Abstract
Rhizomes of Cyperus rotundus have been widely used as a traditional medicine in Asia for the treatment of gynecological diseases. However, there is no scientific evidence demonstrating the effect of C. rotundus rhizomes on endometriosis, which is characterized by the adhesion of endometrial tissues outside the uterus, resulting in chronic and severe pelvic pain. The aim of this study was to investigate the effects of Cyperi rhizoma extract (CRE) on cell adhesion and the expression of pain-related factors (neurotrophins) in endometriotic cells, and to elucidate the underlying molecular mechanisms. CRE inhibited the adhesion of human endometriotic 12Z cells to peritoneal mesothelial Met5A cells using by adhesion assays. The mRNA expression of adhesion molecules [P-cadherin and matrix metalloproteinase (MMP)-2] was downregulated by CRE treatment. In addition, CRE significantly inhibited the mRNA expression of neurotrophins (BDNF, NGF, NT-3 and NT-4/5) in 12Z cells. Moreover, Akt overexpression markedly neutralized the inhibition of cell adhesion by CRE and expression of neurotrophins in 12Z cells. Furthermore, it was found that CRE suppressed NF-kB activation through the Akt pathway. These data suggest that CRE exerts anti-endometriotic activities by the inhibition of cell adhesion and neurotrophin expression, through the negative regulation of the Akt and NF-kB pathways in endometriotic cells.
1. Introduction
Endometriosis is a chronic gynecological disease causing chronic pelvic pain, dysmenorrhea, dyspareunia and infertility [1]. It is characterized by the presence and growth of endometrial tissue outside the uterus in the peritoneal cavity. It affects approximately 6–10% of women of reproductive age and 30–50% of women with chronic pelvic pain [2]. Although the exact etiology of endometriosis remains unclear, retrograde menstruation and implantation theories are widely accepted for endometriosis [2,3,4].
The adhesion of retrograde endometrial fragments onto the pelvic mesothelium is a key step in the initiation of endometriosis formation [5]. Several adhesion molecules, including P-cadherin (a predominant cadherin subtype) and matrix metalloproteinase-2 (MMP-2) are upregulated in ectopic endometrial lesions, and have been suggested to be critical factors in regulating the adhesion of ectopic endometrium to the peritoneal mesothelium and/or extracellular matrix (ECM) [6,7]. Recently, an endometriotic implant surrounded by an inflammatory environment has also been reported to be involved in the generation of endometriosis-associated pelvic pain, by releasing pro-nociceptive mediators [8,9]. Emerging studies have demonstrated that neurotrophins, as a family of nerve growth factors, including brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5), are observed in endometriotic lesions, resulting in pelvic pain in endometriosis via neurogenic inflammation [10,11].
Cyperus rotundus Linn. (Cyperaceae), also known as “nut grass” or “purple nut sedge” is a species of sedge native to Asia, southern and central Europe, and Africa, and it grows naturally in tropical, sub-tropical and temperate regions [12]. In Asia, the rhizome of C. rotundus (Cyperi rhizoma, CR) has been traditionally used as a herbal medicine for the treatment of stomach and bowel disorders, bacterial infection and inflammatory diseases [13]. In traditional Chinese medicine, CR is one of the oldest known medicinal plants used for the treatment of dysmenorrhea, a symptom of endometriosis [14]. Modern pharmacological studies have demonstrated that CR has several pharmacological and biological properties, including anti-inflammatory, anti-diabetic, anti-diarrheal, cytoprotective, anti-microbial, anti-bacterial, anti-oxidant, anti-pyretic, anti-analgesic and neuroprotective activities [15,16,17,18,19,20,21,22]. However, there is no scientific evidence demonstrating the effect of CR on endometriosis, a chronic gynecological disease characterized by the growth and adhesion of endometrial tissues outside the uterus, resulting in chronic and severe pelvic pain [23,24]. In this study, we aimed to investigate the effects of Cyperi rhizoma extract (CRE) on endometriosis, and to elucidate the underlying molecular mechanisms.
2. Materials and Methods
2.1. Plant Material and Preparation of the Extract
The rhizomes of Cyperus rotundus L. were obtained from a domestic Korean market (Kyungdong Crude Drugs Market, Seoul, Korea) in June 2011. The origin of the herbal material was identified by one of the authors (D.S. Jang), and a voucher specimen (CYRO1-2011) was deposited in the lab of Natural Product Medicine, College of Pharmacy, Kyung Hee University. The dried and milled plant material (2.8 kg) was extracted with 10 L of 80% ethanol (EtOH) three times by maceration. The extract was combined and concentrated in vacuo at 40 °C to give an 80% EtOH extract (CRE; 399 g). For in vitro experiments, CRE powder was dissolved in dimethyl sulfoxide (DMSO) (v/v) and filtered through a 0.22 μm disk filter.
2.2. Materials
Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium, Medium 199 (M199), Opti-modified Eagle’s medium (Opti-MEM), fetal bovine serum (FBS), penicillin and streptomycin were obtained from Life Technologies Inc. (Grand Island, NY, USA). Phosphate buffered saline (PBS), DMSO, RNase A, leupeptin, aprotinin and phenylmethylsulfonylfluoride (PMSF) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Molecular Probes Inc. (Eugene, OR, USA). Antibodies against anti-phospho-Akt, anti-phospho-IkBα and anti-phospho-IKKα/β were purchased from Cell Signaling Technology (Beverly, MA, USA). The antibodies against anti-Akt, anti-p65, anti-IkBα, anti-IKKα/β, anti-PARP and anti-ß-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). CellTrackerTM was obtained from Invitrogen (Grand Island, NY, USA).
