Opportunities of Amlodipine as a Potential Candidate in the Evaluation of Drug Compliance during Antihypertensive Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Statistical Methods
2.3. Method Validation
3. Results
3.1. Results from Participant Surveys
3.2. Results of AML and DAML Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Method Validation
| Chromatographic Parameters | |||
|---|---|---|---|
| Column | Acquity UPLC BEH C18 (2.1 × 50 mm, 1.7 μm) | ||
| Mobile phase | A: 0.1% aqueous formic acid B: acetonitrile | ||
| Gradient | linear, 0 min—25% B, 1.5 min—98% B, 3.0 min—98% B, 3.5 min—25% B, 5.0 min—25% B | ||
| Flow rate | 0.4 mL/min | ||
| Column temperature | 40 °C | ||
| Injection volume | 5 µL | ||
| Mass spectrometer parameters | |||
| Ionization | positive electrospray | ||
| Capillary voltage | 3.0 kV | ||
| Ion source temperature | 140 °C | ||
| Desolvation gas (N2) flow | 1000 L/h | ||
| Desolvation temperature | 600 °C | ||
| MRM parameters | |||
| Compound | MRM transition | Cone voltage, V | Collision energy, (eV) |
| AML | 409.0 > 238.0 409.0 > 294.0 | 30 30 | 10 10 |
| DAML | 407.0 > 286.0 | 30 | 25 |
Appendix A.2. Reagents
Appendix A.3. Sample Preparation
Appendix A.4. Sample Preparation for UPLC/MS/MS Analysis
Appendix A.5. Results of AML and DAML Validation
Appendix A.5.1. Selectivity
| Plasma Source | %, Response Blank Plasma vs. Spiked Plasma at LLOQ | |
|---|---|---|
| AML | DAML | |
| 1 | 4.2 | 0.2 |
| 2 | 4.0 | 1.9 |
| 3 | 3.1 | 1.7 |
| 4 | 3.3 | 2.2 |
| 5 | 4.5 | 1.9 |
| 6 | 3.5 | 1.8 |
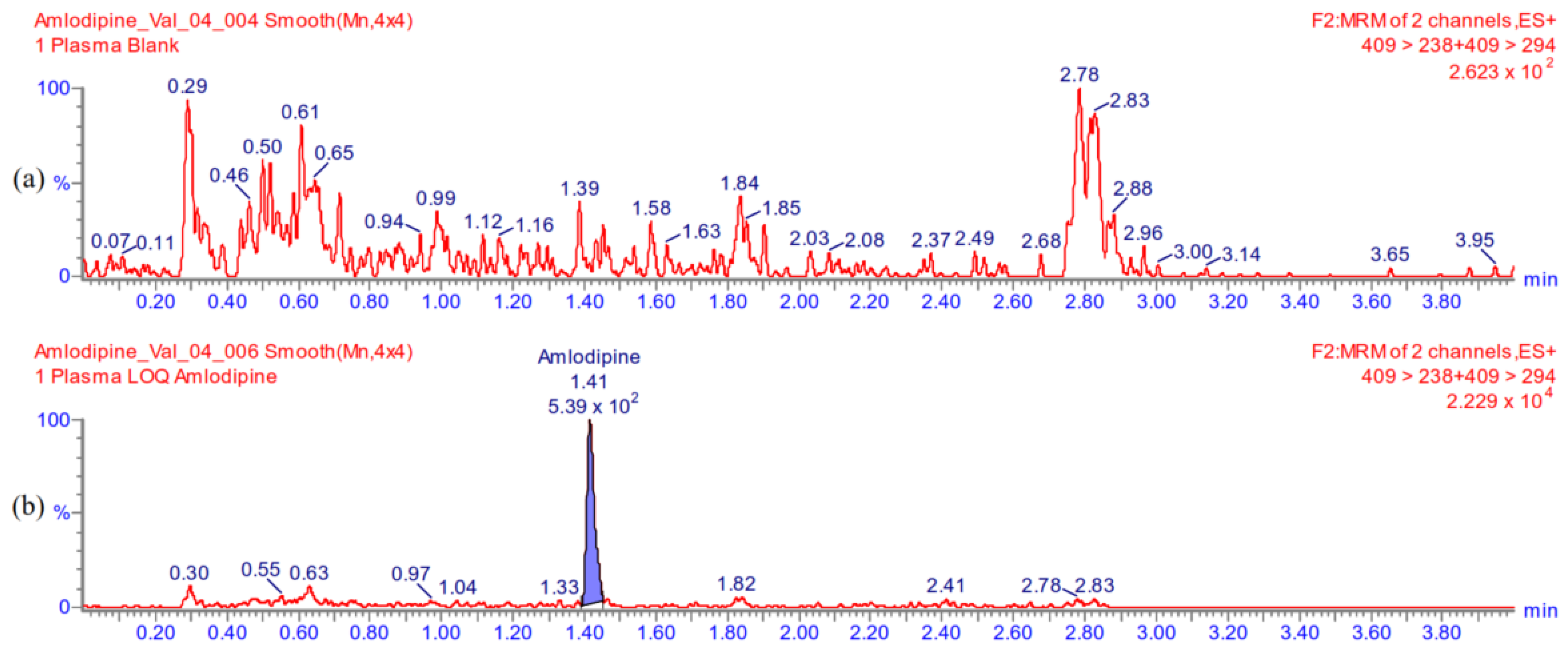
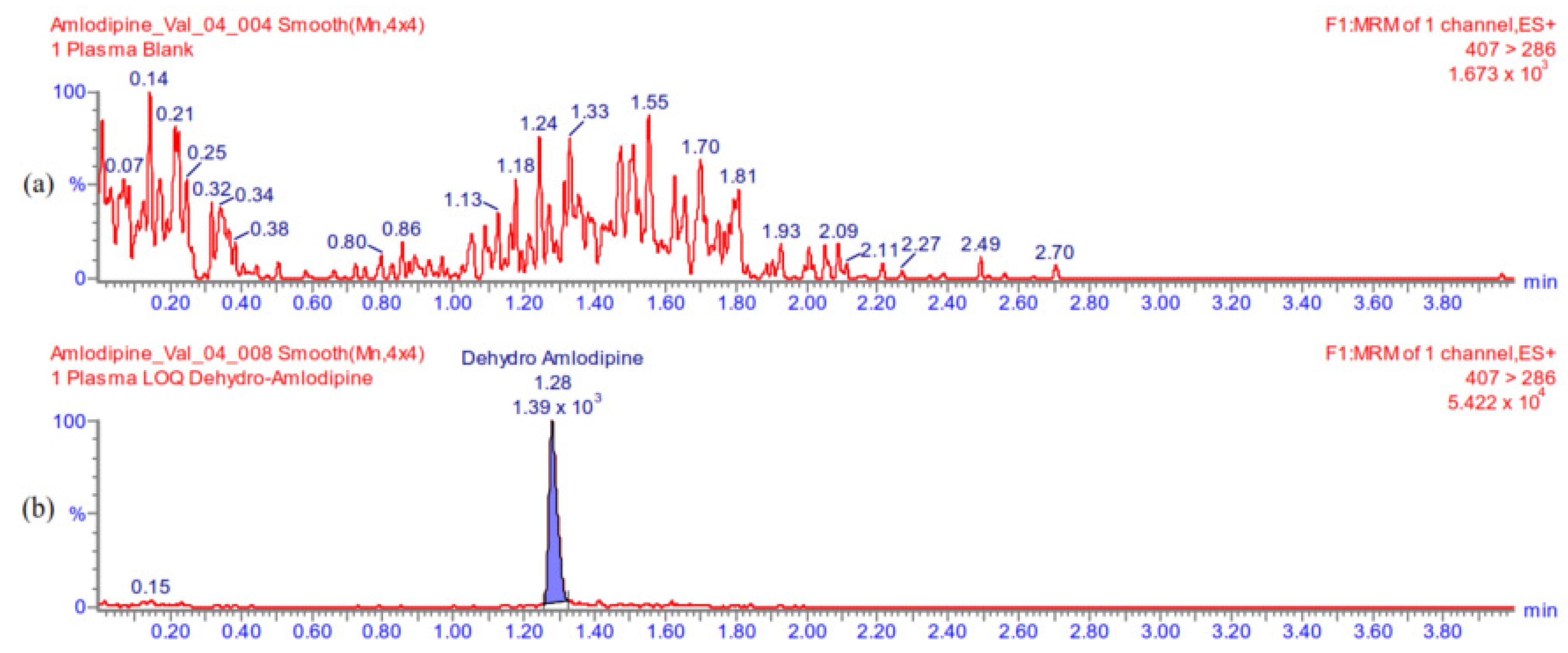
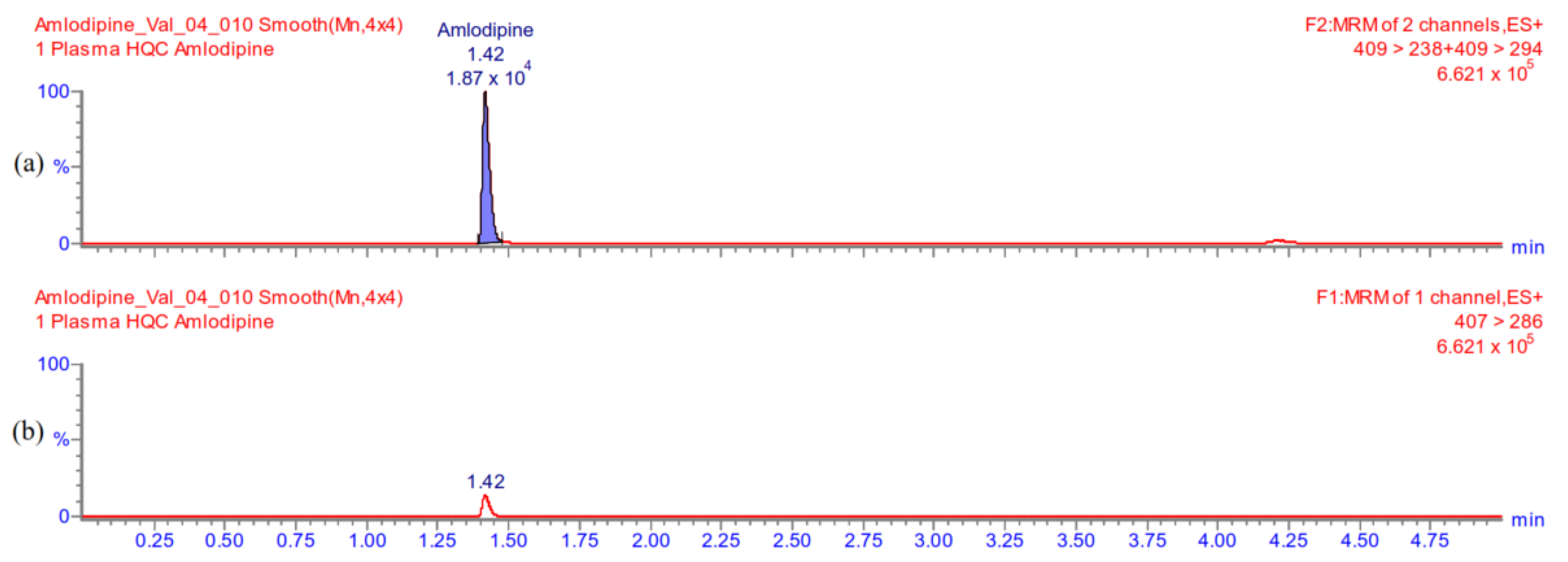
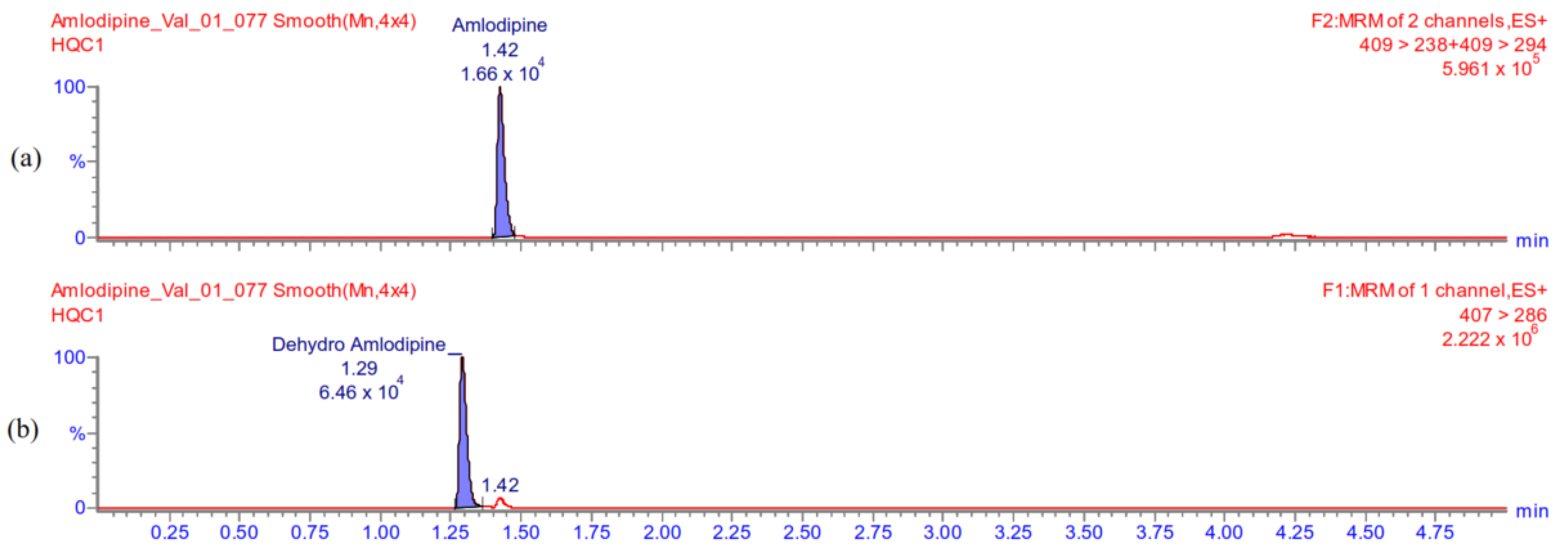
Appendix A.5.2. Carry-Over
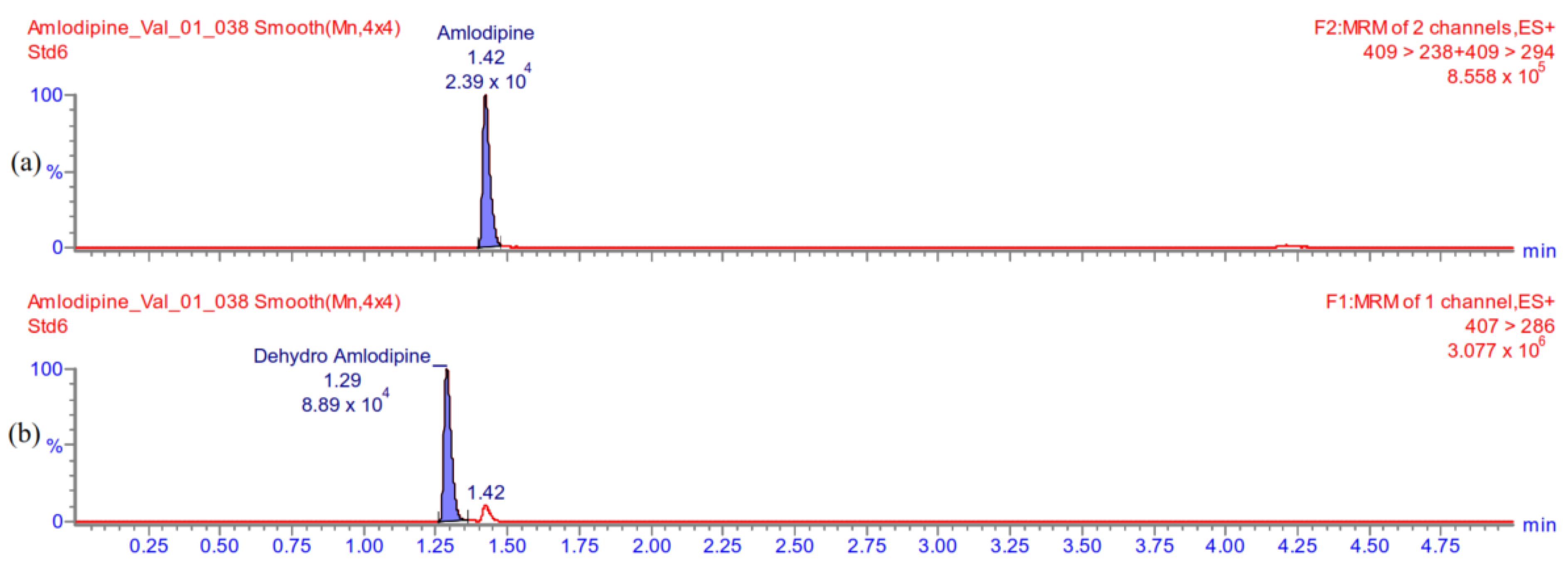
Appendix A.5.3. The Lowest Limit of Quantification
| Plasma Source | Signal to Noise | |
|---|---|---|
| AML | DAML | |
| 1 | 24 | 45 |
| 2 | 25 | 52 |
| 3 | 32 | 58 |
| 4 | 30 | 45 |
| 5 | 22 | 51 |
| 6 | 28 | 56 |
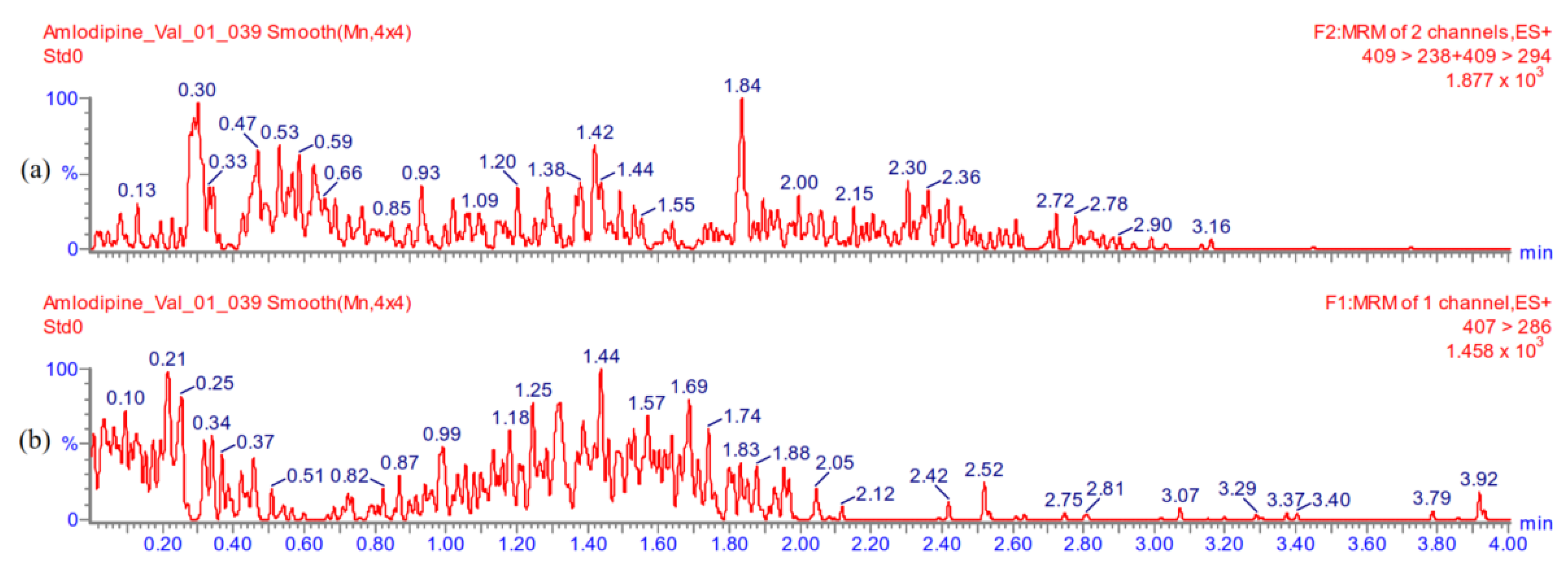
Appendix A.5.4. Linearity
| Standard Level | Nominal Conc., ng/mL | Day 1 | Day 2 | Day 3 | |||
|---|---|---|---|---|---|---|---|
| Back Calc. Conc., ng/mL | Conc. Dev., % | Back Calc. Conc., ng/mL | Conc. Dev., % | Back Calc. Conc., ng/mL | Conc. Dev., % | ||
| 1 | 1 | 0.91 | −8.8 | 1.16 | 15.5 | 1.09 | 9.0 |
| 1.09 | 9.0 | 1.12 | 11.9 | 1.05 | 5.3 | ||
| 1.10 | 10.0 | 1.11 | 11.0 | 1.10 | 10.4 | ||
| 2 | 2 | 2.07 | 3.7 | 1.98 | −1.0 | 2.04 | 2.0 |
| 1.97 | −1.6 | 1.83 | −8.4 | 1.94 | −3.2 | ||
| 1.94 | −3.1 | 1.77 | −11.3 | 2.10 | 5.1 | ||
| 3 | 5 | 4.96 | −0.8 | 4.96 | −0.7 | 4.90 | −2.1 |
| 5.04 | 0.7 | 4.69 | −6.3 | 4.76 | −4.8 | ||
| 5.03 | 0.6 | 4.98 | −0.4 | 4.64 | −7.3 | ||
| 4 | 10 | 9.76 | −2.4 | 9.96 | −0.4 | 9.50 | −5.0 |
| 9.66 | −3.4 | 9.56 | −4.4 | 9.32 | −6.8 | ||
| 9.50 | −5.0 | 9.40 | −6.0 | 9.50 | −5.0 | ||
| 5 | 25 | 24.57 | −1.7 | 24.63 | −1.5 | 24.50 | −2.0 |
