Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Non-Inferiority Margin
2.3. Sample Size
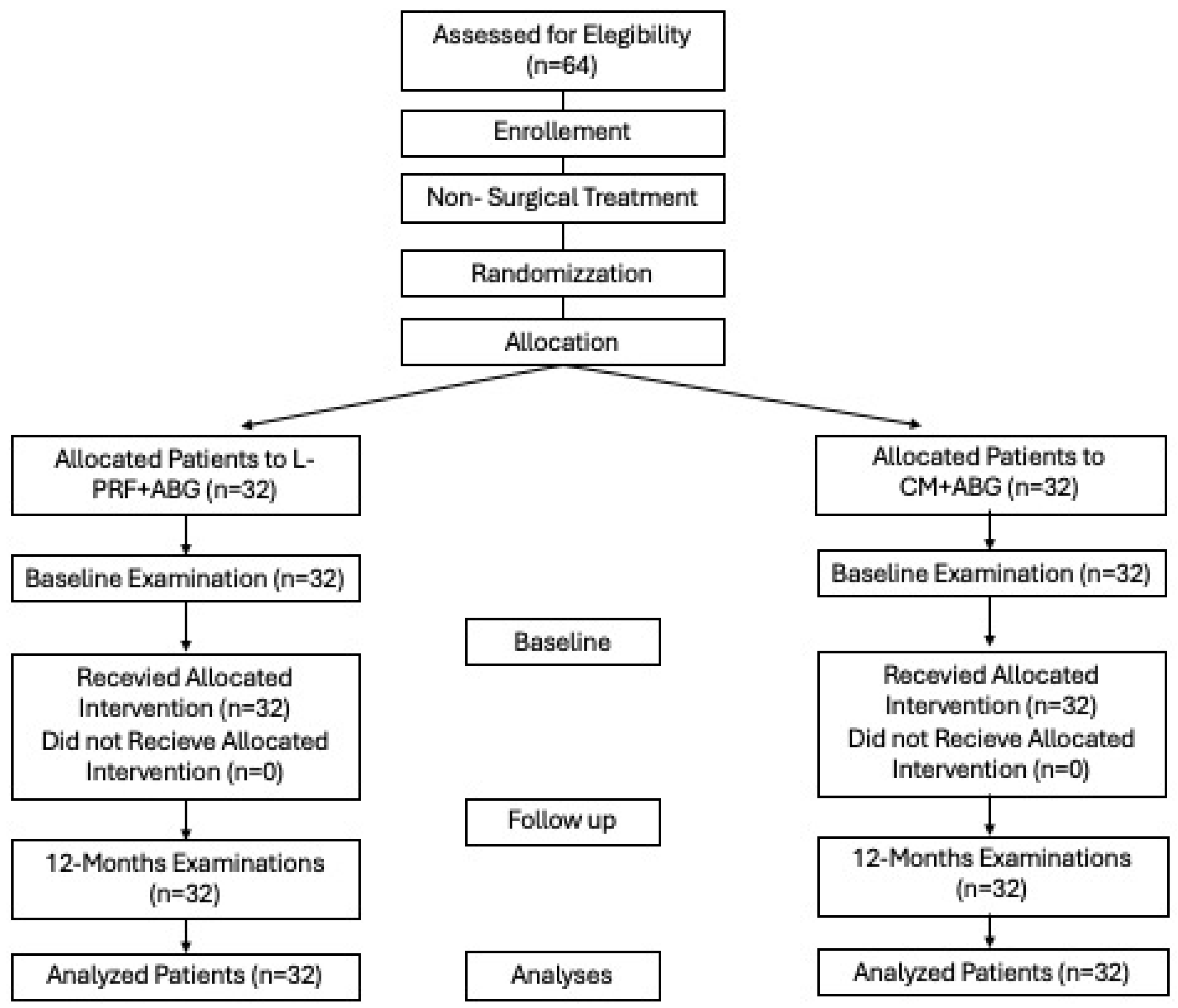
2.4. Study Population
2.5. Patient’s Inclusion and Exclusion Criteria
Randomization and Blinding Protocol
2.6. Clinical Measurements
2.7. Radiographic Measurement
2.8. Platelet-Rich Fibrin Preparation
2.9. Surgical Procedure
2.10. Post-Operative Procedures
2.11. Statistical Analysis
3. Results
3.1. Study Population
3.2. Clinical and Radiographic Outcomes
4. Discussion
4.1. Principal Findings
4.2. Agreement and Disagreement with Previous Findings
4.3. Discussion of Secondary Outcomes
4.4. Clinical Implications
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papapanou, P.N.; Wennström, J.L. The angular bony defect as indicator of further alveolar bone loss. J. Clin. Periodontol. 1991, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Tonetti, M.S. Diagnosis and epidemiology of periodontal osseous lesions. Periodontol. 2000 2000, 22, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Radiographic alveolar bone morphology and progressive periodontitis. J. Periodontol. 2018, 89, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Goldman, H.M.; Cohen, D.W. The Infrabony Pocket: Classification and Treatment†. J. Periodontol. 1958, 29, 272–291. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S.; Consultants, E.W.P.A.M.; EFP Workshop Participants and Methodological Consultants; et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef] [PubMed]
- Suvan, J.; Leira, Y.; Sancho, F.M.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 155–175. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol. 2000 2013, 62, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Nyman, S. Histometric evaluation of periodontal surgery I. The modified Widman flap procedure. J. Clin. Periodontol. 1980, 7, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.; Nyman, S.; Zander, H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J. Clin. Periodontol. 