2.3. Cell Culture
Immortalized human endometriotic epithelial cells (12Z) were established from active endometriotic lesions from women with endometriosis. In addition, the cell line displayed the in vivo properties of the active phase of endometriosis as well as the phenotypic characteristics [25]. The endometriotic 12Z cells were generously provided by Dr. Starzinski-Powitz (Johann-Wolfgang-Goethe-Universitaet, Frankfurt, Germany) and maintained in DMEM/F12 medium supplemented with 5% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin (Life Technologies, Grand Island, NY, USA) in a humidified atmosphere of 5% CO2–95% air at 37 °C. The human mesothelial cell line Met5A were originally from the American Type Culture Collection. The cells were cultured in M199 supplemented with 10% FBS, penicillin (100 U/mL) and streptomycin sulfate (100 μg/mL).
2.4. Transfection
The pcDNA3-Akt-Myr (the constitutively active form of Akt) or the empty vector were obtained from Addgene (Cambridge, MA, USA). After cells were cultured to 60–70% confluence, the cells were transfected with plasmid DNA constructs (2 µg/mL) by polyethylenimine (PEI) in serum-free OPTI-MEM (Invitrogen, CA, USA), according to the manufacturer’s instructions. After 24 h, the medium containing the DNA plasmids was replaced with a fresh DMEM/F12 medium with 5% FBS, and the cells were treated with CRE.
2.5. Cell Viability Assay
Cell viability was assessed using an MTT assay. Briefly, human endometriotic cells (12Z) were seeded at a density of 9 × 104 cells/mL in each well containing 50 μL of DMEM/F12 medium in a 96-well plate. After 24 h, various concentrations of CRE were added. After 24 h, 30 μL of MTT (1 mg/mL stock solution) was added, and the plates were incubated for an additional 4 h. The medium was discarded, and the formazan blue, which was formed in the cells, was dissolved in 50 μL DMSO. The optical density was measured at 540 nm using a microplate spectrophotometer (Spectra Max; Molecular Devices, Sunnyvale, CA, USA).
2.6. Adhesion Assay
The mesothelial Met5A cells were grown to confluence on 96-well plates. Endometriotic cells were detached by trypsinization, washed with phosphate-buffered saline (PBS), and probed with 10 µM CellTrackerTM for 45 min at 37 °C. CellTrackerTM-labeled cells were washed with a DMEM/F12 medium containing 0.1% FBS to remove the free dye, and were added (2 × 104 cells/well) to the mesothelial cells. After incubation at 37 °C for the indicated time, the non-adherent cells were removed by gentle washing, the fluorescence in each well was imaged, and the fluorescence was quantified in pixels using Scion Image Software (Scion Corp, Frederick, MD, USA).
2.7. Nuclear Extraction
Cells were washed with PBS, scraped into 1 mL of ice-cold PBS, and pelleted by centrifugation. The cell pellets were resuspended in hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.2 mM PMSF, 0.5 mM DTT, 10 μg/mL aprotinin) and incubated on ice for 15 min. The cells were then lysed by adding 0.1% Nonidet P-40 and vortexed vigorously for 10 s. Nuclei were pelleted by centrifugation at 12,000× g for 1 min at 4 °C and resuspended in a high salt buffer (20 mM HEPES, pH 7.9, 25% glycerol, 400 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 1 mM NaF, 1 mM Sodium orthovanadate).
2.8. Western Blot Analysis
Cells were washed with ice-cold PBS and extracted in a protein lysis buffer (Intron Biotechnology, Seoul, Korea). Protein concentrations were determined by the Bradford assay. The cell lysates were mixed with 5 Χ SDS sample buffer, boiled for 4 min, and then separated on 10% SDS-PAGE. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes. The membranes were blocked in 2% bovine serum albumin (BSA) for 30 min, washed, and incubated overnight at 4 °C with specific primary antibodies in Tris-buffered saline 2% BSA and 0.1% Tween-20 (TBS-T). The membranes were washed three times to remove the primary antibodies and incubated for 2 h with a horseradish peroxidase-conjugated secondary antibody (1:1000–2000). After washing three times with TBS-T, immuno-positive bands were visualized using the ECL chemiluminescent system (Amersham Pharmacia Biotech, ON, Canada) and analyzed using ImageQuant Las-4000 (GE Healthcare Life Science, WI, USA).
2.9. RNA Isolation and Real-Time RT–PCR Analysis
Total RNA was extracted from cells using the Easy Blue® kits (Intron Biotechnology, Seoul, Korea), according to the manufacturer’s instructions. The total RNA was reverse transcribed into first-strand cDNA (Amersham Pharmacia Biotech, ON, Canada) following the manufacturer’s procedure. The synthesized cDNA was used as a template for polymerase chain reaction (PCR) amplification. Real-time PCR was performed using the Thermal cycler dice real-time PCR system (Takara, Japan). The primers used for SYBR Green real-time RT-PCR were purchased from Bioneer technology (Daejon, Korea) and are listed in Table S1. A dissociation curve analysis of P-cadherin, MMP-2, BDNF, NGF, NT-3, NT-4/5, and GAPDH showed a single peak. PCRs were carried out for 45 cycles using the following conditions: denaturation at 95 °C for 5 s, annealing at 57 °C for 10 s, and elongation at 72 °C for 20 s. The results for P-cadherin, MMP-2, BDNF, NGF, NT-3 and NT-4/5 mRNA were normalized to a control gene GAPDH.
2.10. Statistical Analysis
All the data were expressed as mean ± SD. The data obtained in experiments with multiple treatments were subjected to one-way ANOVA analysis of variance followed by a post hoc Tukey test of significance. Under all circumstances, p < 0.05 was considered to be significant.
