A Clinical Prediction Model of Overall Survival for Patients with Cervical Cancer Aged 25–69 Years
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Development and Validation of the Prediction Model
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
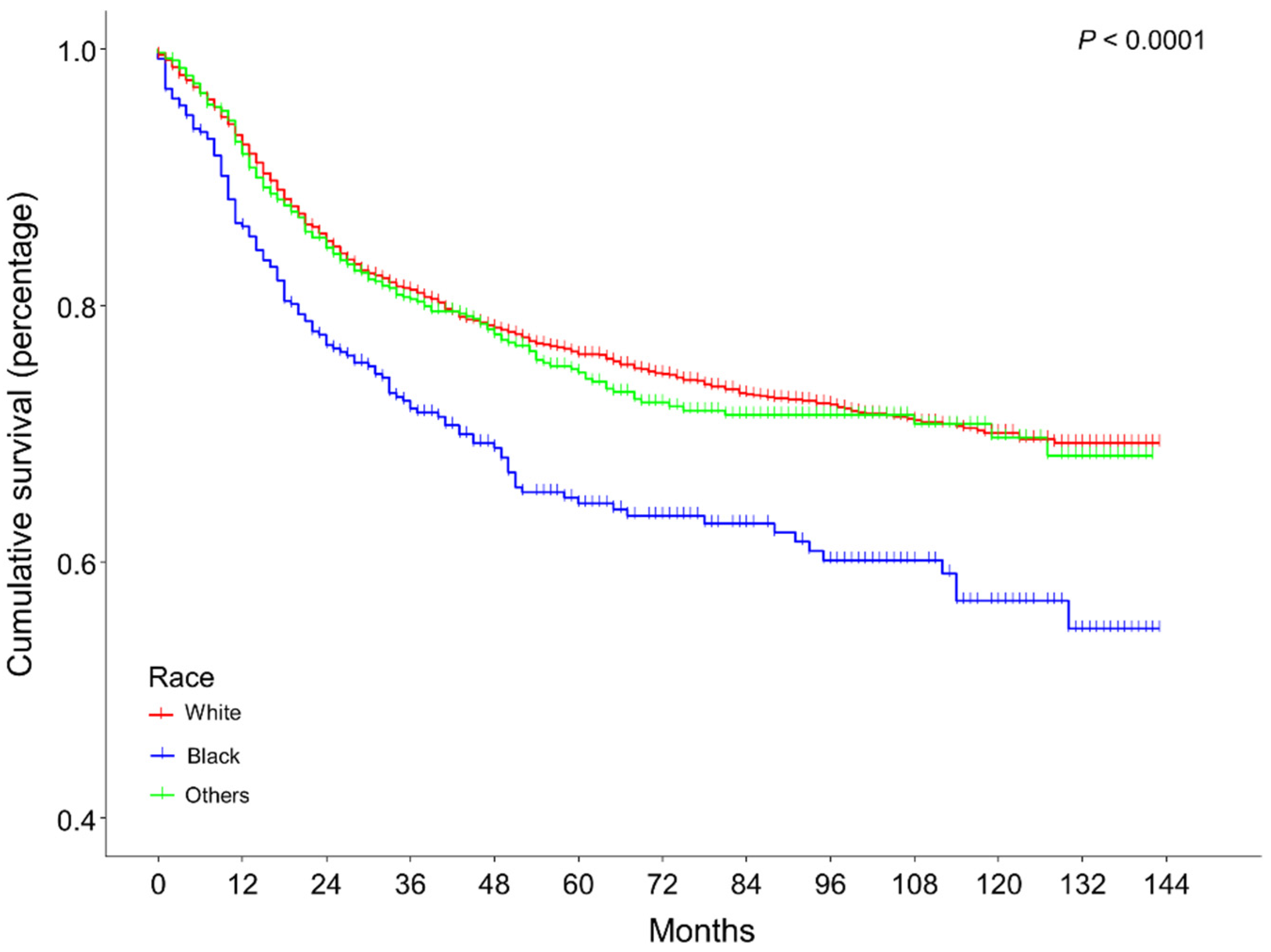
3.2. Cox Regression Analysis
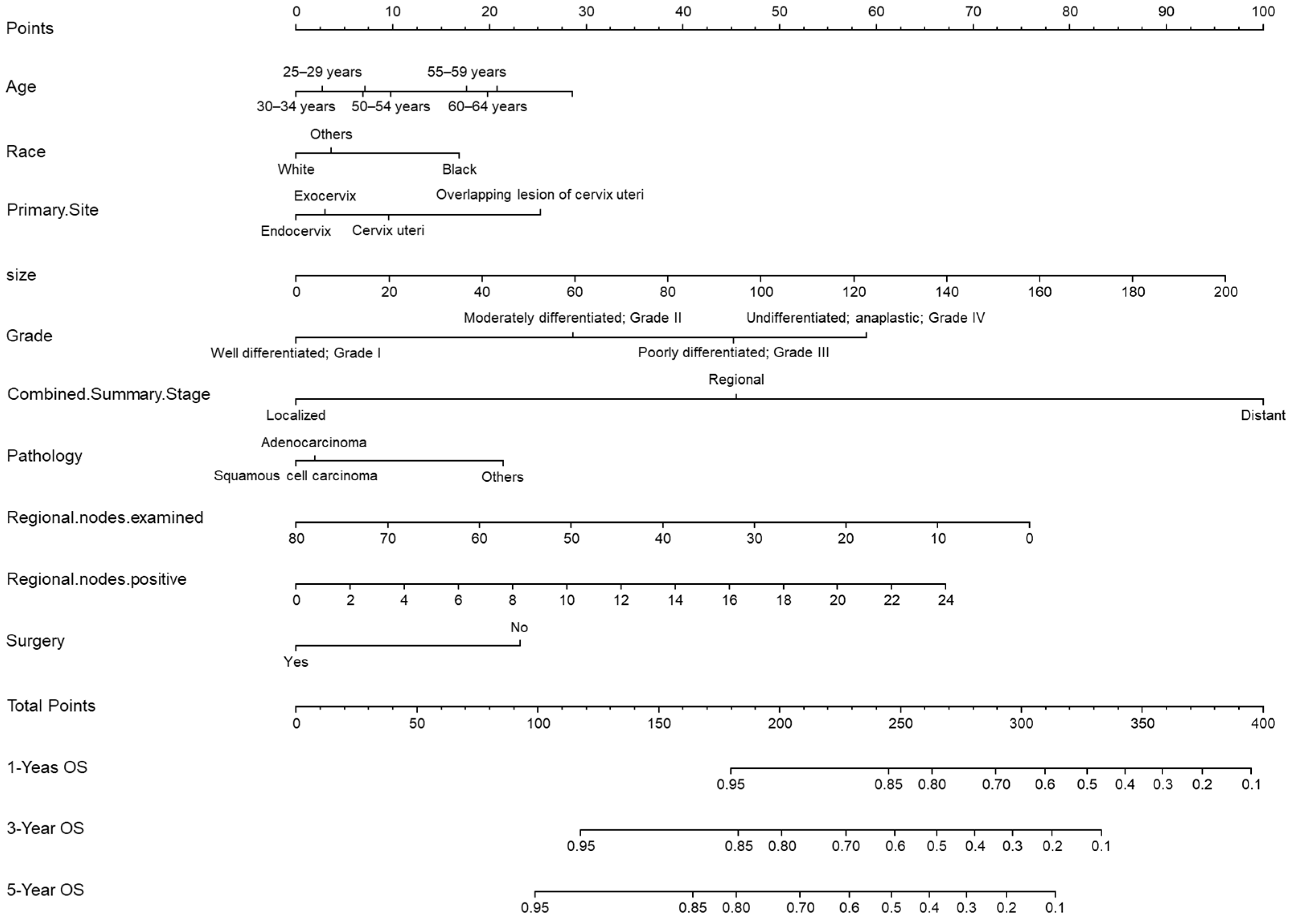
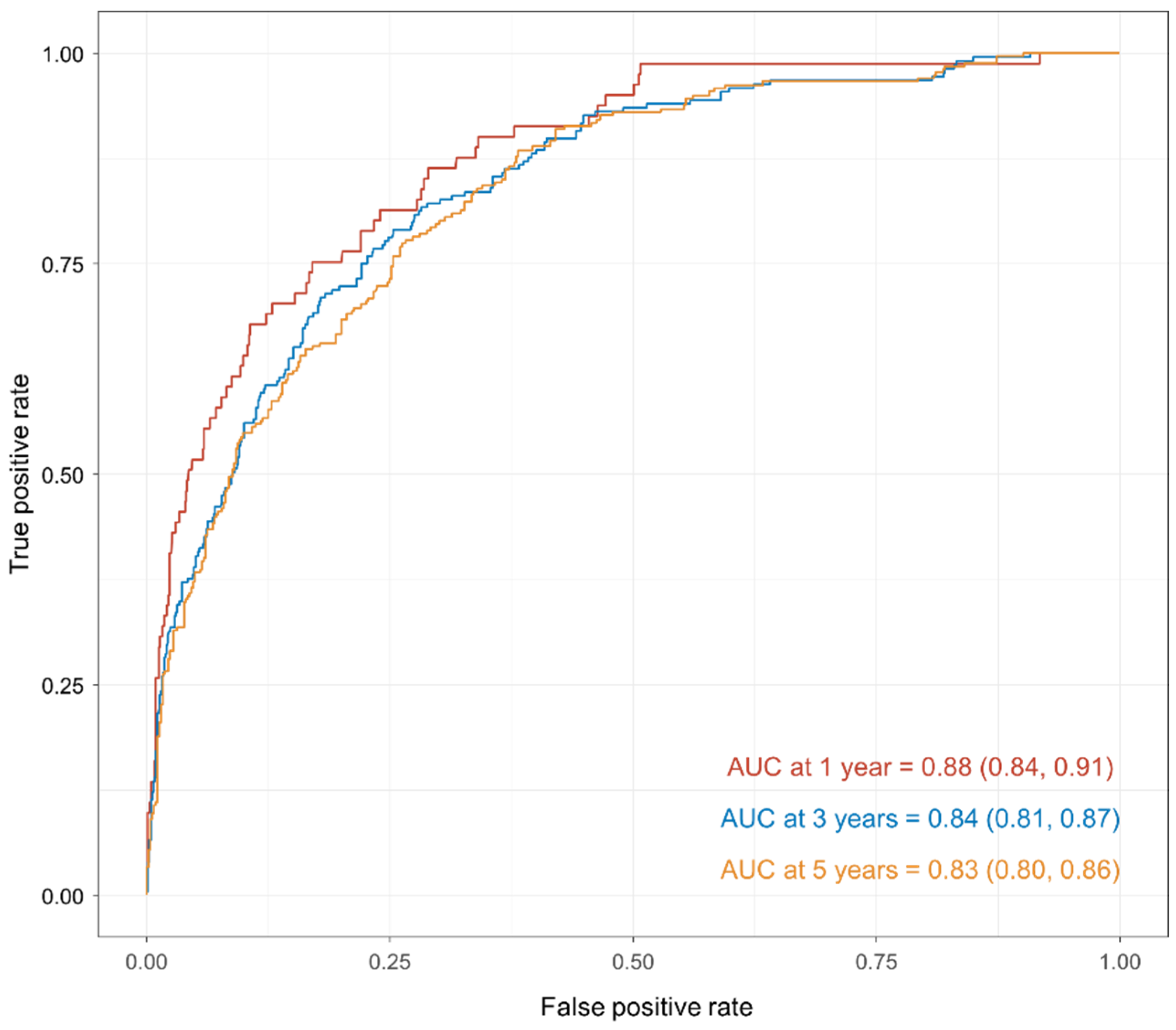
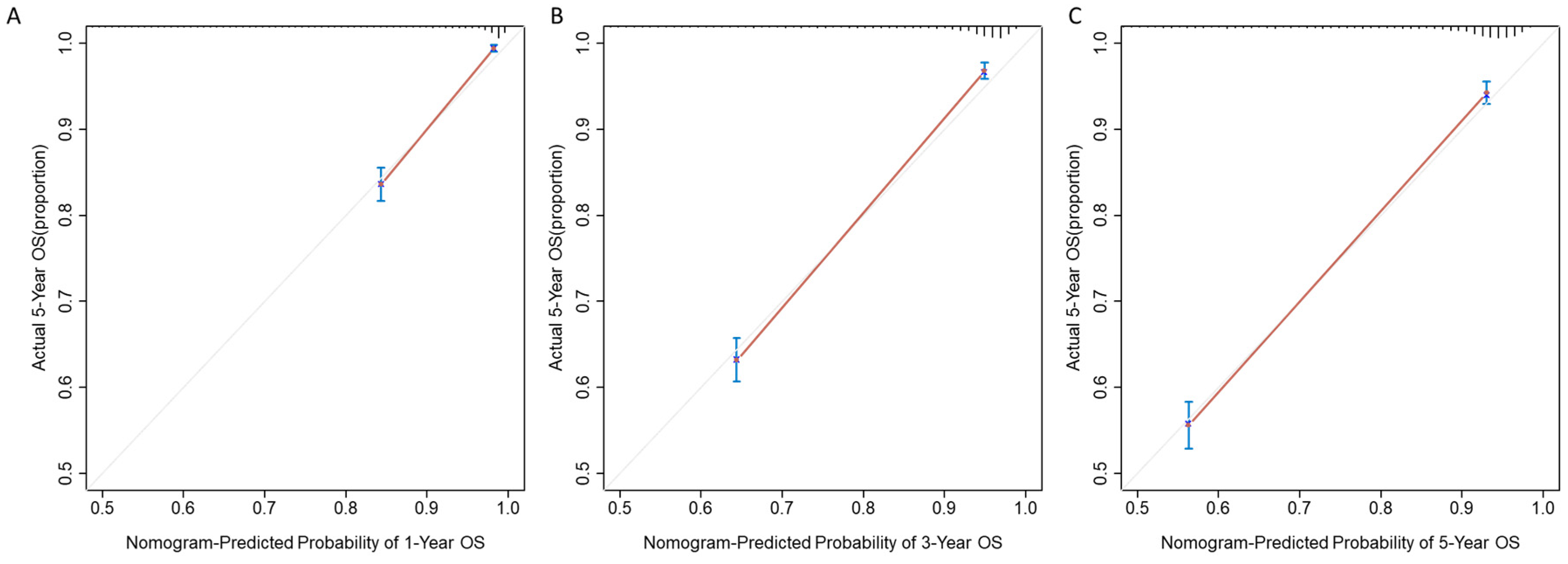
3.3. Development and Validation of the Prediction Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed]
- Small, W., Jr.; Bacon, M.A.; Bajaj, A.; Chuang, L.T.; Fisher, B.J.; Harkenrider, M.M.; Jhingran, A.; Kitchener, H.C.; Mileshkin, L.R.; Viswanathan, A.N.; et al. Cervical cancer: A global health crisis. Cancer 2017, 123, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.-X.; Fang, F. Progress in the Study of Lymph Node Metastasis in Early-stage Cervical Cancer. Curr. Med. Sci. 2018, 38, 567–574. [Google Scholar] [CrossRef]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, I.W. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef]
- Zhou, X.; Ning, Q.; Jin, K.; Zhang, T.; Ma, X. Development and validation of a preoperative nomogram for predicting survival of patients with locally advanced prostate cancer after radical prostatectomy. BMC Cancer 2020, 20, 97. [Google Scholar] [CrossRef]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.-M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef]