1980, 7, 224–231. [Google Scholar] [CrossRef]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 320–351. [Google Scholar] [CrossRef]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal regeneration of human infrabony defects. I. Clinical measures. J. Periodontol. 1993, 64, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal Regeneration of Human Infrabony Defects. II. Re-Entry Procedures and Bone Measures. J. Periodontol. 1993, 64, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Donos, N.; Windisch, P.; Brecx, M.; Gera, I.; Reich, E.; Karring, T. Healing of human intrabony defects following treatment with enamel matrix proteins or guided tissue regeneration. J. Periodontal Res. 1999, 34, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Sultan, D.; Arena, C.; Pelekos, G.; Lin, G.-H.; Tonetti, M. Periodontal infrabony defects: Systematic review of healing by defect morphology following regenerative surgery. J. Clin Periodontol. 2021, 48, 101–114. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, G.P.; Tonetti, M.S. Periodontal regeneration of human intrabony defects with bioresorbable membranes. A controlled clinical trial. J. Periodontol. 1996, 67, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J. Clin. Periodontol. 1986, 13, 604–616. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 82–91. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Ávila, G.; Fernández-Barbero, J.E.; Mesa, F.; O’Valle-Ravassa, F.; Wang, H.L. Clinical and histologic comparison of two different composite grafts for sinus augmentation: A pilot clinical trial. Clin. Oral. Implants Res. 2008, 19, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- Paolantonio, M.; Di Tullio, M.; Giraudi, M.; Romano, L.; Secondi, L.; Paolantonio, G.; Graziani, F.; Pilloni, A.; De Ninis, P.; Femminella, B. Periodontal regeneration by leukocyte and platelet-rich fibrin with autogenous bone graft versus enamel matrix derivative with autogenous bone graft in the treatment of periodontal intrabony defects: A randomized non-inferiority trial. J. Periodontol. 