3. Results
3.1. Cyperi Rhizoma Extract Inhibits Endometriotic Cell Adhesion to Mesothelial Cells
Since the implantation of endometrial fragments at the peritoneum, which is covered by a mesothelial cell layer, is a key step in the establishment of endometriosis [24,26], we investigated the effect of CRE on the adhesion of endometriotic cells to mesothelial cells. As shown in Figure 1A, pre-treatment of human endometriotic 12Z cells with CRE (25, 50, and 100 µg/mL) significantly reduced the adhesion of 12Z cells to human peritoneal mesothelial Met5A cells. The inhibitory effect of CRE on the adhesion of 12Z cells to Met5A cells was not attributed to cytotoxic effects when the endometriotic cells were exposed to CRE up to 100 µg/mL for 24 h (Figure 1B). Furthermore, we investigated the expression of P-cadherin and MMP-2, which have been well known as potent determinants in peritoneal adhesion formation [5], after treatment with CRE in endometriotic 12Z cells. The results of real-time RT-PCR showed that CRE at concentrations of 50 and 100 µg/mL significantly decreased the mRNA expression of P-cadherin and MMP-2 in 12Z cells (Figure 1C).
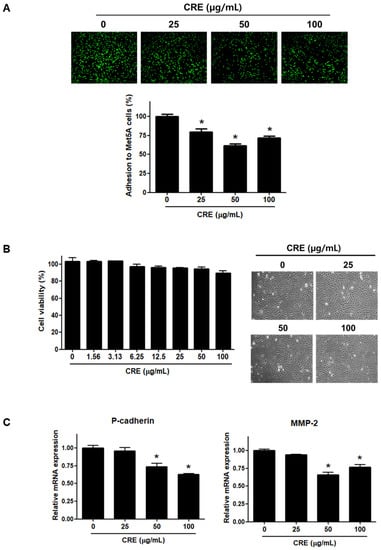
Figure 1.
Effect of CRE on endometriotic cell adhesion to mesothelial cells and expression of adhesion molecules in endometriotic cells. (A) Human endometriotic cells (12Z) were treated with CRE at indicated concentration (25, 50 and 100 µg/mL) for 24 h. The 12Z cells labeled with CellTrackerTM (10 µM) were cultured on Met5A cell layers in a 96-well plate for 1 h. The total fluorescence in each well was measured by fluorescence microphotography. (B) Cell viability was determined by MTT assay. Representative images show the cell morphology of 12Z cells treated with CRE at indicated concentration (25, 50 and 100 µg/mL) for 24 h. (C) The mRNA expression of P-cadherin and MMP-2 was measured by real-time RT-PCR. All expression levels were normalized to GAPDH. Results are the combined data (mean ± SD) from three independent experiments. * p < 0.05 compared with control group.
3.2. Cyperi Rhizoma Extract Decreases the Expression of Neurotrophins in Human Endometriotic Cells
Neurotrophins, such as BDNF, NGF, NT-3 and NT-4/5, are overexpressed in endometriosis and are involved in the pathophysiology of pain generation in women with endometriosis [27]. In this regard, we investigated whether CRE has an inhibitory effect on the expression of neurotrophins in human endometriotic 12Z cells. When 12Z cells were treated with CRE, the mRNA expression of BDNF, NGF, NT-3 and NT-4/5 was inhibited in a dose-dependent manner (Figure 2). BDNF and NT-3 expression was markedly decreased by CRE at concentrations of 25, 50 and 100 µg/mL. NGF expression was slightly reduced by CRE stimulation at concentrations of 25, 50 and 100 µg/mL. NT-4/5 expression was significantly inhibited only at the highest concentration tested (100 µg/mL). Consequently, the highest dose (100 µg/mL) of CRE significantly inhibited the expression of all four tested neurotrophins in this study in endometriotic 12Z cells.
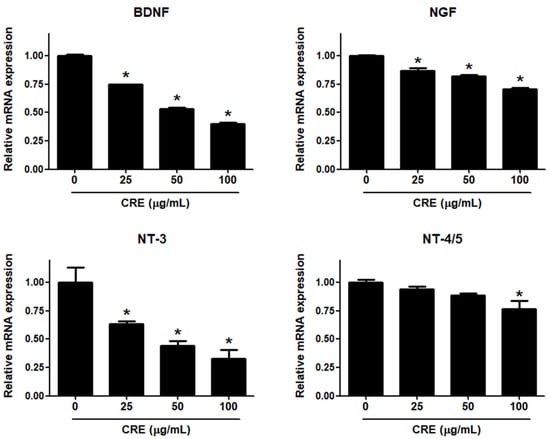
Figure 2.
Effect of CRE on the expression of neurotrophins in endometriotic cells. The 12Z cells were treated with CRE (25, 50 and 100 µg/mL) for 24 h, and then the mRNA expression of neurotrophins (BDNF, NGF, NT-3 and NT-4/5) was measured by real-time RT-PCR. All expression levels were normalized to GAPDH. Results are the combined data (mean ± S.D.) from three independent experiments. * p < 0.05 compared with control group.
3.3. The Akt Pathway Is Involved in Cyperi Rhizoma Extract-Induced Anti-Endometriotic Effects in Human Endometriotic Cells
The Akt pathway is a critical regulator of cell adhesion and migration through the regulation of actin organization and extracellular degradation [28]. Western blot analysis demonstrated that CRE significantly decreased Akt phosphorylation in 12Z cells at 50 and 100 µg/mL. In addition, CRE treatment inhibited the Akt activation in a time-dependent manner (Figure 3). To determine the involvement of the Akt pathway in the CRE-induced anti-endometriotic effect, we performed a cell adhesion assay and real-time RT-PCR analysis after transfection with a constitutively active form of Akt (Akt-Myr) in 12Z cells. As shown in Figure 4A,B, the overexpression of Akt partially reversed CRE-inhibited adhesion of 12Z cells to Met5A cells as well as the expression of P-cadherin and MMP-2 in 12Z cells, which was downregulated by CRE. In addition, the downregulation of neurotrophins by CRE was significantly attenuated by Akt overexpression in 12Z cells (Figure 5). These results suggested that CRE exerts anti-endometriotic effects through the downregulation of Akt activation in endometriotic cells.
