The Biological Activity of Fragmented Computer-Aided Design/Manufacturing Dental Materials before and after Exposure to Acidic Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used and Preparation Procedure
- -
- Samples denoted with A, from each finished CAD/CAM block not exposed to acidic artificial saliva, a powder was obtained using a straight handpiece and a diamond-coated disc double side (Komet USA LLC, Rock Hill, SC, USA). After that, the CAD/CAM block surface remained rough.
- -
- Samples denoted with B, the CAD/CAM blocks with rough surfaces, which remained after samples A were obtained, were immersed in acidic artificial saliva and after 1 month were milled with the diamond-coated disc double side until a powder was obtained;
- -
- Samples denoted with C, other finished CAD/CAM blocks from each group, were immersed in acidic artificial saliva, kept for 1 month, and milled with the diamond-coated disc double side, until fine powders were obtained.
2.2. Artificial Acidic Saliva Preparation and Samples Exposure
2.3. Structural and Morphological Investigations
2.4. Cell Cultures Media and Cell Lines
2.5. MTT Protocol—Mitochondrial Activity Assessment
2.6. LDH Release Method—Cytotoxicity Test
2.7. NO by Griess Assay
2.8. Adherence Test by Scanning Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. The Structural Characterization of the Nonexposed and Exposed CAD/CAM Restorative Materials Blocks to Acidic Artificial Saliva
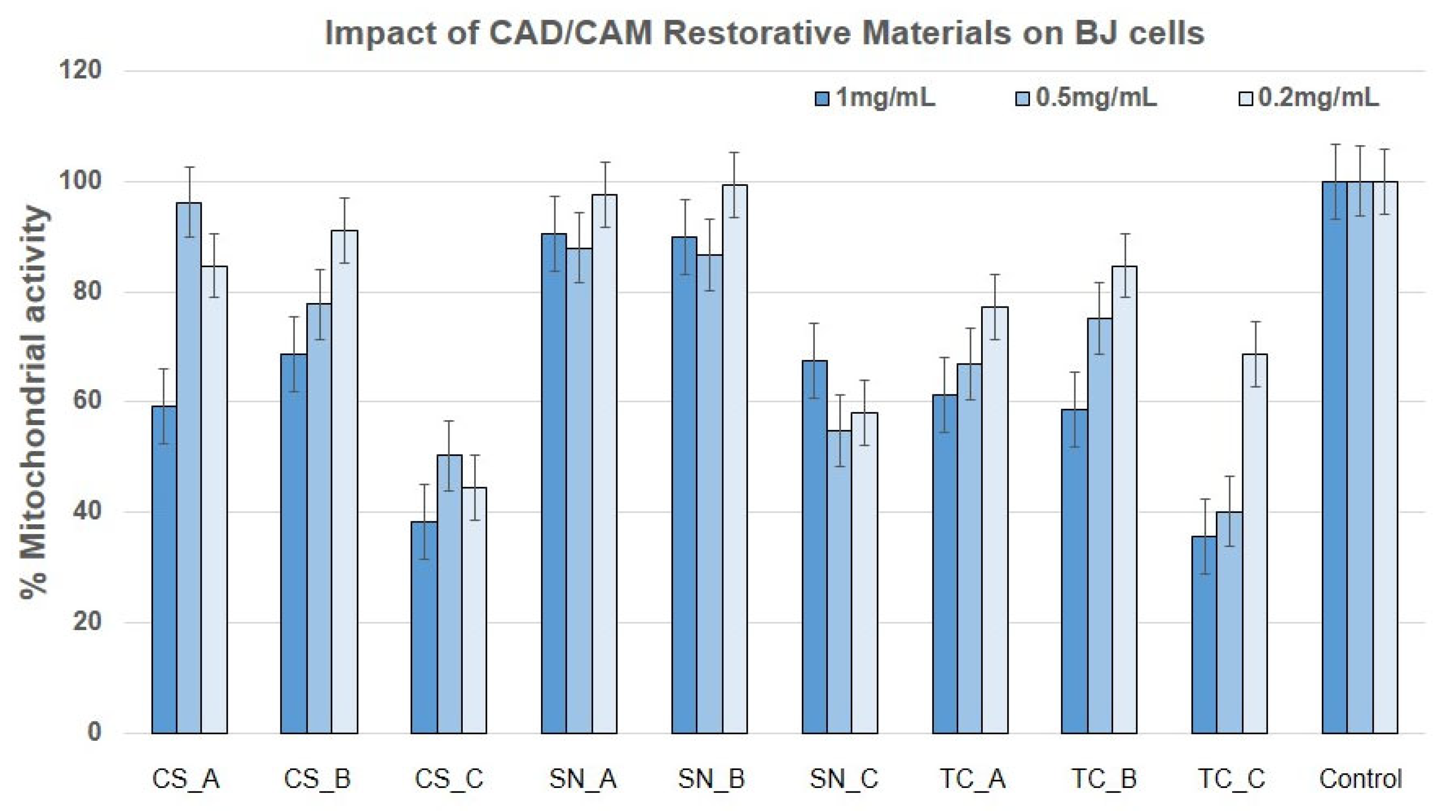
