Prevalence of FMS Diagnosis According to ACR 2016 Revised Criteria in a Pain Therapy Centre in Italy: Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Endpoints
2.3. Ethics
2.4. Data Collection and Statistical Analysis
3. Results
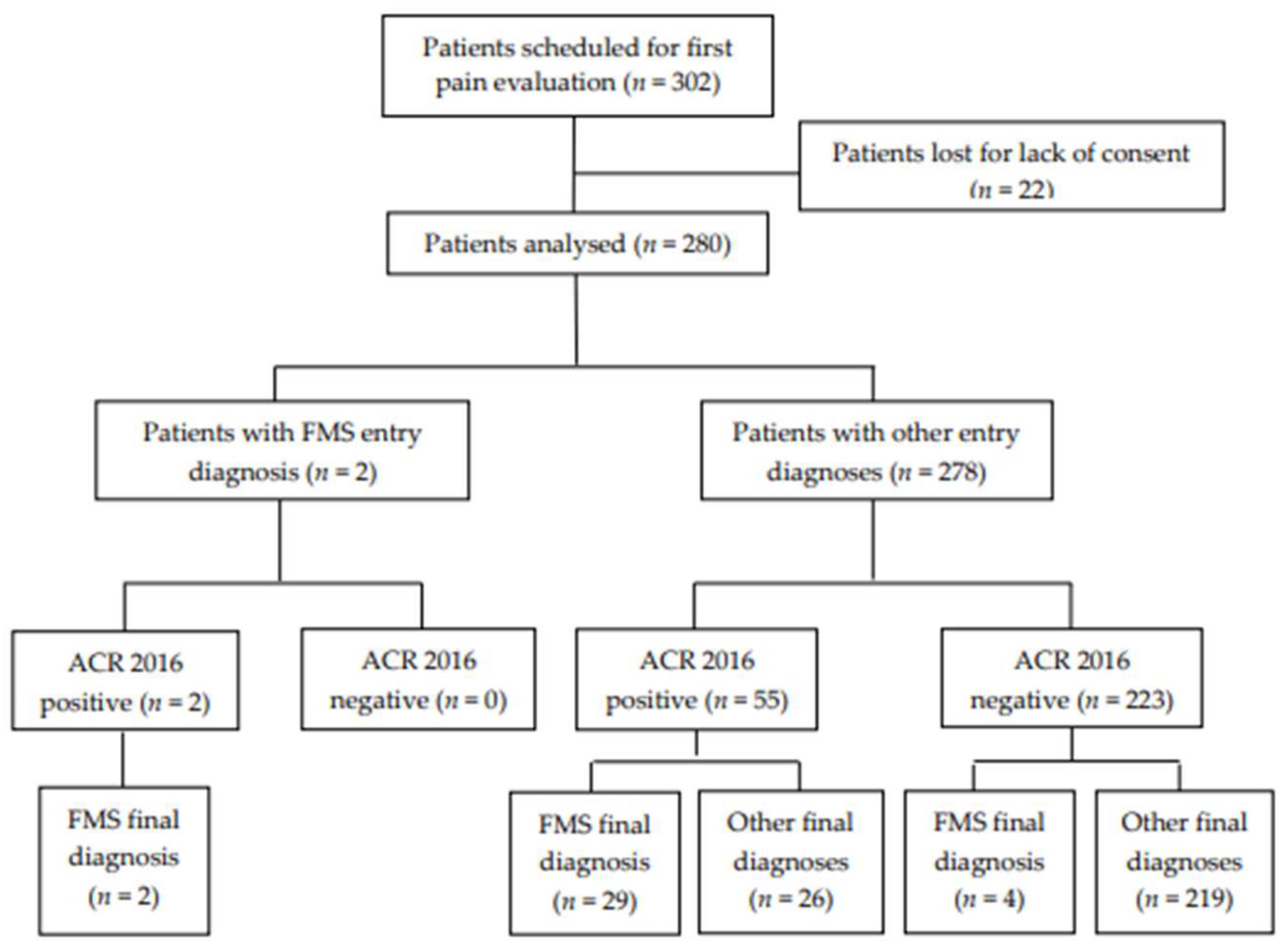
3.1. Study Population
3.2. Primary Endpoint
3.3. Secondary Endpoints
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giorgi, V.; Bazzichi, L.; Batticciotto, A.; Pellegrino, G.; Di Franco, M.; Sirotti, S.; Atzeni, F.; Alciati, A.; Salaffi, F.; Sarzi-Puttini, P. Fibromyalgia: One year in review 2023. Clin. Exp. Rheumatol. 2023, 41, 1205–1213. [Google Scholar] [CrossRef]
- Cohen, H. Controversies and challenges in fibromyalgia: A review and a proposal. Ther. Adv. Musculoskelet. Dis. 2017, 9, 115–127. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Bennett, R.M.; Crofford, L.J.; Dean, L.E.; Clauw, D.J.; Goldenberg, D.L.; Fitzcharles, M.A.; Paiva, E.S.; Staud, R.; Sarzi-Puttini, P.; et al. AAPT Diagnostic Criteria for Fibromyalgia. J. Pain. 2019, 20, 611–628. [Google Scholar] [CrossRef]
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017, 37, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Sarzi-Puttini, P.; Fitzcharles, M.A. Fibromyalgia syndrome: Under-, over- and misdiagnosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S116), 90–97. [Google Scholar]
- Lee, H.J.; Choi, E.; Nahm, F.S.; Choi, S.S.; Kim, Y.H.; Moon, J.Y.; Kim, S.H.; Cho, C.W.; Lee, P.B. Prevalence of fibromyalgia in fourteen Korean tertiary care university hospital pain clinics. J. Pain. Res. 2018, 11, 2417–2423. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Perrot, S.; Häuser, W. Comorbid fibromyalgia: A qualitative review of prevalence and importance. Eur. J. Pain. 2018, 22, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgen, C.P.; Ucles-Juarez, R.; Krutko, D.; Li, Y.; Polli, A.; Syed, A.; Zampese, S.; Reis, F.J.J.; de Zeeuw, J. Knowledge on cause, clinical manifestation and treatment for fibromyalgia among medical doctors: A worldwide survey. Pain. Pract. 2023, 29. [Google Scholar] [CrossRef]
- Häuser, W.; Fitzcharles, M.A. Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 2018, 20, 53–62. [Google Scholar] [CrossRef]
- Ablin, J.N.; Berman, M.; Aloush, V.; Regev, G.; Salame, K.; Buskila, D.; Lidar, Z. Effect of fibromyalgia symptoms on outcome of spinal surgery. Pain. Med. 2017, 18, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Hernández, I.; Pérez-Marín, M.L.Á.; Nunez-Nagy, S.; Pecos-Martín, D.; Gallego-Izquierdo, T.; Sosa-Reina, M.D. Effectiveness of invasive techniques in patients with fibromyalgia: Systematic review and meta-analysis. Pain. Med. 2020, 21, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, J.; Hurley, J.A. Fibromyalgia. [Updated 2023 Jun 11]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wolfe, F.; Walitt, B.; Perrot, S.; Rasker, J.J.; Häuser, W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS ONE 2018, 13, e0203755. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, N.; Haghighi, A.; Bidari, A.; Sharafian Ardekani, Y.; Karimi, M.A. Are general practitioners well informed about fibromyalgia? Int. J. Rheum. Dis. 2017, 20, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Hasselroth, R.; Björling, G.; Faag, C.; Bose, C.N. “Can Someone as Young as You Really Feel That Much Pain?”—A Survey on how people with Fibromyalgia experience healthcare in Sweden. SAGE Open Nurs. 2021, 7, 23779608211026145. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Maloney, E.; Wright, B.; Kennedy, M.; Kallail, K.J.; Rasker, J.J.; Häuser, W.; Wolfe, F. The problematic nature of Fibromyalgia diagnosis in the community. ACR Open Rheumatol. 2019, 1, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Buskila, D. Epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2003, 7, 362–368. [Google Scholar] [CrossRef]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Carbonell, J.A.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubère, J.; et al. Prevalence of Fibromyalgia: A Survey in five European countries. Semin. Arthritis Rheum. 2010, 39, 448–453. [Google Scholar] [CrossRef]
- Ciaffi, J.; Brognara, L.; Gangemi, G.; Vanni, E.; Assirelli, E.; Neri, S.; Casadei, G.; Mazzotti, A.; Di Martino, A.; Faldini, C.; et al. Prevalence and characteristics of fibromyalgia in patients with foot and ankle pain: The experience of an Academic Podiatry Clinic. Medicina 2022, 59, 58. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Wolfe, F.; Schmukler, J.; Jamal, S.; Castrejon, I.; Gibson, K.A.; Srinivasan, S.; Häuser, W.; Pincus, T. Diagnosis of fibromyalgia: Disagreement between fibromyalgia criteria and clinician-based fibromyalgia diagnosis in a University Clinic. Arthritis Care Res. 2019, 71, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Mengshoel, A.M.; Sim, J.; Ahlsen, B.; Madden, S. Diagnostic experience of patients with fibromyalgia—A meta-ethnography. Chronic Illn. 2018, 14, 194–211. [Google Scholar] [CrossRef] [PubMed]
| Patients Evaluated, n | 302 |
|---|---|
| Gender, n (%) | |
| Female | 202 (66.8%) |
| Male | 100 (33.1%) |
| Age, yr. * | 61 (51–75) |
| Entry diagnosis, n (%) | |
| Low back pain | 126 (41.7%) |
| Headache or facial pain | 40 (13.2%) |
| Chronic widespread pain | 32 (10.6%) |
| Cervical or neck pain | 28 (9.2%) |
| Articular pain | 25 (8.2%) |
| Dorsal pain | 13 (4.3%) |
| Abdominal pain | 11 (3.6%) |
| Vascular pain | 10 (3.3%) |
| Neuropathic pain | 8 (2.6%) |
| Pelvic pain | 7 (2.3%) |
| Fibromyalgia | 2 (0.6%) |
| Patients with Positive ACR Criteria, n | 57 |
|---|---|
| Gender, n (%) | |
| Female | 53 (92.9%) |
| Male | 4 (7.02%) |
| F:M ratio | 14:1 |
| Age, yr. * | 57 (43–66) |
| Entry diagnosis, n (%) | |
| Chronic widespread pain | 22 (38.6%) |
