Balloon Pulmonary Angioplasty for Inoperable Chronic Thromboembolic Pulmonary Hypertension: Insights from a Pilot Low-Volume Centre Study and a Comparative Analysis with Other Centres
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Our Study | Ogawa et al. [17] (Japanese) | Brenot et al. [19] (French) | Darocha et al. [24] (Polish) | Olsson et al. [18] (German) | van Thor et al. [25] Netherlands | Hoole et al. [26] (UK) | Atas et al. [27] (Turkey) | |
|---|---|---|---|---|---|---|---|---|
| Centre/ Centres, n | Single centre N = 12 | 7 centers in Japan N = 308 | Single centre N = 184 | 8 centres in Poland N = 156 | 2 centres in Germany N = 56 | 2 centres in Netherlands N = 38 | Single centre N = 30 | Single centre N = 26 |
| Cohort | Inoperable CTEPH | Inoperable CTEPH 76% Post PEA 4.5% Refusal of PEA 13.6% Unfavorable risk/benefit ratio 5.8% | Inoperable CTEPH 81% Post PEA 8% Refusal of PEA 11% | Inoperable CTEPH 54.3% Post PEA 12.7% Refusal of PEA 11% Unfavorable risk/benefit ratio 22% | Inoperable CTEPH 87% Post PEA 13% Refusal of PEA 11% | Inoperable CTEPH 69% Refusal of PEA 13% Unfavorable risk/benefit ratio 18% | Inoperable CTEPH | Inoperable CTEPH 57.6% Post PEA 42.4% |
| Mean number of sessions per patient | 6.08 (3–9) | 4 (1–24) | 5.2 ± 2.4 and 5.7 ± 2.1 | 4.5 (2–7) | 5 (3–8) | 4.5 ± 1.3 | 3 (1–6) | No data |
| Mean time from the first to final procedure | 355.5 (119–624) days | 366.6 ± 394.1 days | 6.1 (4.5–7.5) months from first BPA to re-evaluation | 7.7 (3.4–13.9) month | 7.8 month | No data | 2 (1–5) month | No data |
| Follow-up time interval since last procedure | 258.4 (129–364) days | 425 ± 280.9 days | 3–6 month | 5.9 (3.0–8.0) month | 6 month | 6 month | 3 month | 3 month |
| Patents with PH therapy, % | 100% | 72.1% | 62% | 69.4% | 93% | 82% | 93.3% | 80% |
| 6MWD before BPA, m | 293 ± 151 | 318.1 ± 122.1 | 383 ± 137 | 341 ± 129 | 358 ± 108 | 374 ± 124 | 366 ± 107 | 315 ± 129 |
| 6MWD Follow-up, m | 380 ± 183 | 401.3 ± 104.8 | 434 ± 119 | 423 ± 136 | 391 ± 108 | 422 ± 125 | 440 ± 94 | 411 ± 140 |
| BNP before BPA, pg/mL | 590 ± 445 | 239.5 ± 334.2 | No data | NT pro-BNP 2275 (385–2675) 628 (85–533) | No data | NT pro-BNP 195 (96–18120 154 (71–387) | NT pro-BNP 442 (168–1607) 202 (105–447) | NT pro-BNP 456 189 |