- Xie, G.; Wang, R.; Shang, L.; Qi, C.; Yang, L.; Huang, L.; Yang, W.; Chung, M.C. Calculating the overall survival probability in patients with cervical cancer: A nomogram and decision curve analysis-based study. BMC Cancer 2020, 20, 833. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Liu, W.; Fu, M.; Jiang, W.; Xu, S.; Wang, G.; Chen, F.; Lu, J.; Chen, H.; et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat. Commun. 2021, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Raghav, K.; Hwang, H.; Jácome, A.A.; Bhang, E.; Willett, A.; Huey, R.W.; Dhillon, N.P.; Modha, J.; Smaglo, B.; Matamoros, J.A.; et al. Development and Validation of a Novel Nomogram for Individualized Prediction of Survival in Cancer of Unknown Primary. Clin. Cancer Res. 2021, 27, 3414–3421. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Cotogni, P.; Vullo, S.L.; Pironi, L.; Giardiello, D.; Mariani, L. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann. Oncol. 2015, 26, 2335–2340. [Google Scholar] [CrossRef] [PubMed]
- Polterauer, S.; Grimm, C.; Hofstetter, G.; Concin, N.; Natter, C.; Sturdza, A.; Pötter, R.; Marth, C.; Reinthaller, A.; Heinze, G. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br. J. Cancer 2012, 107, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; El Naqa, I.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. FDG-PET-based prognostic nomograms for locally advanced cervical cancer. Gynecol. Oncol. 