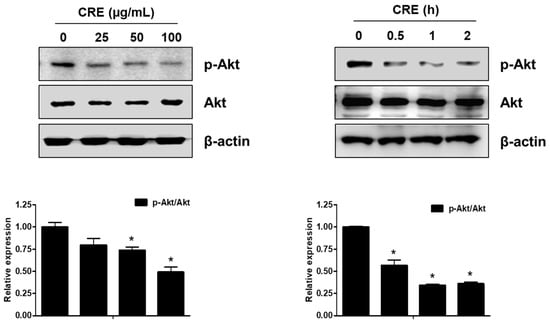
Figure 3.
Effect of CRE on the phosphorylation of Akt in endometriotic cells. 12Z cells were treated with CRE (25, 50 and 100 µg/mL) for 2 h or with CRE (100 µg/mL) for 0.5, 1 and 2 h. Phosphorylated Akt and total Akt were measured by western blot analysis using specific antibodies. β-Actin was used as an internal control. Data are shown as mean band density. Data are presented as the means ± SD of three independent experiments; * p < 0.05 as compared with the control group.
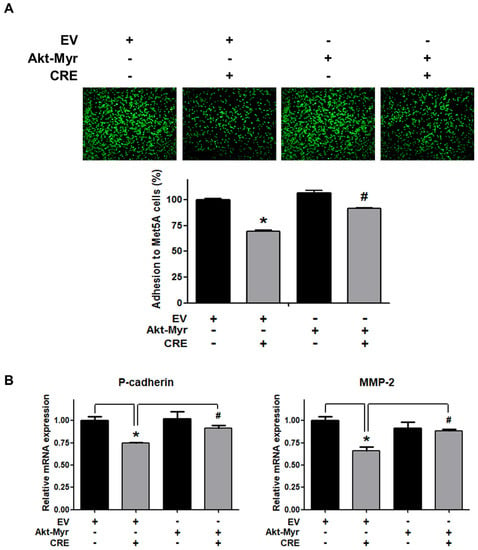
Figure 4.
Involvement of the Akt pathway in endometriotic cell adhesion to mesothelial cells and expression of adhesion molecules in endometriotic cells. 12Z cells were transfected with an empty vector (EV) or a constitutive active form (Akt-Myr), and then the cells were treated with CRE (100 µg/mL). After 24 h, (A) an adhesion assay and (B) a real-time RT-PCR were performed. The mRNA expression of P-cadherin and MMP-2 was normalized to GAPDH. * p < 0.05 compared with EV-transfected none treatment group and # p < 0.05 compared with EV-transfected CRE treatment group.
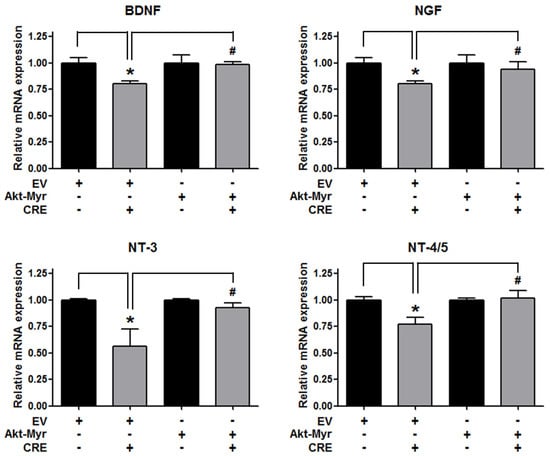
Figure 5.
Involvement of the Akt pathway in the expression of neurotrophins in endometriotic cells. The 12Z cells were transfected with an empty vector (EV) or a constitutive active form (Akt-Myr), and then the cells were treated with CRE (100 µg/mL). After 24 h, real-time RT-PCR was performed to measure the mRNA expression of BDNF, NGF, NT-3 and NT-4/5. The expression levels were normalized to GAPDH. * p < 0.05 compared with EV-transfected none treatment group and # p < 0.05 compared with EV-transfected CRE treatment group.
3.4. Cyperi Rhizoma Extract Inhibits NF-kB Activation through the Akt Pathway
Since NF-kB activation is implicated in the pathogenesis of endometriosis and an endometriosis-associated pro-inflammatory environment [29], we examined the effect of CRE on NF-kB activation in endometriotic 12Z cells. As shown in Figure 6A, CRE markedly reduced the nuclear translocation of p65 NF-kB in 12Z cells. In addition, the phosphorylation of both IkBα and IKKα/β, a key step in the modulation of NF-kB activation, was inhibited by CRE treatment in 12Z cells. To examine the involvement of Akt signaling in CRE-induced NF-kB inactivation in endometriotic cells further, we performed western blot analysis after transfection with Akt-Myr in 12Z cells. As shown in Figure 6B, NF-kB inactivation by CRE was reversed by Akt overexpression. In addition, Akt overexpression could recover the decrease in phosphorylation of IkBα and IKKα/β in the presence of CRE in 12Z cells. These results suggested that NF-kB inactivation by CRE is mediated by the Akt pathway in endometriotic 12Z cells.
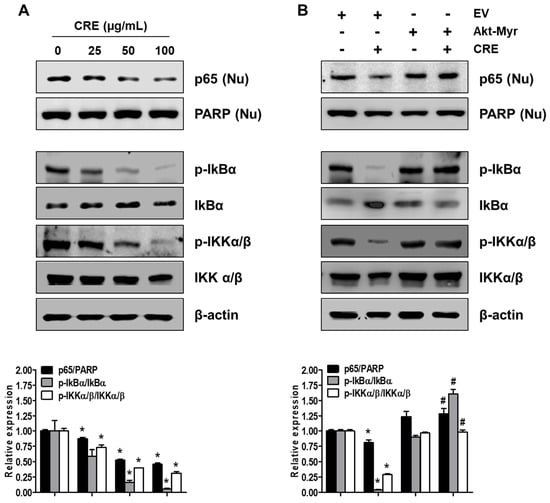
Figure 6.