| 24.64 | −1.4 | 24.43 | −2.3 | 25.22 | 0.9 | ||
| 25.40 | 1.6 | 24.82 | −0.7 | 24.32 | −2.7 | ||
| 6 | 50 | 50.12 | 0.2 | 51.02 | 2.0 | 51.25 | 2.5 |
| 51.27 | 2.5 | 51.26 | 2.5 | 50.91 | 1.8 | ||
| 49.98 | 0.0 | 50.32 | 0.6 | 50.87 | 1.7 | ||
| Calibration curve parameters | |||||||
| Day 1 | Day 2 | Day 3 | |||||
| A | 478.63 | 412.26 | 378.34 | ||||
| B | −55.19 | −96.62 | −98.69 | ||||
| r2 | 0.9994 | 0.9989 | 0.9988 | ||||
| Standard Level | Nominal Conc., ng/mL | Day 1 | Day 2 | Day 3 | |||
|---|---|---|---|---|---|---|---|
| Back Calc. Conc., ng/mL | Conc. Dev., % | Back Calc. Conc., ng/mL | Conc. Dev., % | Back Calc. Conc., ng/mL | Conc. Dev., % | ||
| 1 | 1 | 1.05 | 5.3 | 1.04 | 3.5 | 1.02 | 2.1 |
| 1.05 | 5.0 | 1.03 | 3.1 | 1.03 | 3.0 | ||
| 1.04 | 3.7 | 1.03 | 3.3 | 1.04 | 3.7 | ||
| 2 | 2 | 1.95 | −2.7 | 1.97 | −1.5 | 2.01 | 0.4 |
| 1.94 | −2.9 | 2.00 | 0.1 | 2.03 | 1.4 | ||
| 2.01 | 0.4 | 1.99 | −0.6 | 2.05 | 2.4 | ||
| 3 | 5 | 4.94 | −1.3 | 4.89 | −2.2 | 4.85 | −3.0 |
| 4.98 | −0.4 | 4.92 | −1.6 | 4.89 | −2.2 | ||
| 4.79 | −4.2 | 4.89 | −2.3 | 4.90 | −2.1 | ||
| 4 | 10 | 9.99 | −0.1 | 9.70 | −3.0 | 9.79 | −2.2 |
| 9.77 | −2.3 | 9.99 | −0.1 | 9.78 | −2.2 | ||
| 9.88 | −1.2 | 9.75 | −2.5 | 9.62 | −3.8 | ||
| 5 | 25 | 25.16 | 0.6 | 25.38 | 1.5 | 25.19 | 0.8 |
| 24.72 | −1.1 | 25.55 | 2.2 | 25.22 | 0.9 | ||
| 24.85 | −0.6 | 25.20 | 0.8 | 24.83 | −0.7 | ||
| 6 | 50 | 49.66 | −0.7 | 49.60 | −0.8 | 51.02 | 2.0 |
| 50.33 | 0.7 | 49.35 | −1.3 | 50.17 | 0.3 | ||
| 50.91 | 1.8 | 50.73 | 1.5 | 49.58 | −0.8 | ||
| Calibration curve parameters | |||||||
| Day 1 | Day 2 | Day 3 | |||||
| A | 1750.97 | 1587.51 | 1419.38 | ||||
| B | −235.98 | −264.37 | −233.04 | ||||
| r2 | 0.9998 | 0.9997 | 0.9997 | ||||
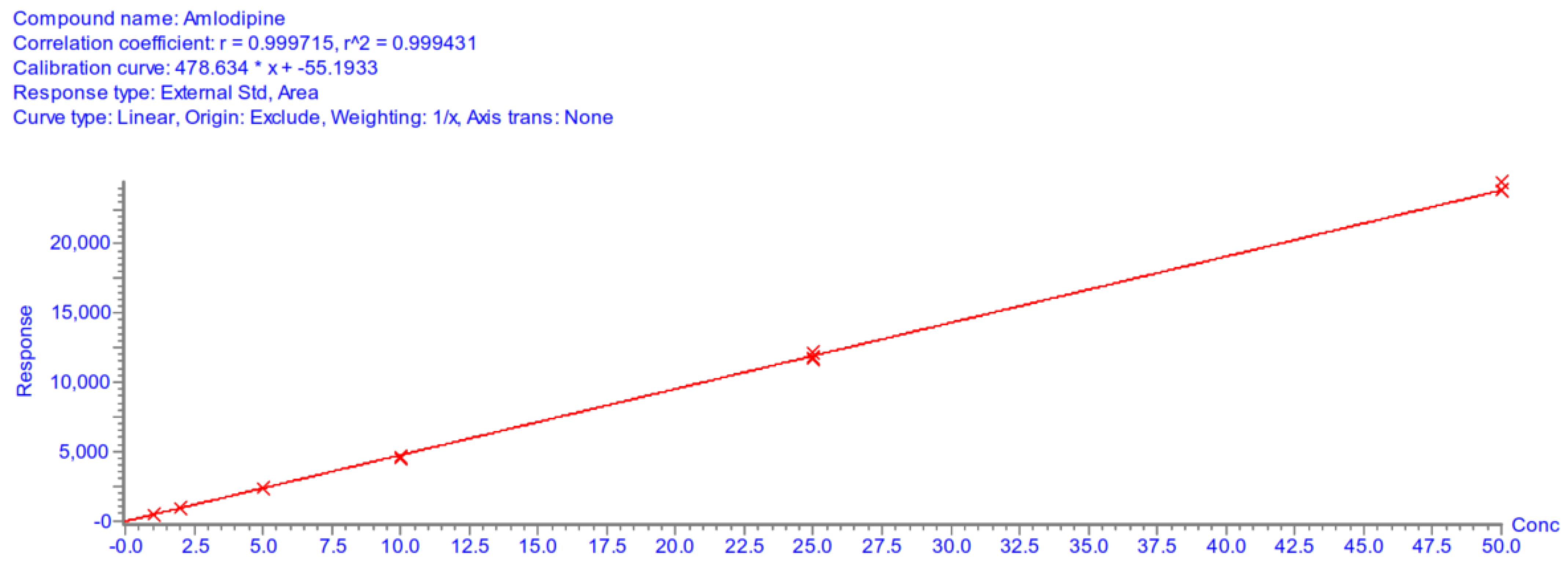
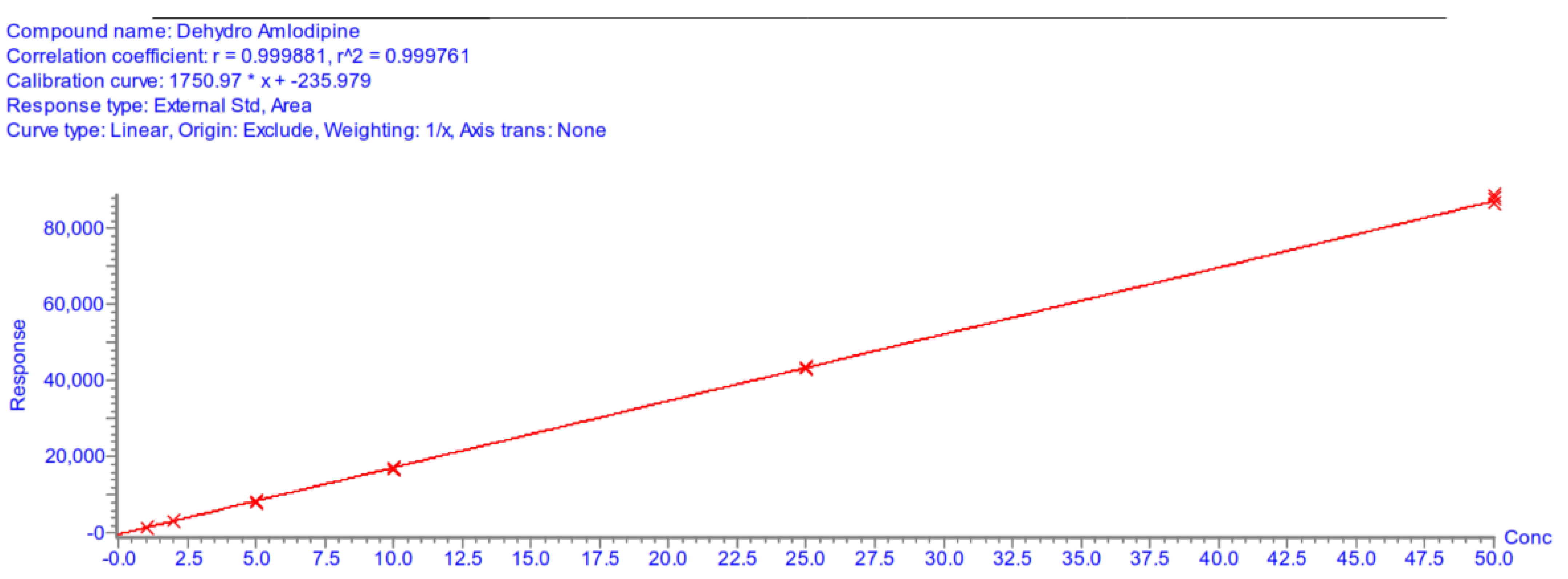
Appendix A.5.5. Accuracy and Precision
| QC Level, Conc., ng/mL | Day 1 | Day 2 | Day 3 | Between-Run | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Conc., ng/mL | RE, % | CV, % | Mean Conc., ng/mL | RE, % | CV, % | Mean Conc., ng/mL | RE, % | CV, % | Mean Conc., ng/mL | RE, % | CV, % | |
| LLOQ 1.0 ng/mL | 0.98 | −1.80 | 3.68 | 1.09 | 9.10 | 6.38 | 1.15 | 15.11 | 3.36 | 1.07 | 7.48 | 8.01 |
| LQC 2.5 ng/mL | 2.31 | −7.40 | 4.70 | 2.64 | 1.70 | 2.69 | 2.62 | 4.96 | 3.72 | 2.49 | −0.25 | 6.45 |
| MQC 20.0 ng/mL | 17.61 | −12.00 | 0.70 | 19.83 | −0.90 | 2.61 | 20.11 | 0.57 | 1.20 | 19.18 | −4.08 | 6.26 |
| HQC 40.0 ng/mL | 35.44 | −11.40 | 0.69 | 39.96 | −0.10 | 1.26 | 41.22 | 3.04 | 1.69 | 38.87 | −2.82 | 6.71 |
| QC Level, Conc., ng/mL | Day 1 | Day 2 | Day 3 | Between-Run | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Conc., ng/mL | RE, % | CV, % | Mean Conc., ng/mL | RE, % | CV, % | Mean Conc., ng/mL | RE, % | CV, % | Mean Conc., ng/mL | RE, % | CV, % | |
| LLOQ 1.0 ng/mL | 1.07 | 7.20 | 2.99 | 1.14 | 14.00 | 2.07 | 1.15 | 15.06 | 4.14 | 1.12 | 12.08 | 4.35 |