3.2. Impact of the CAD/CAM Restorative Materials Powders on Mitochondrial Activity by Means of MTT Assay
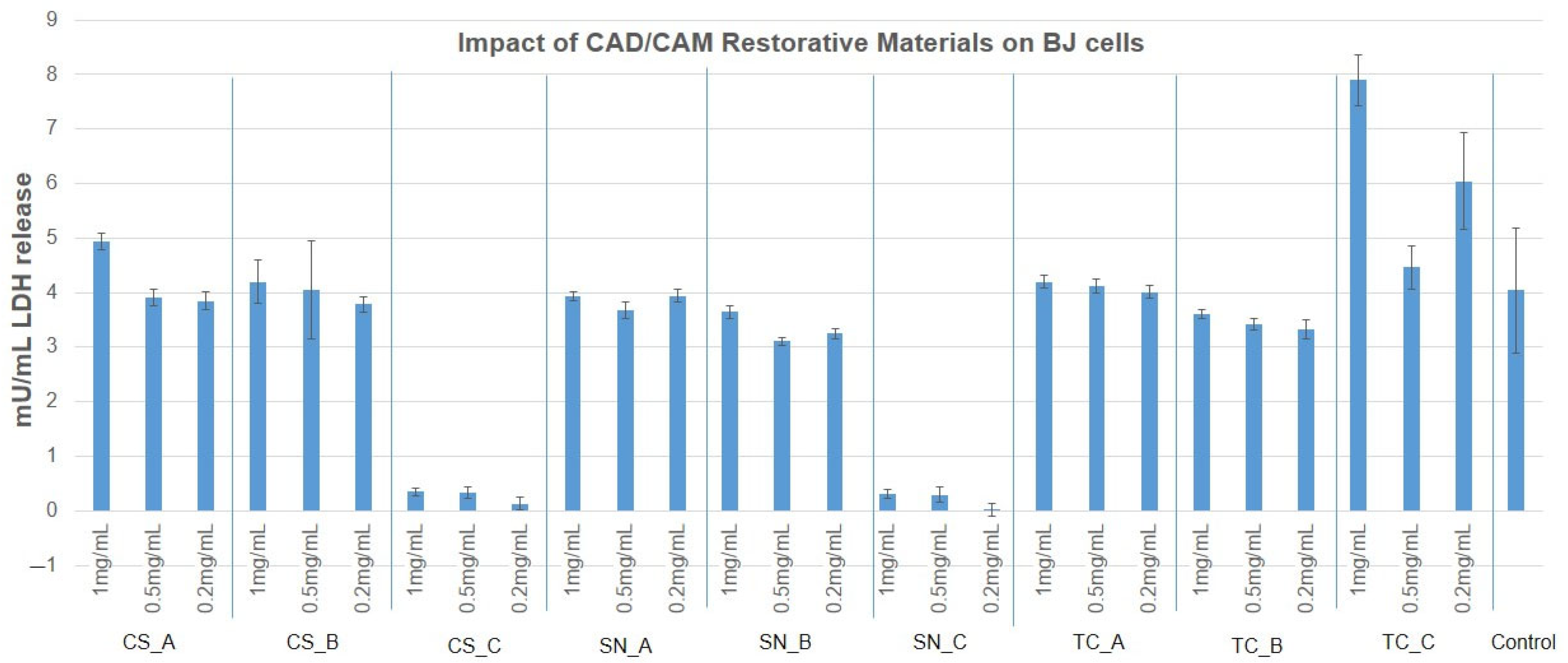
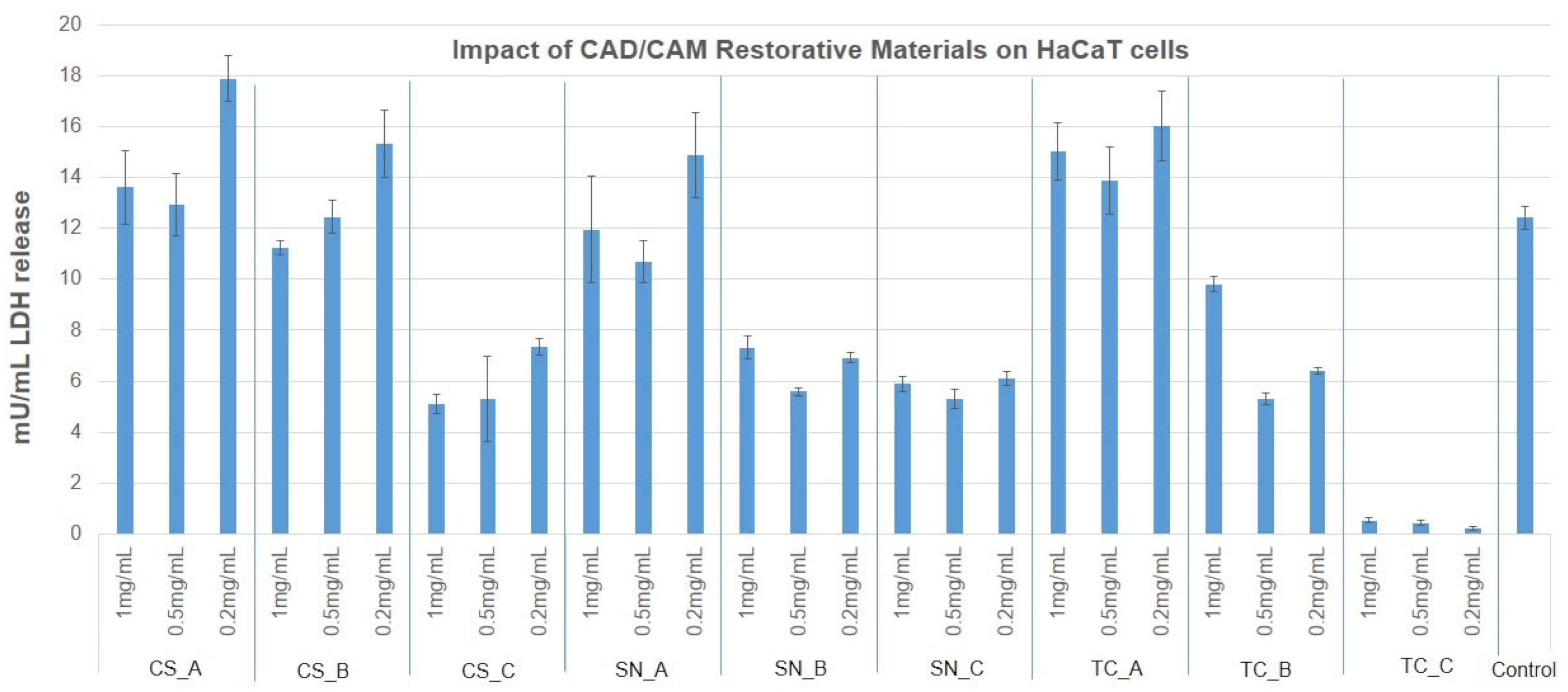
3.3. Cytotoxicity Evaluation of CAD/CAM Restorative Materials Powders via the LDH Release Method
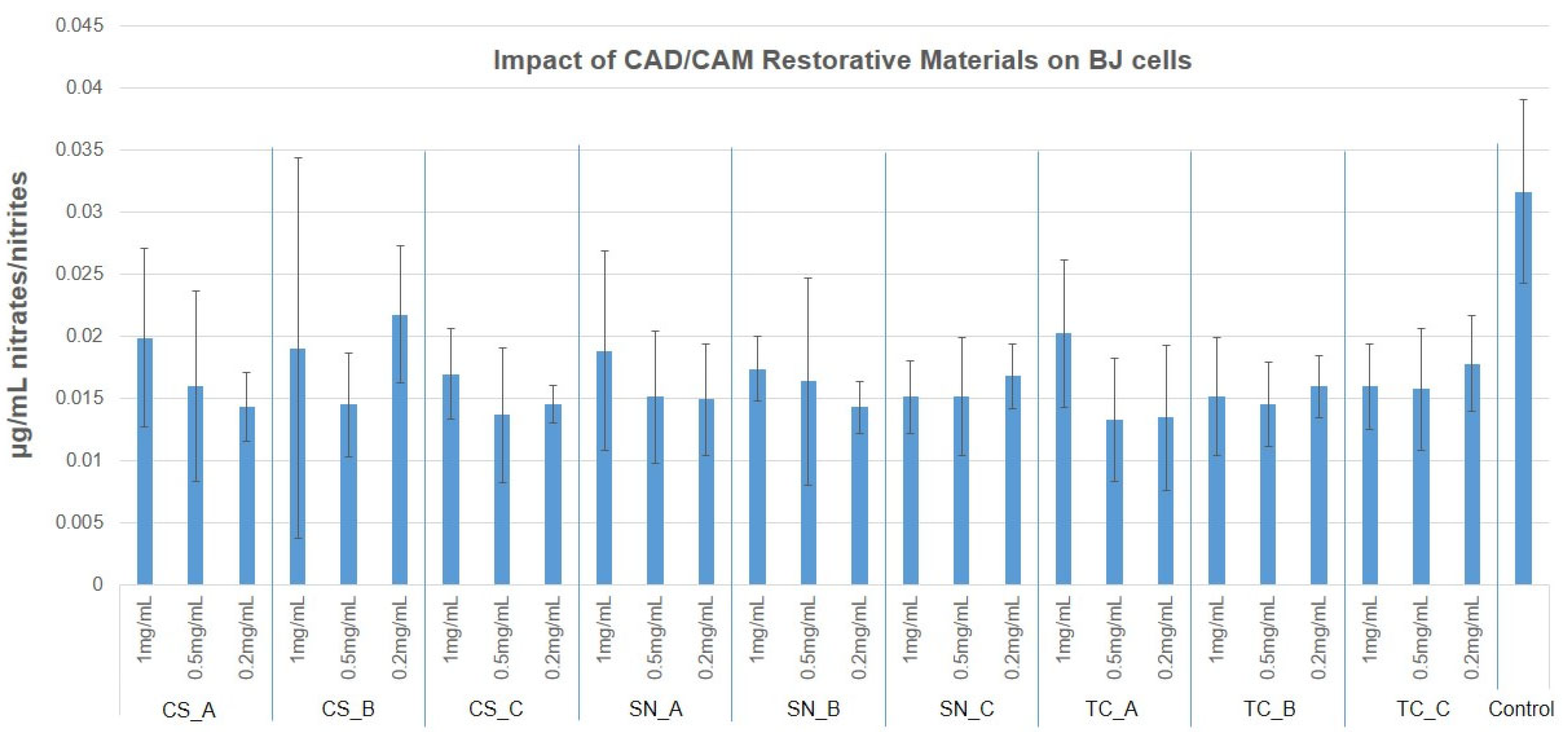
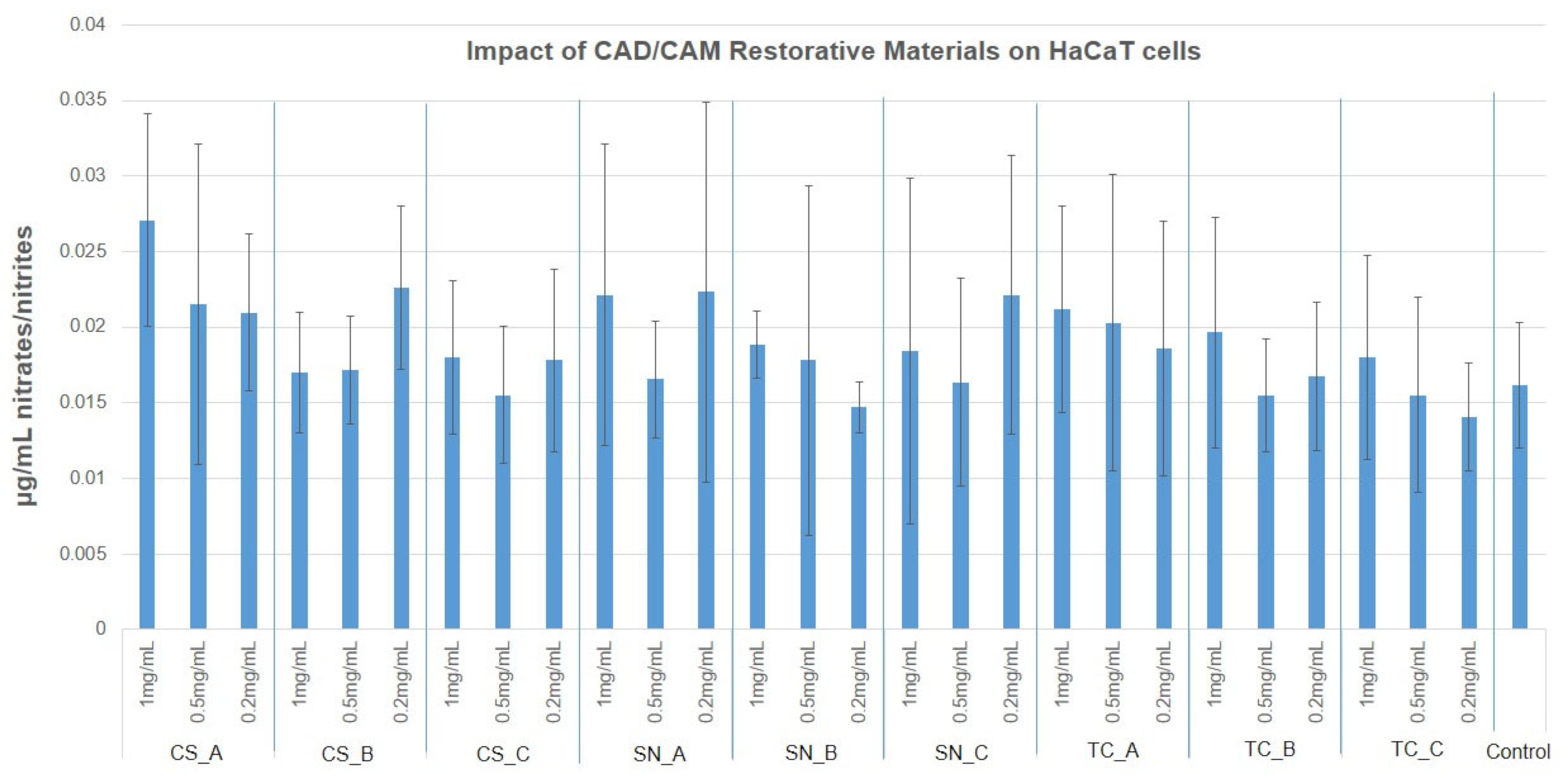
3.4. NO Production via Griess Assay
3.5. The Degree of Adhesion of CAD/CAM Restorative Materials Slices, on Human BJ Cells
4. Discussion
5. Conclusions
- ✓
- As concerns the cytotoxicity evaluation, the restorative materials tested ranged from moderately cytotoxic to slightly cytotoxic to noncytotoxic on the growth of human fibroblast, according to the MTT assay and LDH assay.