2012, 127, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Deutsch, I.; Horowitz, D.P.; Hershman, D.L.; Lewin, S.N.; Lu, Y.-S.; Neugut, A.I.; Herzog, T.J.; Chao, C.K.; Wright, J.D. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer 2012, 118, 3618–3626. [Google Scholar] [CrossRef]
- Ikushima, H.; Takegawa, Y.; Osaki, K.; Furutani, S.; Yamashita, K.; Kawanaka, T.; Kubo, A.; Kudoh, T.; Nishitani, H. Radiation therapy for cervical cancer in the elderly. Gynecol. Oncol. 2007, 107, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Milosevic, M.; Fyles, A.; Pintilie, M.; Viswanathan, A. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int. J. Radiat. Oncol. Byoo. Phys. 2013, 87, 111–119. [Google Scholar] [CrossRef]
- Massad, L.S.; Einstein, M.H.; Huh, W.K.; Katki, H.A.; Kinney, W.K.; Schiffman, M.; Solomon, D.; Wentzensen, N.; Lawson, H.W. Conference, 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J. Low. Genit. Tract Dis. 2013, 17, S1–S27. [Google Scholar] [CrossRef]
- Dilley, S.; Huh, W.; Blechter, B.; Rositch, A.F. It′s time to re-evaluate cervical Cancer screening after age 65. Gynecol. Oncol. 2021, 162, 200–202. [Google Scholar] [CrossRef]
- Quinn, B.A.; Deng, X.; Colton, A.; Bandyopadhyay, D.; Carter, J.S.; Fields, E.C. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy 2019, 18, 29–37. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Alive | Dead | p Value | |
|---|---|---|---|---|