| LQC 2.5 ng/mL | 2.53 | 1.20 | 3.45 | 2.66 | 6.40 | 1.80 | 2.68 | 7.34 | 4.14 | 2.63 | 5.01 | 4.03 |
| MQC 20.0 ng/mL | 18.77 | −6.20 | 1.19 | 20.04 | 0.20 | 1.56 | 20.93 | 4.66 | 0.84 | 19.91 | −0.43 | 4.75 |
| HQC 40.0 ng/mL | 37.83 | −5.40 | 1.10 | 39.98 | −1.60 | 2.10 | 41.92 | 4.79 | 0.85 | 39.71 | −0.73 | 4.59 |
Appendix A.5.6. Matrix Effect
| Analyte | QC Level, Conc., ng/mL | Plasma Source | Matrix Effect | Average Matrix Effect | CV, % |
|---|---|---|---|---|---|
| AML | LQC 2.5 ng/mL | 1 | 1.06 | 1.01 | 3.7 |
| 2 | 1.03 | ||||
| 3 | 1.04 | ||||
| 4 | 0.96 | ||||
| 5 | 1.00 | ||||
| 6 | 0.98 | ||||
| HQC 40.0 ng/mL | 1 | 1.03 | 1.02 | 2.0 | |
| 2 | 1.05 | ||||
| 3 | 1.00 | ||||
| 4 | 1.00 | ||||
| 5 | 1.00 | ||||
| 6 | 1.02 | ||||
| DAML | LQC 2.5 ng/mL | 1 | 1.15 | 1.07 | 4.1 |
| 2 | 1.08 | ||||
| 3 | 1.06 | ||||
| 4 | 1.04 | ||||
| 5 | 1.03 | ||||
| 6 | 1.04 | ||||
| HQC 40.0 ng/mL | 1 | 1.13 | 1.01 | 6.4 | |
| 2 | 0.98 | ||||
| 3 | 0.96 | ||||
| 4 | 1.01 | ||||
| 5 | 0.95 | ||||
| 6 | 1.04 |
Appendix A.5.7. Stability
| QC Level, Conc., ng/mL | AML | DAML | ||
|---|---|---|---|---|
| Average Concentration Found, ng/mL | Stability, % | Average Concentration Found, ng/mL | Stability, % | |
| LQC 2.5 ng/mL | 2.11 | 82.4 | 2.42 | 96.7 |
| HQC 40.0 ng/mL | 34.80 | 87.0 | 36.49 | 91.2 |
| QC Level, Conc., ng/mL | AML | DAML | ||
|---|---|---|---|---|
| Average Concentration Found, ng/mL | Stability, % | Average Concentration Found, ng/mL | Stability, % | |
| LQC 2.5 ng/mL | 2.82 | 112.8 | 2.80 | 112.1 |
| HQC 40.0 ng/mL | 41.39 | 103.5 | 41.67 | 104.2 |
References
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- WHO. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- De Cates, A.N.; Farr, M.R.B.; Wright, N.; Jarvis, M.C.; Rees, K.; Ebrahim, S.; Huffman, M.D. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014, CD009868. [Google Scholar] [CrossRef]
- McCormack, T.; Boffa, R.J.; Jones, N.R.; Carville, S.; McManus, R.J. The 2018 ESC/ESH hypertension guideline and the 2019 NICE hypertension guideline, how and why they differ. Eur. Heart J. 2019, 40, 3456–3458. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, P.A. Amlodipine: An overview of its pharmacodynamic and pharmacokinetic properties. Clin. Cardiol. 1994, 17, III3-6. [Google Scholar] [PubMed]
- Toal, C.B.; Meredith, P.A.; Elliott, H.L. Long-acting dihydropyridine calcium-channel blockers and sympathetic nervous system activity in hypertension: A literature review comparing amlodipine and nifedipine GITS. Blood Press 2012, 21, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vukadinović, D.; Scholz, S.; Messerli, F.H.; Weber, M.A.; Williams, B.; Böhm, M.; Mahfoud, F. Peripheral edema and headache associated with amlodipine treatment: A meta-Analysis of randomized, placebo-controlled trials. J. Hypertens. 2019, 37, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, F.; Li, Q.; Zhu, M.; Du, A.; Tang, W.; Chen, W. Amlodipine metabolism in human liver microsomes and roles of CYP3A4/5 in the dihydropyridine dehydrogenation. Drug Metab. Dispos. 2013, 42, 245–2492014. [Google Scholar] [CrossRef]
