Biochemical Intracystic Biomarkers in the Differential Diagnosis of Pancreatic Cystic Lesions
Abstract
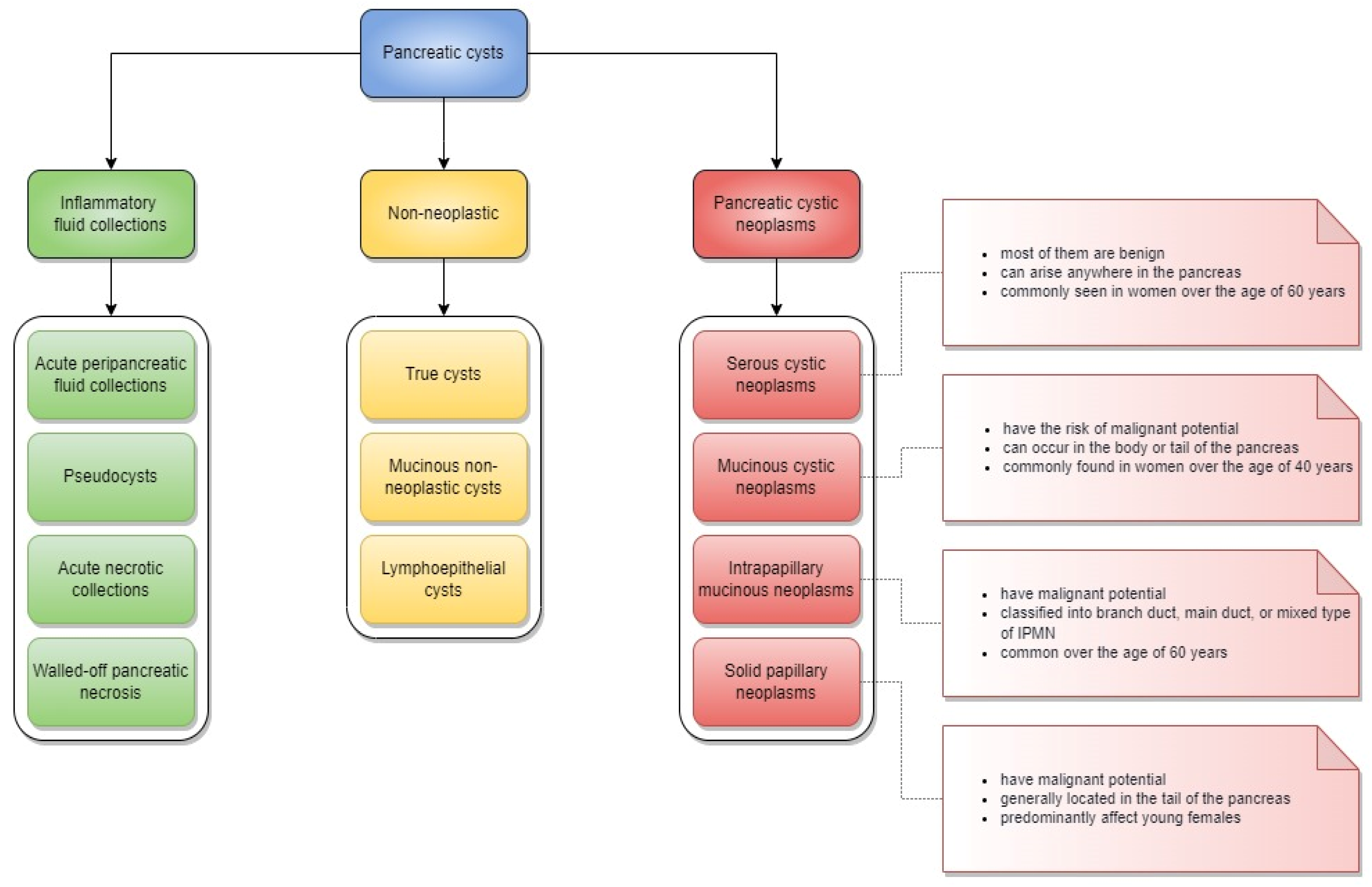
:1. Introduction
2. Materials and Methods
3. Results
3.1. Interpretative Synthesis of Data: Carcinoembryonic Antigen
3.2. Interpretative Synthesis of Data: Glucose
3.3. Interpretative Synthesis of Data: Other Biomarkers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Lanke, G.; Lee, J.H. Similarities and Differences in Guidelines for the Management of Pancreatic Cysts. World J. Gastroenterol. 2020, 26, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Faias, S.; Pereira, L.; Fonseca, R.; Chaves, P.; Dias Pereira, A.; Cravo, M. A Second Endoscopic Ultrasound with Fine-Needle Aspiration for Cytology Identifies High-Risk Pancreatic Cysts Overlooked by Current Guidelines. Diagn. Cytopathol. 2021, 49, 109–118. [Google Scholar] [CrossRef] [PubMed]
- European Study Group on Cystic Tumours of the Pancreas. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Hao, C.; Zhang, G.; Zhang, L. Chapter Eleven—Serum CEA Levels in 49 Different Types of Cancer and Noncancer Diseases. In Glycans and Glycosaminoglycans as Clinical Biomarkers and Therapeutics—Part A; Zhang, L., Ed.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2019; Volume 162, pp. 213–227. [Google Scholar]
- Levy, A.; Popovici, T.; Bories, D.P.-N. Tumor Markers in Pancreatic Cystic Fluids for Diagnosis of Malignant Cysts. Int. J. Biol. Markers 2017, 32, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Lee, J.K.; Lee, K.T.; Lee, K.H.; Woo, Y.S.; Noh, D.H. The Combination of Cyst Fluid Carcinoembryonic Antigen, Cytology and Viscosity Increases the Diagnostic Accuracy of Mucinous Pancreatic Cysts. Gut Liver 2017, 11, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Chahal, P.; Vargo, J.; Sanaka, M.; Jang, S.; Zuccaro, G. Clinical Utility of Intracystic Glucose Levels in Differentiating Mucinous From Non-Mucinous Pancreatic Cysts: 226. Off. J. Am. Coll. Gastroenterol.|ACG 2014, 109, S70. [Google Scholar] [CrossRef]
- Zikos, T.; Pham, K.; Bowen, R.; Chen, A.M.; Banerjee, S.; Friedland, S.; Dua, M.M.; Norton, J.A.; Poultsides, G.A.; Visser, B.C.; et al. Cyst Fluid Glucose Is Rapidly Feasible and Accurate in Diagnosing Mucinous Pancreatic Cysts. Off. J. Am. Coll. Gastroenterol.|ACG 2015, 110, 909–914. [Google Scholar] [CrossRef]
- Simons-Linares, C.R.; Yadav, D.; Lopez, R.; Bhatt, A.; Jang, S.; El-Khider, F.; Sanaka, M.; Stevens, T.; Vargo, J.; Chahal, P. The Utility of Intracystic Glucose Levels in Differentiating Mucinous from Non-Mucinous Pancreatic Cysts. Pancreatology 2020, 20, 1386–1392. [Google Scholar] [CrossRef]
- Yip-Schneider, M.T.; Carr, R.A.; Wu, H.; Schmidt, C.M. Prostaglandin E(2): A Pancreatic Fluid Biomarker of Intraductal Papillary Mucinous Neoplasm Dysplasia. J. Am. Coll. Surg. 2017, 225, 481–487. [Google Scholar] [CrossRef]
- Carr, R.A.; Yip-Schneider, M.T.; Dolejs, S.; Hancock, B.A.; Wu, H.; Radovich, M.; Schmidt, C.M. Pancreatic Cyst Fluid Vascular Endothelial Growth Factor A and Carcinoembryonic Antigen: A Highly Accurate Test for the Diagnosis of Serous Cystic Neoplasm. J. Am. Coll. Surg. 2017, 225, 93–100. [Google Scholar] [CrossRef]
- Ivry, S.L.; Sharib, J.M.; Dominguez, D.A.; Roy, N.; Hatcher, S.E.; Yip-Schneider, M.T.; Schmidt, C.M.; Brand, R.E.; Park, W.G.; Hebrok, M.; et al. Global Protease Activity Profiling Provides Differential Diagnosis of Pancreatic Cysts. Clin. Cancer Res. 2017, 23, 4865–4874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucera, S.; Centeno, B.; Springett, G.; Malafa, M.; Chen, Y.; Weber, J.; Klapman, J. Cyst Fluid Carcinoembryonic Antigen Level Is Not Predictive of Invasive Cancer in Patients with Intraductal Papillary Mucinous Neoplasm of the Pancreas. JOP J. Pancreas 2012, 13, 409–413. [Google Scholar] [CrossRef]
