Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR)
Abstract
:1. Introduction
2. Epidemiology, Prevalence, Risk Factors, and Outcomes of INOCA
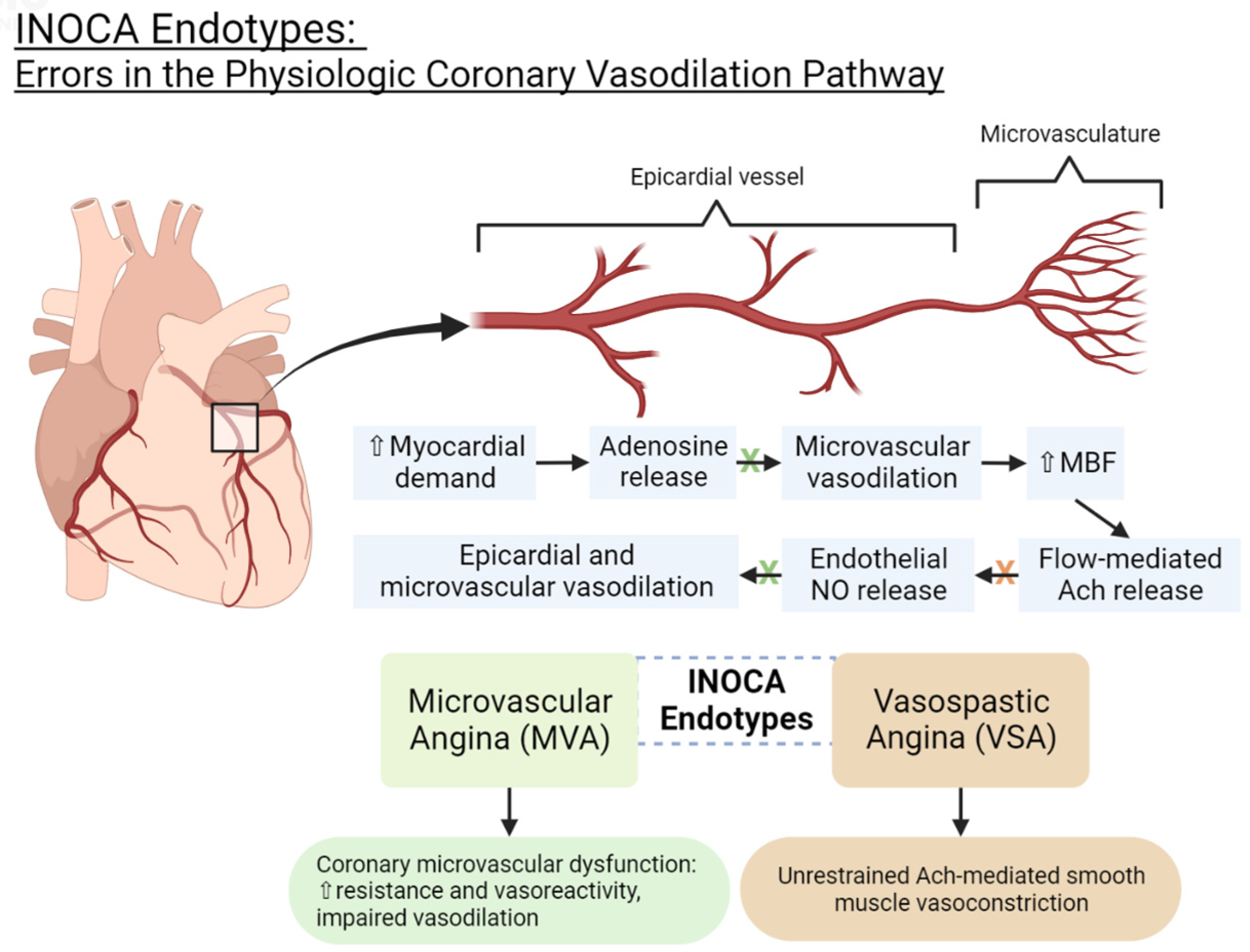
3. INOCA Endotypes: Pathophysiology and Current Diagnostic Criteria
3.1. Microvascular Dysfunction Endotype (MVD)
3.2. Vasospastic Angina Endotype (VSA)
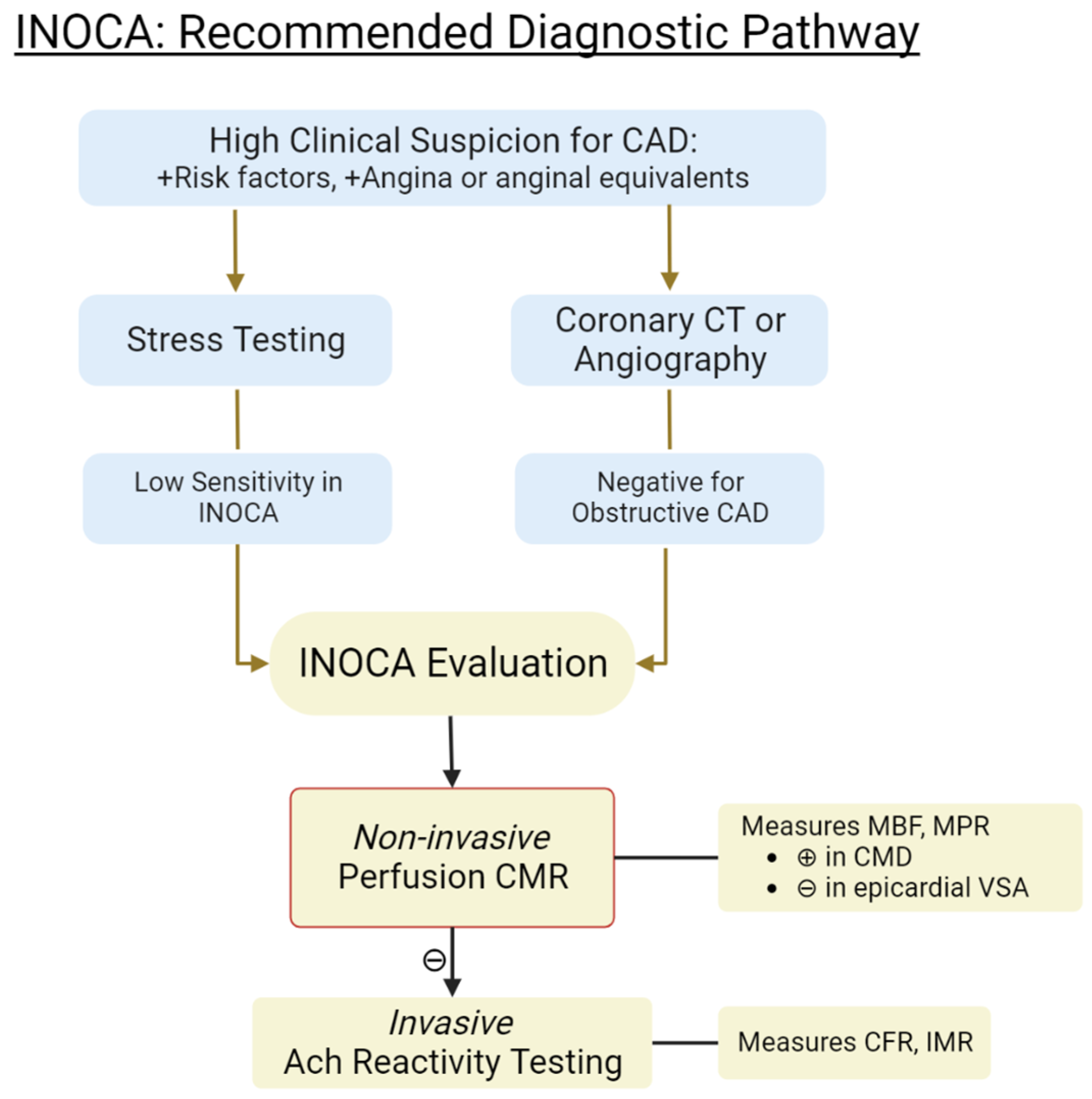
4. Invasive Coronary Reactivity Testing (CRT)
5. Non-Invasive Methods for Evaluating INOCA
5.1. Coronary CT Angiography (CTA)
5.2. Cardiac Positron Emission Tomography (PET)
5.3. Cardiac Magnetic Resonance (CMR)
6. Contemporary Applications of CMR in INOCA Evaluation
6.1. First-Pass Sequence Perfusion Imaging
6.2. Semi-Quantitative CMR
6.3. Quantitative CMR: Dual-Bolus and Dual-Sequence Methods
6.4. Automated In-Line CMR Perfusion Mapping
7. Conclusions
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kemp, H.G.; Kronmal, R.A.; Vlietstra, R.E.; Frye, R.L. Seven year survival of patients with normal or near normal coronary arteriograms: A CASS registry study. J. Am. Coll. Cardiol. 1986, 7, 479–483. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, 23207. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2017, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, R.; Van Assen, M.; Vliegenthart, R.; De Bock, G.H.; Van Der Harst, P.; Oudkerk, M. Diagnostic performance of semi-quantitative and quantitative stress CMR perfusion analysis: A meta-analysis. J. Cardiovasc. Magn. Reson. 2017, 19, 92. [Google Scholar] [CrossRef]
- Kelshiker, M.A.; Seligman, H.; Howard, J.P.; Rahman, H.; Foley, M.; Nowbar, A.N.; Rajkumar, C.A.; Shun-Shin, M.J.; Ahmad, Y.; Sen, S.; et al. Coronary flow reserve and cardiovascular outcomes: A systematic review and meta-analysis. Eur. Heart J. 2022, 43, 1582–1593. [Google Scholar] [CrossRef]
- Pepine, C.J.; Ferdinand, K.C.; Shaw, L.J.; Light-McGroary, K.A.; Shah, R.U.; Gulati, M.; Duvernoy, C.; Walsh, M.N.; Bairey Merz, C.N. The Present and Future Emergence of Nonobstructive Coronary Artery Disease A Woman’s Problem and Need for Change in Definition on Angiography. J. Am. Coll. Cardiol. 2015, 66, 1918–1933. [Google Scholar] [CrossRef]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse Cardiovascular Outcomes in Women With Nonobstructive Coronary Artery Disease: A Report From the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Klotzka, A.; Iwańczyk, S.; Ropacka-Lesiak, M.; Misan, N.; Lesiak, M. Anthracycline-induced microcirculation disorders: AIM PILOT Study. Kardiol. Pol. 2023. [Google Scholar] [CrossRef]