Effect of CRE on the NF-kB activation in endometriotic cells. Nuclear (Nu) and whole cellular proteins were isolated from the cells (A) treated with CRE (25, 50 and 100 µg/mL) for 8 h and (B) transfected with EV or Akt-Myr. The levels of p65 (Nu), p-IkBα, IkBα, p-IKKα/β and IKKα/β were detected by western blot analysis using specific antibodies. PARP and β-actin were used as an internal control for nuclear fractions and whole cell lysates, respectively. Data are shown as mean band density. Data are presented as the means ± SD of three independent experiments; * p < 0.05 as compared with the control group, and # p < 0.05 as compared with EV-transfected CRE treatment group.
4. Discussion
The rhizomes of C. rotundus have been traditionally considered one of the most important herbs for the treatment of obstetric/gynecologic disorders in Asia. In Vietnam and Taiwan, the decoction of C. rotundus is used for treating infertility and dysmenorrhea in women with endometriosis [14,30]. For example, Qi Gong Wan composed of various herbs including Cyperi rhizoma relieves mid-cycle pain, vaginal discharge and adhesions [14]. Although numerous experiences have been reported to show the effect of C. rotundus on gynecologic disorders [14,31], there is little scientific evidence of the effect of C. rotundus on gynecological diseases and the underlying molecular mechanisms of action. Choi et al. examined the effect of water-extracted tubers of C. rotundus on endometrial receptivity both in vitro and in vivo [32]. It was found that the water-extracted tubers of C. rotundus enhanced the adhesion of trophoblastic JAr cells to endometrial cancer Ishikawa cells for blastocyst implantation and improved the number of implantation sites in a mifepristone-induced implantation failure mice model. In a recent retrospective cohort study using the Taiwan National Health Insurance reimbursement database, the rate of endometriosis-related surgery, including hysterectomy and oophorectomy, was significantly lower in patients with endometriosis who took the traditional Chinese medicine formula Gui-zhi-fu-ling-wan, which mainly comprises C. rotundus, than in patients with endometriosis who did not take it [33]. Kim et al. has also demonstrated that Boheotang containing Cyperi rhizoma has an inhibitory effect on recurrent endometriosis after laparoscopic excision and hormone therapy [34]. However, to the best of our knowledge, there is no direct experimental evidence supporting the effect of C. rotundus on endometriosis. In this study, we investigated the effect of CRE on endometriosis using human endometriotic 12Z cells for the first time. The adhesive ability of endometriotic cells has been well observed in endometriotic lesion formation [26]. In this study, we demonstrated that CRE significantly inhibited the adhesion of endometriotic cells to peritoneal mesothelial cells, without cytotoxicity.
Previous phytochemical reports revealed the presence of flavonoids, tannins, alkaloids, glycosides, furochromones and sesquiterpenes in C. rotundus [35,36]. In fact, most of the biological activities of the rhizomes of C. rotundus have been attributed to the sesquiterpenes [36]. It is reported that isocyperol and α-cyperone, known as major sesquiterpene components of the rhizomes of C. rotundus, have various pharmacological activities, such as anti-inflammation, antivirulence, antigenotoxic, antibacterial and anti-depressant effects [37,38,39,40,41,42]. For example, isocyperol and α-cyperone have an inhibitory effect on inflammation through suppression of the NF-κB pathway [37,38]. Zhang et al. showed the inhibitory effects of α-cyperone on adhesion and invasion of avian pathogenic Escherichia coli O78 to chicken type Ⅱ pneumocytes [43]. However, to the best of our knowledge, there is no evidence supporting the effect of active constituents of CRE in gynecologic disorders including endometriosis. In a previous study, we found that α-cyperone is the most abundant and active component of CRE [44,45,46,47]. In these regards, we have examined the anti-endometriotic effect of α-cyperone in human endometriotic 12Z cells. Similar to CRE, α-cyperone significantly inhibited the adhesive ability of 12Z cells to mesothelial Met5A cells (Figure S1). This observation suggests a possibility that the anti-endometriotic effects of CRE are mainly attributed to its major component, α-cyperone. However, further experiments are still needed to draw this conclusion.
Cell surface proteins, including cadherin and MMPs, are expressed in the ectopic endometrium and implicated in the regulation of cell-cell adhesion as well as cell-ECM adhesion during the development of endometriosis [5]. Unlike E-cadherin and N-cadherin, P-cadherin is found to be remarkably increased in peritoneal endometriotic lesions and directly involved in the interaction between endometrial cells and peritoneal cells [6]. MMP-2 is a collagenase-degrading collagen IV, the principal component of basement membranes, and is significantly elevated in the urine of patients with endometriosis, compared to healthy women [48]. A recent study demonstrated that physiological changes, including adhesion, in endometriosis involve abnormal matrix remodeling, which is affected by proteolytic enzymes, such as MMP-2 [49]. In addition, MMPs support the irreversible inactivation of cadherin-catenin complexes [50]. In this study, we showed that CRE inhibited the mRNA expression of P-cadherin and MMP-2 in endometriotic 12Z cells. These results suggest that the downregulation of adhesion molecules, such as P-cadherin and MMP-2 by CRE, may contribute to the inhibitory effect of CRE on the adhesion of endometriotic cells to peritoneal mesothelial cells.
Pelvic pain is one of the major symptoms in women with endometriosis, and is associated with the activation of nociceptive neurons [51]. It has been suggested that the neurotrophins such as BDNF, NGF, NT-3 and NT-4/5 generate pelvic pain in endometriosis by the direct alteration of neurological responsiveness, leading to hyperalgesia and allodynia stimuli [1]. A recent study demonstrated that the level of BDNF in circulation is found to be elevated in women with endometriosis compared to healthy controls, and it decreased after the surgical removal of lesions [10]. In addition, it has been reported in a recent study that specific antibodies against neurotrophins, including NGF, may be used to treat and prevent pain and symptoms associated with endometriosis [52]. In the present study, we elucidated the inhibitory effect of CRE on the expression of BDNF, NGF, NT-3 and NT-4/5 in endometriotic cells. It was found that CRE significantly decreased the mRNA expression of BDNF, NGF, NT-3 and NT-4/5 in 12Z cells. Taken together, CRE may have an inhibitory effect on endometriosis-associated pain by modulating the expression of neurotrophins, which is elevated in women with endometriosis.