- Talar-Wojnarowska, R.; Pazurek, M.; Durko, L.; Degowska, M.; Rydzewska, G.; Smigielski, J.; Janiak, A.; Olakowski, M.; Lampe, P.; Grzelak, P.; et al. Pancreatic Cyst Fluid Analysis for Differential Diagnosis between Benign and Malignant Lesions. Oncol. Lett. 2013, 5, 613–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.G.; Wu, M.; Bowen, R.; Zheng, M.; Fitch, W.L.; Pai, R.K.; Wodziak, D.; Visser, B.C.; Poultsides, G.A.; Norton, J.A.; et al. Metabolomic-Derived Novel Cyst Fluid Biomarkers for Pancreatic Cysts: Glucose and Kynurenine. Gastrointest. Endosc. 2013, 78, 295–302.e2. [Google Scholar] [CrossRef] [Green Version]
- Nagashio, Y.; Hijioka, S.; Mizuno, N.; Hara, K.; Imaoka, H.; Bhatia, V.; Niwa, Y.; Tajika, M.; Tanaka, T.; Ishihara, M.; et al. Combination of Cyst Fluid CEA and CA 125 Is an Accurate Diagnostic Tool for Differentiating Mucinous Cystic Neoplasms from Intraductal Papillary Mucinous Neoplasms. Pancreatology 2014, 14, 503–509. [Google Scholar] [CrossRef]
- Gaddam, S.; Ge, P.S.; Keach, J.W.; Mullady, D.; Fukami, N.; Edmundowicz, S.A.; Azar, R.R.; Shah, R.J.; Murad, F.M.; Kushnir, V.M.; et al. Suboptimal Accuracy of Carcinoembryonic Antigen in Differentiation of Mucinous and Nonmucinous Pancreatic Cysts: Results of a Large Multicenter Study. Gastrointest. Endosc. 2015, 82, 1060–1069. [Google Scholar] [CrossRef]
- Jin, D.; Small, A.; Vollmer, C.; Jhala, N.; Furth, E.; Ginsberg, G.; Kochman, M.; Ahmad, N.; Chandrasekhara, V. A Lower Cyst Fluid CEA Cut-Off Increases Diagnostic Accuracy in Identifying Mucinous Pancreatic Cystic Lesions. JOP J. Pancreas 2015, 16, 271–277. [Google Scholar] [CrossRef]
- Carr, R.A.; Yip-Schneider, M.T.; Simpson, R.E.; Dolejs, S.; Schneider, J.G.; Wu, H.; Ceppa, E.P.; Park, W.; Schmidt, C.M. Pancreatic Cyst Fluid Glucose: Rapid, Inexpensive, and Accurate Diagnosis of Mucinous Pancreatic Cysts. Surgery 2018, 163, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Jabbar, K.S.; Arike, L.; Verbeke, C.S.; Sadik, R.; Hansson, G.C. Highly Accurate Identification of Cystic Precursor Lesions of Pancreatic Cancer Through Targeted Mass Spectrometry: A Phase IIc Diagnostic Study. J. Clin. Oncol. 2018, 36, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Soyer, O.M.; Baran, B.; Ormeci, A.C.; Sahin, D.; Gokturk, S.; Evirgen, S.; Basar, R.; Firat, P.; Akyuz, F.; Demir, K.; et al. Role of Biochemistry and Cytological Analysis of Cyst Fluid for the Differential Diagnosis of Pancreatic Cysts: A Retrospective Cohort Study. Medicine 2017, 96, e5513. [Google Scholar] [CrossRef] [PubMed]
- Faias, S.; Pereira, L.; Roque, R.; Chaves, P.; Torres, J.; Cravo, M.; Pereira, A.D. Excellent Accuracy of Glucose Level in Cystic Fluid for Diagnosis of Pancreatic Mucinous Cysts. Dig. Dis. Sci. 2020, 65, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Bruno, M.; Gaia, S.; Cantamessa, A.; Bragoni, A.; Caropreso, P.; Sacco, M.; Fagoonee, S.; Saracco, G.M.; De Angelis, C. Differential Diagnosis of Pancreatic Cysts: A Prospective Study on the Role of Intra-Cystic Glucose Concentration. Dig. Liver Dis. 2020, 52, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Petrone, M.; Capurso, G.; Locatelli, M.; Crippa, S.; Doglioni, C.; Mariani, A.; Testoni, S.G.; Archibugi, L.; Tacelli, M.; et al. Sa1421 Glucose Levels in Eus-Aspirated Cyst Fluid Have a High Accuracy for the Diagnosis of Mucinous Pancreatic Cystic Lesions. Gastrointest. Endosc. 2020, 91, AB181. [Google Scholar] [CrossRef]
- Smith, Z.L.; Satyavada, S.; Simons-Linares, R.; Mok, S.R.S.; Martinez Moreno, B.; Aparicio, J.R.; Chahal, P. Intracystic Glucose and Carcinoembryonic Antigen in Differentiating Histologically Confirmed Pancreatic Mucinous Neoplastic Cysts. Off. J. Am. Coll. Gastroenterol.|ACG 2021, 117, 478–485. [Google Scholar] [CrossRef]
- Oria, I.; Lariño-Noia, J.; Villaverde, A.; Pizzala, J.E.; Pasqua, A.; Urgiles, D.; De La Iglesia Garcia, D.; Mejuto, R.; Iglesias-Garcia, J.; Mazza, O.C.; et al. Sa1413 cyst fluid glucose obtained by eus-fna is accurate for the diagnosis of mucinous pancreatic cysts. Experience from two terciary care centers. Gastrointest. Endosc. 2020, 91, AB178–AB179. [Google Scholar] [CrossRef]
- Noia, J.L.; Mejuto, R.; Oria, I.; De la Iglesia-García, D.; Villaverde, A.; Voces, A.; Pizzala, J.; Iglesias-García, J.; Urgiles, D.; Marcolongo, M.; et al. Rapid Diagnosis of Mucinous Cystic Pancreatic Lesions by On-Site Cyst Fluid Glucometry. Surg. Endosc. 2022, 36, 2473–2479. [Google Scholar] [CrossRef]