- Gulati, M.; Parwani, P. Myocardial Blood Flow Reserve: The Achilles’ Heel of CAD Prognostication? JACC Cardiovasc. Imaging 2022, 15, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. 1-Year Outcomes of Angina Management Guided by Invasive Coronary Function Testing (CorMicA). JACC Cardiovasc. Interv. 2020, 13, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Heggie, R.; Briggs, A.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. Stratified medicine using invasive coronary function testing in angina: A cost-effectiveness analysis of the British Heart Foundation CorMicA trial. Int. J. Cardiol. 2021, 337, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Maddox, T.M.; Stanislawski, M.A.; Grunwald, G.K.; Bradley, S.M.; Ho, P.M.; Tsai, T.T.; Patel, M.R.; Sandhu, A.; Valle, J.; Magid, D.J.; et al. Nonobstructive Coronary Artery Disease and Risk of Myocardial Infarction. JAMA 2014, 312, 1754. [Google Scholar] [CrossRef]
- Carlos Plana, J.; Jones, P.H. The Use of Statins in Acute Coronary Syndromes: The Mechanisms Behind the Outcomes. Curr. Atheroscler. Rep. 2001, 3, 355–364. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; Elias-Smale, S.E.; van den Oord, S.C.; Ong, P.; de Vos, A.M.J.; van de Hoef, T.P.; Paradies, V.; Smits, P.C.; van Royen, N.; et al. Assessing Microvascular Dysfunction in Angina With Unobstructed Coronary Arteries: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1471–1479. [Google Scholar] [CrossRef]
- Parwani, P.; Kang, N.; Safaeipour, M.; Mamas, M.A.; Wei, J.; Gulati, M.; Naidu, S.S.; Merz, N.B. Contemporary Diagnosis and Management of Patients with MINOCA. Curr. Cardiol. Rep. 2023, 25, 561–570. [Google Scholar] [CrossRef]
- Pargaonkar, V.S.; Lee, J.H.; Chow, E.K.H.; Nishi, T.; Ball, R.L.; Kobayashi, Y.; Kimura, T.; Lee, D.P.; Stefanick, M.L.; Fearon, W.F.; et al. Dose-Response Relationship between Intracoronary Acetylcholine and Minimal Lumen Diameter in Coronary Endothelial Function Testing of Women and Men with Angina and No Obstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2020, 13, 8587. [Google Scholar] [CrossRef]
- Godo, S.; Suda, A.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Coronary Microvascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1625–1637. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Limaye, S.B.; Horowitz, J.D. The Coronary Slow Flow Phenomenon—A New Coronary Microvascular Disorder. Gen. Cardiol. Cardiol. 2002, 97, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Noel, C.; Merz, B.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L.; Vincent, S. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-based Therapies and Research Agenda for the Next Decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef]
