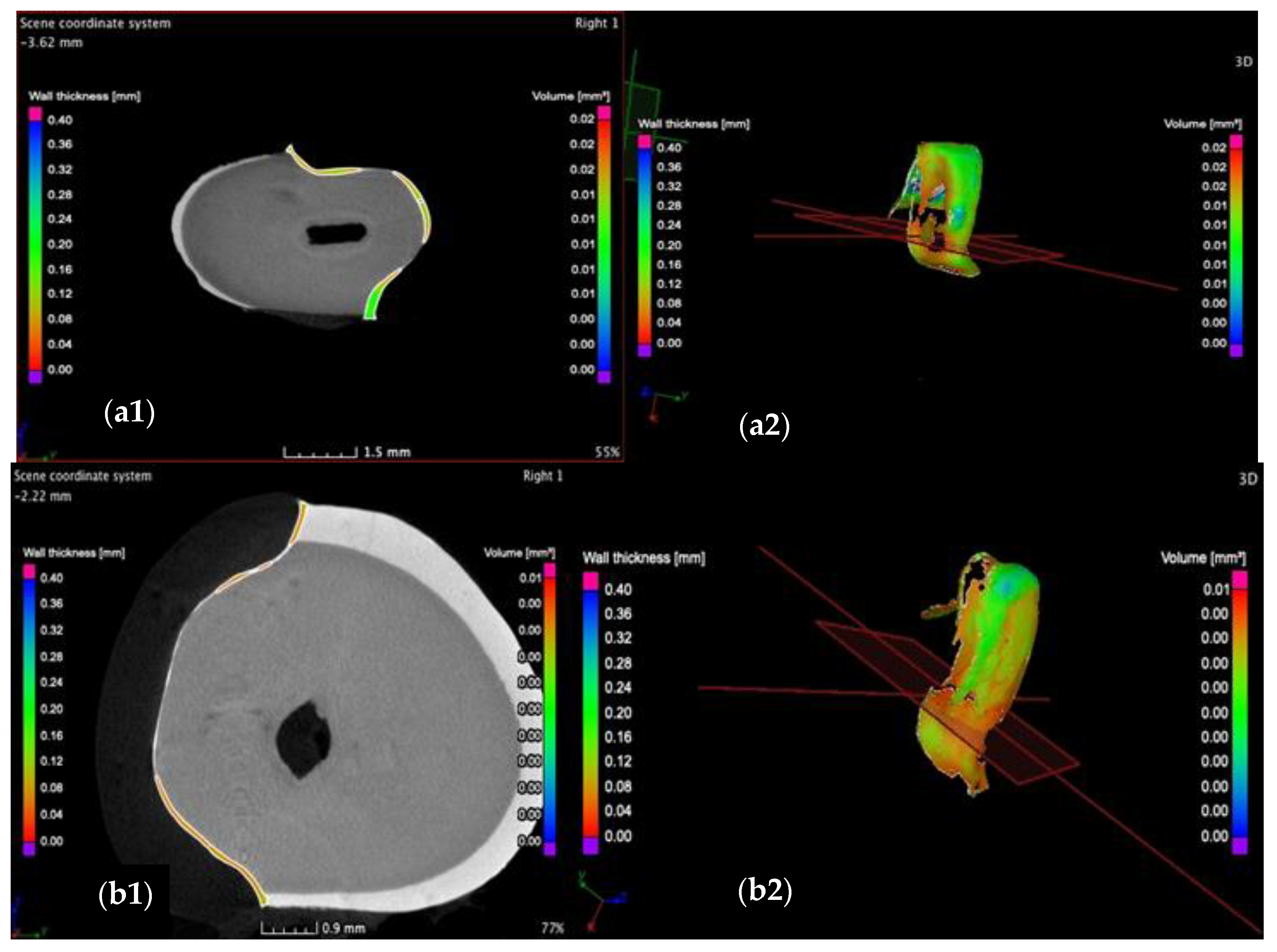
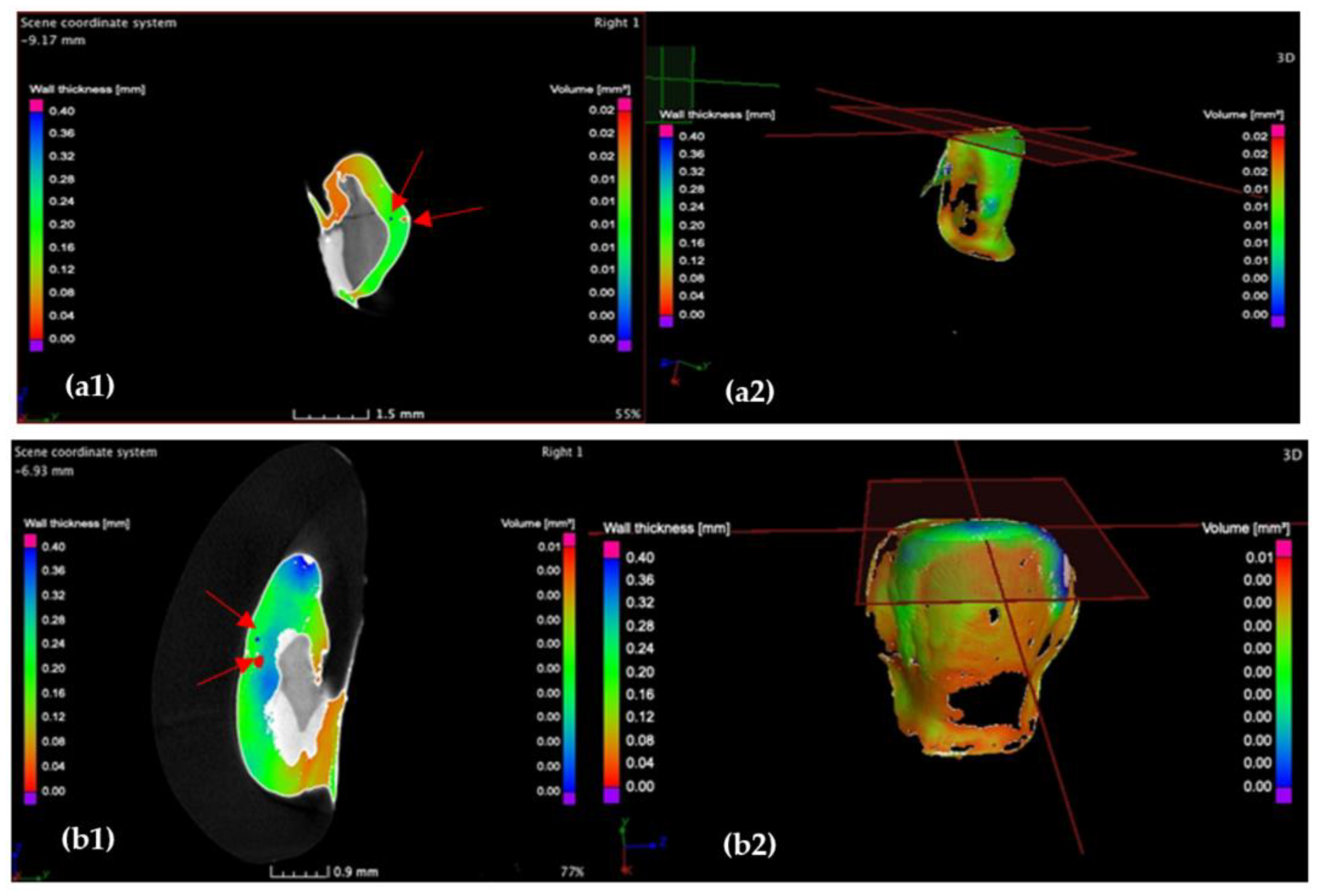
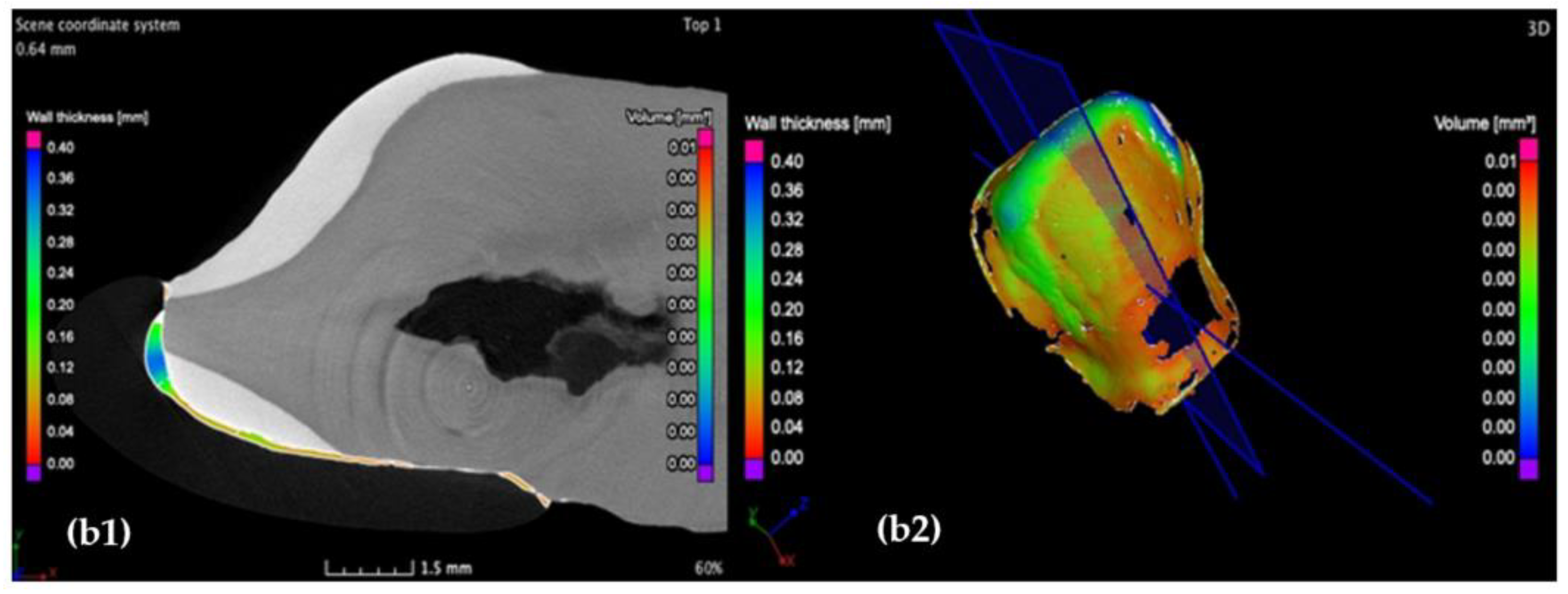