- Alghurair, S.A.; Hughes, C.A.; Simpson, S.H.; Guirguis, L.M. A systematic review of patient self-reported barriers of adherence to antihypertensive medications using the world health organization multidimensional adherence model. J. Clin. Hypertens. 2012, 14, 877–886. [Google Scholar] [CrossRef]
- Iuga, A.O.; McGuire, M.J. Adherence and health care costs. Risk Manag. Health Policy 2014, 7, 35–44. [Google Scholar] [CrossRef]
- Burnier, M.; Egan, B.M. Adherence in Hypertension. Circ. Res. 2019, 124, 1124–1140. [Google Scholar] [CrossRef]
- George, J.; MacDonald, T. Home blood pressure monitoring. Eur. Cardiol. Rev. 2015, 10, 95–101. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency, Guideline on Bioanalytical Method Validation; European Medicines Agency: London, UK, 2012. [Google Scholar]
- Shi, S.; Shen, Z.; Duan, Y.; Ding, S.; Zhong, Z. Association Between Medication Literacy and Medication Adherence Among Patients With Hypertension. Front. Pharmacol. 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Fleig, S.V.; Weger, B.; Haller, H.; Limbourg, F.P. Effectiveness of a Fixed-Dose, Single-Pill Combination of Perindopril and Amlodipine in Patients with Hypertension: A Non-Interventional Study. Adv. Ther. 2018, 35, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Pallangyo, P.; Komba, M.; Mkojera, Z.S.; Kisenge, P.R.; Bhalia, S.; Mayala, H.; Kifai, E.; Richard, M.K.; Khanbhai, K.; Wibonela, S.; et al. Medication Adherence and Blood Pressure Control Among Hypertensive Outpatients Attending a Tertiary Cardiovascular Hospital in Tanzania: A Cross-Sectional Study. Integr. Blood Press. Control 2022, 15, 97–112. [Google Scholar] [CrossRef]
- Verma, A.A.; Khuu, W.; Tadrous, M.; Gomes, T.; Mamdani, M.M. Fixed-dose combination antihypertensive medications, adherence, and clinical outcomes: A population-based retrospective cohort study. PLoS Med. 2018, 15, e1002584. [Google Scholar] [CrossRef]
- Choi, H.Y.; Oh, I.J.; Lee, J.A.; Lim, J.; Kim, Y.S.; Jeon, T.-H.; Cheong, Y.-S.; Kim, D.-H.; Kim, M.-C.; Lee, S.Y. Factors affecting adherence to antihypertensive medication. Korean J. Fam. Med. 2018, 39, 325–332. [Google Scholar] [CrossRef]
- Sheppard, J.P.; Albasri, A.; Gupta, P.; Patel, P.; Khunti, K.; Martin, U.; McManus, R.J.; Hobbs, F.D.R. Measuring adherence to antihypertensive medication using an objective test in older adults attending primary care: Cross-sectional study. J. Hum. Hypertens. 2021, 36, 1106–1112. [Google Scholar] [CrossRef]
- Uchmanowicz, B.; Chudiak, A.; Uchmanowicz, I.; Rosińczuk, J.; Froelicher, E.S. Factors influencing adherence to treatment in older adults with hypertension. Clin. Interv. Aging 2018, ume 13, 2425–2441. [Google Scholar] [CrossRef]