- Fearon, W.F.; Farouque, H.M.; Balsam, L.B.; Caffarelli, A.D.; Cooke, D.T.; Robbins, R.C.; Fitzgerald, P.J.; Yeung, A.C.; Yock, P.G. Comparison of Coronary Thermodilution and Doppler Velocity for Assessing Coronary Flow Reserve. Circulation 2003, 108, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Lak, H.M.; Ranka, S.; Goyal, A. Pharmacologic Stress Testing. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Henzlova, M.J.; Duvall, W.L.; Einstein, A.J.; Travin, M.I.; Verberne, H.J. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J. Nucl. Cardiol. 2016, 23, 606–639. [Google Scholar] [CrossRef]
- Tonet, E.; Pompei, G.; Faragasso, E.; Cossu, A.; Pavasini, R.; Passarini, G.; Tebaldi, M.; Campo, G. Coronary microvascular dysfunction: Pet, cmr and ct assessment. J. Clin. Med. 2021, 10, 1848. [Google Scholar] [CrossRef]
- Mathew, R.C.; Bourque, J.M.; Salerno, M.; Kramer, C.M. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc. Imaging 2020, 13, 1577–1590. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Patel, A.R.; Salerno, M.; Kwong, R.Y.; Singh, A.; Heydari, B.; Kramer, C.M. Stress Cardiac Magnetic Resonance Myocardial Perfusion Imaging: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction: Validation Against Invasive Coronary Physiology. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Lee, K.Y.; Chun, E.J.; Lee, W.W.; Park, E.K.; Chang, H.J.; Choi, S.I. Comparison of stress perfusion MRI and SPECT for detection of myocardial ischemia in patients with angiographically proven three-vessel coronary artery disease. Am. J. Roentgenol. 2010, 195, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Maredia, N.; Fairbairn, T.A.; Kozerke, S.; Greenwood, J.P.; Plein, S. Assessment of ischaemic burden in angiographic three-vessel coronary artery disease with high-resolution myocardial perfusion cardiovascular magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 701–708. [Google Scholar] [CrossRef]
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 978–986. [Google Scholar] [CrossRef]
- Jerosch-Herold, M. Quantification of myocardial perfusion by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 57. [Google Scholar] [CrossRef]
- Nagel, E.; Klein, C.; Paetsch, I.; Hettwer, S.; Schnackenburg, B.; Wegscheider, K.; Fleck, E. Magnetic Resonance Perfusion Measurements for the Noninvasive Detection of Coronary Artery Disease. Circulation 2003, 108, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.E.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction: A national heart, lung, and blood institute-sponsored study from the women’s ischemia syndrome evaluation. Circ. Cardiovasc. Imaging 2015, 8, e002481. [Google Scholar] [CrossRef]