| BNP follow-up, pg/mL | 121 ± 195 | 43.3 ± 76.4 | ||||||
| mPAP before BPA, mmHg | 56 ± 10 | 43.2 ± 11.0 | 44.3 ± 9.8 | 45.1 ± 10.7 | 40 ± 12 | 39.5 ± 11.6 | 44.7 ± 11.0 | 47.5 ± 13.4 |
| mPAP Follow-up, mmHg | 38 ± 10 | 24.3 ± 6.4 | 33.8 ± 9.8 | 30.1 ± 10.2 | 33 ± 11 | 30.6 ± 8.2 | 34.4 ± 8.3 | 38 ± 10.9 |
| PVR before BPA, dyn/s/cm−5 | 784 ± 320 | 853.7 ± 450.7 | 607 ± 218 | 642 ± 341 | 591 ± 286 | 488 ± 376 | 663 ± 281 | 744 ± 376 |
| PVR follow-up, dyn/s/cm−5 | 464 ± 232 | 359.5 ± 222.6 | 371 ± 188 | 324 ± 183 | 440 ± 279 | 264 ± 160 | 436 ± 196 | 464 ± 224 |
| Visit | I BPA | VIII BPA | Follow-Up (11 Month after Last BPA) |
|---|---|---|---|
| Date | 18 June 2020 | 21 April 2022 | 23 March 2023 |
| BNP, pg/mL | 1035.8 | 12.1 | 10.5 |
| PAP (s/d/m), mmHg | 85/30/52 | 37/14/24 | 37/14/24 |
| PWP, mmHg | 8 | 8 | 10 |
| CO (L/min) | 3.06 | 4.18 | 7.11 |
| PVR, Wood units | 12 | 3.1 | 2 |
| 6MWD, metres | 315 | 510 | 510 |
| This was a 40-year-old woman receiving PH-specific treatment with Riociguat. During the entire treatment period, eight sessions of BPA treatments were performed, with a treatment duration of 22 months. Follow-up was performed 11 months after the last BPA. | |||
References
- Delcroix, M.; Torbicki, A.; Gopalan, D.; Sitbon, O.; Klok, F.A.; Lang, I.; Jenkins, D.; Kim, N.H.; Humbert, M.; Jais, X.; et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2021, 57, 2002828. [Google Scholar] [CrossRef]
- Ende-Verhaar, Y.M.; Cannegieter, S.C.; Vonk Noordegraaf, A.; Delcroix, M.; Pruszczyk, P.; Mairuhu, A.T.A.; Huisman, M.V.; Klok, F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017, 49, 1601792. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm. Circ. 2021, 11, 2045894020977300. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D. Pulmonary endarterectomy: The potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Madani, M.; Fadel, E.; D’Armini, A.M.; Mayer, E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160111. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’Armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; Treacy, C.; Ponnaberanam, A.; Condliffe, R.; Sheares, K.; et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: Results from the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef]