- ✓
- As regards the growth of human keratinocytes, the outcomes showed that the samples tested can be considered slightly cytotoxic and noncytotoxic, with the exception of SN_B, which recorded a reduced mitochondrial activity (around 50%).
- ✓
- As concerns NO production, in the case of fibroblasts, this was reduced to half whereas, in the case of keratinocytes, the NO production was slightly increased compared to fibroblasts. Therefore, the tested materials do not determine cell oxidative stress on the nitric pathway.
- ✓
- As regards the adhesion degree, the fibroblasts cells were more affected when the fine was exposed to an acidic environment of each material and was tested, however, when the cells were placed on each compact dental material (CS, SN, and TC), the fibroblasts cells were attached and spreading, preferring particularly the TC material.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmalz, G.; Galler, K.M. Biocompatibility of biomaterials—Lessons learned and considerations for the design of novel materials. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Gabor, A.G.; Duma, V.-F.; Fabricky, M.M.C.; Marsavina, L.; Tudor, A.; Vancea, C.; Negrea, P.; Sinescu, C. Ceramic Scaffolds for Bone Augmentation: Design and Characterization with SEM and Confocal Microscopy. Materials 2022, 15, 4899. [Google Scholar] [CrossRef] [PubMed]
- Fabricky, M.M.C.; Gabor, A.-G.; Milutinovici, R.A.; Watz, C.G.; Avram, S.; Drăghici, G.; Mihali, C.V.; Moacă, E.-A.; Dehelean, C.A.; Galuscan, A.; et al. Scaffold-Type Structure Dental Ceramics with Different Compositions Evaluated through Physicochemical Characteristics and Biosecurity Profiles. Materials 2021, 14, 2266. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kummara, M.R.; Kamal, T.; Alghyamah, A.A.A.; Iftikhar, F.J.; Bano, B.; Khan, N.; Afridi, M.A.; Han, S.S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Mangano, F.J.A.; Shibli, J.A.; Fortin, T. Digital Dentistry. New Mater. Tech. 2016, 2016, 5261247. [Google Scholar] [CrossRef]
- Pabst, A.M.; Walter, C.; Grassmann, L.; Weyhrauch, M.; Brüllmann, D.D.; Ziebart, T.; Scheller, H.; Lehmann, K.M. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2014, 18, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Grenade, C.; de Pauw-Gillet, M.C.; Gailly, P.; Vanheusden, A.; Mainjot, A. Biocompatibility of polymerin filtrated- ceramic-network (PICN) materials with Human Gingival Fibroblasts (HGFs). Dent. Mater. 2016, 32, 1152–1164. [Google Scholar] [CrossRef]
- Tetè, S.; Zizzari, V.L.; Borelli, B.; De Colli, M.; Zara, S.; Sorrentino, R.; Scarano, A.; Gherlone, E.; Cataldi, A.; Zarone, F. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro. Dent. Mater. J. 2014, 33, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Awada, A.; Nathanson, D. Mechanical properties of resin-ceramic CAD/CAM restorative materials. J. Prosthet. Dent. 2015, 114, 587–593. [Google Scholar] [CrossRef]
- Zarone, F.; Ferrari, M.; Mangano, F.G.; Leone, R.; Sorrentino, R. Digitally Oriented Materials: Focus on Lithium Disilicate Ceramics. Int. J. Dent. 2016, 2016, 9840594. [Google Scholar] [CrossRef]
- Horvath, S.D. Key Parameters of Hybrid Materials for CAD/CAM-Based Restorative Dentistry. Compend. Contin. Educ. Dent. 2016, 37, 638–643. [Google Scholar] [PubMed]