| Low back pain | 12 (21%) |
| Headache or facial pain | 7 (12.2%) |
| Articular pain | 5 (8.7%) |
| Cervical or neck pain | 4 (7%) |
| Dorsal pain | 3 (5.2%) |
| Fibromyalgia | 2 (3.5%) |
| Neuropathic pain | 2 (3.5%) |
| ACR 2016 items * | |
| WPI (0–19) | 13 (10–15) |
| SS (0–12) | 9 (6.5–10) |
| VAS (0–100) * | 80 (70–90) |
| Patients with Positive ACR Criteria, n | 57 |
|---|---|
| Final diagnosis, n (%) | |
| FMS | 31 (54.5%) |
| Primary FMS | 19 (61.2%) |
| Concomitant FMS | 12 (38.7%) |
| WP from rheumatic disease | 6 (10.5%) |
| WP from ostheoarthritis | 5 (8.7%) |
| Neuropathic pain | 4 (7%) |
| Migraine | 4 (7%) |
| Mixed pain on upper limbs | 3 (5.2%) |
| LBP from vertebral fracture | 2 (3.5%) |
| Sacroiliac joint pain | 2 (3.5%) |
| Knee pain | 1 (1.7%) |
| Tension-type headache (TTH) | 1 (1.7%) |
| Patients with Negative ACR Criteria, n | 223 |
|---|---|
| Final diagnosis, n (%) | |
| LBP | 91 (40.8%) |
| Headache | 26 (11.6%) |
| Cervical pain | 18 (8%) |
| Chronic abdominal pain | 12 (5.3%) |
| Dorsal pain | 11 (4.9%) |
| Facial pain | 10 (4.4%) |
| Pain from ostheoarthritis | 9 (4%) |
| Vascular pain on lower limbs | 8 (3.5) |
| Cancer pain | 8 (3.5) |
| Neuropathic pain | 8 (3.5) |
| Scapular pain | 6 (2.6%) |
| Coccigodynia | 5 (2.2%) |
| FMS | 4 (1.7%) |
| Primary FMS | 3 (75%) |
| Concomitant FMS | 1 (25%) |
| WP (unspecified) | 3 (1.3%) |
| Thoracic pain | 2 (0.9%) |
| Anal pain | 2 (0.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schweiger, V.; Martini, A.; Nizzero, M.; Bonora, E.; Del Balzo, G.; Gottin, L.; Torroni, L.; Polati, L.; Zuliani, G.; Secchettin, E.; et al. Prevalence of FMS Diagnosis According to ACR 2016 Revised Criteria in a Pain Therapy Centre in Italy: Observational Study. Medicina 2024, 60, 599. https://doi.org/10.3390/medicina60040599
Schweiger V, Martini A, Nizzero M, Bonora E, Del Balzo G, Gottin L, Torroni L, Polati L, Zuliani G, Secchettin E, et al. Prevalence of FMS Diagnosis According to ACR 2016 Revised Criteria in a Pain Therapy Centre in Italy: Observational Study. Medicina. 2024; 60(4):599. https://doi.org/10.3390/medicina60040599
Chicago/Turabian StyleSchweiger, Vittorio, Alvise Martini, Marta Nizzero, Eleonora Bonora, Giovanna Del Balzo, Leonardo Gottin, Lorena Torroni, Luca Polati, Giada Zuliani, Erica Secchettin, and et al. 2024. "Prevalence of FMS Diagnosis According to ACR 2016 Revised Criteria in a Pain Therapy Centre in Italy: Observational Study" Medicina 60, no. 4: 599. https://doi.org/10.3390/medicina60040599
APA StyleSchweiger, V., Martini, A., Nizzero, M., Bonora, E., Del Balzo, G., Gottin, L., Torroni, L., Polati, L., Zuliani, G., Secchettin, E., & Polati, E. (2024). Prevalence of FMS Diagnosis According to ACR 2016 Revised Criteria in a Pain Therapy Centre in Italy: Observational Study. Medicina, 60(4), 599. https://doi.org/10.3390/medicina60040599