- Lee, L.S.; Bellizzi, A.M.; Banks, P.A.; Sainani, N.I.; Kadiyala, V.; Suleiman, S.; Conwell, D.L.; Paulo, J.A. Differentiating Branch Duct and Mixed IPMN in Endoscopically Collected Pancreatic Cyst Fluid via Cytokine Analysis. Gastroenterol. Res. Pract. 2012, 2012, 247309. [Google Scholar] [CrossRef]
- Tun, M.T.; Pai, R.K.; Kwok, S.; Dong, A.; Gupta, A.; Visser, B.C.; Norton, J.A.; Poultsides, G.A.; Banerjee, S.; Van Dam, J.; et al. Diagnostic Accuracy of Cyst Fluid Amphiregulin in Pancreatic Cysts. BMC Gastroenterol. 2012, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Das, K.K.; Xiao, H.; Geng, X.; Fernandez-Del-Castillo, C.; Morales-Oyarvide, V.; Daglilar, E.; Forcione, D.G.; Bounds, B.C.; Brugge, W.R.; Pitman, M.B.; et al. MAb Das-1 Is Specific for High-Risk and Malignant Intraductal Papillary Mucinous Neoplasm (IPMN). Gut 2014, 63, 1626–1634. [Google Scholar] [CrossRef]
- Räty, S.; Sand, J.; Laukkarinen, J.; Vasama, K.; Bassi, C.; Salvia, R.; Nordback, I. Cyst Fluid SPINK1 May Help to Differentiate Benign and Potentially Malignant Cystic Pancreatic Lesions. Pancreatology 2013, 13, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Yip-Schneider, M.T.; Wu, H.; Dumas, R.P.; Hancock, B.A.; Agaram, N.; Radovich, M.; Schmidt, M.C. Vascular Endothelial Growth Factor, a Novel and Highly Accurate Pancreatic Fluid Biomarker for Serous Pancreatic Cysts. J. Am. Coll. Surg. 2014, 218, 608–617. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, C.J.; Weis-Garcia, F.; Bagiella, E.; Tang, L.H.; Allen, P.J. Pancreatic Cyst Fluid Concentration of High-Mobility Group A2 Protein Acts as a Differential Biomarker of Dysplasia in Intraductal Papillary Mucinous Neoplasm. Gastrointest. Endosc. 2016, 83, 1205–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moris, M.; Dawson, D.W.; Jiang, J.; Lewis, J.; Nassar, A.; Takeuchi, K.K.; Lay, A.R.; Zhai, Q.; Donahue, T.R.; Kelly, K.A.; et al. Plectin-1 as a Biomarker of Malignant Progression in Intraductal Papillary Mucinous Neoplasms: A Multicenter Study. Pancreas 2016, 45, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Das, K.K.; Geng, X.; Brown, J.W.; Morales-Oyarvide, V.; Huynh, T.; Pergolini, I.; Pitman, M.B.; Ferrone, C.; Al Efishat, M.; Haviland, D.; et al. Cross Validation of the Monoclonal Antibody Das-1 in Identification of High-Risk Mucinous Pancreatic Cystic Lesions. Gastroenterology 2019, 157, 720–730.e2. [Google Scholar] [CrossRef]
- Simpson, R.E.; Yip-Schneider, M.T.; Flick, K.F.; Wu, H.; Colgate, C.L.; Schmidt, C.M. Pancreatic Fluid Interleukin-1β Complements Prostaglandin E2 and Serum Carbohydrate Antigen 19-9 in Prediction of Intraductal Papillary Mucinous Neoplasm Dysplasia. Pancreas 2019, 48, 1026–1031. [Google Scholar] [CrossRef]
- Siu, L.; Paredes, J.; Kurbatov, V.; Ramachandran, R.; Serafini, F.; Grossman, E.; Gress, F.; Martello, L. Clinical Utility of Cytokine Biomarker Analysis of Pancreatic Cyst Fluid Obtained by Endoscopic Ultrasound Fine Needle Aspiration: A Pilot Study. Pancreas 2019, 48, e60–e61. [Google Scholar] [CrossRef]


| Author | Year | Study |
Patients (n) | Sensitivity (%) |
Specificity (%) |
Accuracy (%) | Main Findings |
|---|---|---|---|---|---|---|---|
| Kucera S. et al. [14] | 2012 |
retrospective cross-sectional study EUS-FNA | 47 |
CEA > 200 ng/mL 52.4% for IPMN |
CEA > 200 ng/mL 42.3% for IPMN |
CEA > 200 ng/mL 46.8% for IPMN | The mean levels of CEA increased as pathology progressed from low-grade dysplasia to moderate and high-grade dysplasia. The mean CEA level decreased when invasive cancer developed. |
| Talar - Wojnarowska R. et al. [15] | 2012 |
prospective study EUS-FNA | 52 |
CEA cut-off point 45 ng/mL 91.8% |
CEA cut-off point 45 ng/mL 63.9% |
CEA cut-off point 45 ng/mL 89.2% | CEA was higher in patients with malignant cysts compared to benign lesions. |
| Park W.G. et al. [16] | 2013 |
retrospective cohort study EUS-FNA and surgical resection | 31 from 45 |
CEA > 192 ng/mL 73% |
CEA > 192 ng/mL 89% |
