REST/NRSF Silencing Modifies Neuronal Gene Expression in siRNA-Treated HeLa Cells: A Preliminary Exploration in the Search for Neuronal Biomarkers of Cervical Cancer
Abstract
:1. Introduction
2. Materials and Methods
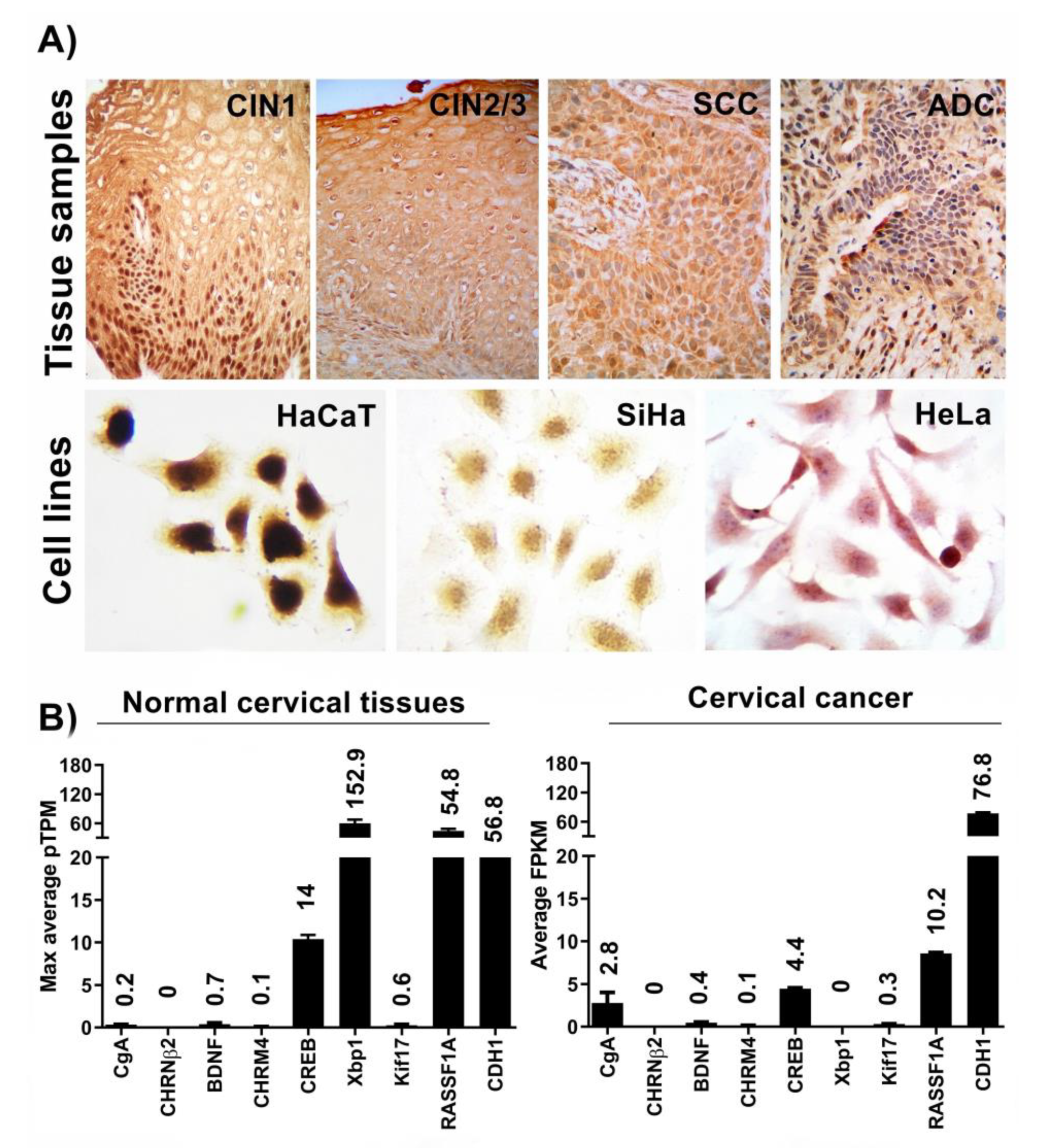
2.1. Immunocytochemistry (ICC) and Immunohistochemistry (IHC)
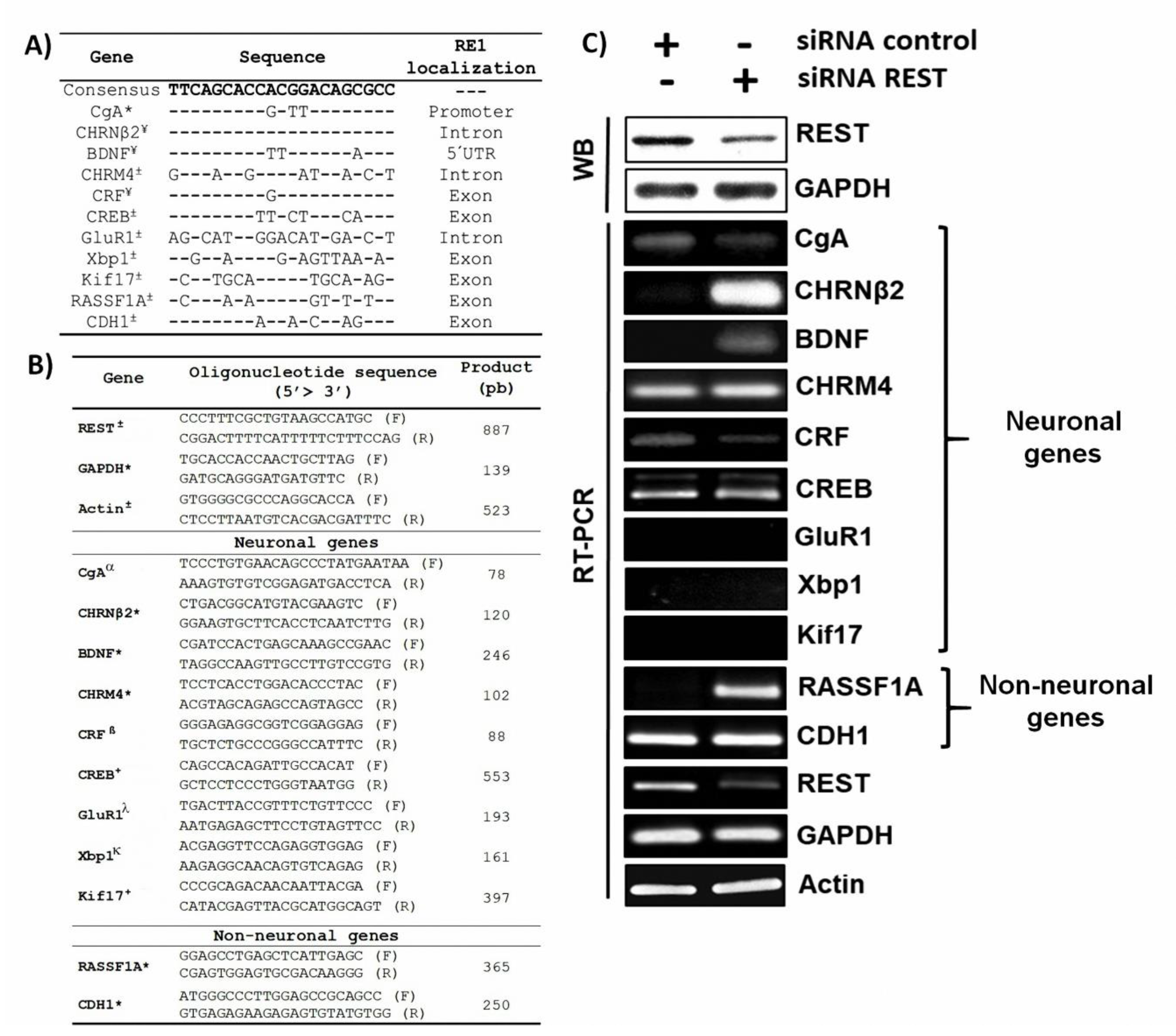
2.2. In Silico Analysis
2.3. HeLa Cell Culture and Transfection
2.4. Western Blot
2.5. RT-PCR
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballas, N.; Grunseich, C.; Lu, D.D.; Speh, J.C.; Mandel, G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 2005, 121, 645–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernsberger, U. Regulation of gene expression during early neuronal differentiation: Evidence for patterns conserved across neuron populations and vertebrate classes. Cell Tissue Res. 2012, 348, 1–27. [Google Scholar] [CrossRef]
- Ortuño-Pineda, C.; Galindo-Rosales, J.M.; Calderón-Salinas, J.V.; Villegas-Sepúlveda, N.; Saucedo-Cárdenas, O.; De Nova-Ocampo, M.; Valdés, J. Binding of hnRNP H and U2AF65 to Respective G-codes and a Poly-Uridine Tract Collaborate in the N50-5′ss Selection of the REST N Exon in H69 Cells. PLoS ONE 2012, 7, e40315. [Google Scholar]
- Zhang, P.; Lathia, J.D.; Flavahan, W.A.; Rich, J.N.; Mattson, M.P. Squelching glioblastoma stem cells by targeting REST for proteasomal degradation. Trends Neurosci. 2009, 32, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimojo, M. Characterization of the nuclear targeting signal of REST/NRSF. Neurosci. Lett. 2006, 398, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shimojo, M.; Chai, Y.G.; Hersh, L.B. Studies on the interaction of REST4 with the cholinergic repressor element-1/neuron restrictive silencer element. Brain Res. Mol. Brain Res. 2000, 80, 88–98. [Google Scholar] [CrossRef]
- Bruce, A.W.; Donaldson, I.J.; Wood, I.C.; Yerbury, S.A.; Sadowski, M.I.; Chapman, M.; Göttgens, B.; Buckley, N.J. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc. Natl. Acad. Sci. USA 2004, 101, 10458–10463. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Kameoka, S.; Gopalakrishnan, V.; Aldape, K.D.; Pan, Z.Z.; Lang, F.F.; Majumder, S. Conversion of myoblasts to physiologically active neuronal phenotype. Genes Dev. 2004, 18, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.