- Consolazio, D.; Gattoni, M.E.; Russo, A.G. Exploring gender differences in medication consumption and mortality in a cohort of hypertensive patients in Northern Italy. BMC Public Health 2022, 22, 768. [Google Scholar] [CrossRef]
- Bhagavathula, A.S.; Shah, S.M.; Aburawi, E.H. Medication adherence and treatment-resistant hypertension in newly treated hypertensive patients in the United Arab Emirates. J. Clin. Med. 2021, 10, 5036. [Google Scholar] [CrossRef]
- Najimi, A.; Mostafavi, F.; Sharifirad, G.; Golshiri, P. Barriers to medication adherence in patients with hypertension: A qualitative study. J. Educ. Health Promot. 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A.; Bandere, D.; Logviss, K.; Šmits, D.; Urtāne, I. Adherence level to arterial hypertension treatment: A cross-sectional patient survey and retrospective analysis of the nhs prescription database. Healthcare 2021, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Shimels, T.; Kassu, R.A.; Bogale, G.; Bekele, M.; Getnet, M.; Getachew, A.; Shewamene, Z.; Abraha, M. Magnitude and associated factors of poor medication adherence among diabetic and hypertensive patients visiting public health facilities in Ethiopia during the COVID-19 pandemic. PLoS ONE 2021, 16, e0249222. [Google Scholar] [CrossRef]
- Abdisa, L.; Alemu, A.; Heluf, H.; Sertsu, A.; Dessie, Y.; Negash, B.; Ayana, G.M.; Letta, S. Factors associated with poor medication adherence during COVID-19 pandemic among hypertensive patients visiting public hospitals in Eastern Ethiopia: A cross-sectional study. BMJ Open 2022, 12, 64284. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Aydın Avcı, İ. The influence of the pandemic on fear of contagion, blood pressure management and adherence to medication in hypertensive older adults in Turkey. J. Hum. Hypertens. 2022, 36, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Rao, R.; Goodman, J.D.H.; Connolly, K.; O’Shaughnessy, K.M. Nonadherence to antihypertensive medications amongst patients with uncontrolled hypertension: A retrospective study. Medicine 2021, 100, e24654. [Google Scholar] [CrossRef] [PubMed]
- Gebreyohannes, E.A.; Bhagavathula, A.S.; Abebe, T.B.; Tefera, Y.G.; Abegaz, T.M. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin. Hypertens. 2019, 25, 1. [Google Scholar] [CrossRef]
- Corrao, G.; Rea, F.; Ghirardi, A.; Soranna, D.; Merlino, L.; Mancia, G. Adherence with antihypertensive drug therapy and the risk of heart failure in clinical practice. Hypertension 2015, 66, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Wagmann, L.; Vollmer, A.C.; Lauder, L.; Mahfoud, F.; Meyer, M.R. Assessing adherence to antihypertensive medication by means of dose-dependent reference plasma concentration ranges and ultra-high performance liquid chromatography-ion trap mass spectrometry analysis. Molecules 2021, 26, 1495. [Google Scholar] [CrossRef]