- Lang, I.; Meyer, B.C.; Ogo, T.; Matsubara, H.; Kurzyna, M.; Ghofrani, H.A.; Mayer, E.; Brenot, P. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160119. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Jansa, P.; Huang, W.C.; Nižnanský, M.; Omara, M.; Lindner, J. Residual pulmonary hypertension after pulmonary endarterectomy: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2018, 156, 1275–1287. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Simonneau, G.; D’Armini, A.M.; Fedullo, P.; Howard, L.S.; Jaïs, X.; Jenkins, D.P.; Jing, Z.-C.; Madani, M.M.; Martin, N.; et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): Results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir. Med. 2017, 5, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; D’Armini, A.M.; Ghofrani, H.A.; Grimminger, F.; Jansa, P.; Kim, N.H.; Mayer, E.; Pulido, T.; Wang, C.; Colorado, P.; et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: Data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir. Med. 2016, 4, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiovasc. Interv. Ther. 2020, 35, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Darocha, S.; Pietura, R.; Pietrasik, A.; Norwa, J.; Dobosiewicz, A.; Piłka, M.; Florczyk, M.; Biederman, A.; Torbicki, A.; Kurzyna, M. Improvement in quality of life and hemodynamics in chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Circ. J. 2017, 81, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Ogo, T. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Curr. Opin. Pulm. Med. 2015, 21, 425–431. [Google Scholar] [CrossRef]
- Zoppellaro, G.; Badawy, M.R.; Squizzato, A.; Denas, G.; Tarantini, G.; Pengo, V. Balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension—A systematic review and meta-analysis. Circ. J. 2019, 83, 1660–1667. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, P.; Caforio, G.; Baratella, E.