Tobacco Smoking Is a Strong Predictor of Failure of Conservative Treatment in Hinchey IIa and IIb Acute Diverticulitis—A Retrospective Single-Center Cohort Study
Abstract
1. Introduction
Aim of the Study
2. Materials and Methods
2.1. Study Design
2.2. Study Variables
2.3. Study Objectives
2.4. Statistical Analysis
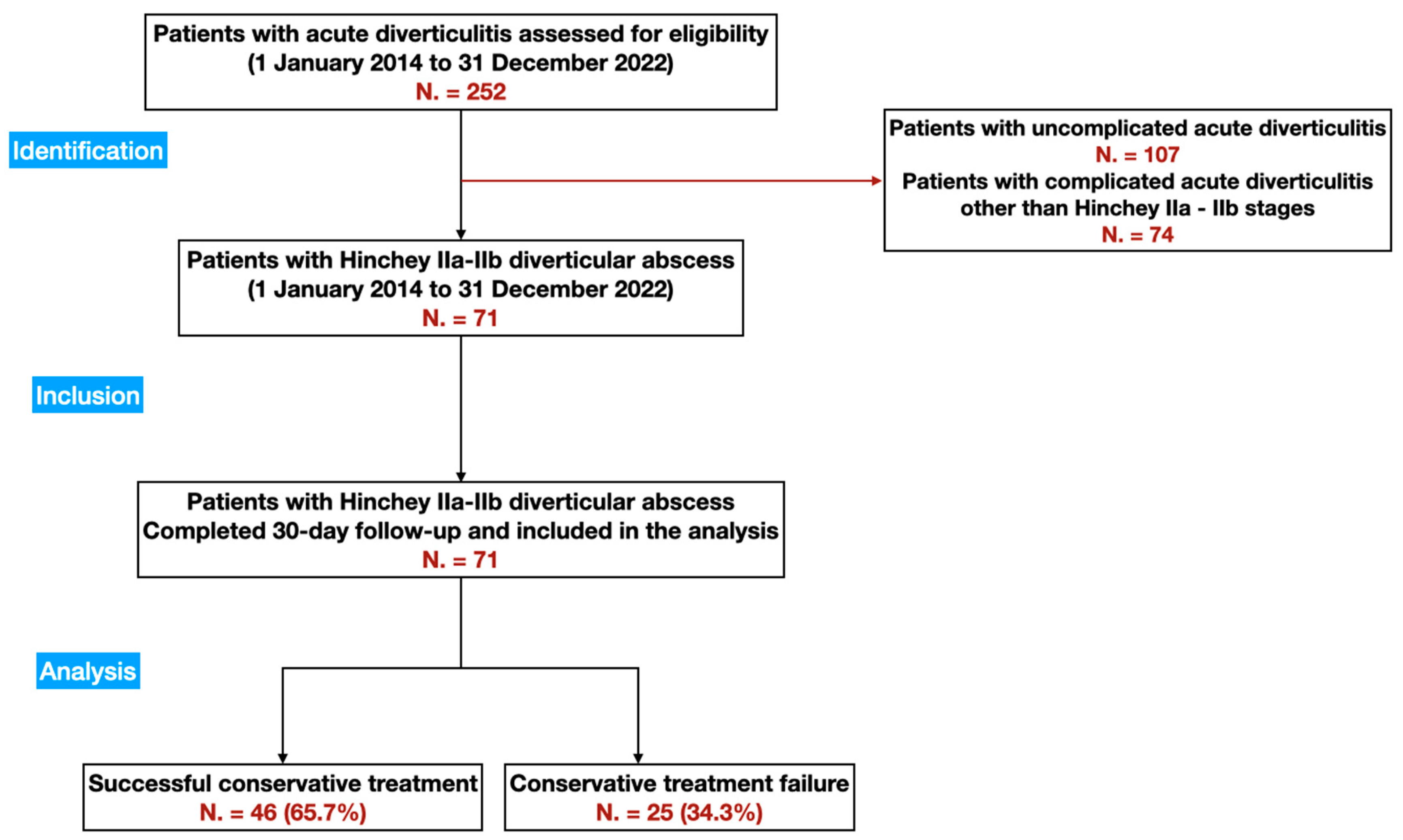
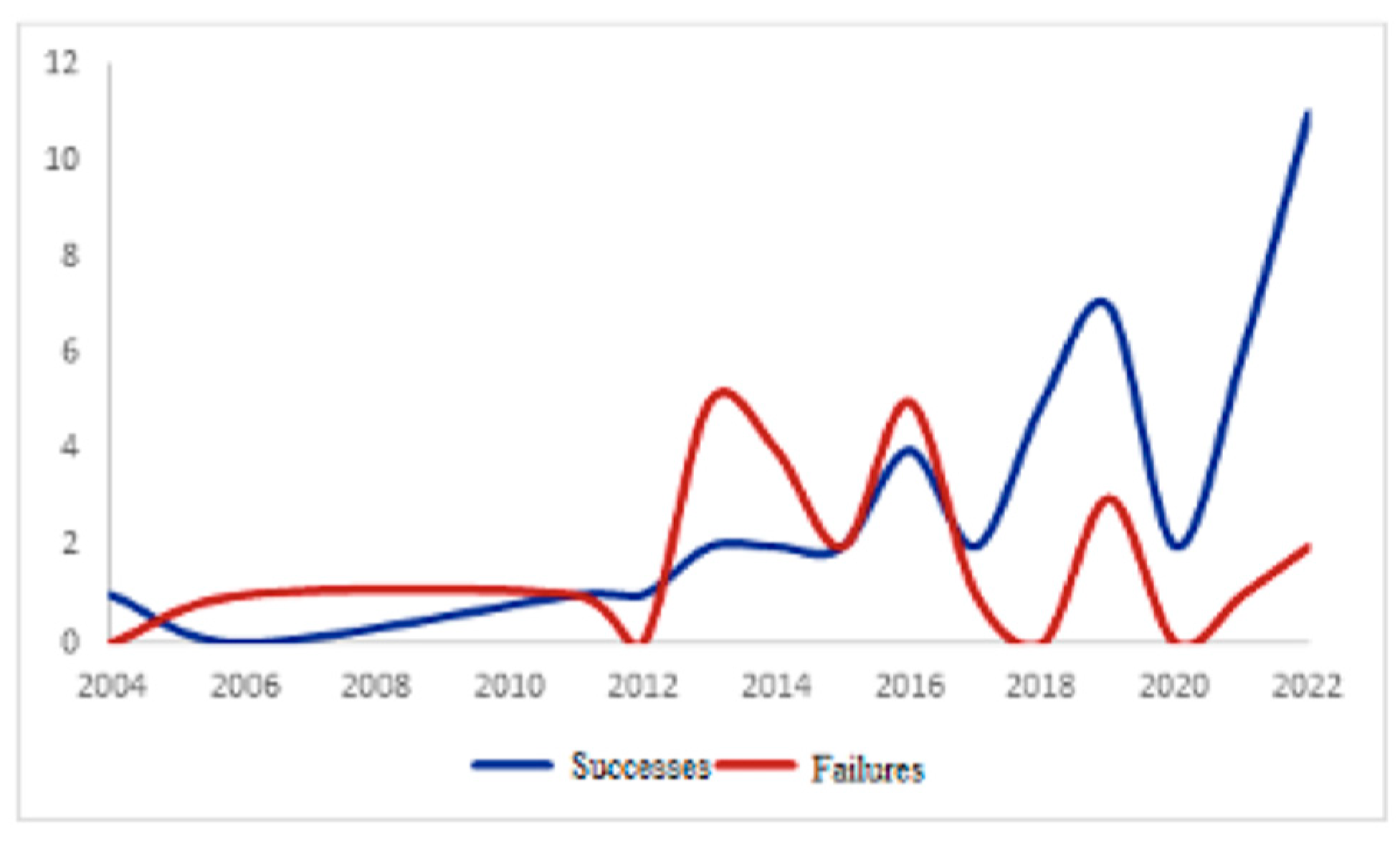
3. Results
3.1. Hospitalization Data
3.2. Data Relating to the Abscess Characteristics on CT Scan
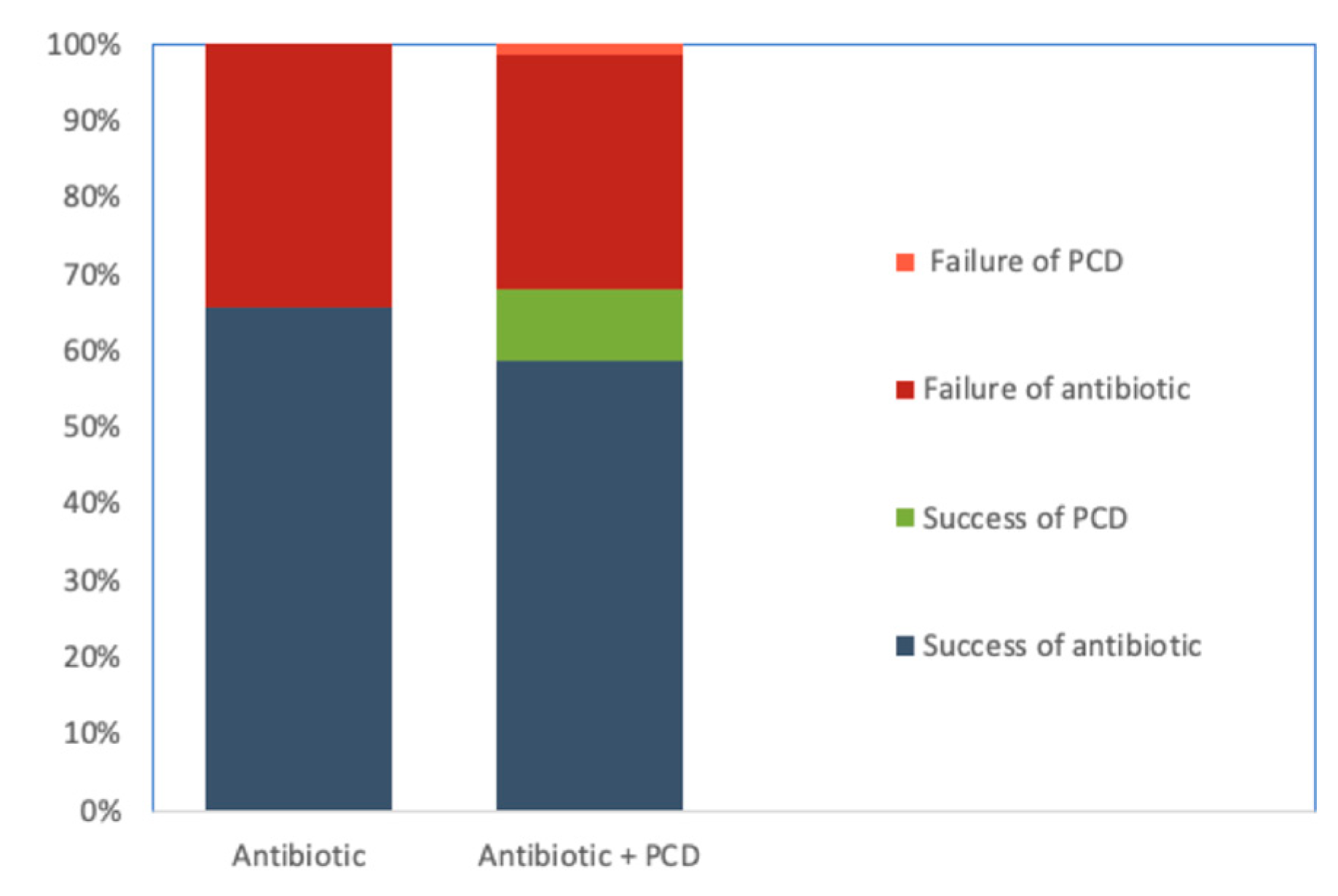
3.3. Management Data
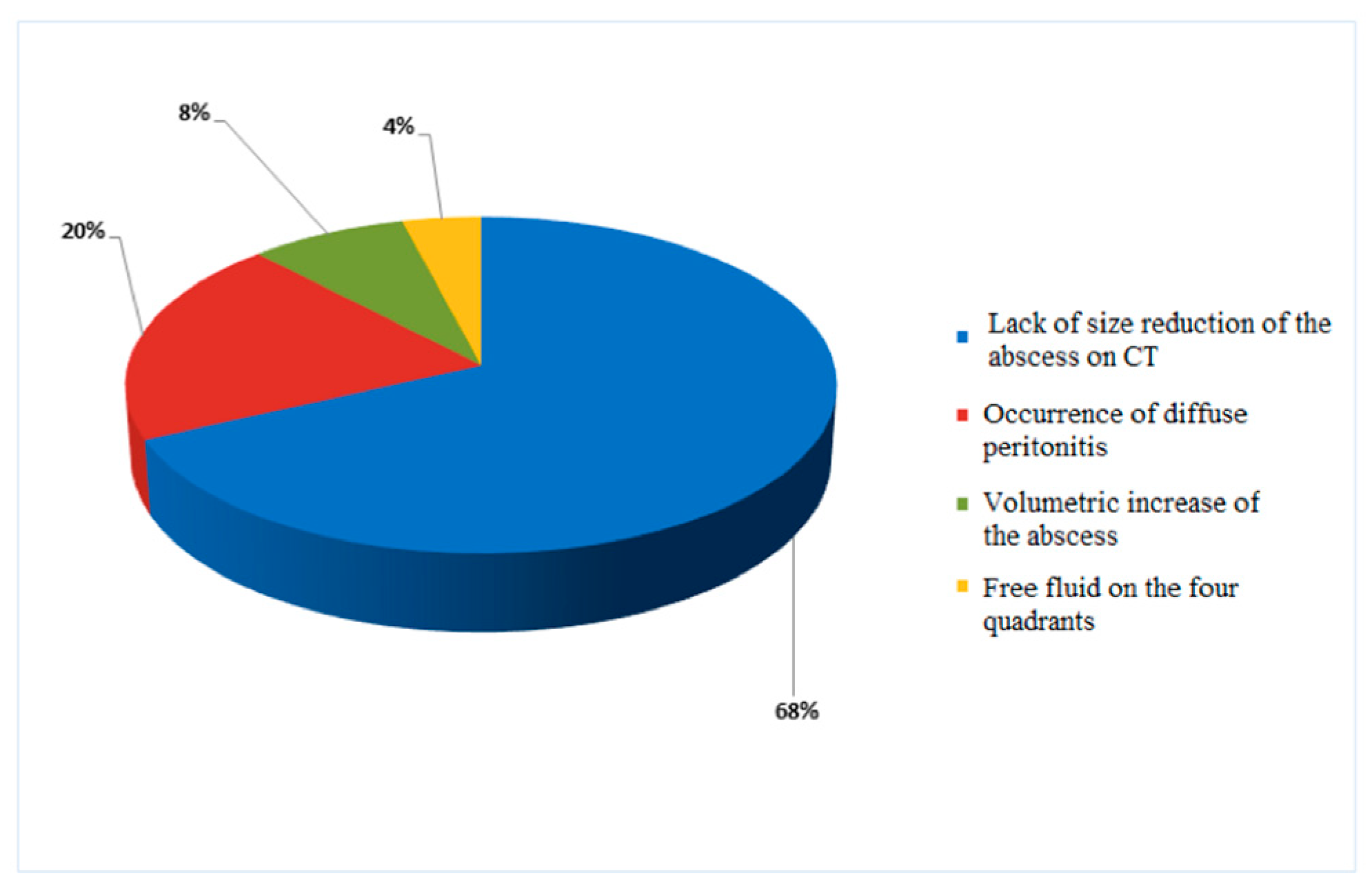
3.4. Clinical Outcomes
3.5. Analysis of the Predictive Factors of Conservative Treatment Failure
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sartelli, M.; Weber, D.G.; Kluger, Y.; Ansaloni, L.; Coccolini, F.; Abu-Zidan, F.; Augustin, G.; Ben-Ishay, O.; Biffl, W.L.; Bouliaris, K.; et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J. Emerg. Surg. 2020, 15, 1–18. [Google Scholar] [CrossRef]
- Pisanu, A.; Vacca, V.; Reccia, I.; Podda, M.; Uccheddu, A. Acute Diverticulitis in the Young: The Same Disease in a Different Patient. Gastroenterol. Res. Pr. 2013, 2013, 867961. [Google Scholar] [CrossRef] [PubMed]
- Weizman, A.V.; Nguyen, G.C. Diverticular Disease: Epidemiology and Management. Can. J. Gastroenterol. 2011, 25, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, N.; Shimamoto, T.; Takahashi, Y.; Sakaguchi, Y.; Kakimoto, H.; Matsuda, R.; Kataoka, Y.; Saito, I.; Tsuji, Y.; Yakabi, S.; et al. Trend and Risk Factors of Diverticulosis in Japan: Age, Gender, and Lifestyle/Metabolic-Related Factors May Cooperatively Affect on the Colorectal Diverticula Formation. PLOS ONE 2015, 10, e0123688. [Google Scholar] [CrossRef]
- Hinchey, E.J.; Schaal, P.G.; Richards, G.K. Treatment of perforated diverticular disease of the colon. Adv. Surg. 1978, 12, 85–109. [Google Scholar]
- Sher, M.E.; Agachan, F.; Bortul, M.; Nogueras, J.J.; Weiss, E.G.; Wexner, S.D. Laparoscopic surgery for diverticulitis. Surg. Endosc. 1997, 11, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, B.R.; de Korte, N.; van der Peet, D.L.; Cuesta, M.A. Review of current classifications for diverticular disease and a translation into clinical practice. Int. J. Color. Dis. 2012, 27, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.M.; Jiang, J.-K.; Lake, J.P.; Ault, G.; Artinyan, A.; Gonzalez-Ruiz, C.; Essani, R.; Beart, R.W., Jr. The Management of Complicated Diverticulitis and the Role of Computed Tomography. Am. J. Gastroenterol. 2005, 100, 910–917. [Google Scholar] [CrossRef]