- Adidja, N.M.; Agbor, V.N.; Aminde, J.A.; Ngwasiri, C.A.; Ngu, K.B.; Aminde, L.N. Non-adherence to antihypertensive pharmacotherapy in Buea, Cameroon: A cross-sectional community-based study. BMC Cardiovasc. Disord. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Fares, H.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Amlodipine in hypertension: A first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart 2016, 3, e000473. [Google Scholar] [CrossRef]
- Ozawa, Y.; Hayashi, K.; Kobori, H. New Generation Calcium Channel Blockers in Hypertensive Treatment. Curr. Hypertens. Rev. 2006, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, D.R. Pharmacokinetics and pharmacodynamics of Amlodipine. Cardiology 1992, 80, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Meredith, P.A.; Elliott, H.L. Clinical Pharmacokinetics of Amlodipine. Clin. Pharmacokinet. 1992, 22, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Koraćević, G.P.; Dakić, S.S.; Veličković-Radovanović, R.M.; Apostolovic, S.; Krstić, N.H.; Tasic, I.; Zdravkovic, M.; Antonijević, N.M.; Damnjanović, G.N.; Kostić, T.L. Amlodipine as an antiischemic drug is superior to long acting nitrates. Open Med. 2015, 10, 50–56. [Google Scholar] [CrossRef]
- Sirikatitham, A.; Panrat, K.; Tanmanee, N.W. Determination of amlodipine in human plasma by electrospray ionization LC-MS/MS method: Validation and its stability studies. Songklanakarin J. Sci. Technol. 2008, 30, 455–462. [Google Scholar]
- Nirogi, R.V.S.; Kandikere, V.N.; Mudigonda, K.; Shukla, M.; Maurya, S. Sensitive and rapid liquid chromatography/tandem mass spectrometry assay for the quantification of amlodipine in human plasma. Biomed. Chromatogr. 2006, 20, 833–842. [Google Scholar] [CrossRef]
- Bhatt, J.; Singh, S.; Subbaiah, G.; Shah, B.; Kambli, S.; Ameta, S. A rapid and sensitive liquid chromatography-tandem mass spectrometry (LS-MS/MS) method for the estimation of amlodipine in human plasma. Biomed. Chromatogr. 2007, 21, 169–175. [Google Scholar] [CrossRef]
- Kristoffersen, L.; Øiestad, E.L.; Opdal, M.S.; Krogh, M.; Lundanes, E.; Christophersen, A.S. Simultaneous determination of 6 beta-blockers, 3 calcium-channel antagonists, 4 angiotensin-II antagonists and 1 antiarrhytmic drug in post-mortem whole blood by automated solid phase extraction and liquid chromatography mass spectrometry. Method developm. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 850, 147–160. [Google Scholar] [CrossRef]
- Ma, Y.; Qin, F.; Sun, X.; Lu, X.; Li, F. Determination and pharmacokinetic study of amlodipine in human plasma by ultra performance liquid chromatography-electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 1540–1545. [Google Scholar] [CrossRef]