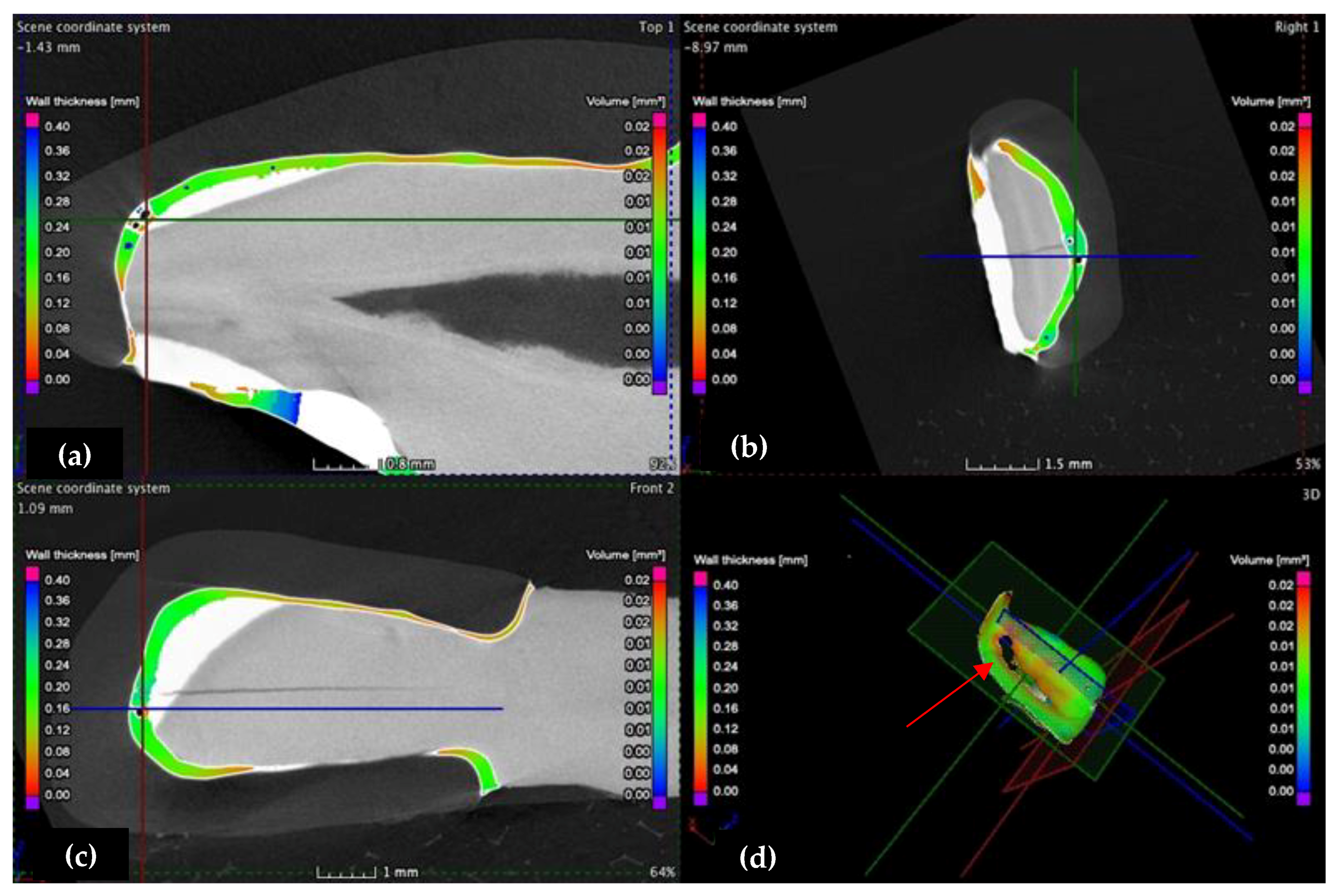
3.2. Micro-CT
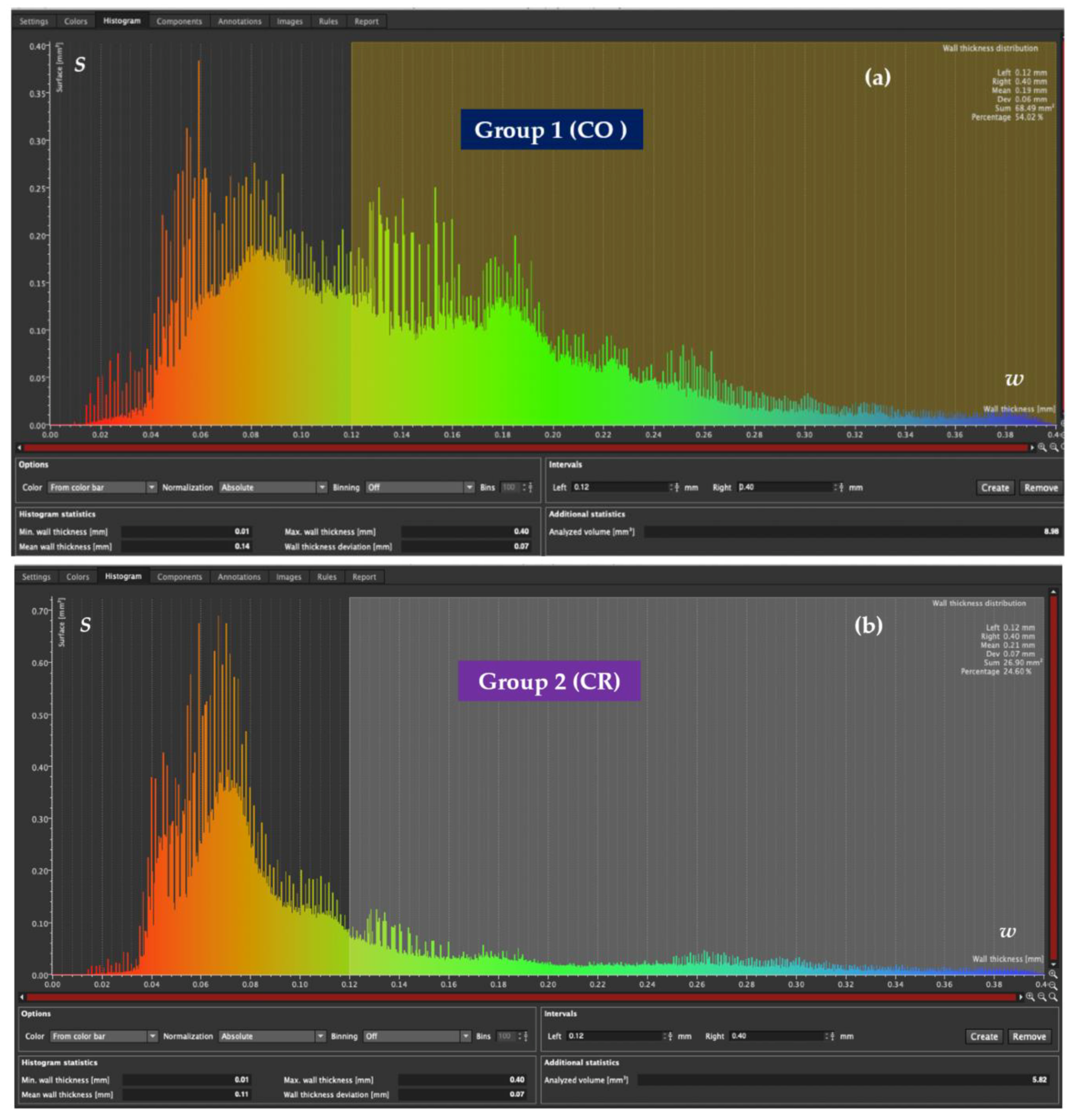
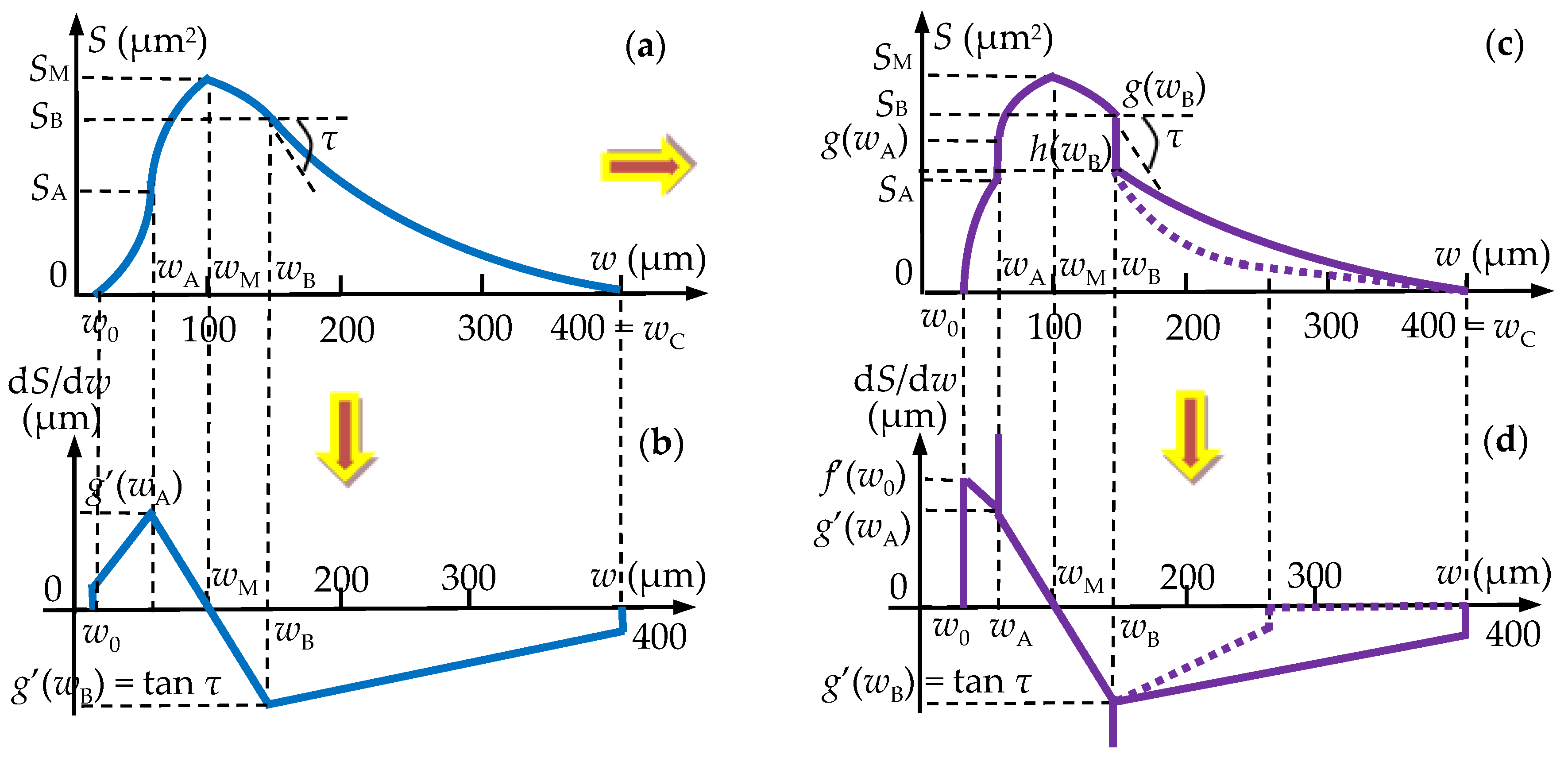
3.3. Mathematical Modeling of the Micro-CT Results
3.4. Porosity Results
4. Discussion
5. Conclusions
- (i)
- Crenelated (CR) ceramic dental veneers, with their marginal sinusoidal contour, displayed a higher marginal adaptation (60 μm) than the conventional (CO) (with linear margins) veneers (230 μm).