The PI3K/Akt pathway is well known to regulate various cellular events, such as cell growth, proliferation, apoptosis and differentiation stimulated by intra- and extracellular signals [53]. Recently, the role of PI3K/Akt signaling in endometriosis has been implicated. Yin et al. demonstrated that the over activation of the PI3K/Akt signaling pathway contributes to the decrease in decidualization of stromal cells from endometriosis [54]. In addition, rapid activation of PI3K/Akt mediated the growth factor- and estrogen-induced migration of endometrial stromal cells [55]. Moreover, it has been suggested that the activation of Akt signaling is involved in the expression of P-cadherin and MMP-2 [56,57]. In the present study, we found that the inhibitory effect of CRE on endometriotic cell adhesion and neurotrophin expression in endometriotic cells is mediated by the Akt pathway. Notably, it has been demonstrated that the activation of Akt affects the phosphorylation of IKKβ and the translocation of the NF-kB p65 subunit [58,59]. Many in vitro and in vivo evidences show that the constitutive activation of NF-kB in endometriotic lesions from patients with endometriosis promotes inflammation, cell proliferation, adhesion invasion and angiogenesis in an endometriosis-associated pro-inflammatory environment [60,61,62,63]. In these regards, we investigated the involvement of NF-kB activation in the effect of CRE on cell adhesion and neurotrophin expression in endometriotic cells through the Akt pathway. The results suggest that NF-kB signaling is likely to mediate the anti-endometriotic effect of CRE through the Akt pathway.
5. Conclusions
In summary, we demonstrated the inhibitory effect of CRE on endometriosis via the inhibition of cell adhesion and neurotrophin expression through negative regulation of Akt and NF-kB pathways in human endometriotic cells.
These results provide scientific evidence that CRE could be a candidate for multidisciplinary therapy, demonstrating a reduction of the adhesion of endometriotic fragments and pain relief in patients with endometriosis. The in vivo effect of CRE on endometriosis should be further investigated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina58030335/s1, Figure S1: Effect of α-cyperone on endometriotic cell adhesion to mesothelial cells, Table S1: Primer sequences for real-time RT-PCR analysis, Supplementary Material and Methods: Preparation of the α-cyperone.
Author Contributions
Conceptualization, J.-H.A. and J.-H.C.; formal analysis, J.-M.C., E.-S.K., J.-H.Y., Y.-J.C.; resources, D.S.J.; writing—original draft preparation, J.-H.A.; writing—review and editing, J.-H.A. and J.-H.C.; supervision, J.-H.A. and J.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the National Research Foundation of Korea(NRF) grant funded by the Korean government (MSIT) (NRF-2020R1I1A3073660).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have declared no conflict of interest.
References
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Czyzyk, A.; Podfigurna, A.; Szeliga, A.; Meczekalski, B. Update on endometriosis pathogenesis. Minerva Ginecol. 2017, 69, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrao, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef] [Green Version]
- Young, V.J.; Brown, J.K.; Saunders, P.T.; Horne, A.W. The role of the peritoneum in the pathogenesis of endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef]
- Chen, G.T.; Tai, C.T.; Yeh, L.S.; Yang, T.C.; Tsai, H.D. Identification of the cadherin subtypes present in the human peritoneum and endometriotic lesions: Potential role for P-cadherin in the development of endometriosis. Mol. Reprod. Dev. 2002, 62, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yang, Y.I.; Ahn, J.H.; Lee, J.G.; Lee, K.T.; Choi, J.H. Deer (Cervus elaphus) antler extract suppresses adhesion and migration of endometriotic cells and regulates MMP-2 and MMP-9 expression. J. Ethnopharmacol. 2012, 140, 391–397. [Google Scholar] [CrossRef]
- Morotti, M.; Vincent, K.; Becker, C.M. Mechanisms of pain in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 8–13. [Google Scholar] [CrossRef]
- Pezet, S.; McMahon, S.B. Neurotrophins: Mediators and modulators of pain. Annu. Rev. Neurosci. 2006, 29, 507–538. [Google Scholar] [CrossRef]
- Wessels, J.M.; Kay, V.R.; Leyland, N.A.; Agarwal, S.K.; Foster, W.G. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil. Steril. 2016, 105, 119–128.e5. [Google Scholar] [CrossRef] [Green Version]
- Streiter, S.; Fisch, B.; Sabbah, B.; Ao, A.; Abir, R. The importance of neuronal growth factors in the ovary. Mol. Hum. Reprod. 2016, 22, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, S.J.; Mondal, K.; Shilpi, J.A.; Rahman, M.T. Antidiarrhoeal activity of Cyperus rotundus. Fitoterapia 2006, 77, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Dang, G.K.; Parekar, R.R.; Kamat, S.K.; Scindia, A.M.; Rege, N.N. Antiinflammatory activity of Phyllanthus emblica, Plumbago zeylanica and Cyperus rotundus in acute models of inflammation. Phytother. Res. 2011, 25, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qu, F. Treating gynaecological disorders with traditional Chinese medicine: A review. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 494–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.B.; Palit, T.K.; Singh, N.; Bhargava, K.P. Pharmacological studies to isolate the active constituents from Cyperus rotundus possessing anti-inflammatory, anti-pyretic and analgesic activities. Indian J. Med. Res. 1971, 59, 76–82. [Google Scholar]