- Aldahian, N.; Khan, R.; Mustafa, M.; Vohra, F.; Alrahlah, A. Influence of Conventional, CAD-CAM, and 3D Printing Fabrication Techniques on the Marginal Integrity and Surface Roughness and Wear of Interim Crowns. Appl. Sci. 2021, 11, 8964. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite Blocks for Dental CAD/CAM Applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odayni, A.B.; Alfotawi, R.; Khan, R.; Saeed, S.W.; Al-Kahtani, A.; Aouak, T.; Alrahlah, A. Synthesis of chemically modified BisGMA analog with low viscosity and potential physical and biological properties for dental resin composite. Dent. Mater. 2019, 35, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Magne, P.; Stanley, K.; Schlichting, L.H. Modeling of ultrathin occlusal veneers. Dent. Mater. 2012, 28, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Magne, P.; Cheung, R. Numeric simulation of occlusal interferences in molars restored with ultrathin occlusal veneers. J. Prosthet. Dent. 2017, 117, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Gorrita, M.; Herráez-Galindo, C.; Torres-Lagares, D.; Serrera-Figallo, M.-Á.; Gutiérre-Pérez, J.-L. Biocompatibility of Polymer and Ceramic CAD/CAM Materials with Human Gingival Fibroblasts (HGFs). Polymers 2019, 11, 1446. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, G.; Camurri Piloni, A.; Nicolin, V.; Turco, G.; Di Lenarda, R. Chairside CAD/CAM Materials. Current Trends of Clinical Uses. Biology 2021, 10, 1170. [Google Scholar] [CrossRef]
- Sonmez, N.; Gultekin, P.; Turp, V.; Akgungor, G.; Sen, D.; Mijiritsky, E. Evaluation of five CAD/CAM materials by microstructural characterization and mechanical tests: A comparative in vitro study. BMC Oral Health 2018, 18, 5. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, C.; Rask, H.; Awada, A. Mechanical properties of resin-ceramic CAD-CAM materials after accelerated aging. J. Prosthet. Dent. 2018, 119, 954–958. [Google Scholar] [CrossRef]
- Giordano, R. Materials for chairside CAD/CAM-produced restorations. J. Am. Dent. Assoc. 2006, 137 (Suppl. 1), 14S–21S. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, C.; Vanini, L.; Rondoni, G.D.; De Angelis, F. Wear properties of dental ceramics and porcelains compared with human enamel. J. Prosthet. Dent. 2016, 115, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ankyu, S.; Nakamura, K.; Harada, A.; Hong, G.; Kanno, T.; Niwano, Y.; Örtengren, U.; Egusa, H. Fatigue analysis of computer-aided design/computer-aided manufacturing resin-based composite vs. lithium disilicate glass-ceramic. Eur. J. Oral. Sci. 2016, 124, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Grzebieluch, W.; Mikulewicz, M.; Kaczmarek, U. Resin Composite Materials for Chairside CAD/CAM Restorations: A Comparison of Selected Mechanical Properties. J. Healthc. Eng. 2021, 2021, 8828954. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Vohra, F.; Albogami, S.M.; Alkhammash, N.M.; Alnashwan, M.A.; Almutairi, N.S.; Aali, K.A.; Alrabiah, M.; Abduljabbar, T. Marginal Misfit of 3D-Printed (Selective Laser Sintered), CAD-CAM and Lost Wax Technique Cobalt Chromium Copings with Shoulder and Chamfer Finish Lines: An In-Vitro Study. Medicina 2022, 58, 1313. [Google Scholar] [CrossRef]
- Muhammad, S.; Zafar, A.; Alnazzawi, A.; Alrahabi, M.; Fareed, A.M.; Najeeb, S.; Khurshid, Z. Nanotechnology and nanomaterials in dentistry. In Advanced Dental Biomaterials; Chapter 18; Woodhead Publishing: Sawston, UK, 2019; pp. 477–505. [Google Scholar] [CrossRef]
- Nashaat, Y.; Sabry, H.; Hassan, S.A. Evaluation of the Cytotoxicity and apoptotic effect of Nano triple antibiotic paste with Nano anti-inflammatory drug as an intracanal medicament. Eur. Endod. J. 2021, 6, 82–89. [Google Scholar] [CrossRef]
- Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.; Popoviciu, N.O.; Stanescu, I.I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into their Modern Manufacturing Techniques. Materials 2020, 13, 2894. [Google Scholar] [CrossRef]
- Holban, A.-M.; Farcasiu, C.; Andrei, O.-C.; Grumezescu, A.M.; Farcasiu, A.-T. Surface Modification to Modulate Microbial Biofilms Applications in Dental Medicine. Materials 2021, 14, 6994. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W.; Hartsfield, J.K. (Eds.) Nanobiomaterials in Clinical Dentistry, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Zaharia, C.; Duma, V.-F.; Sinescu, C.; Socoliuc, V.; Craciunescu, I.; Turcu, R.P.; Marin, C.N.; Tudor, A.; Rominu, M.; Negrutiu, M.-L. Dental Adhesive Interfaces Reinforced with Magnetic Nanoparticles: Evaluation and Modeling with Micro-CT versus Optical Microscopy. Materials 2020, 13, 3908. [Google Scholar] [CrossRef]
- Saeed, W.S.; Alotaibi, D.H.; Al-Odayni, A.-B.; Haidyrah, A.S.; Al-Owais, A.A.; Khan, R.; De Vera, M.A.T.; Alrahlah, A.; Aouak, T. Poly(ethylene-Co-vinyl Alcohol)/Titanium Dioxide Nanocomposite: Preparation and Characterization of Properties for Potential Use in Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 3449. [Google Scholar] [CrossRef]
- Mok, Z.H.; Proctor, G.; Thanou, M. Emerging nanomaterials for dental treatments. Emerg. Top. Life Sci. 2020, 4, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Beleidy, M.; Alaa El-din, Y. Biocompatibility and Surface Roughness of Different Sustainable Dental Composite Blocks: Comprehensive In Vitro Study. ACS Omega 2022, 7, 34258–34267. [Google Scholar] [CrossRef] [PubMed]
- Noreen Wuersching, S.; Hickel, R.; Edelhoff, D.; Kollmuss, M. Initial biocompatibility of novel resins for 3D printed fixed dental prostheses. Dent. Mater. 2022, 38, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Daguano, J.K.M.B.; Milesi, M.T.B.; Rodas, A.C.D.; Weber, A.F.; Sarkis, J.E.S.; Hortellani, M.A.; Zanotto, E.G. In vitro biocompatibility of new bioactive lithia-silica glass-ceramics. Mater. Sci. Eng. C 2019, 94, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wegehaupt, F.J.; Wiegand, A.; Roos, M.; Attin, T.; Buchalla, W. Erosion and abrasion of tooth-colored restorative materials and human enamel. J. Dent. 2009, 37, 913–922. [Google Scholar] [CrossRef] [Green Version]
- El-Serag, H.B.; Sweet, S.; Winchester, C.C.; Dent, J. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014, 63, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Milleding, P.; Haraldsson, C.; Karlsson, S. Ion leaching from dental ceramics during static in vitro corrosion testing. J Biomed Mater Res. 2002, 61, 541–550. [Google Scholar] [CrossRef]
- Munksgaard, E.C.; Freund, M. Enzymatic hydrolysis of (di)methacrylates and their polymers. Scand. J. Dent. Res 1990, 98, 261–267. [Google Scholar] [CrossRef]
- Koda, T.; Tsuchiya, H.; Yamauchi, M.; Ohtani, S.; Takagi, N.; Kawano, J. Leachability of denture-base acrylic resins in artificial saliva. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1990, 6, 13–16. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Papadopoulos, T.; Garefis, P. Molecular toxicology of substances released from resin-based dental restorative materials. Int. J. Mol. Sci. 2009, 10, 3861–3899. [Google Scholar] [CrossRef] [Green Version]
- Ille, C.; Moacă, E.A.; Pop, D.; Goguță, L.; Opriș, C.; Pîrvulescu, I.L.; Avram, L.; Faur, A.; Jivănescu, A. Compressive strength evaluation of thin occlusal veneers from different CAD/CAM materials, before and after acidic saliva exposure. Odontology 2022. [Google Scholar] [CrossRef]