2020, 91, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.S.; Narula, S.C.; Sharma, R.K.; Tewari, S.; Yadav, R. Clinical evaluation of guided tissue regeneration combined with autogenous bone or autogenous bone mixed with bioactive glass in intrabony defects. J. Oral. Sci. 2011, 53, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Rexhepi, I.; Paolantonio, M.; Romano, L.; Serroni, M.; Santamaria, P.; Secondi, L.; Paolantonio, G.; Sinjari, B.; De Ninis, P.; Femminella, B. Efficacy of inorganic bovine bone combined with leukocyte and platelet-rich fibrin or collagen membranes for treating unfavorable periodontal infrabony defects: Randomized non-inferiority trial. J. Periodontol. 2021, 92, 1576–1587. [Google Scholar] [CrossRef]
- Aghazadeh, A.; Persson, G.R.; Renvert, S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: Results after 12 months. J. Clin. Periodontol. 2012, 39, 666–673. [Google Scholar] [CrossRef]
- Bezerra, B.T.; Pinho, J.N.A.; Figueiredo, F.E.D.; Brandão, J.R.M.C.B.; Ayres, L.C.G.; da Silva, L.C.F. Autogenous Bone Graft Versus Bovine Bone Graft in Association With Platelet-Rich Plasma for the Reconstruction of Alveolar Clefts: A Pilot Study. Cleft Palate Craniofacial J. 2019, 56, 134–140. [Google Scholar] [CrossRef] [PubMed]
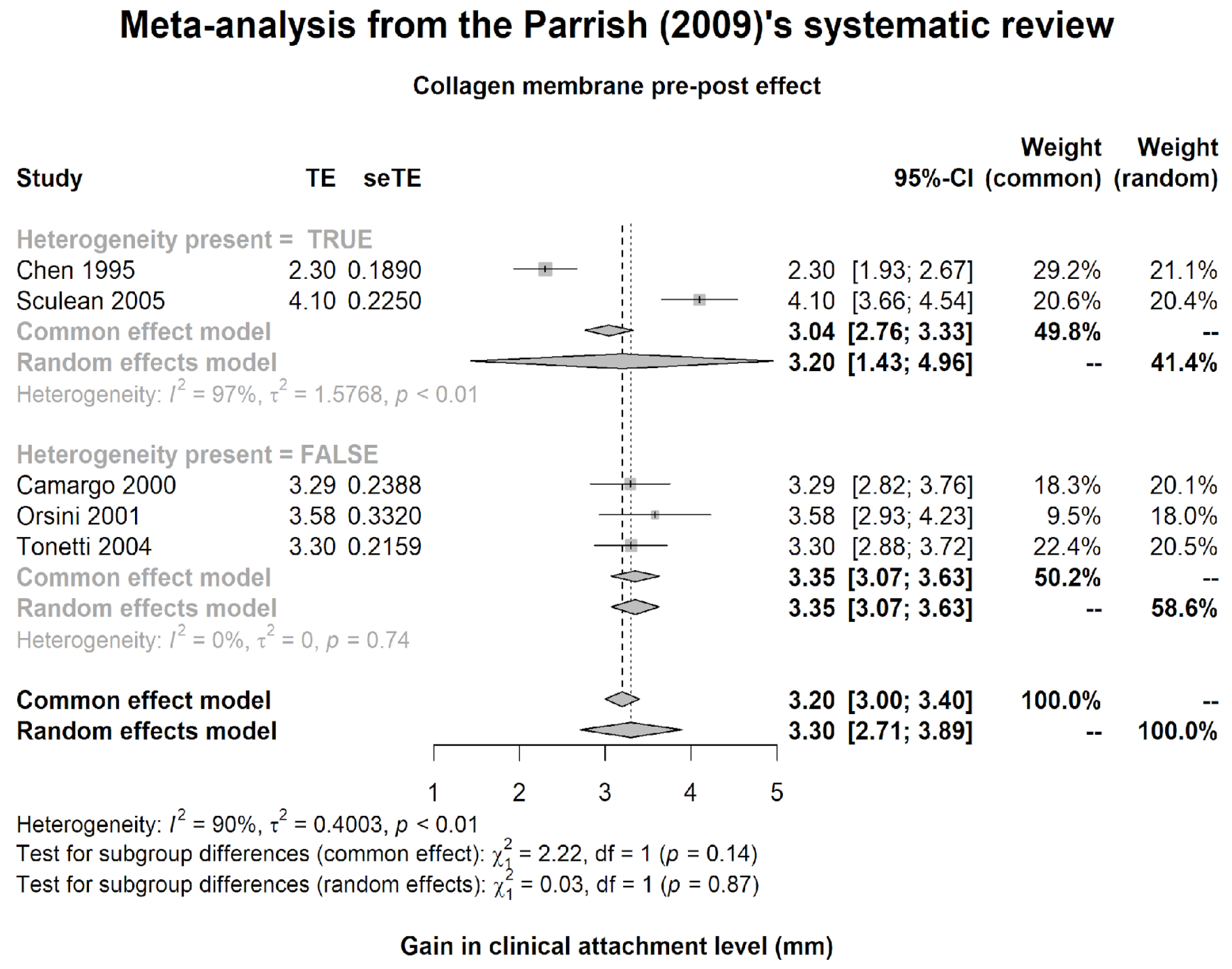
- Parrish, L.C.; Miyamoto, T.; Fong, N.; Mattson, J.S.; Cerutis, D.R. Non-bioabsorbable vs. bioabsorbable membrane: Assessment of their clinical efficacy in guided tissue regeneration technique. A systematic review. J. Oral. Sci. 2009, 51, 383–400. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Cei, S.; Cairo, F.; Baggiani, A.; Miccoli, M.; Gabriele, M.; Tonetti, M. Clinical performance of access flap surgery in the treatment of the intrabony defect. A systematic review and meta-analysis of randomized clinical trials. J. Clin. Periodontol. 2012, 39, 145–156. [Google Scholar] [CrossRef]
- FDA. Non-Inferiority Clinical Trials. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/non-inferiority-clinical-trials (accessed on 21 May 2024).