CEA > 192 ng/mL 77% | CEA > 192 ng/mL in combination with glucose < 66 mg/dl showed better diagnostic accuracy in differentiating mucinous from non-mucinous cysts compared to the above markers alone. |
|
Nagashio Y. et al. [17] | 2014 |
retrospective study EUS-FNA and surgical resection | 68 |
CEA cut-off point 67.3 ng/mL 89.2% |
CEA cut-off point 67.3 ng/mL 77.8% |
CEA cut-off point 67.3 ng/mL 88.4% | CEA can be a helpful marker in differentiating mucinous from non-mucinous cysts, but not malignant from benign cystic lesions. |
| Yadav D. et al. [8] | 2014 |
retrospective study fluid aspiration method was not mentioned | 17 |
CEA ≥ 184 ng/mL 36% CEA ≥ 184 ng/mL with glucose ≤ 21 mg/dL 100% |
CEA ≥ 184 ng/mL 100% CEA ≥ 184 ng/mL with glucose ≤ 21 mg/dL 83% |
CEA ≥ 184 ng/mL 70% no data | Patients with non-mucinous cysts (pseudocysts) had higher levels of intracystic glucose. The differentiation based on CEA levels was not that good. The use of a combination of glucose ≤ 21 or CEA ≥ 184 did not improve diagnoses. |
| Gaddam S. et al. [18] | 2015 |
retrospective study surgical resection | 226 |
CEA cut-off point 105 ng/mL 70% CEA cut-off point 192 ng/mL 61% |
CEA cut-off point 105 ng/mL 63% CEA cut-off point 192 ng/mL 77% |
CEA cut-off point 105 ng/mL 77% CEA cut-off point 192 ng/mL 61% | CEA had clinically suboptimal accuracy in distinguishing MCN from NMCN. |
| Jin D.X. et al. [19] | 2015 |
retrospective study surgical resection | 86 | no data | no data |
CEA cut-off point 30.7 ng/mL 87.2% for differentiating mucinous from non-mucinous cysts CEA cut-off point 30.7 ng/mL 82.7% for differentiating IPMN from non-mucinous cysts |
CEA level was significantly higher in mucinous cysts compared with non-mucinous cysts and in IPMN compared with non-mucinous cysts. CEA levels were not significantly different between malignant and non-malignant mucinous cysts. |
| Zikos T. et al. [9] | 2015 |
prospective study methods of collecting fluid from the cysts were not mentioned | 65 |
CEA > 192 ng/mL 77% CEA > 192 ng/mL with glucose < 50 mg/dL 100% |
CEA > 192 ng/mL 83% CEA > 192 ng/mL with glucose < 50 mg/dL 33% | no data | CEA in combination with glucose showed greater sensitivity but less specificity than using CEA alone. Glucose, whether measured with a laboratory test, glucometer, or reagent strip, was significantly lower in mucinous cysts compared to non-mucosal cysts. |
| Oh S.H. et al. [7] | 2016 |
retrospective study EUS-FNA | 48 |
CEA cut-off point 48.6 ng/mL 72.4% |
CEA cut-off point 48.6 ng/mL 94.7% |
CEA cut-off point 48.6 ng/mL 81.3% | CEA was the best single test for identifying mucinous cysts. The addition of cytology and string symptom assessment to the fluid CEA increased the overall accuracy in the diagnosis of mucinous cysts. |
| Carr R.A. et al. [12] | 2017 |
retrospective study EUS-FNA and surgical resection and ERCP | 149 |
CEA ≤ 10 ng/mL 95.5% |
CEA ≤ 10 ng/mL 81.5% |
CEA ≤ 10 ng/mL 94.5% | VEGF-A was a very accurate test for SCN. The combination of VEGF-A and CEA approached the gold standard in the diagnosis of pancreatic lesions. |
| Carr R.A. et al. [20] | 2017 |
retrospective study EUS-FNA and surgical resection | 153 |
CEA > 192 ng/mL 58% |
CEA > 192 ng/mL 96% |
CEA > 192 ng/mL 69% | Glucose had a significant diagnostic advantage over CEA. |
| Ivry S.L. et al. [13] | 2017 |
retrospective study EUS-FNA and surgical resection | 89 |
CEA cut-off point 192 ng/mL 65% |
CEA cut-off point 192 ng/mL 94% |
CEA cut-off point 192 ng/mL 86.5% | CEA was significantly elevated in the mucinous cysts. The activities of cathepsin E and gastricsin strongly increased in the fluid of mucinous vs. non-mucinous cysts. Best results were achieved when gastricsin and CEA were combined. |
| Jabbar K.S. et al. [21] | 2017 |
prospective cohort study EUS-FNA | 105 |
CEA cut-off point 1000 ng/mL 54% |
CEA cut-off point 1000 ng/mL 90% |
CEA cut-off point 1000 ng/mL 84% | MUC5AC plus PSCA yielded a significantly higher percentage of correct HGD/cancer scores than CEA and cytology. |
| Levy A. et al. [6] | 2017 |
retrospective study EUS-FNA | 115 |
CEA cut-off point 317 µg/L 89% |