- Singh, P.; Khosa, R.L.; Mishra, G.; Jha, K.K. Antidiabetic activity of ethanolic extract of Cyperus rotundus rhizomes in streptozotocin-induced diabetic mice. J. Pharm. Bioallied Sci. 2015, 7, 289–292. [Google Scholar] [CrossRef]
- Seo, W.G.; Pae, H.O.; Oh, G.S.; Chai, K.Y.; Kwon, T.O.; Yun, Y.G.; Kim, N.Y.; Chung, H.T. Inhibitory effects of methanol extract of Cyperus rotundus rhizomes on nitric oxide and superoxide productions by murine macrophage cell line, RAW 264.7 cells. J. Ethnopharmacol. 2001, 76, 59–64. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, T.W.; Kim, K.H.; Byun, H.; Ryu, B.; Lee, K.T.; Jang, D.S.; Choi, J.H. 6-Acetoxy Cyperene, a Patchoulane-type Sesquiterpene Isolated from Cyperus rotundus Rhizomes Induces Caspase-dependent Apoptosis in Human Ovarian Cancer Cells. Phytother. Res. 2015, 29, 1330–1338. [Google Scholar] [CrossRef]
- Zhu, M.; Luk, H.H.; Fung, H.S.; Luk, C.T. Cytoprotective effects of Cyperus rotundus against ethanol induced gastric ulceration in rats. Phytother. Res. 1997, 11, 392–394. [Google Scholar] [CrossRef]
- Kamala, A.; Middha, S.K.; Gopinath, C.; Sindhura, H.S.; Karigar, C.S. In vitro Antioxidant Potentials of Cyperus rotundus L. Rhizome Extracts and Their Phytochemical Analysis. Pharmacogn. Mag. 2018, 14, 261–267. [Google Scholar] [CrossRef]
- Mannarreddy, P.; Denis, M.; Munireddy, D.; Pandurangan, R.; Thangavelu, K.P.; Venkatesan, K. Cytotoxic effect of Cyperus rotundus rhizome extract on human cancer cell lines. Biomed. Pharmacother. 2017, 95, 1375–1387. [Google Scholar] [CrossRef]
- Jia, H.; Liu, Y.; Yu, M.; Shang, H.; Zhang, H.; Ma, L.; Zhang, T.; Zou, Z. Neuroprotective Effect of Cyperi rhizome against Corticosterone-Induced PC12 Cell Injury via Suppression of Ca2+ Overloading. Metabolites 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and endometriosis. Front. Biosci. 2016, 21, 941–948. [Google Scholar]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am. J. Pathol. 2001, 159, 1839–1852. [Google Scholar] [CrossRef] [Green Version]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamada, Y.; Morioka, S.; Niiro, E.; Shigemitsu, A.; Ito, F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch. Gynecol. Obstet. 2014, 289, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Fabi, F.; Grenier, K.; Parent, S.; Adam, P.; Tardif, L.; Leblanc, V.; Asselin, E. Regulation of the PI3K/Akt pathway during decidualization of endometrial stromal cells. PLoS ONE 2017, 12, e0177387. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramos, R.; Van Langendonckt, A.; Defrere, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.C.; Kao, C.W.; Lin, C.C.; Liao, Y.N.; Wu, B.Y.; Hung, I.L.; Hu, W.L. Chinese Herbal Products for Female Infertility in Taiwan: A Population-Based Cohort Study. Medicine 2016, 95, e3075. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.K. Folk herbal remedies for diarrhoea and dysentery in central Nepal. Fitoterapia 1993, 64, 243–250. [Google Scholar] [CrossRef]
- Choi, H.J.; Chung, T.W.; Park, M.J.; Jung, Y.S.; Lee, S.O.; Kim, K.J.; Ha, K.T. Water-extracted tubers of Cyperus rotundus L. enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin αVβ3 and αVβ5. J. Ethnopharmacol. 2017, 208, 16–23. [Google Scholar] [CrossRef]
- Su, S.Y.; Muo, C.H.; Sung, F.C.; Morisky, D.E. Reduction of surgery rate in endometriosis patients who take Chinese medicine: A population-based retrospective cohort study. Complement. Ther. Med. 2014, 22, 632–639. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoo, J.E. Inhibitory effect of traditional Korean medicine on the recurrent endometriosis after laparoscopic excision: A case report. Integr. Med. Res. 2018, 7, 296–301. [Google Scholar] [CrossRef]
- Xu, X.T.; Deng, Z.P.; Zhong, H.; Yao, Q.Q. Research progress on chemical constituents and pharmacological activities of Cyperus rotundus L. Qilu Pharm. Aff. 2012, 31, 473–475. [Google Scholar]
- Gamal, M.A.; Hani, K.M.K.; Sabrin, I.R.M. A Review: Compounds Isolated from Cyperus Species (Part II):Terpenoidal. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 83–99. [Google Scholar]
- Seo, Y.J.; Jeong, M.; Lee, K.T.; Jang, D.S.; Choi, J.H. Isocyperol, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced inflammatory responses via suppression of the NF-kappaB and STAT3 pathways and ROS stress in LPS-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2016, 38, 61–69. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, S.J.; Jun, B.G.; Lee, K.T.; Hong, S.P.; Oh, M.S.; Jang, D.S.; Choi, J.H. alpha-Cyperone, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced COX-2 expression and PGE2 production through the negative regulation of NFkappaB signalling in RAW 264.7 cells. J. Ethnopharmacol. 2013, 147, 208–214. [Google Scholar] [CrossRef]
- Luo, M.; Qiu, J.; Zhang, Y.; Wang, J.; Dong, J.; Li, H.; Leng, B.; Zhang, Q.; Dai, X.; Niu, X.; et al. alpha-cyperone alleviates lung cell injury caused by Staphylococcus aureus via attenuation of alpha-hemolysin expression. J. Microbiol. Biotechnol. 2012, 22, 1170–1176. [Google Scholar] [CrossRef]