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lerda, S.; Geri, P.; Biolo, M.; et al. Chronic thromboembolic pulmonary hypertension: An observational study. Medicina 2022, 58, 1094. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.; Yan, P.; He, T.; Liu, B.; Wu, S.; Qian, Z.; Li, C.; Cao, Y.; Zhang, M. Balloon pulmonary angioplasty vs. pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Heart Fail. Rev. 2021, 26, 897–917. [Google Scholar] [CrossRef]
- Kennedy, M.K.; Kennedy, S.A.; Tan, K.T.; de Perrot, M.; Bassett, P.; McInnis, M.C.; Thenganatt, J.; Donahoe, L.; Granton, J.; Mafeld, S. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Cardiovasc. Interv. Radiol. 2023, 46, 5–18. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: Results of a multicenter registry. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef] [PubMed]
- Brenot, P.; Jaïs, X.; Taniguchi, Y.; Garcia Alonso, C.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1802095. [Google Scholar] [CrossRef] [PubMed]
- Darocha, S.; Roik, M.; Kopeć, G.; Araszkiewicz, A.; Furdal, M.; Lewandowski, M.; Jacheć, W.; Grabka, M.; Banaszkiewicz, M.; Pietrasik, A.; et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: A multicentre registry. EuroIntervention 2022, 17, 1104–1111. [Google Scholar] [CrossRef]
- Olsson, K.M.; Wiedenroth, C.B.; Kamp, J.C.; Breithecker, A.; Fuge, J.; Krombach, G.A.; Haas, M.; Hamm, C.; Kramm, T.; Guth, S.; et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: The initial German experience. Eur. Respir. J. 2017, 49, 1602409. [Google Scholar] [CrossRef] [PubMed]
- van Thor, M.C.J.; Lely, R.J.; Braams, N.J.; ten Klooster, L.; Beijk, M.A.M.; Heijmen, R.H.; Heuvel, D.A.F.v.D.; Rensing, B.J.W.M.; Snijder, R.J.; Noordegraaf, A.V.; et al. Safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension in the Netherlands. Neth. Heart J. 2020, 28, 81–88. [Google Scholar] [CrossRef]
- Hoole, S.P.; Coghlan, J.G.; Cannon, J.E.; Taboada, D.; Toshner, M.; Sheares, K.; Fletcher, A.J.; Martinez, G.; Ruggiero, A.; Screaton, N.; et al. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: The UK experience. Open Heart 2020, 7, e001144. [Google Scholar] [CrossRef]