- Ramani, A.V.; Sengupta, P.; Mullangi, R. Development and validation of a highly sensitive and robust LC-ESI-MS/MS method for simultaneous quantitation of simvastatin acid, amlodipine and valsartan in human plasma: Application to a clinical pharmacokinetic study. Biomed. Chromatogr. 2009, 23, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhan, Y.; Ge, Z.; Wei, P.; Ouyang, P. Liquid chromatography-mass spectrometry method for the determination of amlodipine in human plasma and its application in a bioequivalence study. Arzneimittel Forschung Drug Res. 2009, 59, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Jangala, H.; Vats, P.; Khuroo, A.H.; Monif, T. Development and Validation of a LC-MS/MS Method for the Simultaneous Estimation of Amlodipine and Valsartan in Human Plasma: Application to a Bioequivalence Study. Sci. Pharm. 2014, 82, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Bae, Y.-J.; Kim, H.S.; Cha, H.-J.; Yun, J.-S.; Shin, J.-S.; Seong, W.-K.; Lee, Y.-M.; Han, K.-M. Measurement of human cytochrome P450 enzyme induction based on mesalazine and mosapride citrate treatments using a luminescent assay. Biomol. Ther. 2015, 23, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.; Zhang, H. In vitro evaluation of reversible and irreversible cytochrome P450 inhibition: Current status on methodologies and their utility for predicting drug-drug interactions. AAPS J. 2008, 10, 410–424. [Google Scholar] [CrossRef]
- Danafar, H.; Hamidi, M. Method validation of amlodipine and atorvastatin by liquid chromatography–mass spectrometry (LC–MS) method in human plasma. Cogent Med. 2016, 3, 1129790. [Google Scholar] [CrossRef]
- Meyyanathan, S.N.; Muralidharan, S.; Rajan, S.; Gopal, K.; Suresh, B. A Simple Sample Preparation with HPLC—UV Method for Estimation of Amlodipine from Plasma: Application to Bioequivalence Study. Open Chem. Biomed. Methods J. 2008, 1, 22–27. [Google Scholar] [CrossRef]
- Lv, C.-M.; Wei, C.-M.; Bu, F.-L.; Chen, R.; Wang, X.-L.; Li, R.; Wang, B.-J.; Guo, R.-C. Determination of Amlodipine in Human Plasma by LC-MS/MS and Its Bioequivalence Study in Healthy Chinese Subjects. Pharmacol. Pharm. 2013, 4, 191–200. [Google Scholar] [CrossRef]
- Taguchi, R.; Naito, T.; Sato, H.; Kawakami, J. Validated LC-MS/MS method for the simultaneous determination of amlodipine and its major metabolites in human plasma of hypertensive patients. Ther. Drug Monit. 2017, 39, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Lin, S.; Fang, L.; Lou, S.; Zhao, D.; Zhu, J.; Yang, Q.; Wang, Y. Evaluation of pharmacokinetics and safety with bioequivalence of Amlodipine in healthy Chinese volunteers: Bioequivalence Study Findings. J. Clin. Lab. Anal. 2020, 34, e23228. [Google Scholar] [CrossRef]
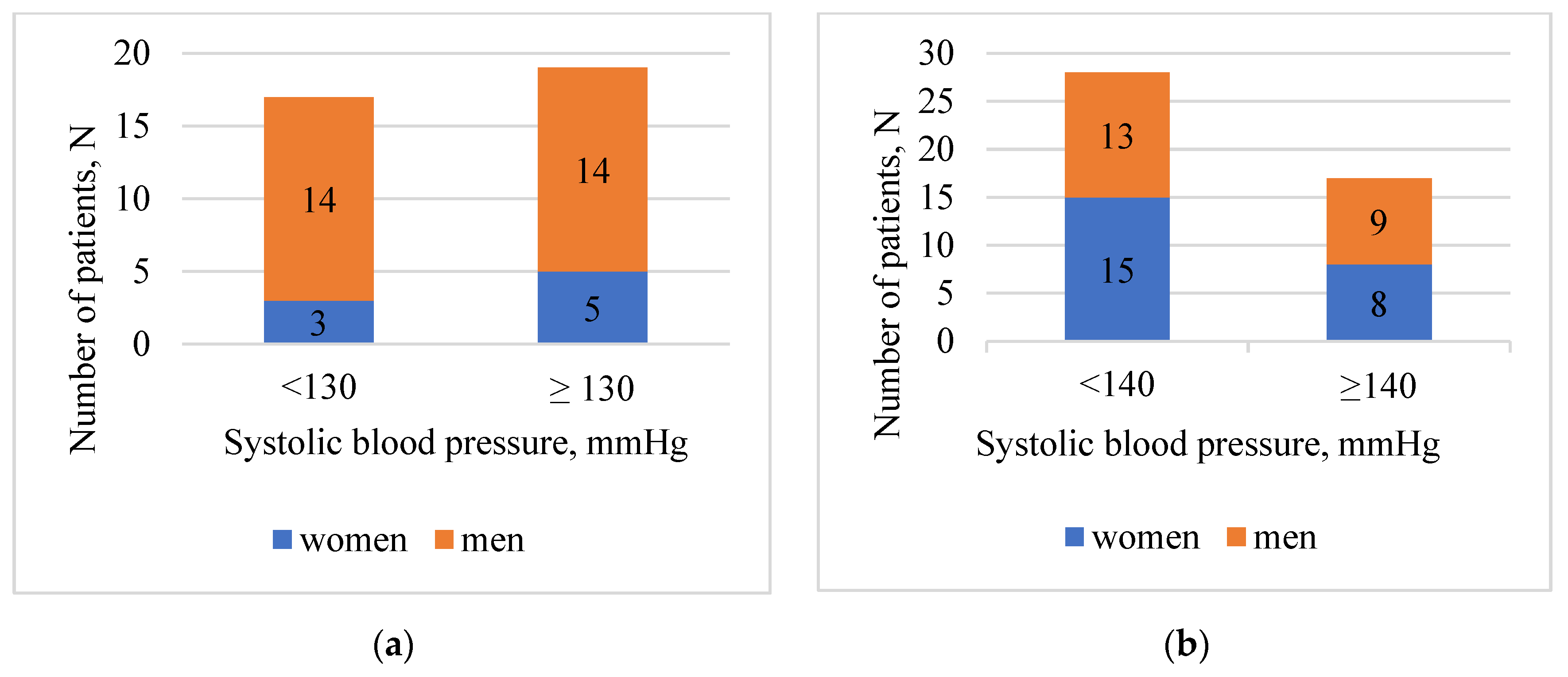
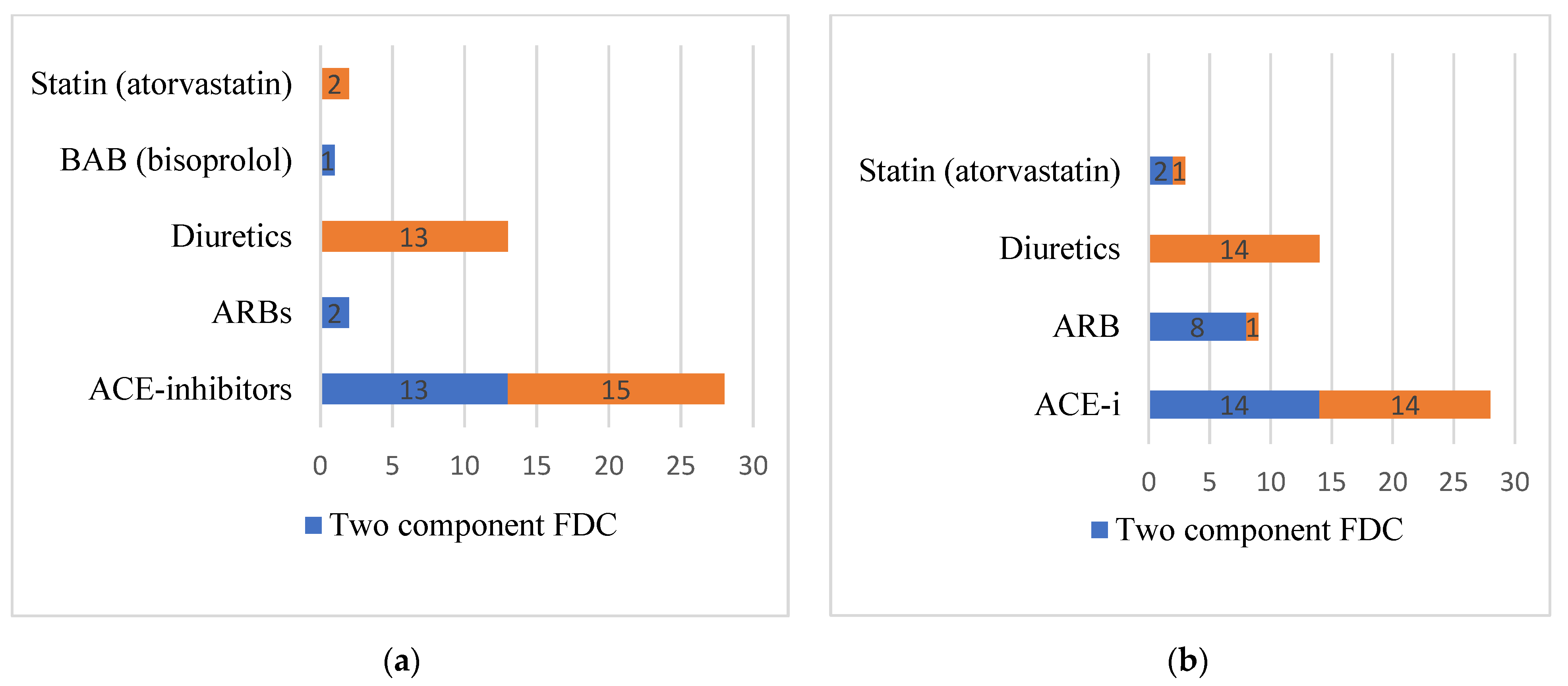
| Characteristics | Value |
|---|---|
| Women, n (%) | 31 (38.7) |
| Men, n (%) | 50 (61.7) |
| Mean age, years ± SD: | 66.6 ± 9.1 |
| 69.8 ± 8.0 |
| 64.6 ± 9.2 |
| Antihypertensive drug combination containing AML, n (%) | |
| 11 (13.6) |
| 40 (49.4) |
| 30 (37.0) |
| Dose of AML (mg), n (%) | |
| 7 (8.6) |
| 50 (61.7) |
| 24 (30.9) |
| Results | Age Group | |||
|---|---|---|---|---|