- Altman, D.G. Practical Statistics for Medical Research; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Julious, S.A. Sample sizes for clinical trials with normal data. Stat. Med. 2004, 23, 1921–1986. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Pini-Prato, G.; Cortellini, P. Periodontal regeneration of human intrabony defects. IV. Determinants of healing response. J. Periodontol. 1993, 64, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Heubel, B.; Nohe, A. The Role of BMP Signaling in Osteoclast Regulation. J. Dev. Biol. 2021, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Huberty, C.J.; Morris, J.D. Multivariate Analysis Versus Multiple Univariate Analyses. Psychol. Bull. 1989, 105, 302–308. [Google Scholar] [CrossRef]
- Rubin, M. When to adjust alpha during multiple testing: A consideration of disjunction, conjunction, and individual testing. Synthese 2021, 199, 10969–11000. [Google Scholar] [CrossRef]
- Derringer, J. A simple correction for non-independent tests. PsyArXiv 2018, 12761885. [Google Scholar] [CrossRef]
- Nyholt, D.R. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am. J. Hum. Genet. 2004, 74, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Equivalence Tests: A Practical Primer for t Tests, Correlations, and Meta-Analyses. Soc. Psychol. Personal. Sci. 2017, 8, 355–362. [Google Scholar] [CrossRef]
- Gassling, V.; Purcz, N.; Braesen, J.-H.; Will, M.; Gierloff, M.; Behrens, E.; Açil, Y.; Wiltfang, J. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: A preliminary study. J. Cranio-Maxillofac. Surg. 2013, 41, 76–82. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Bornstein, M.M.; Carrel, J.