- Atas, H.; Mutlu, B.; Akaslan, D.; Kocakaya, D.; Kanar, B.; Inanc, N.; Karakurt, S.; Cimsit, C.; Yildizeli, B. Balloon pulmonary angioplasty in patients with inoperable or recurrent/residual chronic thromboembolic pulmonary hypertension: A single-centre initial experience. Heart Lung Circ. 2022, 31, 520–529. [Google Scholar] [CrossRef]
- Ejiri, K.; Ogawa, A.; Fujii, S.; Ito, H.; Matsubara, H. Vascular injury is a major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2018, 11, e005884. [Google Scholar] [CrossRef]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.-A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef]
- Shimokawahara, H.; Ogawa, A.; Mizoguchi, H.; Yagi, H.; Ikemiyagi, H.; Matsubara, H. Vessel stretching is a cause of lumen enlargement immediately after balloon pulmonary angioplasty: Intravascular ultrasound analysis in patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2018, 11, e006010. [Google Scholar] [CrossRef]
- Wang, W.; Wen, L.; Song, Z.; Shi, W.; Wang, K.; Huang, W. Balloon pulmonary angioplasty vs riociguat in patients with inoperable chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Clin. Cardiol. 2019, 42, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, X.; Brenot, P.; Bouvaist, H.; Jevnikar, M.; Canuet, M.; Chabanne, C.; Chaouat, A.; Cottin, V.; De Groote, P.; Favrolt, N.; et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): A multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir. Med. 2022, 10, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Matsubara, H.; Shinke, T.; Abe, K.; Kohsaka, S.; Hosokawa, K.; Taniguchi, Y.; Shimokawahara, H.; Yamada, Y.; Kataoka, M.; et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): An open-label, randomised controlled trial. Lancet Respir. Med. 2022, 10, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Wiedenroth, C.B.; Ghofrani, H.A.; Adameit, M.S.D.; Breithecker, A.; Haas, M.; Kriechbaum, S.; Rieth, A.; Hamm, C.W.; Mayer, E.; Guth, S.; et al. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2018, 8, 2045894018783996. [Google Scholar] [CrossRef]