CEA cut-off point 317 µg/L 93% |
CEA cut-off point 317 µg/L 93% | CEA in cyst fluid was higher in mucinous cysts than in non-mucinous ones. |
| Soyer O.M. et al. [22] | 2017 |
retrospective cohort study EUS-FNA | 96 |
CEA cut-off point 207 ng/mL 72.7% |
CEA cut-off point 207 ng/mL 97.7% |
CEA cut-off point 207 ng/mL 89.5% |
CEA and CA 72.4 levels for benign-mucinous and malignant cysts were significantly higher than for non-mucinous cysts. The levels of CEA and CA 72-4 in the cystic fluid are highly accurate in distinguishing mucinous from non-mucinous cysts, but with cytology, their accuracy increases. |
| Faias S. et al. [23] | 2019 |
retrospective study EUS-FNA | 82 |
CEA > 192 ng/mL 72% |
CEA > 192 ng/mL 96% |
CEA > 192 ng/mL 84.2% | Pseudocysts presented low glucose identically to mucinous cysts; only glucose with CEA allowed differential diagnosis. |
| Ribaldone D.G. et al. [24] | 2020 |
prospective study EUS-FNA | 56 |
CEA > 192 ng/mL 54.8% for mucinous cysts CEA < 5 ng/mL 72% for non-mucinous cysts |
CEA > 192 ng/mL 100% for mucinous cysts CEA < 5 ng/mL 87.1% for non-mucinous cysts |
CEA > 192 ng/mL 75% for mucinous cysts CEA < 5 ng/mL 80.4% for non-mucinous cysts | Glucose was more sensitive than CEA in the differential diagnosis of mucinous versus non-mucinous pancreatic cysts. |
| Rossi G. et al. [25] | 2020 |
prospective study EUS-FNA | 48 |
CEA ≥ 192 ng/mL 37.5% |
CEA ≥ 192 ng/mL 100% |
CEA ≥ 192 ng/mL 69% | Glucose was a valid and simple tool for the differential diagnosis of mucinous vs. non-mucinous lesions. It was more accurate than CEA levels. |
| Simons-Linares C.R. et al. [10] | 2020 |
prospective cohort study EUS-FNA | 113 |
CEA ≥ 192 ng/mL 50% CEA ≥ 192 ng/mL with glucose ≤ 21 mg/dl 93% |
CEA ≥ 192 ng/mL 92% CEA ≥ 192 ng/mL with glucose ≤ 21 mg/dL 92% | no data | Glucose outperformed CEA for differentiating mucinous from non-mucinous pancreatic cysts. |
| Smith Z. L. et al. [26] | 2022 |
prospective cohort study EUS-FNA | 93 |
CEA ≥ 192 ng/mL 62.7% |
CEA ≥ 192 ng/mL 88.2% |
CEA ≥ 192 ng/mL 81% | Glucose was superior to CEA for differentiating MCNP when analyzed from freshly obtained fluid of cysts with histologic diagnoses. |
| Author | Year | Study | Patients (n) | Sensitivity (%) | Specificity (%) | Accuracy (%) | Main Findings |
|---|---|---|---|---|---|---|---|
| Park W.G. et al. [16] | 2013 |
retrospective cohort study EUS-FNA and surgical resection |
26—I cohort 19—II cohort together 45 |
glucose cut-off point 66 mg/dL 94% |
glucose cut-off point 66 mg/dL 64% |
glucose cut-off point 66 mg/dL 88% |
Metabolomic abundance for glucose and kynurenine was significantly lower in mucinous cysts compared to non-mucinous cysts. Neither could differentiate premalignant from malignant cysts. Glucose and kynurenine levels were significantly elevated for serous cystadenomas. |
| Yadav D. et al. [8] | 2014 |
retrospective study fluid aspiration method was not mentioned | 17 |
glucose ≤ 21 mg/dL 100% |
glucose ≤ 21 mg/dL 83% |
glucose ≤ 21 mg/dL 87% | Patients with non-mucinous cysts (pseudocysts) had higher levels of intracystic glucose. |
| Zikos T. et al. [9] | 2015 |
prospective study methods of collecting fluid from the cysts were not mentioned | 65 |
laboratory—glucose < 50 mg/dL 95% glucometer—glucose < 50 mg/dL 88% reagent strip — glucose 81% |
laboratory—glucose < 50 mg/dL 57% glucometer—glucose < 50 mg/dL 78% reagent strip — glucose 74% | no data | Glucose, whether measured with a laboratory test, glucometer, or reagent strip, was significantly lower in mucinous cysts compared to pancreatic non-mucosal cysts. |
| Carr R.A. et al. [20] | 2017 |
retrospective study EUS-FNA and surgical resection | 153 |
glucose ≤ 50 mg/dL 92% for mucinous cysts |
glucose ≤ 50 mg/dL 87% for mucinous cysts |
glucose ≤ 50 mg/dL 90% for mucinous cysts |
Glucose in the cystic fluid was lower in mucinous cysts compared to non-mucinous cysts. Glucose outperformed CEA. |
| Faias S. et al. [23] | 2019 |
retrospective study EUS-FNA | 82 |
glucose < 50 mg/dL 89% |
glucose < 50 mg/dL 86% |