-M.; Greenway, D.J.; Johnson, R.; Street, M.; Belyaev, N.D.; Deuchars, J.; Bee, T.; Wilde, S.; Buckley, N.J. Distinct Profiles of REST Interactions with Its Target Genes at Different Stages of Neuronal Development. Mol. Biol. Cell 2005, 16, 5630–5638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson, J.M.; Edgson, J.L.; Woll, P.J.; Quinn, J.P. A splice variant of the neuron-restrictive silencer factor repressor is expressed in small cell lung cancer: A potential role in derepression of neuroendocrine genes and a useful clinical marker. Cancer Res. 2000, 60, 1840–1844. [Google Scholar] [PubMed]
- Wagoner, M.P.; Gunsalus, K.T.W.; Schoenike, B.; Richardson, A.L.; Friedl, A.; Roopra, A. The transcription factor REST is lost in aggressive breast cancer. PLoS Genet. 2010, 6, e1000979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, C.; Ceder, J.; Iglesias-Gato, D.; Chuan, Y.-C.; Pang, S.T.; Bjartell, A.; Martinez, R.M.; Bott, L.; Helczynski, L.; Ulmert, D.; et al. REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer. Nucleic Acids Res. 2014, 42, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.P.; Bubb, V.J.; Marshall-Jones, Z.V.; Coulson, J.M. Neuron restrictive silencer factor as a modulator of neuropeptide gene expression. Regul. Pept. 2002, 108, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Coulson, J.M.; Fiskerstrand, C.E.; Woll, P.J.; Quinn, J.P. Arginine vasopressin promoter regulation is mediated by a neuron-restrictive silencer element in small cell lung cancer. Cancer Res. 1999, 59, 5123–5127. [Google Scholar] [PubMed]
- Volkova, L.V.; Pashov, A.I.; Omelchuk, N.N. Cervical Carcinoma: Oncobiology and Biomarkers. Int. J. Mol. Sci. 2021, 22, 12571. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Sarabia, K.; Alarcón-Romero, L.C.; Flores-Alfaro, E.; Illades-Aguiar, B.; Vences-Velázquez, A.; Mendoza-Catalán, M.A.; Navarro-Tito, N.; Valdés, J.; Moreno-Godínez, M.E.; Ortuño-Pineda, C. Significant decrease of a master regulator of genes (REST/NRSF) in high-grade squamous intraepithelial lesion and cervical cancer. Biomed. J. 2021, 44, S171–S178. [Google Scholar] [CrossRef] [PubMed]