- Ravnestad, H.; Andersen, R.; Birkeland, S.; Svalebjørg, M.; Lingaas, P.S.; Gude, E.; Gullestad, L.; Kvitting, J.E.; Broch, K.; Andreassen, A.K. Pulmonary endarterectomy and balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: Comparison of changes in hemodynamics and functional capacity. Pulm. Circ. 2023, 13, e12199. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Miyagawa, K.; Nakayama, K.; Kinutani, H.; Shinke, T.; Okada, K.; Okita, Y.; Hirata, K.-I.; Emoto, N. Balloon pulmonary angioplasty: An additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention 2014, 10, 518–525. [Google Scholar] [CrossRef]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef]
- Inami, T.; Kataoka, M.; Yanagisawa, R.; Ishiguro, H.; Shimura, N.; Fukuda, K.; Yoshino, H.; Satoh, T. Long-term outcomes after percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circulation 2016, 134, 2030–2032. [Google Scholar] [CrossRef]
| Study Population, n | 26 |
|---|---|
| Mean age, years | 61.68 (40–80) |
| Male, n (%) | 16 (61.5) |
| Previous DVT, n (%) | 12 (46.2) |
| History of PE, n (%) | 21 (80.8) |
| Recurrent PE, n (%) | 3 (11.5) |
| Current smoker, n (%) | 3 (11.5) |
| Comorbidities, n (%) | 26 (100) |
| ➢ Hypertension | 22 (84.6) |
| ➢ Dyslipidaemia | 14 (53.8) |
| ➢ Diabetes | 3 (11.5) |
| ➢ Obesity | 3 (11.5) |
| ➢ Cancer | 1 (3.8) |
| ➢ Coronary artery disease | 4 (15.4) |
| ➢ Chronic kidney disease | 2 (7.7) |
| ➢ COPD | 3 (11.5) |
| Targeted PH medical treatment, n (%) | 23 (88.5) |
| ➢ Riociguat | 19 (73.1) |
| ➢ Riociguat and Bosentan | 2 (7.7) |
| ➢ Sildenafil and Ambrisentan | 1 (3.8) |
| ➢ Sildenafil | 1 (3.8) |
| ➢ One medication | 21 (80.8) |
| ➢ Two medications | 2 (7.7) |
| Oral anticoagulants, n (%) | 26 (100) |
| ➢ Warfarin | 4 (15.4) |
| ➢ Rivaroxaban | 12 (46.2) |
| ➢ Apixaban | 6 (23.1) |
| ➢ Edoxaban | 4 (15.4) |
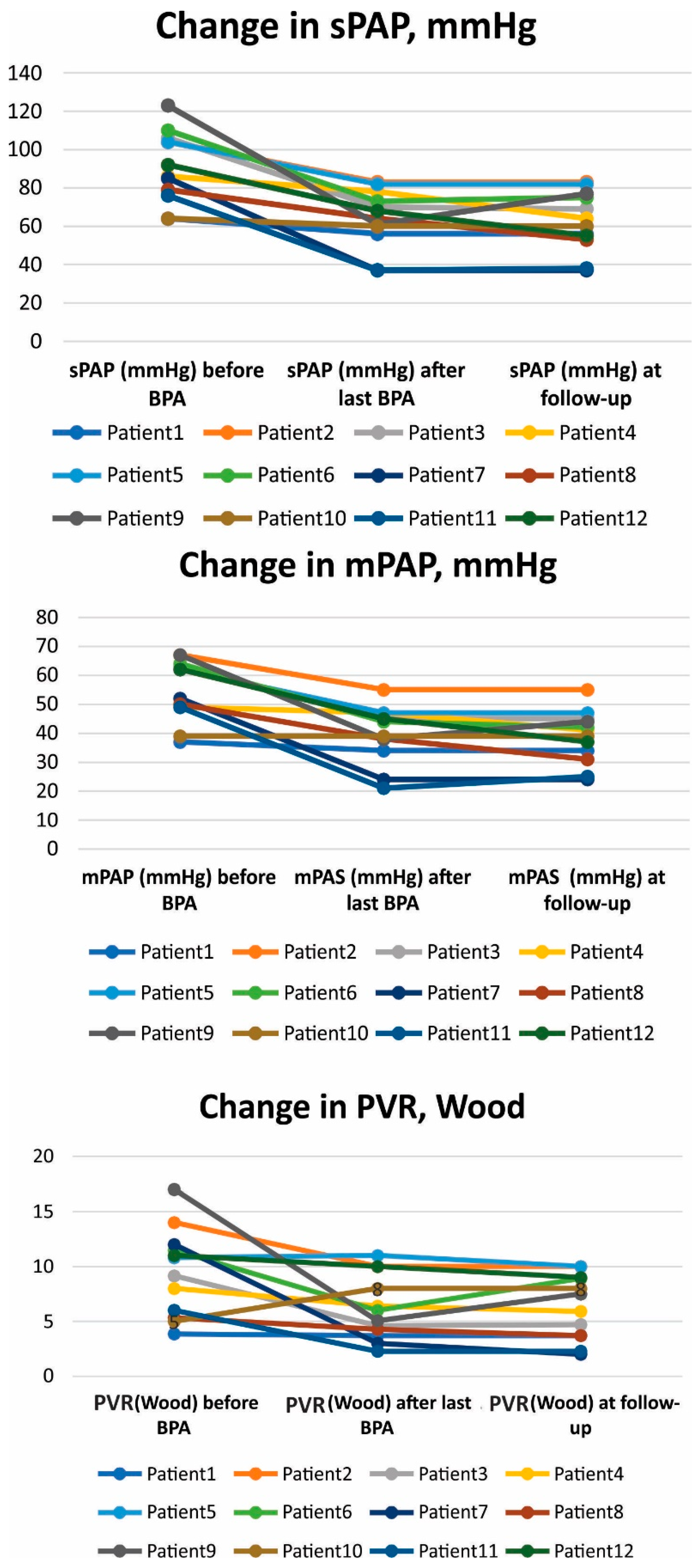
| No/Gender | Age | Specific Treatment | Times of BPA | 6 MWD (m) | BNP | sPAP (mmHg) | mPAP (mmHg) | CO (L/min) | PWR (Wood) | Mean X-ray Exposure (mGy) | Mean Procedure Time (min) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After BPA | At Follow-Up | Baseline | After BPA | At Follow-Up | Baseline | After BPA | At Follow-Up | Baseline | After BPA | At Follow-Up | Baseline | After BPA | At Follow-Up | Baseline | After BPA | At Follow-Up | ||||||