- (ii)
- The internal adaptation was considerably better for the CR group in comparison with CO, as the clinically accepted cement thickness/width of up to 120 μm covered 81.5% of the tooth surface for CR compared to 64.5% for CO. Such a thinner and more uniform layer of luting cement creates the premises for better protection of restorations from cement shrinkage, microleakage, tensile forces beneath the veneers and, eventually, debonding or fracture-aspects to be investigated in future work.
- (iii)
- The homogeneity of the luting cement was similar for CR and CO veneers.
- (iv)
- Micro-CT proved to be reliable and precise to evaluate the internal adaptation of the restorations, as well as the porosities localized in the luting cement. It has the advantage of providing 3D information on the entire volume of dental cement (i.e., the interface between dental support and veneers). The more common and accessible tool, optical microscopy, provided valuable data regarding the marginal adaptation, with a good agreement with the micro-CT results, but cannot evaluate internal adaptation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Areas S (μm2) of the Luting Cement Surfaces as Functions of Their Widths w (μm)
c = w0 [τA·wA − SA·(2wA − w0)/(wA − w0)]/(wA − w0)
r = wC · [τ· wB + SB · (wC − 2wB)/(wB − wC)]/(wB − wC).
r = wP · [τ· wB + (SB − SP) · (wP − 2wB)/(wB − wP)]/(wB − wP).
References
- Mitchell, D.; Mitchell, L. Oxford Handbook of Clinical Dentistry, 6th ed.; Oxford University Press: Oxford, UK, 2014; pp. 42–54. [Google Scholar]
- Pincus, C.L. Building Mouth Personality. A Paper Presented; California State Dental Association: San Jose, CA, USA, 1937. [Google Scholar]
- Buonocore, M.G. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch. Oral Biol. 1968, 13, 61–67. [Google Scholar] [CrossRef]
- Simonsen, R.J. Tensile Bond Strengths of Etched Porcelain. J. Dent. Res. 1983, 62, 67–78. [Google Scholar]
- Calamia, J.R. Etched Porcelain Laminate Restorations: A 20-year Retrospective—Part 1. AACD Monogr. 2005, 2, 137–145. [Google Scholar]
- Gürel, G. Atlas of Porcelain Laminate Veneers. In The Science and Art of Porcelain Laminate Veneers; Quintessence Publishing Co. Ltd.: Baden-Baden, Germany, 2003; pp. 258–285. [Google Scholar]
- Castelnuovo, J.; Than, A.; Philips, K.; Nicholls, J. Fracture load and mode of failure of ceramic veneers with different preparations. J. Prosthet. Dent. 2010, 83, 171–180. [Google Scholar] [CrossRef]
- Ghiorghe, C.A.; Iovan, G.; Andrian, S. Studies on adhesion of composite resins and glass-ionomer cements in open-sandwich technique. Rev. Chim. 2017, 68, 1890. [Google Scholar] [CrossRef]
- Burke, F.J. Survival Rates for Porcelain Laminate Veneers with Special Reference to the Effect of Preparation in Dentin: A Literature Review. J. Esthet. Restor. Dent. 2012, 24, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Buso, L.; Hilgert, E.; Neisser, M.P.; Bottino, M.A. Marginal fit of electroformed copings before and after the coction of the porcelain. Braz. J. Oral Sci. 2010, 3, 409–413. [Google Scholar]
- Manhart, J.; Chen, H.; Hamm, G.; Hickel, R. Buonocore Memorial Lecture. Review of the clinical survival of direct and indirect restorations in posterior teeth of the permanent dentition. Oper. Dent. 2004, 29, 481–508. [Google Scholar] [PubMed]
- Pera, P.; Gilodi, S.; Bassi, F.; Carossa, S. In vitro marginal adaptation of alumina porcelain ceramic crowns. J. Prosthet. Dent. 1994, 72, 585–590. [Google Scholar] [CrossRef]
- McLean, J.W.; von Fraunhofer, J.A. The estimation of cement film thickness by an in vivo technique. Br. Dent. J. 1971, 131, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Beschnidt, S.M.; Strub, J.R. Evaluation of the marginal accuracy of different all-ceramic crown systems after simulation in the artificial mouth. J. Oral Rehabil. 1999, 26, 582–593. [Google Scholar] [CrossRef]
- Tai-Min, L.; Perng-Ru, L.; Lance, C.R. Fracture resistance and marginal discrepancy of porcelain laminate veneers influenced by preparation design and restorative material in vitro. J. Dent. 2012, 40, 202–209. [Google Scholar]
- Beuer, F.; Schweiger, J.; Edelhoff, D. Digital dentistry: An overview of recent developments for CAD/CAM generated restorations. Br. Dent. J. 2008, 204, 505–511. [Google Scholar] [CrossRef]