-P.; Buser, D.; Bernard, J.-P. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: Histologic and histomorphometric results. Int. J. Periodontics Restor. Dent. 2014, 34, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chandradas, N.; Ravindra, S.; Rangaraju, V.; Jain, S.; Dasappa, S. Efficacy of platelet rich fibrin in the treatment of human intrabony defects with or without bone graft: A randomized controlled trial. J. Int. Soc. Prev. Community Dent. 2016, 6, S153–S159. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Bajaj, P.; Rao, N.S.; Agarwal, E.; Naik, S.B. Platelet-Rich Fibrin Combined With a Porous Hydroxyapatite Graft for the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M. Combined periodontal regenerative technique in human intrabony defects by collagen membranes and anorganic bovine bone. A controlled clinical study. J. Periodontol. 2002, 73, 158–166. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Schwarz, F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials—Biological foundation and preclinical evidence: A systematic review. J. Clin. Periodontol. 2008, 35, 106–116. [Google Scholar] [CrossRef]
- Listgarten, M.A.; Rosenberg, M.M. Histological study of repair following new attachment procedures in human periodontal lesions. J. Periodontol. 1979, 50, 333–344. [Google Scholar] [CrossRef] [PubMed]
- UWikesjö, M.E.; Nilvéus, R.E.; Selvig, K.A. Significance of early healing events on periodontal repair: A review. J. Periodontol. 1992, 63, 158–165. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Ellegaard, B.; Löe, H. New attachment of periodontal tissues after treatment of intrabony lesions. J. Periodontol. 1971, 42, 648–652. [Google Scholar] [CrossRef]