| 1/M | 67 | riociguat | 6 | 510 | 565 | 600 | 49 | 21 | 36 | 64 | 56 | 56 | 37 | 34 | 34 | 6.23 | 5.4 | 5.4 | 3.85 | 3.7 | 3.7 | 679 | 119 |
| 2/M | 59 | riociguat, bosentan | 6 | 120 | 180 | 120 | 975 | 418 | 387 | 104 | 83 | 83 | 67 | 55 | 55 | 3.8 | 4 | 4 | 14 | 10 | 10 | 809 | 118 |
| 3/F | 54 | riociguat | 7 | 325 | 300 | 300 | 275 | 75.9 | 70 | 106 | 70 | 69 | 63 | 45 | 45 | 5.57 | 7.33 | 7 | 9.15 | 4.6 | 4.7 | 565 | 84 |
| 4/M | 75 | sildenafil | 9 | 320 | 360 | 360 | 797 | 85 | 36 | 86 | 78 | 64 | 49 | 47 | 41 | 4.58 | 5.1 | 4.7 | 8 | 6.4 | 5.9 | 521 | 103 |
| 5/M | 65 | riociguat, bosentan | 5 | 475 | 505 | 435 | 909 | 317 | 650 | 104 | 82 | 82 | 62 | 47 | 47 | 4.5 | 3.16 | 3.1 | 10.8 | 11 | 11 | 269 | 90 |
| 6/F | 66 | riociguat | 8 | 300 | 390 | 376 | 369 | 160 | 36.1 | 110 | 73 | 75 | 64 | 44 | 42 | 4.27 | 4.5 | 3.57 | 11.47 | 6 | 8.9 | 545 | 107 |
| 7/F | 40 | riociguat | 8 | 315 | 510 | 510 | 1035 | 12.1 | 10.5 | 85 | 37 | 37 | 52 | 24 | 24 | 3.06 | 7.11 | 7 | 12 | 3 | 2 | 216 | 105 |
| 8/M | 52 | riociguat | 7 | 450 | 540 | 570 | 213 | 40 | 40 | 79 | 64 | 53 | 50 | 38 | 31 | 6,19 | 3.9 | 5.13 | 5.33 | 4.28 | 3.7 | 535 | 107.5 |
| 9/M | 60 | riociguat | 6 | 10 | 520 | 420 | 1186 | 46.3 | 51.1 | 123 | 61 | 77 | 67 | 38 | 44 | 3.15 | 5,4 | 4,0 | 17 | 5.5 | 7.5 | 318 | 100 |
| 10/F | 80 | riociguat | 4 | 240 | 240 | 240 | 161 | 21 | 26.6 | 64 | 60 | 60 | 39 | 39 | 39 | 4.17 | 2.8 | 3 | 5 | 8 | 8 | 323 | 102 |
| 11/M | 70 | riiociguat | 4 | 330 | 340 | 340 | 22 | 40.5 | 14.4 | 76 | 37 | 38 | 49 | 21 | 25 | 6.3 | 7 | 7.08 | 6 | 2.28 | 2.28 | 659 | 105 |
| 12/F | 72 | riociguat | 3 | 120 | 285 | 285 | 1091 | 97 | 97 | 92 | 68 | 55 | 62 | 45 | 37 | 4.69 | 3.65 | 2.76 | 11 | 10 | 9 | 515 | 101 |
| N = 12 | Before BPA | After last BPA | p * | Follow-Up | p # |
|---|---|---|---|---|---|
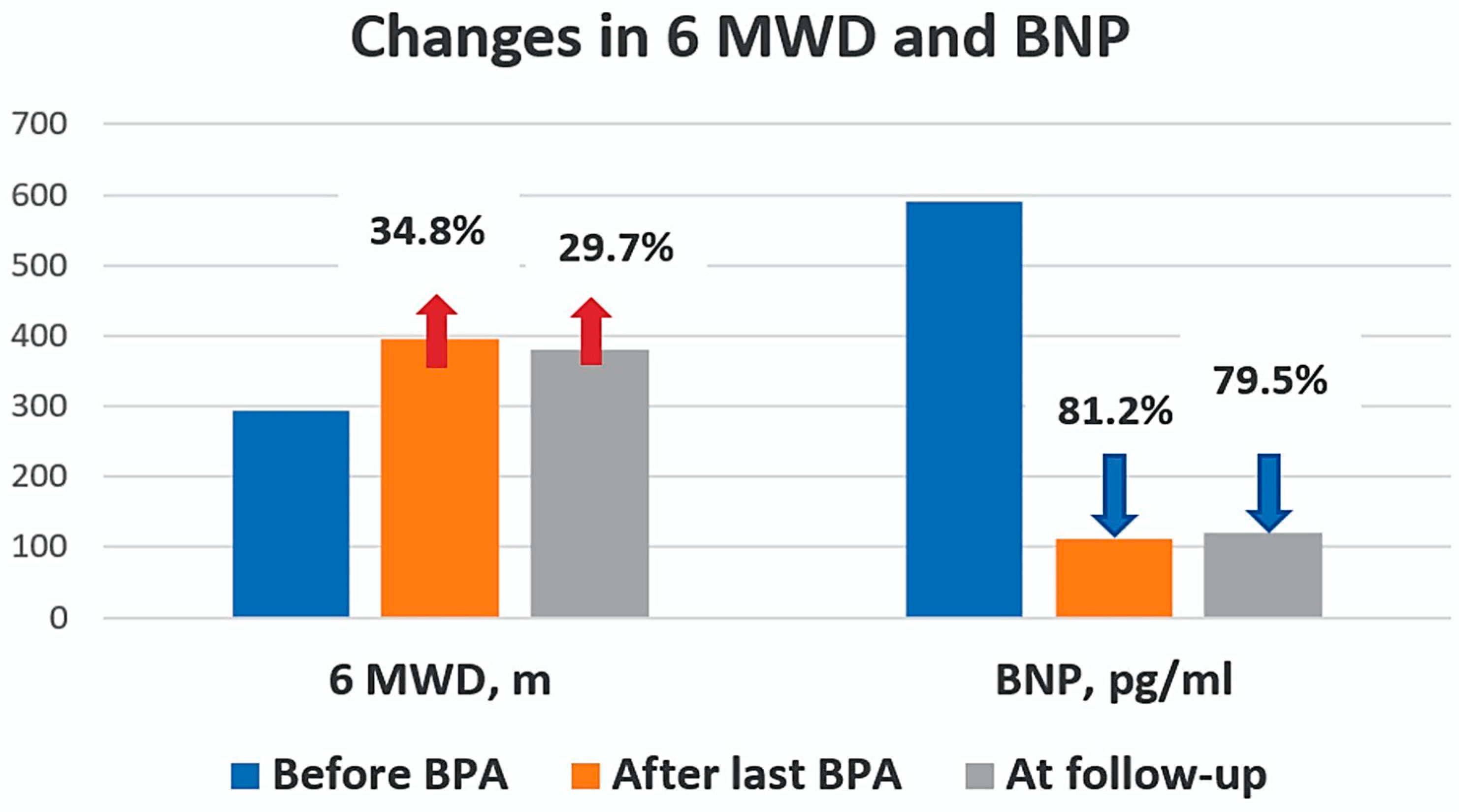
| 6MWD, m | 292.9 ± 151 | 394.5 ± 130 | 0.015 | 379.6 ± 138 | 0.043 |
| BNP, pg/ml | 590 ± 445 | 111.1 ± 128 | <0.001 | 121.2 ± 195.2 | <0.001 |
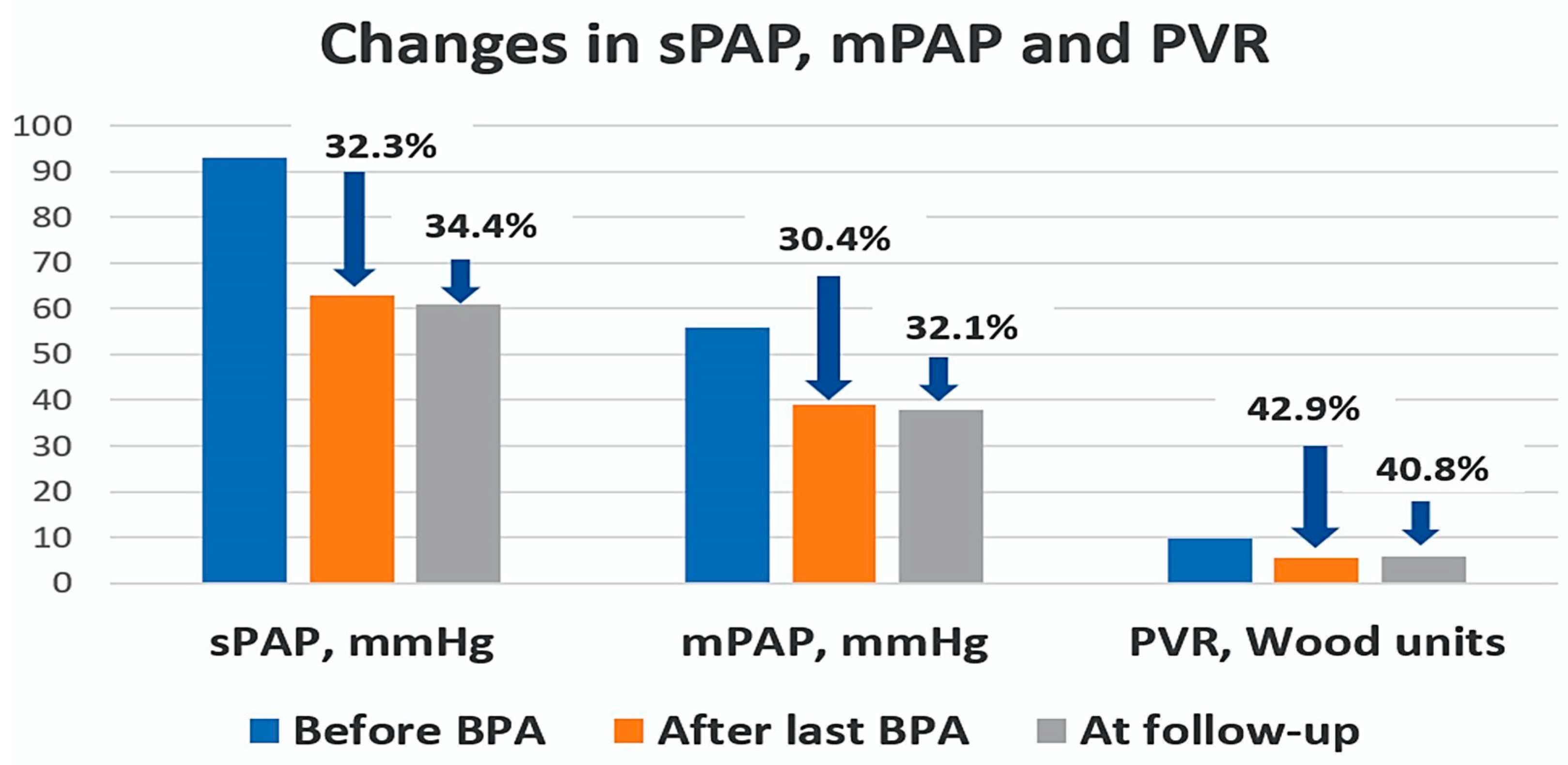
| sPAP, mmHg | 92.5 ± 18 | 62.7 ± 15.6 | <0.001 | 60.7 ± 15.7 | <0.001 |