- Durmishi, Y.; Gervaz, P.; Brandt, D.; Bucher, P.; Platon, A.; Morel, P.; Poletti, P.A. Results from percutaneous drainage of Hinchey stage II diverticulitis guided by computed tomography scan. Surg. Endosc. 2006, 20, 1129–1133. [Google Scholar] [CrossRef]
- Vennix, S.; Musters, G.D.; Mulder, I.M.; A Swank, H.; Consten, E.C.; Belgers, E.H.; A van Geloven, A.; Gerhards, M.F.; Govaert, M.J.; van Grevenstein, W.M.; et al. Laparoscopic peritoneal lavage or sigmoidectomy for perforated diverticulitis with purulent peritonitis: A multicentre, parallel-group, randomised, open-label trial. Lancet 2015, 386, 1269–1277. [Google Scholar] [CrossRef]
- Pellino, G.; Podda, M.; Wheeler, J.; Davies, J.; Di Saverio, S. Laparoscopy and resection with primary anastomosis for perforated diverticulitis: Challenging old dogmas. Updat. Surg. 2020, 72, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lambrichts, D.P.V.; Vennix, S.; Musters, G.D.; Mulder, I.M.; A Swank, H.; Hoofwijk, A.G.M.; Belgers, E.H.J.; Stockmann, H.B.A.C.; Eijsbouts, Q.A.J.; Gerhards, M.F.; et al. Hartmann’s procedure versus sigmoidectomy with primary anastomosis for perforated diverticulitis with purulent or faecal peritonitis (LADIES): A multicentre, parallel-group, randomised, open-label, superiority trial. Lancet Gastroenterol. Hepatol. 2019, 4, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Hoek, V.T.; Edomskis, P.P.; Stark, P.W.; Lambrichts, D.P.V.; Draaisma, W.A.; Consten, E.C.J.; Lange, J.F.; Bemelman, W.A.; LADIES Trial Collaborators. Laparoscopic peritoneal lavage versus sigmoidectomy for perforated diverticulitis with purulent peritonitis: Three-year follow-up of the randomised LOLA trial. Surg. Endosc. 2022, 36, 7764–7774. [Google Scholar] [CrossRef] [PubMed]