glucose < 50 mg/dL 86% | Pseudocysts presented low glucose, identically to mucinous cysts. Glucose combined with CEA allowed differential diagnosis. |
| Oria I. et al. [27] | 2020 |
prospective study EUS-FNA | 75 |
glucose ≤ 50 mg/dL 89.4% |
glucose ≤ 50 mg/dL 76.2% |
glucose ≤ 50 mg/dL 84% | Glucose was a very accurate, rapid, and inexpensive test for the diagnosis of mucinous PCLs. |
| Ribaldone D.G. et al. [24] | 2020 |
prospective study EUS-FNA | 56 |
glucose < 50 mg/dL 93.6% for mucinous cysts glucose ≥ 50 mg/mL 96% for non-mucinous cysts |
glucose < 50 mg/dL 96% for mucinous cysts glucose ≥ 50 mg/mL 93.6% for non-mucinous cysts |
glucose < 50 mg/dL 94.6% for mucinous cysts glucose ≥ 50 mg/mL 94.6% for non-mucinous cysts | Glucose was more sensitive than CEA in the differential diagnosis of mucinous versus non-mucinous pancreatic cysts. |
| Rossi G. et al. [25] | 2020 |
prospective study EUS-FNA | 48 | glucose ≤ 30 mg/dL 91.3% |
glucose ≤ 30 mg/dL 100% | glucose ≤ 30 mg/dL 95% | Glucose level in the cyst fluid obtained during EUS with FNA represented a valid and simple tool for the differential diagnosis of mucinous vs. non-mucinous lesions and was more accurate than CEA. |
| Simons-Linares C.R. et al. [10] | 2020 |
prospective cohort study EUS-FNA | 113 |
glucose ≤ 41 mg/dL 92% glucose ≤ 21 mg/dL 88% |
glucose ≤ 41 mg/dL 92% glucose ≤ 21 mg/dL 97% |
glucose ≤ 41 mg/dL 95% no data | Glucose outperformed CEA for differentiating mucinous from non-mucinous pancreatic cysts. |
| Noia J. L. et al. [28] | 2021 |
retrospective study EUS-FNA | 72 (40 in the derivation cohort and 32 in the validation cohort) |
glucose cut-off point 73 mg/dL 89% for derivation cohort 100% for validation cohort |
glucose cut-off point 73 mg/dL 90% for derivation cohort 71% for validation cohort | no data | On-site glucometry was a feasible, accurate, and reproducible method for the characterization of PCLs after EUS-FNA. It showed an excellent correlation with laboratory glucose values. |
| Smith Z. L. et. al. [26] | 2022 |
prospective cohort study EUS-FNA | 93 |
glucose ≤ 25 mg/dL 88.1% |
glucose ≤ 25 mg/dL 91.2% |
glucose ≤ 25 mg/dL 96% | Glucose was superior to CEA for differentiating MCNP when analyzed from the freshly obtained fluid of cysts with histologic diagnoses. |
| Author | Year | Marker | Study |
Patients (n) | Sensitivity (%) |
Specificity (%) |
Accuracy (%) | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Lee L.S. et al. [29] | 2012 | TGF-β1 G-CSF |
prospective study EUS-FNA and ERCP | 10 | no data | no data | no data | Intracystic TGF-β1 and G-CSF were suggested to be potential diagnostic biomarkers that could distinguish mixed IPMN from BD-IPMN. |
| Talar - Wojnarowska R. et al. [15] | 2012 | CA 19-9 |
prospective study EUS-FNA | 52 |
CA 19-9 cut-off point 37 U/mL 81.3% |
CA 19-9 cut-off point 37 U/mL 69.4% |
CA 19-9 cut-off point 37 U/mL 87.3% | CA 19-9 was considered to be less specific compared to CEA, particularly for the detection of mucinous cysts. CA 19-9 had higher sensitivity and specificity than CEA in the detection of pancreatic cystadenocarcinomas. |
| Talar - Wojnarowska R. et al. [15] | 2012 | amylase |
prospective study EUS-FNA | 52 |
amylase 62.5% |
amylase 69.4% | amylase 68.4% |
Amylase can be useful for the confirmation of pseudocyst diagnosis, particularly in patients with a history of pancreatitis. Mean amylase levels in benign lesions were higher compared to malignant cysts. |
| Tun M.T. et al. [30] | 2012 | AREG |
retrospective study EUS-FNA surgical resection | 33 |
AREG > 300 pg/mL 83% for cancer or high-grade dysplasia |
AREG > 300 pg/mL 73% for cancer or high-grade dysplasia |
AREG > 300 pg/mL 78% for cancer or high-grade dysplasia | AREG levels were significantly higher in cancerous and high-grade dysplastic cysts compared to benign mucinous cysts. |
| Das K.K. et al. [31] | 2013 | mAb Das-1 |
retrospective cohort study EUS-FNA and surgical resection | 94 + 38 |
mAb Das-1 in high risk/malignant IPMNs 85% in resection tissue 89% in liquid from EUS-FNA |
mAb Das-1 in high risk/malignant IPMNs 95% in resection tissue 100% in liquid from EUS-FNA | no data | mAb Das-1 reacted with high specificity to tissue and cyst fluid from high-risk/malignant IPMNs. |