| N | 4116 | 3071 | 1045 | |
| Age | <0.001 | |||
| 25–29 years | 220 (5.3%) | 180 (5.9%) | 40 (3.8%) | |
| 30–34 years | 461 (11.2%) | 397 (12.9%) | 64 (6.1%) | |
| 35–39 years | 562 (13.7%) | 466 (15.2%) | 96 (9.2%) | |
| 40–44 years | 657 (16.0%) | 523 (17.0%) | 134 (12.8%) | |
| 45–49 years | 576 (14.0%) | 428 (13.9%) | 148 (14.2%) | |
| 50–54 years | 547 (13.3%) | 377 (12.3%) | 170 (16.3%) | |
| 55–59 years | 434 (10.5%) | 290 (9.4%) | 144 (13.8%) | |
| 60–64 years | 366 (8.9%) | 234 (7.6%) | 132 (12.6%) | |
| 65–69 years | 293 (7.1%) | 176 (5.7%) | 117 (11.2%) | |
| Race | <0.001 | |||
| White | 3056 (74.2%) | 2314 (75.4%) | 742 (71.0%) | |
| Black | 387 (9.4%) | 251 (8.2%) | 136 (13.0%) | |
| Others | 673 (16.4%) | 506 (16.5%) | 167 (16.0%) | |
| Primary site: | <0.001 | |||
| Cervix uteri | 3031 (73.6%) | 2179 (71.0%) | 852 (81.5%) | |
| Endocervix | 926 (22.5%) | 769 (25.0%) | 157 (15.0%) | |
| Exocervix | 98 (2.4%) | 81 (2.6%) | 17 (1.6%) | |
| Overlapping lesion | 61 (1.5%) | 42 (1.4%) | 19 (1.8%) | |
| Tumor size | 30.0 (12.0, 54.2) | 22.0 (9.0, 43.5) | 54.0 (37.0, 71.0) | <0.001 |
| Grade | <0.001 | |||
| Grade I | 658 (16.0%) | 604 (19.7%) | 54 (5.2%) | |
| Grade II | 1920 (46.6%) | 1518 (49.4%) | 402 (38.5%) | |
| Grade III | 1418 (34.5%) | 886 (28.9%) | 532 (50.9%) | |
| Grade IV | 120 (2.9%) | 63 (2.1%) | 57 (5.5%) | |
| Combined summary stage | <0.001 | |||
| Regional | 1422 (34.5%) | 906 (29.5%) | 516 (49.4%) | |