- Mora-López, L.; Ruiz-Edo, N.; Estrada-Ferrer, O.; Piñana-Campón, M.L.; Labró-Ciurans, M.; Escuder-Perez, J.; Sales-Mallafré, R.; Rebasa-Cladera, P.; Navarro-Soto, S.; Serra-Aracil, X.; et al. Efficacy and Safety of Nonantibiotic Outpatient Treatment in Mild Acute Diverticulitis (DINAMO-study). Ann. Surg. 2021, 274, e435–e442. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, R.; Mortensen, L.Q.; Burcharth, J.; Pommergaard, H.-C.; Rosenberg, J. Treatment of patients with acute colonic diverticulitis complicated by abscess formation: A Multicentre, Randomised, Open-label, Noninferiority Trial. A systematic review. Int. J. Surg. 2016, 35, 201–208. [Google Scholar] [CrossRef]
- Ahmadi, N.; Ravindran, P.; Kim, T.; Ayoubi, S.E.; Byrne, C.M.; Young, C.J. C-reactive protein trajectory in the first 48 hours predicts the need for intervention in conservative management of acute diverticulitis. ANZ J. Surg. 2020, 90, 2036–2040. [Google Scholar] [CrossRef]
- Al-Masrouri, S.; Garfinkle, R.; Al-Rashid, F.; Zhao, K.; Morin, N.; Ghitulescu, G.A.; Vasilevsky, C.-A.; Boutros, M. Readmission for Treatment Failure After Nonoperative Management of Acute Diverticulitis: A Nationwide Readmissions Database Analysis. Dis. Colon Rectum 2020, 63, 217–225. [Google Scholar] [CrossRef]
- Lee, H.; Gachabayov, M.; Rojas, A.; Felsenreich, D.M.; Tsarkov, P.; Bergamaschi, R. Systematic review of failure of nonoperative management in complicated sigmoid diverticulitis with abscess. Langenbeck’s Arch. Surg. 2020, 405, 277–281. [Google Scholar] [CrossRef]
- Fowler, H.; Gachabayov, M.; Vimalachandran, D.; Clifford, R.; Orangio, G.R.; Bergamaschi, R. Failure of nonoperative management in patients with acute diverticulitis complicated by abscess: A systematic review. Int. J. Color. Dis. 2021, 36, 1367–1383. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; STROBhe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Gayam, V.; Koirala, S.; Garlapati, P.R.; Mandal, A.K. Outcomes of diverticulitis in patients with tobacco smoking: A propensity-matched analysis of nationwide inpatient sample. Int. J. Color. Dis. 2021, 36, 1033–1042. [Google Scholar] [CrossRef]
- Hjern, F.; A Wolk, A.; Håkansson, N. Smoking and the risk of diverticular disease in women. Br. J. Surg. 2011, 98, 997–1002. [Google Scholar] [CrossRef]