- Mohammed, I.; Alwahab, Z.N. An evaluation of the effect of artificial saliva with different ph on shear bond strength of veneering ceramic to metal and zirconia substructure (in vitro study). World J. Pharm. Res. 2017, 6, 30–44. [Google Scholar] [CrossRef]
- Moacă, E.A.; Watz, C.G.; Flondor Ionescu, D.; Păcurariu, C.; Tudoran, L.B.; Ianoș, R.; Socoliuc, V.; Drăghici, G.A.; Iftode, A.; Liga, S.; et al. Biosynthesis of Iron Oxide Nanoparticles: Physico-Chemical Characterization and Their In Vitro Cytotoxicity on Healthy and Tumorigenic Cell Lines. Nanomaterials 2022, 12, 2012. [Google Scholar] [CrossRef]
- Pop, D.; Buzatu, R.; Moaca, E.A.; Watz, C.G.; Cînta-Pînzaru, S.; Barbu Tudoran, L.; Nekvapil, F.; Avram, S.; Dehelean, C.A.; Cretu, M.O.; et al. Development and Characterization of Fe3O4@Carbon Nanoparticles and Their Biological Screening Related to Oral Administration. Materials 2021, 14, 3556. [Google Scholar] [CrossRef]
- Suciu, M.; Porav, S.; Radu, T.; Rosu, M.C.; Lazar, M.D.; Macavei, S.; Socaci, C. Photodynamic effect of light emitting diodes on E. coli and human skin cells induced by a graphene-based ternary composite. J. Photochem. Photobiol. B Biol. 2021, 223, 112298. [Google Scholar] [CrossRef]
- Elshahawy, W.M.; Watanabe, I.; Kramer, P. In vitro cytotoxicity evaluation of elemental ions released from different prosthodontic materials. Dent. Mater. 2009, 25, 1551–1555. [Google Scholar] [CrossRef]
- Elshahawy, W.; Shohieb, F.; Yehia, H.; Etman, W.; Watanabe, I.; Kramer, P. Cytotoxic effect of elements released clinically from gold and CAD-CAM fabricated ceramic crowns. Tanta Dent. J. 2014, 11, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Atay, A.; Gürdal, I.; Çetıntas, V.B.; Üşümez, A.; Cal, E. Effects of New Generation All-Ceramic and Provisional Materials on Fibroblast Cells. J. Prosthodont. 2018, 28, e383–e394. [Google Scholar] [CrossRef]
- ISO 10993; Biological Evaluation of Medical Devices—ISO 10993, Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 1999.
- Dal Piva, A.; Contreras, L.; Ribeiro, F.C.; Anami, L.C.; Camargo, S.; Jorge, A.; Bottino, M.A. Monolithic Ceramics: Effect of Finishing Techniques on Surface Properties, Bacterial Adhesion and Cell Viability. Oper. Dent. 2018, 43, 315–325. [Google Scholar] [CrossRef]
- Milleding, P.; Karlsson, S.; Nyborg, L. On the surface elemental composition of non-corroded and corroded dental ceramic materials in vitro. J. Mater. Sci. Mater. Med. 2003, 14, 557–566. [Google Scholar] [CrossRef]
- Elshahawy, W. Chapter 15: Biocompatibility. In Advances in Ceramics Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment, 1st ed.; InTech Open: London, UK, 2011; pp. 359–374. [Google Scholar]
- Patil, A.; Jebaseelan, D. 3-D Surface Morphological Characterization of CAD/CAM Milled Dental Zirconia: An In Vitro Study of the Effect of Post-Fabrication Processes. Materials 2022, 15, 4685. [Google Scholar] [CrossRef]
- Sjögren, G.; Sletten, G.; Dahl, J.E. Cytotoxicity of dental alloys, metals, and ceramics assessed by Millipore filter, agar overlay, and MTT tests. J. Prosthet. Dent. 2000, 84, 229–236. [Google Scholar] [CrossRef]
- Van Landuyt, K.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Gupta, S.; Saxena, P.; Pant, V.; Pant, A. Release and toxicity of dental resin composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar]