- Nibali, L.; Pometti, D.; Tu, Y.K.; Donos, N. Clinical and radiographic outcomes following non-surgical therapy of periodontal infrabony defects: A retrospective study. J. Clin. Periodontol. 2011, 38, 50–57. [Google Scholar] [CrossRef]
- Haney, J.M.; Nilvéus, R.E.; McMillan, P.J.; Wikesjö, U.M.E. Periodontal repair in dogs: Expanded polytetrafluoroethylene barrier membranes support wound stabilization and enhance bone regeneration. J. Periodontol. 1993, 64, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Wikesj, U.M.E.; Nilvéus, R. Periodontal repair in dogs: Effect of wound stabilization on healing. J. Periodontol. 1990, 61, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Baldini, N.; De Sanctis, M.; Ferrari, M. Deproteinized bovine bone in periodontal and implant surgery. Dent. Mater. 2011, 27, 61–70. [Google Scholar] [CrossRef]
- Garrett, S. Periodontal regeneration around natural teeth. Ann. Periodontol. 1996, 1, 621–666. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, G.P.; Tonetti, M. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int. J. Periodontics Restor. Dent. 1999, 19, 589–599. [Google Scholar]
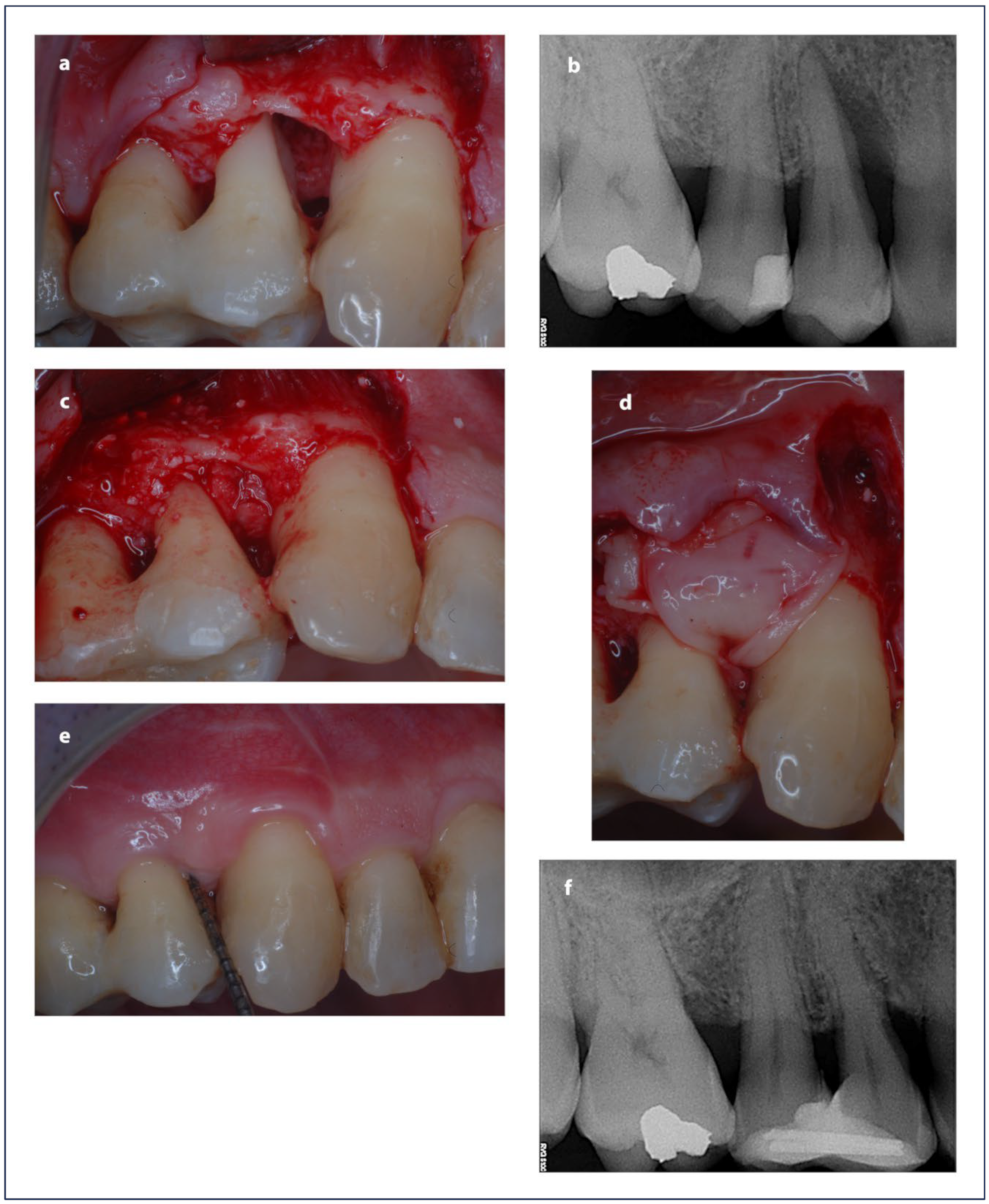
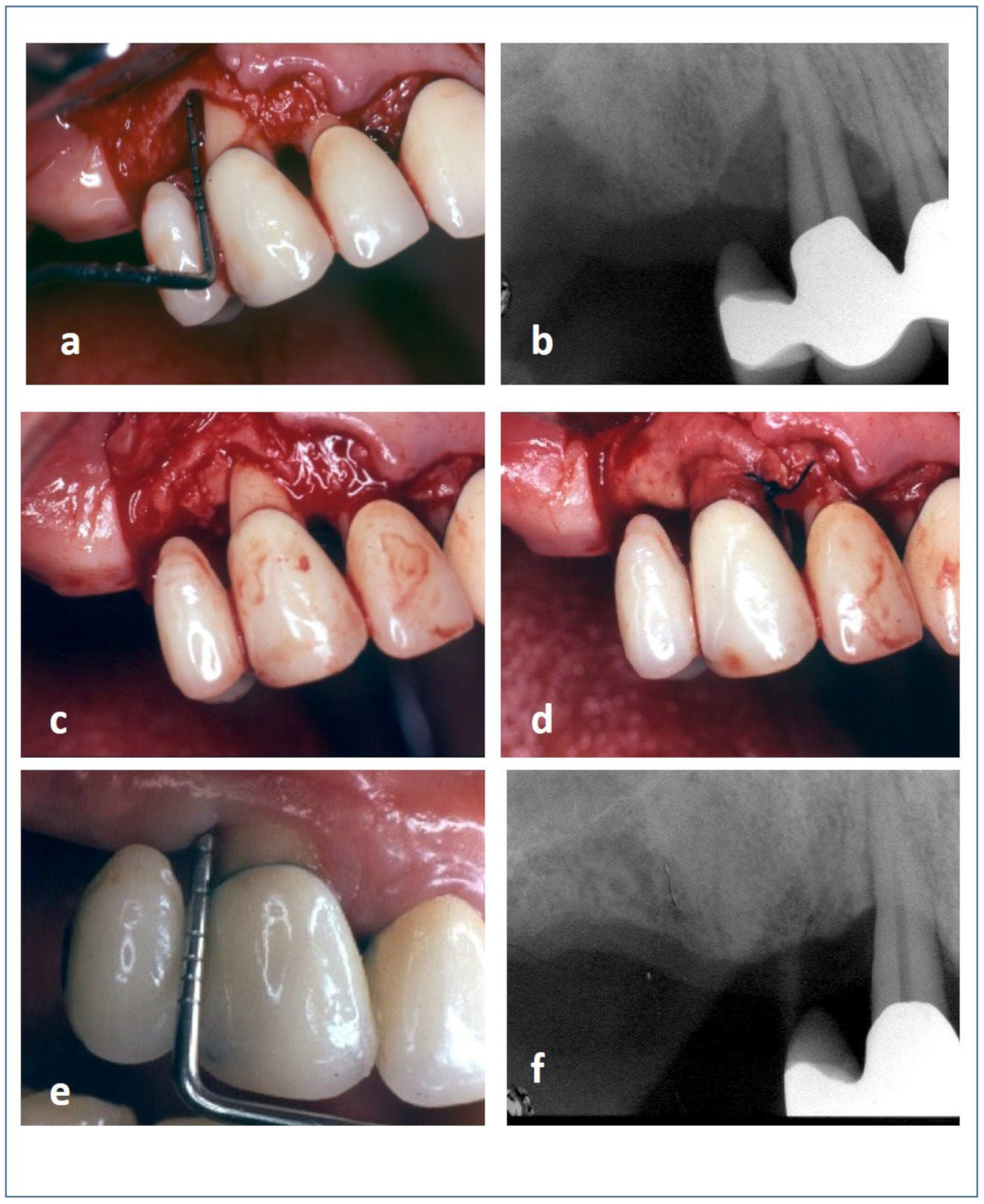


| Parameter | Treatment | Baseline | 12 Months | Baseline–12 Months | Baseline–12 Months |
|---|---|---|---|---|---|
| Observed | Observed | Observed | Estimated Marginal | ||
| Mean ± SD (95% CI) | Mean ± SD (95% CI) | Mean ± SD (95% CI) | Mean ± SD (95% CI) | ||
| PPD | L-PRF+ABG | 7.47 ± 1.5 (6.93 to 8.01) | 3.19 ± 0.78 (2.91 to 3.47) | 4.28 ± 1.22 (3.84 to 4.72) | 3.93 ± 0.65 (3.7 to 4.16) |