| <65 Years (%) | ≥65 Years (%) | |||
| Total | 36 | 45 | ||
| Female | 8 (22.2) | 23 (51.1) | ||
| Male | 28 (77.8) | 22 (48.9) | ||
| BMI, kg/m2 | Women | 18.5–24.99 | 0 (0) | 1 (2.2) |
| >25–29.99 | 4 (11.1) | 10 (22.2) | ||
| ≥30 | 4 (11.1) | 12 (26.7) | ||
| Men | 18.5–24.99 | 5 (13.9) | 6 (13.3) | |
| >25–29.99 | 11 (30.6) | 8 (2.2) | ||
| ≥30 | 12 (33.3) | 8 (17.8) | ||
| Respondents that reached target SBP (measurement in hospital) | 17 (47.2) | 28 (62.2) | ||
| Respondents that reached target SBP (measurement at home *) | 14 (38.8) | 32 (71.1) | ||
| Respondents that reached target DBP (measurement in hospital) | 14 (38.9) | 36 (80.0) | ||
| Respondents that reached target DBP (measurement at home *) | 11 (35.5) | 43 (97.7) | ||
| AML formulations of patients with reached target SBP (<130 mm Hg at the age <65 years; <140 mm Hg at the age ≥65) | Single | 3 (8.3) | 4 (8.9) | |
| Two component FDC | 7 (19.4) | 17 (37.8) | ||
| Three component FDC | 7 (19.4) | 7 (15.6) | ||
| Respondents that failed to reach target SYS blood pressure (measurement in hospital) | 19 (52.8) | 17 (37.8) | ||
| Respondents that failed to reach target SYS blood pressure (measurement at home *) | 17 (54.8) | 12 (26.7) | ||
| Respondents that failed to reach target DBP (measurement in hospital) | 22 (61.1) | 9 (20.0) | ||
| Respondents that failed to reach target DBP (measurement at home *) | 20 (64.5) | 1 (2.3) | ||
| AML formulations of patients with non-reached target SBP (>130 mm Hg at the age <65 years; >140 mm Hg at the age ≥65) | Single | 2 (5.6) | 2 (4.4) | |
| Two component FDC | 9 (25.0) | 7 (15.6) | ||
| Three component FDC | 8 (22.2) | 8 (17.8) | ||
| Respondents that forget to take hypertension medications | Women | 2 (5.6) | 7 (15.6) | |
| Men | 14 (38.9) | 8 (17.8) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kustovs, D.; Urtāne, I.; Sevostjanovs, E.; Moreino, E.; Trušinskis, K. Opportunities of Amlodipine as a Potential Candidate in the Evaluation of Drug Compliance during Antihypertensive Therapy. Medicina 2023, 59, 340. https://doi.org/10.3390/medicina59020340
Kustovs D, Urtāne I, Sevostjanovs E, Moreino E, Trušinskis K. Opportunities of Amlodipine as a Potential Candidate in the Evaluation of Drug Compliance during Antihypertensive Therapy. Medicina. 2023; 59(2):340. https://doi.org/10.3390/medicina59020340
Chicago/Turabian StyleKustovs, Dmitrijs, Inga Urtāne, Eduards Sevostjanovs, Eva Moreino, and Kārlis Trušinskis. 2023. "Opportunities of Amlodipine as a Potential Candidate in the Evaluation of Drug Compliance during Antihypertensive Therapy" Medicina 59, no. 2: 340. https://doi.org/10.3390/medicina59020340
APA StyleKustovs, D., Urtāne, I., Sevostjanovs, E., Moreino, E., & Trušinskis, K. (2023). Opportunities of Amlodipine as a Potential Candidate in the Evaluation of Drug Compliance during Antihypertensive Therapy. Medicina, 59(2), 340. https://doi.org/10.3390/medicina59020340