- Baig, M.R.; Tan, K.B.; Nicholls, J.I. Evaluation of the marginal fit of a zirconia ceramic computer-aided machined (CAM) crown system. J. Prosthet. Dent. 2010, 104, 216–227. [Google Scholar] [CrossRef]
- Pak, H.S.; Han, J.S.; Lee, J.B. Influence of porcelain veneering on the marginal fit of Digident and Lava CAD/CAM zirconia ceramic crowns. J. Adv. Prosthodont. 2010, 2, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azar, B.; Eckert, S. The marginal fit of lithium disilicate crowns: Press vs. CAD/CAM. Braz. Oral Res. 2018, 32, 46–67. [Google Scholar] [CrossRef] [Green Version]
- Papadiochou, S.; Pissiotis, A. Marginal adaptation and CAD-CAM technology: A systematic review of restorative material and fabrication techniques. J. Prosthet. Dent. 2018, 115, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Van Meerbeek, B.; Lambrechts, P.; Vanherle, G. Porcelain veneers: A review of the literature. J. Dent. 2010, 28, 163–177. [Google Scholar] [CrossRef]
- May, L.G.; Kelly, J.R.; Bottino, M.A.; Hill, T. Effects of cement thickness and bonding on the failure loads of CAD/CAM ceramic crowns: Multi-physics FEA modeling and monotonic testing. Dent. Mater. 2012, 28, 99–109. [Google Scholar] [CrossRef]
- Abdel-Azim, T.; Rogers, K.; Elathamna, E.; Zandinejad, A.; Metz, M.; Morton, D. Comparison of the marginal fit of lithium disilicate crowns fabricated with CAD/CAM technology by using conventional impressions and two intraoral digital scanners. J. Prosthet. Dent. 2015, 114, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Tidehag, P.; Ottosson, K.; Sjögren, G. Accuracy of ceramic restorations made using an in-office optical scanning technique: An in vitro study. Oper. Dent. 2014, 39, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Arezoobakhsh, A. Comparison of marginal and internal fit of 3-unit zirconia frameworks fabricated with CAD-CAM technology using direct and indirect digital scans. J. Prosthet. Dent. 2020, 123, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroiu, A.C.; Sinescu, C.; Rominu, M.; Negrutiu, M.L.; Rusu, L.C.; Levai, C.M. Crenelated Dental Facets. Romanian Patent RO 131840 B1, A 2017 00038, 30 December 2020. [Google Scholar]
- Maroiu, A.C.; Sinescu, C.; Romînu, M.; Negrutiu, M.L. Evaluation of the adhesion of a novel design of veneers to dental materials. Rev. Chim. 2017, 68, 2125–2128. [Google Scholar] [CrossRef]
- Zaharia, C.; Duma, V.-F.; Sinescu, C.; Socoliuc, V.; Craciunescu, I.; Turcu, R.P.; Marin, C.N.; Tudor, A.; Rominu, M.; Negrutiu, M.-L. Dental adhesive interfaces reinforced with magnetic nanoparticles: Evaluation and modeling with micro-CT versus optical microscopy. Materials 2020, 13, 3908. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, R.; Conti, A.; Sinjari, B.; Spadone, S.; Pecci, R.; Palombo, M.; Komlev, V.S.; Ortore, M.G.; Tromba, G.; Capuani, S.; et al. Multimodal-3D imaging based on μMRI and μCT techniques bridges the gap with histology in visualization of the bone regeneration process. J. Tissue Eng. Regen. Med. 2018, 12, 750–761. [Google Scholar] [CrossRef]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef] [Green Version]
- Luca, R.; Todea, C.D.; Duma, V.-F.; Bradu, A.; Podoleanu, A. Quantitative assessment of rat bone regeneration using complex master–slave optical coherence tomography. Quant. Imaging Med. Surg. 2019, 9, 782–798. [Google Scholar] [CrossRef]
- Schlenz, M.A.; Vogler, J.; Schmidt, A.; Rehmann, P.; Wöstmann, B. New Intraoral Scanner-Based Chairside Measurement Method to Investigate the Internal Fit of Crowns: A Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 2182. [Google Scholar] [CrossRef] [Green Version]
- Magne, P.; Schlichting, L.H.; Maia, H.P.; Baratieri, L.N. In vitro fatigue resistance of CAD/CAM composite resin and ceramic posterior occlusal veneers. J. Prosthet. Dent. 2010, 104, 149–157. [Google Scholar] [CrossRef]
- Drukteinis, S.; Bilvinaite, G.; Tusas, P.; Shemesh, H.; Peciuliene, V. Microcomputed Tomographic Assessment of the Single Cone Root Canal Fillings Performed by Undergraduate Student, Postgraduate Student and Specialist Endodontist. J. Clin. Med. 2021, 10, 1080. [Google Scholar] [CrossRef]