- Thomas, G.A.; Rhodes, J.; Ingram, J.R. Mechanisms of Disease: Nicotine—A review of its actions in the context of gastrointestinal disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, A.; Shembekar, M.; Church, J.S.; Vashisht, R.; Springall, R.G.; Nott, D.M. Smoking, hypertension, and colonic anastomotic healing; a combined clinical and histopathological study. Gut 1996, 38, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A. Medical hypothesis: Speculating on the pathogenesis of acute diverticulitis. Ann. Gastroenterol. 2018, 31, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Prescott, N.J.; Pessoa-Lopes, P.; Mathew, C.G.; Sanderson, J.; Hart, A.L.; et al. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota*. Inflamm. Bowel Dis. 2012, 18, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Niikura, R.; Nagata, N.; Akiyama, J.; Shimbo, T.; Uemura, N. Hypertension and concomitant arteriosclerotic diseases are risk factors for colonic diverticular bleeding: A case–control study. Int. J. Color. Dis. 2012, 27, 1137–1143. [Google Scholar] [CrossRef]
- Okamoto, T.; Watabe, H.; Yamada, A.; Hirata, Y.; Yoshida, H.; Koike, K. The association between arteriosclerosis related diseases and diverticular bleeding. Int. J. Color. Dis. 2012, 27, 1161–1166. [Google Scholar] [CrossRef]
- Azodi, O.S.; Lindström, D.; Adami, J.; Bellocco, R.; Linder, S.; Wladis, A. Impact of body mass index and tobacco smoking on outcome after open appendicectomy. Br. J. Surg. 2008, 95, 751–757. [Google Scholar] [CrossRef]
- Oba, T.; Yamada, T.; Matsuda, A.; Otani, M.; Matsuda, S.; Ohta, R.; Yoshida, H.; Sato, N.; Hirata, K. Patient backgrounds and short-term outcomes of complicated appendicitis differ from those of uncomplicated appendicitis. Ann. Gastroenterol. Surg. 2021, 6, 273–281. [Google Scholar] [CrossRef]
- Turunen, P.; Wikström, H.; Carpelan-Holmström, M.; Kairaluoma, P.; Kruuna, O.; Scheinin, T. Smoking Increases the Incidence of Complicated Diverticular Disease of the Sigmoid Colon. Scand. J. Surg. 2010, 99, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.K.; Sylla, P.; Abou-Khalil, M.; Arolfo, S.; Berler, D.; Curtis, N.J.; Dolejs, S.C.; Garfinkle, R.; Gorter-Stam, M.; Hashimoto, D.A.; et al. EAES and SAGES 2018 consensus conference on acute diverticulitis management: Evidence-based recommendations for clinical practice. Surg. Endosc. 2019, 33, 2726–2741. [Google Scholar] [CrossRef] [PubMed]