| Park W.G. et al. [16] | 2013 | kynurenine |
retrospective cohort study EUS-FNA and surgical resection |
26—I cohort 19—II cohort |
kynurenine cut-off point 185,650 100% kynurenine cut-off point 34,000 90% |
kynurenine cut-off point 185,650 80% kynurenine cut-off point 34,000 100% |
kynurenine cut-off point 185,650 94% kynurenine cut-off point 34,000 92% | Kynurenine levels were significantly elevated in SCA lesions compared to lesions that were not SCAs. |
| Räty S. et al. [32] | 2013 | SPINK1 |
prospective study surgical resection | 61 |
SPINK1 cut-off point 118 μg/L 85% for differentiating MCA or main/mixed type IPMN from SCA or side branch IPMN SPINK1 cut-off point 146 μg/L 93% for differentiating < 3 cm MCA or main duct IPMN from SCA or side branch IPMN |
SPINK1 cut-off point 118 μg/L 84% for differentiating MCA or main/mixed type IPMN from SCA or side branch IPMN SPINK1 cut-off point 146 μg/L 89% for differentiating < 3 cm MCA or main duct IPMN from SCA or side branch IPMN |
SPINK1 cut-off point 118 μg/L 94% for differentiating MCA or main/mixed type IPMN from SCA or side branch IPMN SPINK1 cut-off point 146 μg/L 98% for differentiating < 3 cm MCA or main duct IPMN from SCA or side branch IPMN | SPINK1 may be a possible marker in the differential diagnosis of benign and potentially malignant pancreatic cystic lesions. |
| Yip-Schneider M.T. et al. [33] | 2014 |
VEGF-A VEGF-C |
prospective study surgical resection | 87 |
VEGF-A cut-off point 8500 pg/mL 100% VEGF-C cut-off point 200 pg/mL 100% |
VEGF-A cut-off point 8500 pg/mL 97% VEGF-C cut-off point 200 pg/mL 90% | no data | VEGF-A and VEGF-C were significantly upregulated in SCN compared with all other diagnoses. |
| DiMaio C.J. et al. [34] | 2015 | HMGA2 protein |
retrospective study surgical resection | 31 | no data | no data | no data | Significantly higher concentrations of HMGA2 protein in the cystic fluid were found in IPMN with HGD compared to changes with LGD or MD. |
| Moris M. et al. [35] | 2016 | plectin-1 |
retrospective study EUS-FNA and surgical resection | 104 |
plectin-1 75% in PDA vs. non- PDA IPMNs |
plectin-1 85% in PDA vs. non- PDA IPMNs |
plectin-1 79% in PDA vs. non- PDA IPMNs | Plectin-1 distinguished IPMN with invasive adenocarcinoma from non-invasive IPMN, but was insufficient for discriminating HGD IPMN from LGD IPMNs. |
| Carr R.A. et al. [12] | 2017 | VEGF-A |
retrospective study EUS-FNA, surgical resection, ERCP | 149 |
VEGF-A > 5000 pg/mL 100% VEGF-A with CEA 99.5% |
VEGF-A > 5000 pg/mL 83.7% VEGF-A with CEA 100% |
VEGF-A > 5000 pg/mL 98.3% VEGF-A with CEA 99.3% | Although VEGF-A was a very accurate test for SCN, a combination of VEGF-A and CEA approached the gold standard in the diagnosis of pancreatic lesions. |
| Ivry S.L. et al. [13] | 2017 |
sathepsin E gastricsin |
retrospective study EUS-FNA and surgical resection | 110 |
cathepsin E 70% gastricsin 93% gastricsin with CEA 98% |
cathepsin E 92% gastricsin 100% gastricsin with CEA 100% |
cathepsin E 82.8% gastricsin 97.9% gastricsin with CEA 99.8% | Activity of cathepsin E and gastricsin increased in the fluid of mucinous vs. non-mucinous cysts; the best results were obtained when combined with gastricsin and CEA. |
| Jabbar K.S. et al. [21] | 2017 | MUC5AC with PSCA |
Prospective cohort study EUS-FNA |
105 68 |
MUC5AC with PSCA cut-off point 12 fmol/µL (summed protein concentration levels) 95% MUC5AC with MUC2 cut-off point 0.01 fmol/µL (summed protein concentration levels) 96% |
MUC5AC with PSCA cut-off point 12 fmol/µL (summed protein concentration levels) 96% MUC5AC with MUC2 cut-off point 0.01 fmol/µL (summed protein concentration levels) 100% |
MUC5AC with PSCA cut-off point 12 fmol/µL (summed protein concentration levels) 96% MUC5AC with MUC2 cut-off point 0.01 fmol/µL (summed protein concentration levels) 97% |
MUC5AC plus PSCA achieved a significantly higher percentage of correct HGD/cancer scores than CEA and cytology. Panel of peptides from mucin-5AC and mucin-2 could discriminate premalignant/malignant lesions from benign. |