- Knott, K.D.; Fernandes, J.L.; Moon, J.C. Automated Quantitative Stress Perfusion in a Clinical Routine. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.D.; Seraphim, A.; Augusto, J.B.; Xue, H.; Chacko, L.; Aung, N.; Petersen, S.E.; Cooper, J.A.; Manisty, C.; Bhuva, A.N.; et al. The Prognostic Utility of AI Perfusion Mapping. Circulation 2020, 141, 1285–1291. [Google Scholar] [CrossRef]
- Kellman, P.; Hansen, M.S.; Nielles-Vallespin, S.; Nickander, J.; Themudo, R.; Ugander, M.; Xue, H. Myocardial perfusion cardiovascular magnetic resonance: Optimized dual sequence and reconstruction for quantification. J. Cardiovasc. Magn. Reson. 2017, 7, 43. [Google Scholar] [CrossRef]
| Diagnosis | Diagnostic Criteria |
|---|---|
| Microvascular dysfunction (MVD) * |
|
| Vasospastic angina (VSA) ** |
|
| Non-cardiac chest pain |
|
| Protocol | Assessment | Reference Standard | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|
| First-pass perfusion qualitative assessment | Visual assessment | CFR < 2.5 | 0.60 | 58% | 83% |
| (0.46–0.69) | (46–69%) | (65–94%) | |||
| Semiquantitative CMR | MPR < 1.84 | ≥1 CRT abnormality | 0.78 | 73% | 74% |
| (0.68–0.88) | (64–82%) | (58–90%) | |||
| Dual-bolus fully quantitative perfusion CMR | MPRENDO ≤ 2.41 | CFR < 2.5 | 0.90 | 95% | 72% |
| (0.82–0.97) | (83–99%) | (52–87%) | |||
| MPR ≤ 2.19 | CFR < 2.5 | 0.88 | 70% | 90% | |
| (0.78–0.96) | (53–83%) | (74–98%) | |||
| Automated in-line CMR perfusion mapping | Regional stress MBF ≤ 2.19 | IMR ≥ 25 | 0.73 | 71% | 70% |
| (0.63–0.84) | |||||
| Regional MPR ≤ 2.06 | IMR ≥ 25 | 0.68 | 44% | 92% | |
| (0.56–0.80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, A.; Kang, N.; Chung, J.; Gupta, A.R.; Parwani, P. Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR). Medicina 2023, 59, 1570. https://doi.org/10.3390/medicina59091570
Chang A, Kang N, Chung J, Gupta AR, Parwani P. Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR). Medicina. 2023; 59(9):1570. https://doi.org/10.3390/medicina59091570
Chicago/Turabian StyleChang, Andrew, Nicolas Kang, Joseph Chung, Aakash Rai Gupta, and Purvi Parwani. 2023. "Evaluation of Ischemia with No Obstructive Coronary Arteries (INOCA) and Contemporary Applications of Cardiac Magnetic Resonance (CMR)" Medicina 59, no. 9: 1570. https://doi.org/10.3390/medicina59091570