- Singh, N.; Kulshrestha, V.K.; Gupta, M.B.; Bhargava, K.P. Apharmacological study of Cyperus rotundus. Indian J. Med. Res. 1970, 58, 103–109. [Google Scholar]
- Jin, J.H.; Lee, D.U.; Kim, Y.S.; Kim, H.P. Anti-allergic activity of sesquiterpenes from the rhizomes of Cyperus rotundus. Arch. Pharm. Res. 2011, 34, 223–228. [Google Scholar] [CrossRef]
- Xia, B.; Tong, Y.; Xia, C.; Chen, C.; Shan, X. alpha-Cyperone Confers Antidepressant-Like Effects in Mice via Neuroplasticity Enhancement by SIRT3/ROS Mediated NLRP3 Inflammasome Deactivation. Front. Pharmacol. 2020, 11, 577062. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Lv, S.; Wu, S.C.; Guo, X.; Xia, F.; Hu, X.R.; Song, Z.; Zhang, C.; Qin, Q.Q.; Fu, B.D.; et al. Inhibitory effects of alpha-cyperone on adherence and invasion of avian pathogenic Escherichia coli O78 to chicken type II pneumocytes. Vet. Immunol. Immunopathol. 2014, 159, 50–57. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.J.; Kim, H.J.; Jang, Y.P.; Oh, M.S.; Jang, D.S. New patchoulane-type sesquiterpenes from the rhizomes of Cyperus rotundus. Bull. Korean Chem. Soc. 2012, 33, 3115–3118. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Ryu, B.; Kim, H.-Y.; Yang, Y.-I.; Ham, J.; Choi, J.-H.; Jang, D.S. Sesquiterpenes from the rhizomes of Cyperus rotundus and their potential to inhibit LPS-induced nitric oxide production. Bull. Korean Chem. Soc. 2013, 34, 2207–2210. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.; Kim, H.M.; Lee, J.-S.; Cho, Y.J.; Oh, M.S.; Choi, J.-H.; Jang, D.S. Sesquiterpenes from rhizomes of Cyperus rotundus with cytotoxic activities on human cancer cells in vitro. Helv. Chim. Acta 2015, 98, 1372–1380. [Google Scholar] [CrossRef]
- Sim, Y.; Choi, J.G.; Gu, P.S.; Ryu, B.; Kim, J.H.; Kang, I.; Jang, D.S.; Oh, M.S. Identification of Neuroactive Constituents of the Ethyl Acetate Fraction from Cyperi Rhizoma Using Bioactivity-Guided Fractionation. Biomol. Ther. 2016, 24, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Osteen, K.G.; Yeaman, G.R.; Bruner-Tran, K.L. Matrix metalloproteinases and endometriosis. Semin. Reprod. Med. 2003, 21, 155–164. [Google Scholar] [CrossRef]
- Yang, W.V.; Au, H.K.; Chang, C.W.; Chen, H.W.; Chen, P.H.; Chen, C.C.; Tang, Y.L.; Wang, I.T.; Tzeng, C.R. Matrix remodeling and endometriosis. Reprod. Med. Biol. 2005, 4, 93–99. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Albergaria, A.; Sousa, B.; Correia, A.L.; Bracke, M.; Seruca, R.; Schmitt, F.C.; Paredes, J. Extracellular cleavage and shedding of P-cadherin: A mechanism underlying the invasive behaviour of breast cancer cells. Oncogene 2010, 29, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Howard, F.M. Endometriosis and mechanisms of pelvic pain. J. Minim. Invasive Gynecol. 2009, 16, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Howe, D.C.; Pullen, N.; Shelton, D.L. Treatment of Endometriosis. 2010. Available online: https://patents.google.com/patent/WO2010029497A1/zh (accessed on 1 October 2020).
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Pavone, M.E.; Lu, Z.; Wei, J.; Kim, J.J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 2012, 97, E35–E43. [Google Scholar] [CrossRef] [Green Version]
- Gentilini, D.; Busacca, M.; Di Francesco, S.; Vignali, M.; Vigano, P.; Di Blasio, A.M. PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol. Hum. Reprod. 2007, 13, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Li, C.; He, C.; Ren, B.; Deng, Q.; Gao, W.; Wang, B. Aurora-A modulates MMP-2 expression via AKT/NF-kappaB pathway in esophageal squamous cell carcinoma cells. Acta Biochim. Biophys. Sin. 2016, 48, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.F.; Ribeiro, A.S.; Dionisio, M.R.; Sousa, B.; Nobre, A.R.; Albergaria, A.; Santiago-Gomez, A.; Mendes, N.; Gerhard, R.; Schmitt, F.; et al. P-cadherin signals through the laminin receptor alpha6beta4 integrin to induce stem cell and invasive properties in basal-like breast cancer cells. Oncotarget 2014, 5, 679–692. [Google Scholar] [CrossRef] [Green Version]
- Ozes, O.N.; Mayo, L.D.; Gustin, J.A.; Pfeffer, S.R.; Pfeffer, L.M.; Donner, D.B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 1999, 401, 82–85. [Google Scholar] [CrossRef]
- Sizemore, N.; Leung, S.; Stark, G.R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell. Biol. 1999, 19, 4798–4805. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.G.; Morin, M.; Sengers, V.; Metz, C.; Roger, T.; Maheux, R.; Akoum, A. Tumour necrosis factor-alpha up-regulates macrophage migration inhibitory factor expression in endometrial stromal cells via the nuclear transcription factor NF-kappaB. Hum. Reprod. 2006, 21, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Ramos, R.; Donnez, J.; Defrere, S.; Leclercq, I.; Squifflet, J.; Lousse, J.C.; Van Langendonckt, A. Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol. Hum. Reprod. 2007, 13, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Ramos, R.; Rocco, J.; Rojas, C.; Sovino, H.; Poch, A.; Kohen, P.; Alvarado-Diaz, C.; Devoto, L. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil. Steril. 2012, 97, 645–651. [Google Scholar] [CrossRef]
- Grund, E.M.; Kagan, D.; Tran, C.A.; Zeitvogel, A.; Starzinski-Powitz, A.; Nataraja, S.; Palmer, S.S. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol. Pharmacol. 2008, 73, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).