| Levy A. et al. [6] | 2017 | CA 19-9 |
retrospective study EUS-FNA | 115 |
CA 19-9 cut-off point 21.395 kU/l 66% |
CA 19-9 cut-off point 21.395 kU/L 78% |
CA 19-9 cut-off point 21.395 kU/L 76% | CA 19-9 was higher in mucinous cysts than non-mucinous ones. |
| Levy A. et al. [6] | 2017 | CA 72-4 |
retrospective study EUS-FNA | 115 |
CA 72-4 cut-off point 7.0 kU/l 94% |
CA 72-4 cut-off point 7.0 kU/L 73% |
CA 72-4 cut-off point 7.0 kU/L 87% | CA 72-4 was higher in mucinous cysts than in non-mucinous cysts. |
| Levy A. et al. [6] | 2017 | amylase |
retrospective study EUS-FNA | 115 |
amylase cut-off point 3.073 U/L 80% |
amylase cut-off point 3.073 U/L 54% |
amylase cut-off point 3.073 U/L 68% | Amylase levels, which indicate pancreatic duct communication, were higher in PCs than in mucinous cysts. |
| Levy A. et al. [6] | 2017 | lipase |
retrospective study EUS-FNA | 115 |
lipase cut-off point 39.260 U/L 88% |
lipase cut-off point 39.260 U/l 45 % |
lipase cut-off point 39.260 U/L 63% | Lipase levels, which indicate pancreatic duct communication, were higher in PCs than in mucinous cysts. |
| Soyer O.M. et al. [22] | 2017 | CEA and CA 72-4 |
retrospective cohort study EUS-FNA | 96 |
CA 72-4 cut-off point 3.32 ng/mL 80% |
CA 72-4 cut-off point 3.32 ng/mL 69.5% |
CA 72-4 cut-off point 3.32 ng/mL 73.6% |
CEA and CA 72-4 levels for benign-mucinous and malignant cysts were significantly higher than for non-mucinous cysts. Levels of CEA and CA 72-4 in the cystic fluid were highly accurate in distinguishing mucinous from non-mucinous cysts; with cytology, their accuracy increases further. |
| Yip-Schneider M.T. et al. [11] | 2017 | PGE-2 |
prospective study surgical resection | 100 |
PGE2 cut-off point 1.1 pg/µL 63% PGE2 cut-off point 0.5 pg/µL with CEA > 192 ng/mL 78% |
PGE2 cut-off point 1.1 pg/µL 79% PGE2 cut-off point 0.5 pg/µL with CEA > 192 ng/mL 100% |
PGE2 cut-off point 1.1 pg/µL 71% PGE2 cut-off point 0.5 pg/µL with CEA > 192 ng/mL 86% |
PGE2 was an indicator of IPMN dysplasia, especially in selected patients with preoperative pancreatic cyst fluid CEA > 192ng/mL. PGE2 levels in high-grade/invasive IPMN were significantly higher than in low/moderate-grade IPMN. |
| Das K.K. et al. [36] | 2019 | mAb Das-1 |
retrospective study surgical resection | 169 |
mAb Das-1 cut-off optical density value 0.104 88% |
mAb Das-1 cut-off optical density value 0.104 99% |
mAb Das-1 cut-off optical density value 0.104 95% | Authors validated the ability of an ELISA with the monoclonal antibody Das-1 to detect PCLs at risk for malignancy with high levels of sensitivity and specificity. |
| Simpson R.E. et al. [37] | 2019 | IL-1β and PGE2 |
retrospective study EUS-FNA and surgical resection | 92 |
IL-1
β
> 20 pg/mL 64.3% PGE2 > 1100 pg/mL 60% IL-1β with PGE2 42.9% |
IL-1
β
> 20 pg/mL 83.8% PGE2 > 1100 pg/mL 78.7% IL-1β with PGE2 89.2% |
IL-1
β
> 20 pg/mL 73.4% PGE2 > 1100 pg/mL 69.6% IL-1β with PGE2 64.6% | IL-1β and PGE2 levels were higher in high-grade/invasive IPMN than in low/moderate-grade IPMN. |
| Siu L. et al. [38] | 2019 |
IL-1α, IL-5, IL-10, and GM-CSF |
prospective study EUS-FNA and surgical resection | 23 | no data | no data | no data | IL-1α and IL-5 had higher concentrations in non-mucinous cysts, while IL-10 and GM-CSF had higher concentrations in mucinous cysts. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wietrzykowska-Grishanovich, D.; Pawlik, E.; Neubauer, K. Biochemical Intracystic Biomarkers in the Differential Diagnosis of Pancreatic Cystic Lesions. Medicina 2022, 58, 994. https://doi.org/10.3390/medicina58080994
Wietrzykowska-Grishanovich D, Pawlik E, Neubauer K. Biochemical Intracystic Biomarkers in the Differential Diagnosis of Pancreatic Cystic Lesions. Medicina. 2022; 58(8):994. https://doi.org/10.3390/medicina58080994
Chicago/Turabian StyleWietrzykowska-Grishanovich, Dominika, Ewa Pawlik, and Katarzyna Neubauer. 2022. "Biochemical Intracystic Biomarkers in the Differential Diagnosis of Pancreatic Cystic Lesions" Medicina 58, no. 8: 994. https://doi.org/10.3390/medicina58080994