- Elagili, F.; Stocchi, L.; Ozuner, G.; Kiran, R.P. Antibiotics alone instead of percutaneous drainage as initial treatment of large diverticular abscess. Tech. Coloproctology 2015, 19, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Garfinkle, R.; Kugler, A.; Pelsser, V.; Vasilevsky, C.-A.; Morin, N.; Gordon, P.; Feldman, L.; Boutros, M. Diverticular Abscess Managed With Long-term Definitive Nonoperative Intent Is Safe. Dis. Colon Rectum 2016, 59, 648–655. [Google Scholar] [CrossRef]
- Devaraj, B.; Liu, W.; Tatum, J.; Cologne, K.; Kaiser, A.M. Medically Treated Diverticular Abscess Associated With High Risk of Recurrence and Disease Complications. Dis. Colon Rectum 2016, 59, 208–215. [Google Scholar] [CrossRef]
| Variable | Sample Size (Total Examined) | N. | % | Mean | Standard Deviation | Median | Interquartile Range |
|---|---|---|---|---|---|---|---|
| Age (year) | 71 | 71 | 61.6 | 14.4 | 63.0 | 19.0 | |
| Sex (Male) | 71 | 35 | 49.3 | ||||
| BMI (Kg/m2) | 35 | 35 | 26.5 | 4.4 | 26.2 | 5.3 | |
| Charlson’s Comorbidity Index | 71 | 71 | 2.1 | 1.8 | 2.0 | 2.0 | |
| Diabetes (N. %) | 71 | 5 | 7.0 | ||||
| Chronic renal failure (N. %) | 71 | 4 | 5.6 | ||||
| Therapy with corticosteroids (N. %) | 71 | 3 | 4.2 | ||||
| Heart failure (N. %) | 71 | 1 | 1.4 | ||||
| Obesity (N. %) | 35 | 7 | 9.7 | ||||
| Coagulopathy (N. %) | 71 | 1 | 1.4 | ||||
| Arterial hypertension (N. %) | 71 | 30 | 42.2 | ||||
| Chronic Obstructive Pulmonary Disease (COPD) (N. %) | 71 | 3 | 4.2 | ||||
| Coronaropathy (N. %) | 71 | 8 | 11.2 | ||||
| Renal failure (Creatinine > 1.2 mg/dL) (N. %) | 71 | 17 | 23.9 | ||||
| Smoking (N. %) | 71 | 11 | 15.4 | ||||
| Alcohol abuse (N. %) | 71 | 7 | 9.8 | ||||
| Leukocytes ×103/μL | 71 | 71 | 14.0 | 4.5 | 13.6 | 5.8 | |
| CRP mg/L | 41 | 41 | 130.0 | 92.3 | 107.0 | 130.0 | |
| Anemia (Hb < 12 g/dL) (N. %) | 71 | 14 | 19.7% | ||||
| Platelets × 103/μL | 71 | 71 | 287 | 94.1 | 260 | 130 | |
| Procalcitonin ng/mL | 43 | 17 | 6.7 | 24.1 | 0.2 | 0.4 | |
| Fever (>38 °C) (N. %) | 71 | 15 | 21.1 | ||||
| Systolic blood pressure (mmHg) | 71 | 71 | 132 | 17.4 | 130 | 20.0 | |
| Tachycardia (>100 bpm) (N. %) | 71 | 22 | 31.0 | ||||
| Patients with previous episodes of acute diverticulitis | 71 | 13 | 18.31 | ||||
| Diameter of the abscess on CT (mm) | 71 | 71 | 51.8 | 33.2 | 45.0 | 39.0 | |