| CM+ABG | 7.44 ± 1.52 (6.89 to 7.99) | 2.94 ± 0.62 (2.71 to 3.16) | 4.5 ± 1.37 (4.01 to 4.99) | 4.16 ± 0.66 (3.93 to 4.4) | |
| diff | p = 0.93 | p = 0.16 | p = 0.50 | p = 0.079 | |
| CAL | L-PRF+ABG | 8.28 ± 1.11 (7.88 to 8.68) | 4.84 ± 0.85 (4.54 to 5.15) | 3.44 ± 0.8 (3.15 to 3.73) | 3.38 ± 0.67 (3.14 to 3.61) |
| CM+ABG | 8.66 ± 1.54 (8.1 to 9.21) | 4.91 ± 0.73 (4.64 to 5.17) | 3.75 ± 1.37 (3.26 to 4.24) | 3.32 ± 0.76 (3.05 to 3.59) | |
| diff | p = 0.27 | p = 0.75 | p = 0.27 | p = 0.718 | |
| GR | L-PRF+ABG | 1 ± 0.57 (0.8 to 1.2) | 1.66 ± 0.48 (1.48 to 1.83) | 0.66 ± 0.48 (0.48 to 0.83) | 0.59 ± 0.45 (0.43 to 0.75) |
| CM+ABG | 1.22 ± 0.79 (0.93 to 1.5) | 1.97 ± 0.54 (1.77 to 2.16) | 0.78 ± 0.71 (0.53 to 1.04) | 0.85 ± 0.45 (0.69 to 1.01) | |
| diff | p = 0.21 | p = 0.017 | p = 0.41 | p = 0.029 | |
| DBL | L-PRF+ABG | 9.62 ± 1.04 (9.25 to 10) | 6.69 ± 0.97 (6.34 to 7.04) | 2.94 ± 0.76 (2.66 to 3.21) | 3.04 ± 0.68 (2.8 to 3.29) |
| CM+ABG | 10.25 ± 1.57 (9.69 to 10.81) | 7.53 ± 1.22 (7.09 to 7.97) | 2.72 ± 0.85 (2.41 to 3.03) | 2.61 ± 0.68 (2.37 to 2.85) | |
| diff | p = 0.65 | p = 0.003 | p = 0.28 | p = 0.015 |
| Parameter | Treatment | ANCOVA | ANOVA |
|---|---|---|---|
| Estimated Mean ± SE (90% CI) | Observed Mean ± SE 90% CI | ||
| CAL Gain | L-PRF+ABG | 3.38 ± 0.118 (3.18 to 3.57) | 3.44 ± 0.198 (3.11 to 3.77) |
| CM+ABG | 3.32 ± 0.134 (3.10 to 3.54) | 3.75 ± 0.198 (3.42 to 4.08) | |
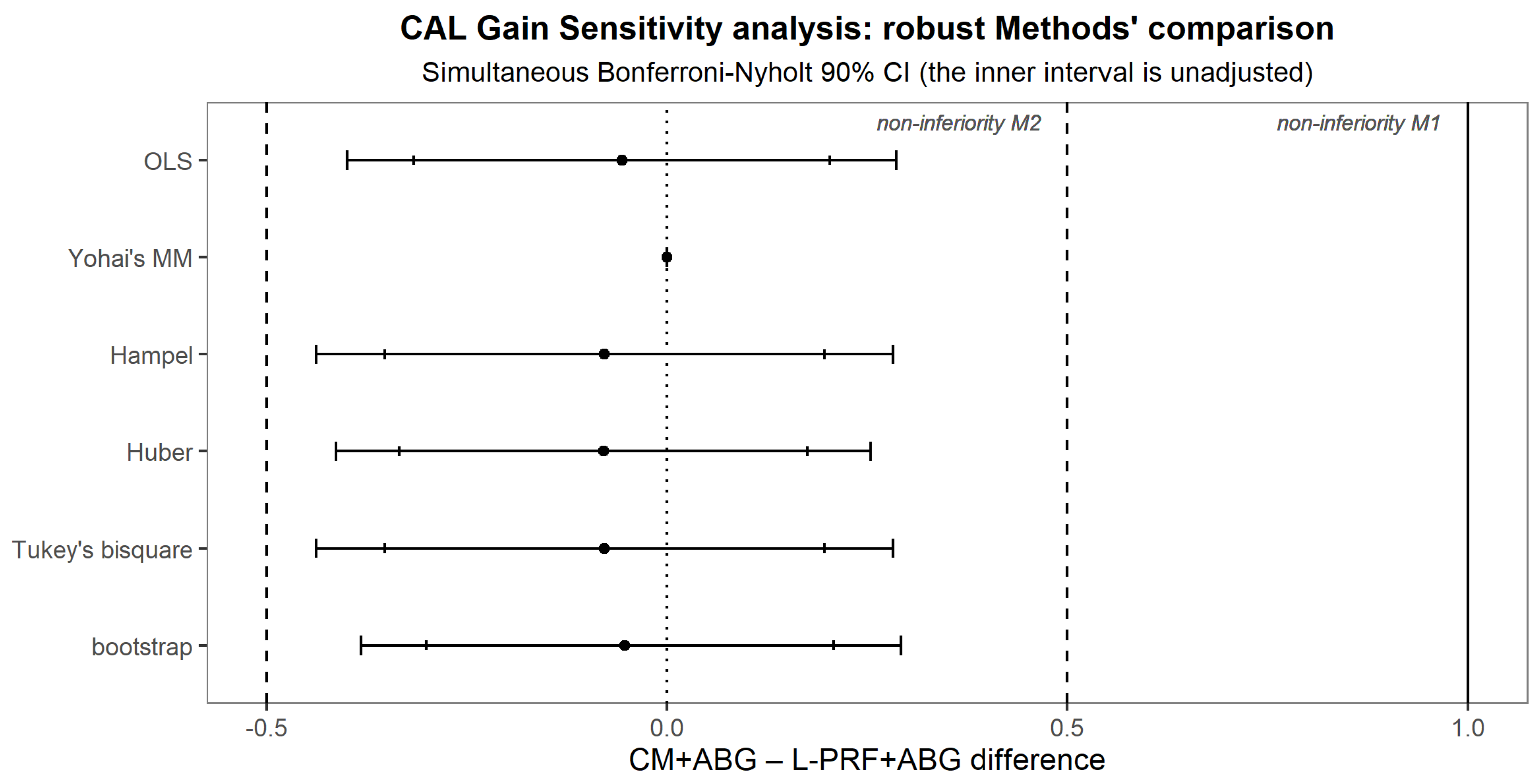
| Mean Difference | Estimated Mean ± SE (90% CI) (Simultaneous Bonferroni-Nyholt 90% CI) | ||
| CAL Gain | CM+ABG–L-PRF+ABG | −0.0564 ± 0.155 (−0.316 to 0.203) (−0.399 to 0.287) | 0.312 ± 0.28 (−0.155 to 0.789) |