- Hanby, A.M.; Walker, C.; Tavassoli, F.A.; Devilee, P. Pathology and Genetics: Tumours of the Breast and Female Genital Organs. WHO Classification of Tumours series-volume IV. Lyon, France: IARC Press: 2003. 250pp. Breast Cancer Res. 2004, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, K.; Ishii, J.; Sakaeda, M.; Arimasu, Y.; Shimoyamada, H.; Sato, H.; Miyata, C.; Kamma, H.; Aoki, I.; Yazawa, T. Differences of molecular expression mechanisms among neural cell adhesion molecule 1, synaptophysin, and chromogranin A in lung cancer cells: Expression mechanisms of LNET markers. Pathol. Int. 2012, 62, 232–245. [Google Scholar] [CrossRef]
- Schoenherr, C.J.; Paquette, A.J.; Anderson, D.J. Identification of potential target genes for the neuron-restrictive silencer factor. Proc. Natl. Acad. Sci. USA 1996, 93, 9881–9886. [Google Scholar] [CrossRef] [Green Version]
- Chteinberg, E.; Sauer, C.M.; Rennspiess, D.; Beumers, L.; Schiffelers, L.; Eben, J.; Haugg, A.; Winnepenninckx, V.; Kurz, A.K.; Speel, E.-J.; et al. Neuroendocrine Key Regulator Gene Expression in Merkel Cell Carcinoma. Neoplasia 2018, 20, 1227–1235. [Google Scholar] [CrossRef]
- Janitzky, K.; Peine, A.; Kröber, A.; Yanagawa, Y.; Schwegler, H.; Roskoden, T. Increased CRF mRNA expression in the sexually dimorphic BNST of male but not female GAD67 mice and TMT predator odor stress effects upon spatial memory retrieval. Behav. Brain Res. 2014, 272, 141–149. [Google Scholar] [CrossRef]
- Bo, J.; Zhang, W.; Sun, X.; Yang, Y.; Liu, X.; Jiang, M.; Ma, Z.; Gu, X. The cyclic AMP response element-binding protein antisense oligonucleotide induced anti-nociception and decreased the expression of KIF17 in spinal cord after peripheral nerve injury in mice. Int. J. Clin. Exp. Med. 2014, 7, 5181–5191. [Google Scholar] [PubMed]
- Challenor, M.; Doig, R.O.H.; Fuller, P.; Giacci, M.; Bartlett, C.; Wale, C.H.; Cozens, G.S.; Hool, L.; Dunlop, S.; Iyer, K.S.; et al. Prolonged glutamate excitotoxicity increases GluR1 immunoreactivity but decreases mRNA of GluR1 and associated regulatory proteins in dissociated rat retinae in vitro. Biochimie 2015, 112, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xing, P.; Cui, W.; Wang, W.; Cui, Y.; Ying, G.; Wang, X.; Li, B. Acute Endoplasmic Reticulum Stress-Independent Unconventional Splicing of XBP1 mRNA in the Nucleus of Mammalian Cells. Int. J. Mol. Sci. 2015, 16, 13302–13321. [Google Scholar]