| Hinchey grade IIa (N. %) | 71 | 33 | 46.5 | ||||
| Hinchey grade IIb (N. %) | 71 | 38 | 53.5 | ||||
| Presence of air bubbles in the abscess (N. %) | 71 | 38 | 53.5 | ||||
| Presence of retroperitoneal air bubbles (N. %) | 71 | 2 | 3.1 | ||||
| Presence of extraluminal air (N. %) | 71 | 8 | 11.9 | ||||
| Presence of free pelvic fluid (N. %) | 71 | 9 | 13.4 | ||||
| CT-guided percutaneous drainage of the abscess (N. %) | 71 | 8 | 11.3 | ||||
| Time between the start of conservative therapy and failure (Days) | 71 | 25 | 13.6 | 8.4 | 12 | 8.0 | |
| Duration of symptoms before admission (Days) | 71 | 71 | 5.8 | 6.6 | 3 | 7.0 | |
| Time spent in the emergency department (Minutes) | 71 | 65 | 447 | 1054 | 285 | 262 | |
| Time of admission (Hours) | 71 | ||||||
| 06.01–12.00 | 5 | 7.1 | |||||
| 12.01–18.00 | 25 | 35.7 | |||||
| 18.01–23.59 | 24 | 34.3 | |||||
| 0.00–06.00 | 16 | 22.8 | |||||
| Day of the admission | |||||||
| Weekday | 59 | 83.1 | |||||
| Weekend | 12 | 16.9 |
| Variable | Success Group n = 46 (65.7%) | Failure Group n = 25 (34.3%) | p Value | Odds Ratio | Mean Difference | 95% CI |
|---|---|---|---|---|---|---|
| Age (years) ± mean difference (MD) | 63.9 ± 14.7 | 57.0 ± 13.1 | 0.056 | 6.96 | −0.18; 0.99 | |
| Sex (Male) n. (%) | 21 (45.6%) | 14 (56.0%) | 0.405 | 0.660 | 0.25; 1.76 | |
| BMI (Kg/m2) ± mean difference (MD) | 26.5 ± 4.6 | 25.8 ± 3.5 | 0.615 | 0.74 | −0.50; 0.85 | |
| Charlson’s Comorbidity Index ± mean difference (MD) | 2.0 ± 0.2 | 2.0 ± 0.3 | 0.524 | 0.28 | −0.34; 0.65 | |
| Diabetes | 4 (8.7%) | 1 (4.0%) | 0.460 | 0.438 | 0.05; 4.14 | |
| Chronic renal failure | 3 (6.5%) | 1 (4.0%) | 0.660 | 0.597 | 0.06; 6.06 | |
| Corticosteroid therapy | 2 (4.3%) | 1 (4.0%) | 0.945 | 0.917 | 0.08; 10.60 | |
| Heart failure | - | 1 (4.0%) | 0.172 | 5.690 | 0.22; 142 | |
| Obesity | 4 (8.7%) | 3 (12.0%) | 0.797 | 0.804 | 0.15; 4.25 | |
| Coagulopathy | - | 1 (4.0%) | 0.172 | 5.690 | 0.22; 145 | |
| Hypertension | 22 (47.8%) | 8 (32.0%) | 0.197 | 0.513 | 0.19; 1.42 | |
| Chronic Obstructive Pulmonary Disease (COPD) | 1 (2.2) | 2 (8.0%) | 0.244 | 3.910 | 0.34; 45.50 | |
| Renal failure (N. %) | 12 (26.1%) | 5 (20.0%) | 0.566 | 0.708 | 0.21; 2.31 | |
| Coronaropathy | 6 (13.0%) | 2 (8.0%) | 0.521 | 0.580 | 0.11; 3.11 | |