| PPD Reduction | CM+ABG–L-PRF+ABG | 0.232 ± 0.13 (0.015 to 0.449) (−0.055 to 0.519) | 0.219 ± 0.325 (−0.323 to 0.761) |
| DBL Gain | CM+ABG–L-PRF+ABG | −0.433 ± 0.173 (−0.721 to −0.145) (−0.814 to −0.052) | −0.219 ± 0.202 (−0.555 to 0.118) |
| GR Increase | CM+ABG–L-PRF+ABG | 0.255 ± 0.114 (0.0645 to 0.445) (0.003 to 0.506) | 0.125 ± 0.151 (−0.128 to 0.378) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balice, G.; Paolantonio, M.; De Ninis, P.; Rexhepi, I.; Serroni, M.; Frisone, A.; Romano, L.; Sinjari, B.; Murmura, G.; Femminella, B. Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study. Medicina 2024, 60, 1091. https://doi.org/10.3390/medicina60071091
Balice G, Paolantonio M, De Ninis P, Rexhepi I, Serroni M, Frisone A, Romano L, Sinjari B, Murmura G, Femminella B. Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study. Medicina. 2024; 60(7):1091. https://doi.org/10.3390/medicina60071091
Chicago/Turabian StyleBalice, Giuseppe, Michele Paolantonio, Paolo De Ninis, Imena Rexhepi, Matteo Serroni, Alessio Frisone, Luigi Romano, Bruna Sinjari, Giovanna Murmura, and Beatrice Femminella. 2024. "Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study" Medicina 60, no. 7: 1091. https://doi.org/10.3390/medicina60071091
APA StyleBalice, G., Paolantonio, M., De Ninis, P., Rexhepi, I., Serroni, M., Frisone, A., Romano, L., Sinjari, B., Murmura, G., & Femminella, B. (2024). Treatment of Unfavorable Intrabony Defects with Autogenous Bone Graft in Combination with Leukocyte- and Platelet-Rich Fibrin or Collagen Membranes: A Non-Inferiority Study. Medicina, 60(7), 1091. https://doi.org/10.3390/medicina60071091