- Nahand, J.S.; Taghizadeh-Boroujeni, S.; Karimzadeh, M.; Borran, S.; Pourhanifeh, M.H.; Moghoofei, M.; Bokharaei-Salim, F.; Karampoor, S.; Jafari, A.; Asemi, Z.; et al. microRNAs: New prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J. Cell Physiol. 2019, 234, 17064–17099. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, F.; Limones-González, J.E.; Mendoza-Almanza, B.; Esparza-Ibarra, E.L.; Gallegos-Flores, P.I.; Ayala-Luján, J.L.; Godina-González, S.; Salinas, E.; Mendoza-Almanza, G. Understanding Cervical Cancer through Proteomics. Cells 2021, 10, 1854. [Google Scholar] [CrossRef]
- Yi, J.W.; Jang, M.; Kim, S.J.; Kim, S.S.; Rhee, J.E. Degradation of p53 by natural variants of the E6 protein of human papillomavirus type 16. Oncol. Rep. 2013, 29, 1617–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Ricciardi, R.P. Transformation by E1A oncoprotein involves ubiquitin-mediated proteolysis of the neuronal and tumor repressor REST in the nucleus. J. Virol. 2012, 86, 5594–5602. [Google Scholar] [CrossRef] [Green Version]
- Bessis, A.; Champtiaux, N.; Chatelin, L.; Changeux, J.P. The neuron-restrictive silencer element: A dual enhancer/silencer crucial for patterned expression of a nicotinic receptor gene in the brain. Proc. Natl. Acad. Sci. USA 1997, 94, 5906–5911. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Sarabia, K.; Alarcón-Romero, L.D.C.; Mendoza-Catalán, M.Á.; Carpio-Pedroza, J.C.; Castañeda-Saucedo, E.; Ortuño-Pineda, C. REST/NRSF Silencing Modifies Neuronal Gene Expression in siRNA-Treated HeLa Cells: A Preliminary Exploration in the Search for Neuronal Biomarkers of Cervical Cancer. Medicina 2023, 59, 537. https://doi.org/10.3390/medicina59030537
Cortés-Sarabia K, Alarcón-Romero LDC, Mendoza-Catalán MÁ, Carpio-Pedroza JC, Castañeda-Saucedo E, Ortuño-Pineda C. REST/NRSF Silencing Modifies Neuronal Gene Expression in siRNA-Treated HeLa Cells: A Preliminary Exploration in the Search for Neuronal Biomarkers of Cervical Cancer. Medicina. 2023; 59(3):537. https://doi.org/10.3390/medicina59030537
Chicago/Turabian StyleCortés-Sarabia, Karen, Luz Del Carmen Alarcón-Romero, Miguel Ángel Mendoza-Catalán, Juan Carlos Carpio-Pedroza, Eduardo Castañeda-Saucedo, and Carlos Ortuño-Pineda. 2023. "REST/NRSF Silencing Modifies Neuronal Gene Expression in siRNA-Treated HeLa Cells: A Preliminary Exploration in the Search for Neuronal Biomarkers of Cervical Cancer" Medicina 59, no. 3: 537. https://doi.org/10.3390/medicina59030537