| Tobacco Smoking | 3 (6.5%) | 8 (32.0%) | 0.007 | 7.330 | 1.55; 34.70 | |
| Alcohol drinking | 2 (4.3%) | 5 (20.0%) | 0.071 | 4.770 | 0.79; 28.70 | |
| Leukocytes × 103/μL ± mean difference (MD) | 14.1 ± 4.9 | 13.7 ± 3.6 | 0.716 | 0.42 | −0.40; 0.59 | |
| CRP mg/L ± mean difference (MD) | 101.4 ± 17.8 | 144.1 ± 21.7 | 0.345 | −15.30 | −0.91; 0.52 | |
| Anemia (Hb < 12 g/dL) | 13.0 ± 1.4 | 13.2 ± 1.7 | 0.665 | −0.18 | −0.60; 0.38 | |
| Platelets × 103/μL ± mean difference (MD) | 258 ± 13.8 | 299 ± 18.2 | 0.087 | −32.11 | −0.93; 0.06 | |
| Procalcitonin ng/mL ± mean difference (MD) | 0.1 ± 6.6 | 0.3 ± 0.1 | 0.601 | 7.14 | −1.20; 1.76 | |
| Fever (>38 °C) | 19 (41.3%) | 6 (24.0%) | 0.662 | 1.301 | 0.40; 4.19 | |
| Systolic blood pressure (mmHg) ± mean difference (MD) | 130 ± 16.5 | 135 ± 19.1 | 0.254 | −1.52 | −0.81; 0.22 | |
| Tachycardia (>100 bpm) | 12 (26.1%) | 10 (40.0%) | 0.226 | 1.892 | 0.67; 5.32 | |
| Number of previous episodes of acute diverticulitis (previous episodes) | 36 (78.2%) (0 episodes) 4 (8.7%) (1 episode) 6 (13.0%) (>1 episodes) | 22 (88.0%) (0 episodes) 3 (12.0%) (1 episode) - (>1 episodes) | 0.163 | 0.14; 2.41 | ||
| Diameter of the abscess on CT (mm) ± mean difference (MD) | 40.0 ± 4.3 | 50.0 ± 10.1 | 0.195 | −14.43 | −1.01; 0.13 | |
| Number of abscesses on CT | 38 (82.6%) (1 abscess) 5 (10.9%) (2 abscesses) 3 (6.5%) (>2 abscesses) | 23 (92.0%) (1 abscess) 2 (8.0%) (2 abscesses) - (>2 abscesses) | 0.521 | 0.600 | 0.11; 3.16 | |
| Hinchey stage IIa | 21 (45.6%) | 6 (24.0%) | 0.081 | 0.376 | 0.12; 1.11 | |
| Hinchey stage IIb | 25 (54.4%) | 19 (76.0%) | ||||
| Presence of air bubbles in the abscess | 22 (47.8%) | 12 (48.0%) | 0.848 | 0.900 | 0.31; 2.64 | |
| Number of air bubbles in the abscess | 24 (52.2%) (0 bubbles) 11 (23.9%) (1 bubble) 11 (23.9%) (>1 bubble) | 8 (32.0%) (0 bubbles) 4 (16.0%) (1 bubble) 8 (32.0%) (>1 bubble) | 0.839 | 1.320 | 0.44; 3.96 | |
| Presence of retroperitoneal air bubbles | - | 2 (8.0%) | 0.025 | 13.300 | 1.61; 291.0 | |
| Presence of extraluminal air at distance | 3 (6.5%) | 5 (20.0%) | 0.043 | 4.480 | 1.96; 20.91 | |
| Presence of free pelvic fluid | 6 (13.0%) | 3 (12.0%) | 0.890 | 1.110 | 0.25; 4.95 | |
| CT-guided percutaneous drainage of the abscess | 7 (15.2%) | 1 (4.0%) | 0.153 | 0.232 | 0.03;2.01 | |