- Ivković, N.; Božović, D.; Ristić, S.; Mirjanić, V.; Janković, O. The residual monomer in dental acrylic resin and its adverse effects. Contemp. Mater. 2013, 1, 84–91. [Google Scholar] [CrossRef]
- Yao, J.; Li, J.; Wang, Y.; Huang, H. Comparison of the flexural strength and marginal accuracy of traditional and CAD/CAM interim materials before and after thermal cycling. J. Prosthet. Dent. 2014, 112, 649–657. [Google Scholar] [CrossRef]

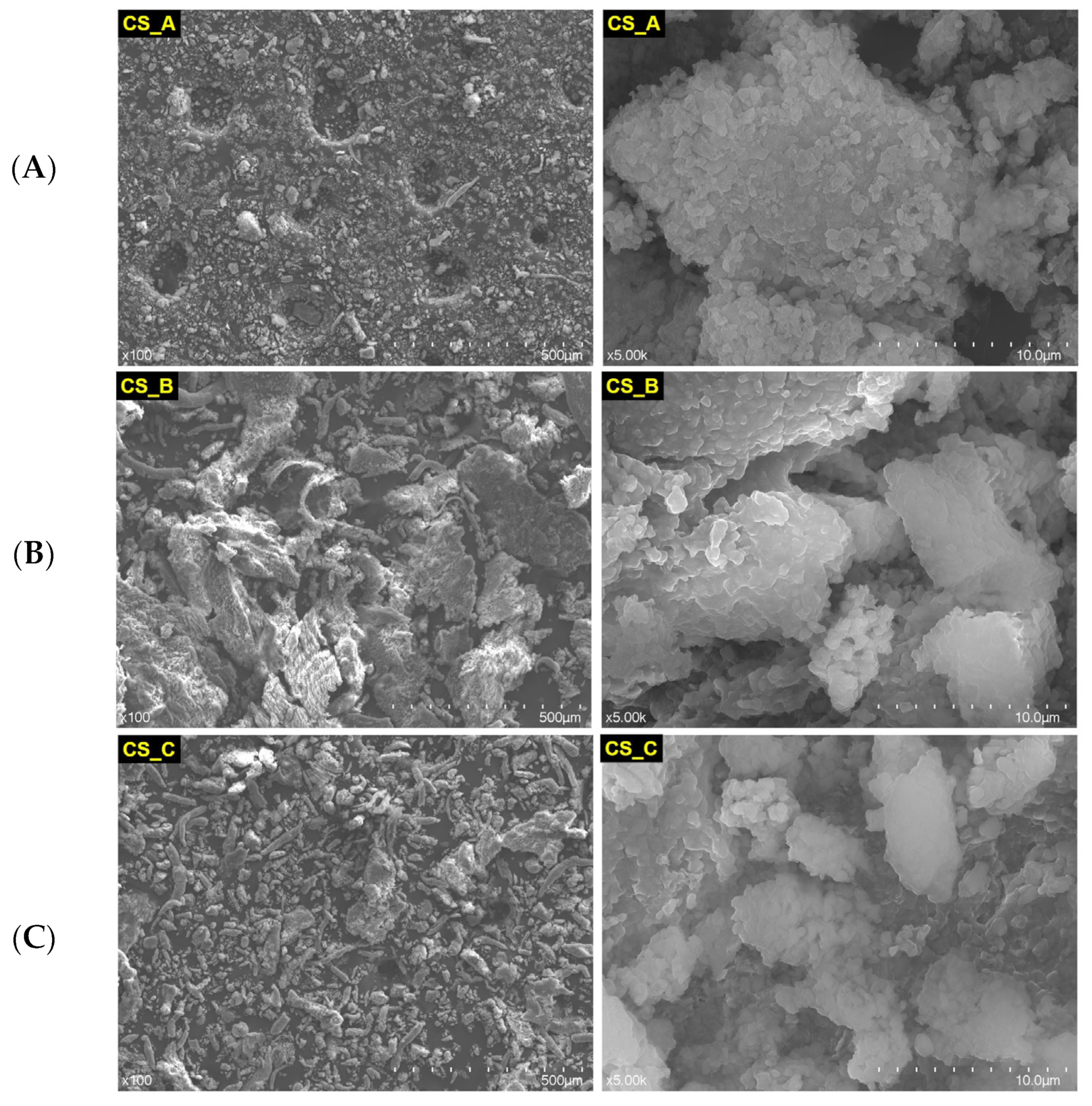

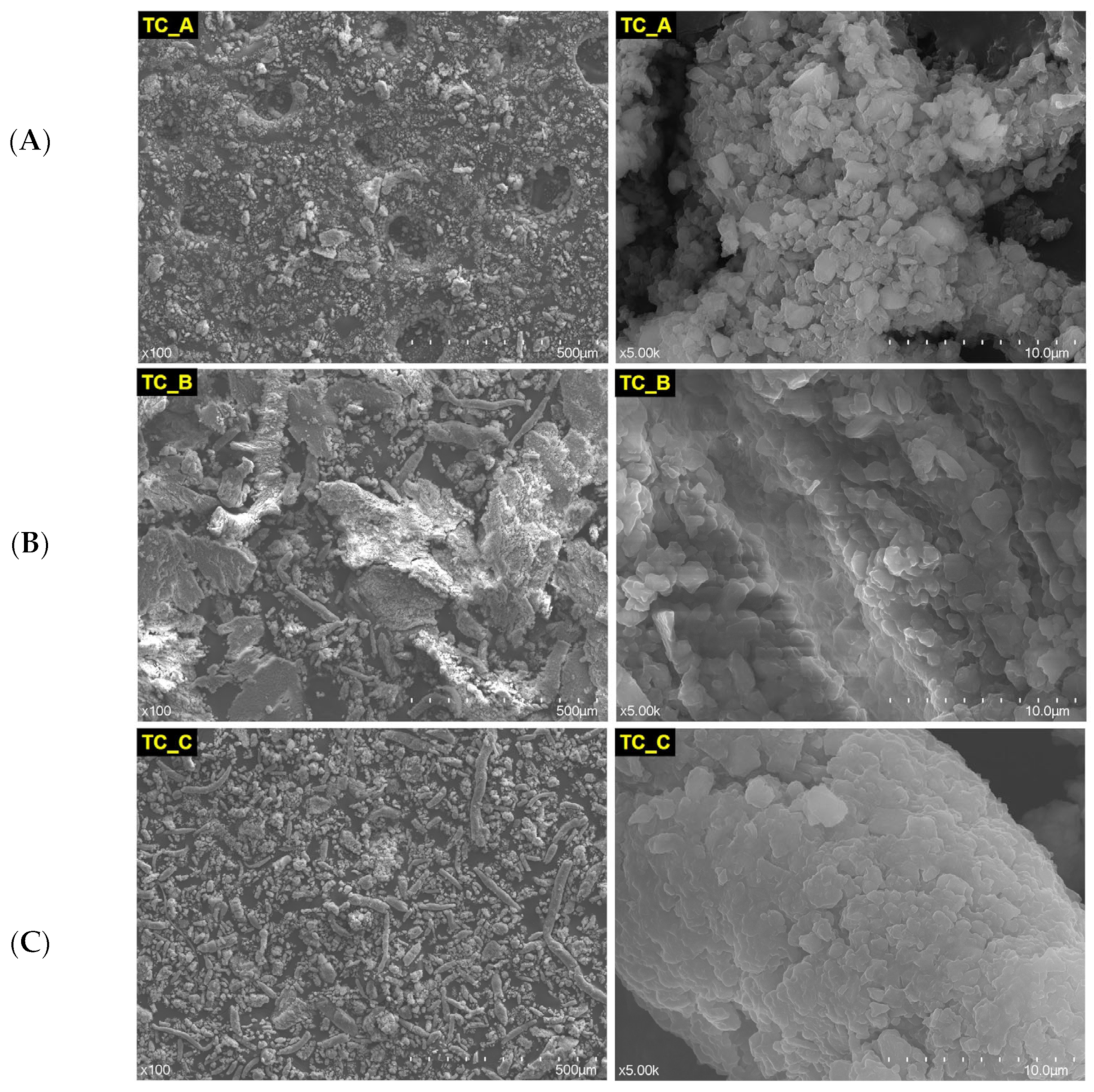
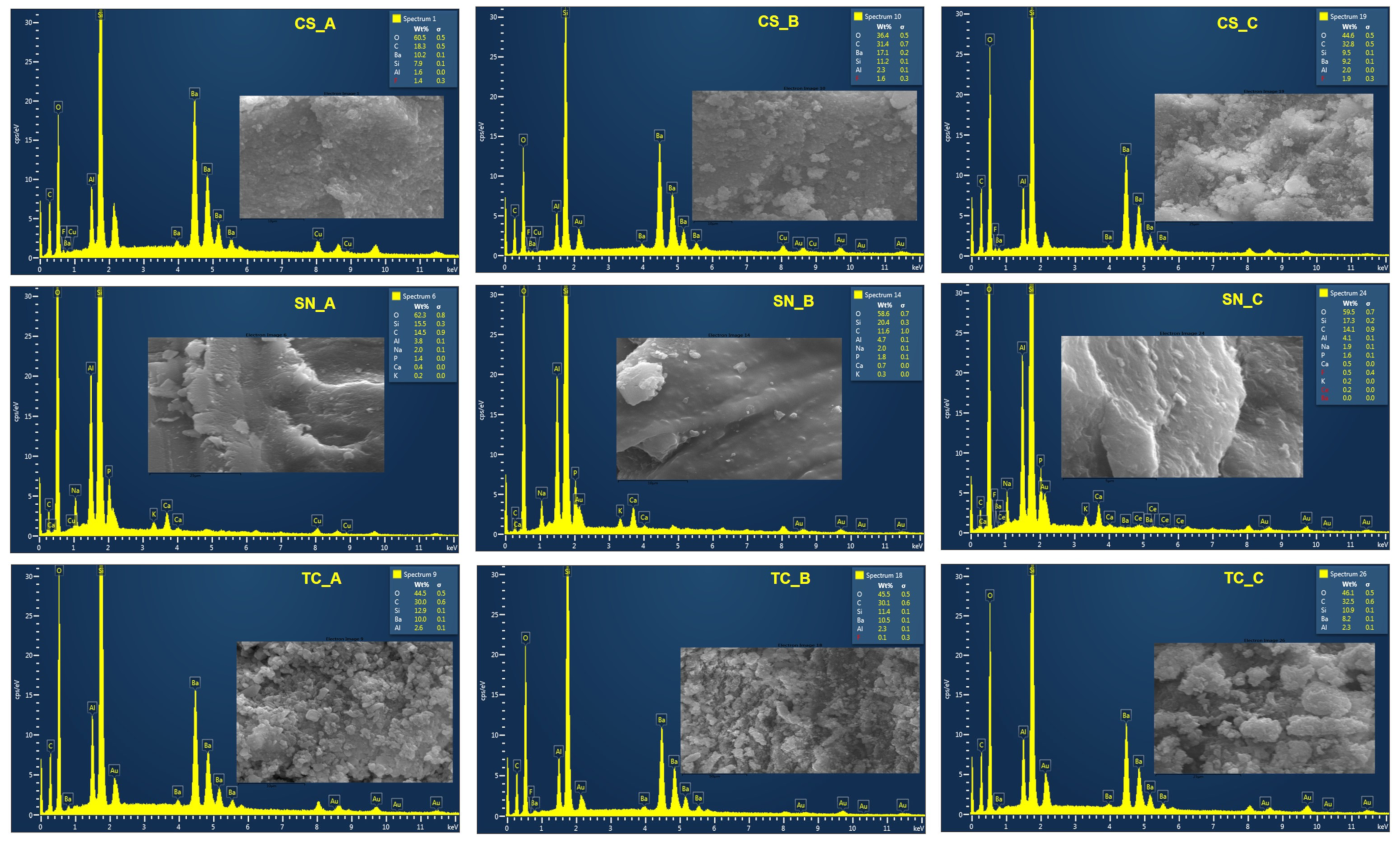


| Restorative Material | Batch No. | Classification | Composition |
|---|---|---|---|
| Cerasmart (CS) | 2109066 | nanoceramic resin | 71% silica and barium glass nanoparticles |
| Straumann Nice (SN) | PA354 | glass ceramic | 70% SiO2, 11% Li2O, 11% Al2O3, 3% K2O, 2% Na2O, 8% P2O5, 0.5% ZrO2, 2% CaO and 9% coloring oxides |
| Tetric CAD (TC) | Y07670 | composite resin | barium glass (<1 mm) and silicon dioxide fillers (<20 nm) |
| CAD/CAM Restorative Material Group | Tested Specimen/ Denomination | Sample Description |
|---|---|---|
| Cerasmart (CS) | CS_nonexposed CS_A | Powder from the finished CAD/CAM block of CS, nonexposed to acidic artificial saliva |
| CS_rough exposed CS_B | Powder from the rough CAD/CAM block of CS, immersed in acidic artificial saliva for 1 month, at 37 °C | |
| CS_fine exposed CS_C | Powder from the finished CAD/CAM block of CS, immersed in acidic artificial saliva for 1 month, at 37 °C | |
| Straumann Nice (SN) | SN_nonexposed SN_A | Powder from the finished CAD/CAM block of SN, nonexposed to acidic artificial saliva |
| SN_rough exposed SN_B | Powder from the rough CAD/CAM block of SN, immersed in acidic artificial saliva for 1 month, at 37 °C | |
| SN_fine exposed SN_C | Powder from the finished CAD/CAM block of SN, immersed in acidic artificial saliva for 1 month, at 37 °C | |
| Tetric CAD (TC) | TC_nonexposed TC_A | Powder from the finished CAD/CAM block of TC, nonexposed to acidic artificial saliva |
| TC_rough exposed TC_B | Powder from the rough CAD/CAM block of TC, immersed in acidic artificial saliva for 1 month, at 37 °C | |
| TC_fine exposed TC_C | Powder from the finished CAD/CAM block of TC, immersed in acidic artificial saliva for 1 month, at 37 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ille, C.E.; Moacă, E.-A.; Suciu, M.; Barbu-Tudoran, L.; Negruțiu, M.-L.; Jivănescu, A. The Biological Activity of Fragmented Computer-Aided Design/Manufacturing Dental Materials before and after Exposure to Acidic Environment. Medicina 2023, 59, 104. https://doi.org/10.3390/medicina59010104
Ille CE, Moacă E-A, Suciu M, Barbu-Tudoran L, Negruțiu M-L, Jivănescu A. The Biological Activity of Fragmented Computer-Aided Design/Manufacturing Dental Materials before and after Exposure to Acidic Environment. Medicina. 2023; 59(1):104. https://doi.org/10.3390/medicina59010104
Chicago/Turabian StyleIlle, Codruța Eliza, Elena-Alina Moacă, Maria Suciu, Lucian Barbu-Tudoran, Meda-Lavinia Negruțiu, and Anca Jivănescu. 2023. "The Biological Activity of Fragmented Computer-Aided Design/Manufacturing Dental Materials before and after Exposure to Acidic Environment" Medicina 59, no. 1: 104. https://doi.org/10.3390/medicina59010104
APA StyleIlle, C. E., Moacă, E.-A., Suciu, M., Barbu-Tudoran, L., Negruțiu, M.-L., & Jivănescu, A. (2023). The Biological Activity of Fragmented Computer-Aided Design/Manufacturing Dental Materials before and after Exposure to Acidic Environment. Medicina, 59(1), 104. https://doi.org/10.3390/medicina59010104