- Duma, V.-F.; Sinescu, C.; Bradu, A.; Podoleanu, A. Optical Coherence Tomography Investigations and Modeling of the Sintering of Ceramic Crowns. Materials 2019, 12, 947. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, T. Marginal adaptation of Spinell InCeram and feldspathic porcelain laminate veneers. Dent. Res. J. 2016, 13, 239–244. [Google Scholar] [CrossRef]
- Beuer, F.; Aggstaller, H.; Edelhoff, D.; Gernet, W.; Sorensen, J. Marginal and internal fits of fixed dental prostheses zirconia retainers. Dent. Mater. 2012, 25, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hasanzade, M.; Shirani, M.; Afrashtehfar, K.I.; Naseri, P.; Alikhasi, M. In Vivo and In Vitro Comparison of Internal and Marginal Fit of Digital and Conventional Impressions for Full-Coverage Fixed Restorations: A Systematic Review and Meta-analysis. J. Evid. Based Dent. Pract. 2019, 19, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Hasanzade, M.; Aminikhah, M.; Afrashtehfar, K.I.; Alikhasi, M. Marginal and internal adaptation of single crowns and fixed dental prostheses by using digital and conventional workflows: A systematic review and meta-analysis. J. Prosthet. Dent. 2020. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, D.P.; Ruse, N.D.; Carvalho, R.M.; Wyatt, C.C. Assessment of the Internal Fit of Lithium Disilicate Crowns Using Micro-CT. J. Prosthodont. 2015, 24, 381–386. [Google Scholar] [CrossRef]
- Mostafa, N.Z.; Ruse, N.D.; Ford, N.L.; Carvalho, R.M.; Wyatt, C.C.L. Marginal Fit of Lithium Disilicate Crowns Fabricated Using Conventional and Digital Methodology: A Three-Dimensional Analysis. J. Prosthodont. 2018, 27, 145–152. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.; Alkhatatbeh, R. A comparison of the marginal and internal fit of porcelain laminate veneers fabricated by pressing and CAD-CAM milling and cemented with 2 different resin cements. J. Prosthet. Dent. 2019, 121, 470–476. [Google Scholar] [CrossRef]
- Yuce, M.; Ulusoy, M. Comparison of Marginal and Internal Adaptation of Heat-Pressed and CAD/CAM Porcelain Laminate Veneers and a 2-Year Follow-Up. J. Prosthet. Dent. 2019, 28, 504–510. [Google Scholar] [CrossRef]
- Schlenz, M.A.; Schmidt, A.; Rehmann, P.; Niem, T.; Wöstmann, B. Microleakage of composite crowns luted on CAD/CAM-milled human molars: A new method for standardized in vitro tests. Clin. Oral Investig. 2019, 23, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.A. A rationale for comparison of plaque-retaining properties of crown systems. J. Prosthetic Dent. 1989, 62, 264–269. [Google Scholar] [CrossRef]
- Sinjari, B.; Santilli, M.; D’Addazio, G.; Rexhepi, I.; Gigante, A.; Caputi, S.; Traini, T. Influence of Dentine Pre-Treatment by Sandblasting with Aluminum Oxide in Adhesive Restorations. An In Vitro Study. Materials 2020, 13, 3026. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M. Internal adaptation, marginal accuracy and microleakage of a pressable versus a machinable ceramic laminate veneers. J. Dent. 2012, 40, 670–677. [Google Scholar] [CrossRef] [PubMed]
- De Jager, N.; Pallav, P.; Feilzer, A.J. The apparent increase of the Young’s modulus in thin cement layer. Dent. Mat. 2004, 20, 457–462. [Google Scholar] [CrossRef]
- Alicia, M.; Jose, C. Porosity of resin cements and resin-modified glass-ionomers. Am. J. Dent. 2001, 14, 17–21. [Google Scholar]
- Drexler, W.; Fujimoto, J.G. (Eds.) Optical Coherence Tomography: Technology and Applications; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [Green Version]
- Erdelyi, R.-A.; Duma, V.-F.; Sinescu, C.; Dobre, G.M.; Bradu, A.; Podoleanu, A. Dental Diagnosis and Treatment Assessments: Between X-rays Radiography and Optical Coherence Tomography. Materials 2020, 13, 4825. [Google Scholar] [CrossRef]
- Erdelyi, R.-A.; Duma, V.-F.; Sinescu, C.; Dobre, G.M.; Bradu, A. Podoleanu, Optimization of X-ray Investigations in Dentistry using Optical Coherence Tomography. Sensors 2021, 21, 4554. [Google Scholar] [CrossRef]
- Demian, D.; Duma, V.-F.; Sinescu, C.; Negrutiu, M.L.; Cernat, R.; Topala, F.I.; Hutiu, G.; Bradu, A.; Podoleanu, A.G. Design and testing of prototype handheld scanning probes for optical coherence tomography. J. Eng. Med. 2014, 228, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Monroy, G.L.; Won, J.; Spillman, D.R.; Dsouza, R.; Boppart, S.A. Clinical translation of handheld optical coherence tomography: Practical considerations and recent advancements. J. Biomed. Opt. 2017, 22, 121715. [Google Scholar] [CrossRef] [Green Version]