| Duration of symptoms before hospital admission (>3 Days) (N. %) | 26 (56.5%) | 18 (72.0%) | 0.199 | 1.980 | 0.69; 5.65 | |
| Time spent in the emergency department (minutes) ± mean difference (MD) | 303.0 ± 187.2 | 203.0 ± 40.8 | 0.111 | 276.68 | −0.27; 0.79 | |
| Day of the admission | 0.608 | 1.39 | 0.39; 4.95 | |||
| Weekdays | 39 (84.8%) | 20 (80.0%) | ||||
| Weekends | 7 (15.2%) | 5 (20.0%) |
| Variables | p-Value | Adjusted Odds Ratio | 95% CI |
|---|---|---|---|
| Age (years) | 0.461 | 0.973 | 0.90; 1.05 |
| Tobacco smoking | 0.006 | 32.693 | 2.69; 397.27 |
| Alcohol drinking | 0.140 | 5.466 | 0.57; 52.29 |
| Hinchey stage (2a/2b) | 0.595 | 2.190 | 0.12; 39.53 |
| Presence of retroperitoneal air bubbles | 0.995 | 1.078 | 0.18; 12.37 |
| Presence of extraluminal air at distance | 0.428 | 2.919 | 0.20; 41.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzi, V.; Locci, E.; Carta, A.; Pilia, T.; Frongia, F.; Gessa, E.; Podda, M.; Pisanu, A. Tobacco Smoking Is a Strong Predictor of Failure of Conservative Treatment in Hinchey IIa and IIb Acute Diverticulitis—A Retrospective Single-Center Cohort Study. Medicina 2023, 59, 1236. https://doi.org/10.3390/medicina59071236
Murzi V, Locci E, Carta A, Pilia T, Frongia F, Gessa E, Podda M, Pisanu A. Tobacco Smoking Is a Strong Predictor of Failure of Conservative Treatment in Hinchey IIa and IIb Acute Diverticulitis—A Retrospective Single-Center Cohort Study. Medicina. 2023; 59(7):1236. https://doi.org/10.3390/medicina59071236
Chicago/Turabian StyleMurzi, Valentina, Eleonora Locci, Alessandro Carta, Tiziana Pilia, Federica Frongia, Emanuela Gessa, Mauro Podda, and Adolfo Pisanu. 2023. "Tobacco Smoking Is a Strong Predictor of Failure of Conservative Treatment in Hinchey IIa and IIb Acute Diverticulitis—A Retrospective Single-Center Cohort Study" Medicina 59, no. 7: 1236. https://doi.org/10.3390/medicina59071236
APA StyleMurzi, V., Locci, E., Carta, A., Pilia, T., Frongia, F., Gessa, E., Podda, M., & Pisanu, A. (2023). Tobacco Smoking Is a Strong Predictor of Failure of Conservative Treatment in Hinchey IIa and IIb Acute Diverticulitis—A Retrospective Single-Center Cohort Study. Medicina, 59(7), 1236. https://doi.org/10.3390/medicina59071236