| Localized | 2257 (54.8%) | 2054 (66.9%) | 203 (19.4%) | |
| Distant | 437 (10.6%) | 111 (3.6%) | 326 (31.2%) | |
| Pathology | <0.001 | |||
| Squamous cell carcinoma | 2564 (62.3%) | 1874 (61.0%) | 690 (66.0%) | |
| Adenocarcinoma | 952 (23.1%) | 807 (26.3%) | 145 (13.9%) | |
| Others | 600 (14.6%) | 390 (12.7%) | 210 (20.1%) | |
| Surgical treatment | <0.001 | |||
| No | 1257 (30.5%) | 633 (20.6%) | 624 (59.7%) | |
| Yes | 2859 (69.5%) | 2438 (79.4%) | 421 (40.3%) |
| HR (95%CI) | p Value | |
|---|---|---|
| Age | ||
| 25–29 years | Reference | |
| 30–34 years | 0.96 [0.64, 1.42] | 0.823 |
| 35–39 years | 1.08 [0.74, 1.56] | 0.687 |
| 40–44 years | 1.10 [0.77, 1.57] | 0.599 |
| 45–49 years | 1.39 [0.98, 1.98] | 0.065 |
| 50–54 years | 1.14 [0.81, 1.62] | 0.448 |
| 55–59 years | 1.31 [0.92, 1.87] | 0.133 |
| 60–64 years | 1.37 [0.96, 1.96] | 0.083 |
| 65–69 years | 1.59 [1.11, 2.28] | 0.012 |
| Race | ||
| White | Reference | |
| Black | 1.37 [1.14, 1.65] | 0.001 |
| Others | 1.07 [0.90, 1.27] | 0.434 |
| Primary site | <0.001 | |
| Cervix uteri | Reference | |
| Endocervix | 0.84 [0.69, 1.01] | 0.069 |
| Exocervix | 0.89 [0.55, 1.44] | 0.629 |
| Overlapping lesion | 1.27 [0.80, 2.01] | 0.308 |
| Tumor size | 1.01 [1.01, 1.01] | <0.001 |
| Grade | ||
| Grade I | Reference | |
| Grade II | 1.66 [1.24, 2.22] | 0.001 |
| Grade III | 2.21 [1.65, 2.95] | <0.001 |
| Grade IV | 2.83 [1.92, 4.16] | <0.001 |
| Combined summary stage | ||