| mPAP, mmHg | 56 ± 10 | 39 ± 10 | <0.001 | 37.8 ± 9.6 | <0.001 |
| CO, L/min | 4.7 ± 1.2 | 5.3 ± 1.3 | 0.749 | 5.0 ± 1.55 | 1 |
| PVR, Wood units | 9.8 ± 4 | 5.6 ± 2.6 | <0.001 | 5.8 ± 2.9 | 0.001 |
| Procedures, n | 100 |
|---|---|
| Range of number of procedures, mean per patient | 3.84 (1–9) |
| Mean duration of procedure, hours | 1:43 ± 0:23 |
| Mean radiation exposure, mGy | 496 ± 180 |
| Complications, n (%) | 17 (17) |
| ➢ Contrast extravasation and leakage into bronchi, n (%) | 3 (3) |
| ➢ Haemoptysis, n (%) | 3 (3) |
| ➢ Vessel dissection, n (%) | 10 (10) |
| ➢ Hyperperfusion pulmonary oedema, n (%) | 1 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanauskiene, T.; Cesna, S.; Grigoniene, E.; Gumbiene, L.; Daubaraite, A.; Ivanauskaite, K.; Glaveckaite, S. Balloon Pulmonary Angioplasty for Inoperable Chronic Thromboembolic Pulmonary Hypertension: Insights from a Pilot Low-Volume Centre Study and a Comparative Analysis with Other Centres. Medicina 2024, 60, 461. https://doi.org/10.3390/medicina60030461
Ivanauskiene T, Cesna S, Grigoniene E, Gumbiene L, Daubaraite A, Ivanauskaite K, Glaveckaite S. Balloon Pulmonary Angioplasty for Inoperable Chronic Thromboembolic Pulmonary Hypertension: Insights from a Pilot Low-Volume Centre Study and a Comparative Analysis with Other Centres. Medicina. 2024; 60(3):461. https://doi.org/10.3390/medicina60030461
Chicago/Turabian StyleIvanauskiene, Taida, Sigitas Cesna, Egle Grigoniene, Lina Gumbiene, Aurelija Daubaraite, Kaste Ivanauskaite, and Sigita Glaveckaite. 2024. "Balloon Pulmonary Angioplasty for Inoperable Chronic Thromboembolic Pulmonary Hypertension: Insights from a Pilot Low-Volume Centre Study and a Comparative Analysis with Other Centres" Medicina 60, no. 3: 461. https://doi.org/10.3390/medicina60030461
APA StyleIvanauskiene, T., Cesna, S., Grigoniene, E., Gumbiene, L., Daubaraite, A., Ivanauskaite, K., & Glaveckaite, S. (2024). Balloon Pulmonary Angioplasty for Inoperable Chronic Thromboembolic Pulmonary Hypertension: Insights from a Pilot Low-Volume Centre Study and a Comparative Analysis with Other Centres. Medicina, 60(3), 461. https://doi.org/10.3390/medicina60030461