- Alenezi, A.; Alsweed, M.; Alsidrani, S.; Chrcanovic, B.R. Long-Term Survival and Complication Rates of Porcelain Laminate Veneers in Clinical Studies: A Systematic Review. J. Clin. Med. 2021, 10, 1074. [Google Scholar] [CrossRef] [PubMed]






| Type of Sample | N | Mean width w (μm) | Standard Deviation (μm) | Standard Error Mean (μm) |
|---|---|---|---|---|
| Conventional (CO) | 12 | 235.9 | 89.38 | 7.45 |
| Crenelated (CR) | 12 | 64.3 | 26.25 | 2.19 |
| Intervals of Cement width w (μm) | Average Covered Surface S (%) | |
|---|---|---|
| Group 1, CO Veneers | Group 2, CR Veneers | |
| 0–120 | 64.55 | 81.56 |
| 120–400 | 35.45 | 18.31 |
| Group | N | Mean ± Standard Deviation (%) | Standard Error Mean (%) | Mean Rank | Sum of Ranks | p |
|---|---|---|---|---|---|---|
| (1) CO veneers | 12 | 64.56 ± 9.728 | 2.808 | 6.92 | 83.00 | <0.001 s |
| (2) CR veneers | 12 | 81.57 ± 4.619 | 1.333 | 18.08 | 217.00 |
| Group | Parameters w (μm); S (103 μm2) | Area S (μm2) of the Surface of Adhesive Layer with a Certain width w (μm) | ||
|---|---|---|---|---|
| w0 < w < wA | wA < w < wB | wB < w < wC = wmax | ||
| 1 (CO) | w0 = 30; S0 = 0 | f(w) = (w − w0){τ(wA − wM)(w − wA)/(wB − wM) − SA [w − (2wA − w0)]/(wA − w0)}/(wA − w0) f(w) = −62.47 · w2 + 62,499 · w − 56,239 | g(w) = SM+ τ· (w − wM)2/[2(wB − wM)] g(w) = −14.43 · w2 + 2.31 · w − 179,815.03 | h(w) = (w − wC){τ(w − wB) − SB · [w + (wC − 2wB)]/(wB − wC)}/(wB − wC) h(w) = 1.78 · w2 + 356.52 · w + 142,299.3 |
| wA = 40; SA = 180 | ||||
| wM = 60; SM = 360 | ||||
| wB = 80; SB = 300 | ||||
| wC = 400 = wmax; S3 = 0 | ||||
| Group | Parameters w (μm); S (103 μm2) | Area S (μm2) of the Surface of Adhesive Layer with a Certain Width w (μm) | |||
|---|---|---|---|---|---|
| w0 < w < wA | wA < w < wB | wB < w < wP | wB < w < wC = wmax | ||
| 2 (CR) | w0 = 10; S0 = 0 | The same expression for f(w) as in Table 4 f(w) = −1999.9 · w2 + 143,987.9 · w − 899,930.7 | The same expression for g(w) as in Table 4 g(w) = −0.043 · w2 + 5.19 · w + 358,441.15 | h(w) = (w − wP){τ(w − wB) − (SB − SP) · [w + (wP − 2wB)]/(wB − wP)}/(wB − wP) h(w) = 53.06 · w2 − 594,687.08 · w + 79,294.92 | j(w) = SP · (−w + wC)/(wC − wP) j(w) = −160 · w + 64,000 |
| wA = 50; SA = 100 | |||||
| wM = 80; SM = 180 | |||||
| wB = 100; SB = 160 | |||||
| wP = 150; SP = 40 | |||||
| wC = 400 = wmax; S3 = 0 | |||||
| Group | N | Mean (106 μm3) | Standard Deviation (106 μm3) | Min. (106 μm3) | Max. (106 μm3) | Mean Rank | Sum of Ranks |
|---|---|---|---|---|---|---|---|
| (1) CO veneers | 12 | 3.42276 | 1.022662 | 2.442 | 4.906 | 12.00 | 144.00 |
| (2) CR veneers | 12 | 3.94780 | 2.833850 | 1.175 | 7.829 | 13.00 | 156.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Măroiu, A.-C.; Sinescu, C.; Duma, V.-F.; Topală, F.; Jivănescu, A.; Popovici, P.M.; Tudor, A.; Romînu, M. Micro-CT and Microscopy Study of Internal and Marginal Gap to Tooth Surface of Crenelated versus Conventional Dental Indirect Veneers. Medicina 2021, 57, 772. https://doi.org/10.3390/medicina57080772
Măroiu A-C, Sinescu C, Duma V-F, Topală F, Jivănescu A, Popovici PM, Tudor A, Romînu M. Micro-CT and Microscopy Study of Internal and Marginal Gap to Tooth Surface of Crenelated versus Conventional Dental Indirect Veneers. Medicina. 2021; 57(8):772. https://doi.org/10.3390/medicina57080772
Chicago/Turabian StyleMăroiu, Alexandra-Cristina, Cosmin Sinescu, Virgil-Florin Duma, Florin Topală, Anca Jivănescu, Paul Mircea Popovici, Anca Tudor, and Mihai Romînu. 2021. "Micro-CT and Microscopy Study of Internal and Marginal Gap to Tooth Surface of Crenelated versus Conventional Dental Indirect Veneers" Medicina 57, no. 8: 772. https://doi.org/10.3390/medicina57080772
APA StyleMăroiu, A.-C., Sinescu, C., Duma, V.-F., Topală, F., Jivănescu, A., Popovici, P. M., Tudor, A., & Romînu, M. (2021). Micro-CT and Microscopy Study of Internal and Marginal Gap to Tooth Surface of Crenelated versus Conventional Dental Indirect Veneers. Medicina, 57(8), 772. https://doi.org/10.3390/medicina57080772