| Regional | Reference | |
| Localized | 0.43 [0.36, 0.52] | <0.001 |
| Distant | 2.69 [2.32, 3.13] | <0.001 |
| Pathology | ||
| Squamous cell carcinoma | Reference | |
| Adenocarcinoma | 1.03 [0.84, 1.26] | 0.813 |
| Others | 1.46 [1.22, 1.73] | <0.001 |
| Regional nodes positive | 1.02 [0.99, 1.06] | 0.226 |
| Surgical treatment | <0.001 | |
| No | Reference | |
| Yes | 0.57 [0.49, 0.67] | <0.001 |
| AUC (95%CI) | Sensitivity | Specificity | |
|---|---|---|---|
| 1 year | 0.88 (0.84, 0.91) | 0.85 | 0.71 |
| 3 years | 0.84 (0.81, 0.87) | 0.71 | 0.76 |
| 5 years | 0.83 (0.80, 0.86) | 0.47 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Lu, Q.; Liu, G. A Clinical Prediction Model of Overall Survival for Patients with Cervical Cancer Aged 25–69 Years. Medicina 2023, 59, 600. https://doi.org/10.3390/medicina59030600
Fan W, Lu Q, Liu G. A Clinical Prediction Model of Overall Survival for Patients with Cervical Cancer Aged 25–69 Years. Medicina. 2023; 59(3):600. https://doi.org/10.3390/medicina59030600
Chicago/Turabian StyleFan, Wenli, Qin Lu, and Guokun Liu. 2023. "A Clinical Prediction Model of Overall Survival for Patients with Cervical Cancer Aged 25–69 Years" Medicina 59, no. 3: 600. https://doi.org/10.3390/medicina59030600
APA StyleFan, W., Lu, Q., & Liu, G. (2023). A Clinical Prediction Model of Overall Survival for Patients with Cervical Cancer Aged 25–69 Years. Medicina, 59(3), 600. https://doi.org/10.3390/medicina59030600





