The Relationship of Adherence to the Mediterranean Diet with Disease Activity and Quality of Life in Crohn’s Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Ricciardi, W.; Iavicoli, I. Occupational risk factors in inflammatory bowel disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2838–2851. [Google Scholar] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Poullis, A.; Foster, R.; Shetty, A.; Fagerhol, M.K.; Mendall, M.A. Bowel inflammation as measured by fecal calprotectin: A link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Kamm, M.A.; Colombel, J.F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Paknahad, Z.; Sheklabadi, E.; Derakhshan, Y.; Bagherniya, M.; Chitsaz, A. The effect of the Mediterranean diet on cognitive function in patients with Parkinson’s disease: A randomized clinical controlled trial. Complement. Ther. Med. 2020, 50, 102366. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Taylor, L.; Shommu, N.; Ghosh, S.; Reimer, R.; Panaccione, R.; Kaur, S.; Hyun, J.E.; Cai, C.; Deehan, E.C.; et al. A Diversified Dietary Pattern Is Associated With a Balanced Gut Microbial Composition of Faecalibacterium and Escherichia/Shigella in Patients With Crohn’s Disease in Remission. J. Crohns Colitis 2020, 14, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohns Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Arpón, A.; Riezu-Boj, J.I.; Milagro, F.I.; Marti, A.; Razquin, C.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Casas, R.; Fitó, M.; et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J. Physiol. Biochem. 2016, 73, 445–455. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Schwärzler, J.; Mayr, L.; Vich Vila, A.; Grabherr, F.; Niederreiter, L.; Philipp, M.; Grander, C.; Meyer, M.; Jukic, A.; Tröger, S.; et al. PUFA-Induced Metabolic Enteritis as a Fuel for Crohn’s Disease. Gastroenterology 2022, 162, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 1, 514. [Google Scholar] [CrossRef] [PubMed]
- Alrubaiy, L.; Cheung, W.Y.; Dodds, P.; Hutchings, H.A.; Russell, I.T.; Watkins, A.; Williams, J.G. Development of a short questionnaire to assess the quality of life in Crohn’s disease and ulcerative colitis. J. Crohns Colitis 2015, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Energy and Protein Requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ. Tech. Rep. Ser. 1985, 724, 1–206. [Google Scholar]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nic Suibhne, T.; Raftery, T.C.; McMahon, O.; Walsh, C.; O’Morain, C.; O’Sullivan, M. High prevalence of overweight and obesity in adults with Crohn’s disease: Associations with disease and lifestyle factors. J. Crohns Colitis 2013, 7, e241–e248. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Harmsen, W.S.; Aniwan, S.; Tremaine, W.J.; Abu Dayyeh, B.K.; Loftus, E.V. Prevalence and Impact of Obesity on Disease-specific Outcomes in a Population-based Cohort of Patients with Ulcerative Colitis. J. Crohns Colitis 2021, 15, 1816–1823. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, C.M.; Yoo, J.H. Obesity and novel management of inflammatory bowel disease. World J. Gastroenterol. 2023, 29, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Zietek, T.; Rath, E. Inflammation Meets Metabolic Disease: Gut Feeling Mediated by GLP-1. Front. Immunol. 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Karmiris, K.; Koutroubakis, I.E.; Xidakis, C.; Polychronaki, M.; Voudouri, T.; Kouroumalis, E.A. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel. Dis. 2006, 12, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Ananthakrishnan, A.N.; Konijeti, G.G.; Higuchi, L.M.; Fuchs, C.S.; Richter, J.M.; Chan, A.T. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm. Bowel. Dis. 2015, 21, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.M.; Chen, Y.; Casey, K.; Olen, O.; Ludvigsson, J.F.; Carbonnel, F.; Oldenburg, B.; Gunter, M.J.; Tjønneland, A.; Grip, O.; et al. Obesity is Associated With Increased Risk of Crohn’s disease, but not Ulcerative Colitis: A Pooled Analysis of Five Prospective Cohort Studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1048–1058. [Google Scholar] [CrossRef]
- Holtmann, M.H.; Krummenauer, F.; Claas, C.; Kremeyer, K.; Lorenz, D.; Rainer, O.; Vogel, I.; Böcker, U.; Böhm, S.; Büning, C.; et al. Significant differences between Crohn’s disease and ulcerative colitis regarding the impact of body mass index and initial disease activity on responsiveness to azathioprine: Results from a European multicenter study in 1,176 patients. Dig. Dis. Sci. 2010, 55, 1066–1078. [Google Scholar] [CrossRef]
- Dai, Z.H.; Xu, X.T.; Ran, Z.H. Associations Between Obesity and the Effectiveness of Anti-Tumor Necrosis Factor-α Agents in Inflammatory Bowel Disease Patients: A Literature Review and Meta-analysis. Ann. Pharmacother. 2020, 54, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Radford-Smith, G.; Banks, M.; Lord, A.; Chachay, V. Dietary intake of patients with inflammatory bowel disease aligns poorly with traditional Mediterranean diet principles. Nutr. Diet. 2022, 79, 229–237. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Vilović, M.; Živković, P.M.; Tadin Hadjina, I.; Rušić, D.; Bukić, J.; Borovac, J.A.; Božić, J. Mediterranean Diet Adherence and Dietary Attitudes in Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 3429. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Almutairdi, A.; Shommu, N.; Fedorak, R.; Ghosh, S.; Reimer, R.A.; Panaccione, R.; Raman, M. Cross-Sectional Analysis of Overall Dietary Intake and Mediterranean Dietary Pattern in Patients with Crohn’s Disease. Nutrients 2018, 10, 1761. [Google Scholar] [CrossRef]
- Jamieson, A.E.; Fletcher, P.C.; Schneider, M.A. Seeking control through the determination of diet: A qualitative investigation of women with irritable bowel syndrome and inflammatory bowel disease. Clin Nurse Spec. 2007, 21, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, F.; Bouin, M.; D’Aoust, L.; Lemoyne, M.; Presse, N. Food avoidance in patients with inflammatory bowel disease: What, when and who? Clin. Nutr. 2018, 37, 884–889. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Fiorindi, C.; Dinu, M.; Gavazzi, E.; Scaringi, S.; Ficari, F.; Nannoni, A.; Sofi, F.; Giudici, F. Adherence to mediterranean diet in patients with inflammatory bowel disease. Clin. Nutr. ESPEN 2021, 46, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Papada, E.; Amerikanou, C.; Forbes, A.; Kaliora, A.C. Adherence to Mediterranean diet in Crohn’s disease. Eur. J. Nutr. 2020, 59, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel. Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Çelik, K.; Güveli, H.; Erzin, Y.; Kenge, E.B.; Özlü, T. The Effect of Adherence to Mediterranean Diet on Disease Activity in Patients with Inflammatory Bowel Disease. Turk. J. Gastroenterol. 2023, 34, 714–719. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; Elashry, H.; Salamah, A.; Maher, S.; Abd-Elsalam, S.M.; Hasan, S. Adherence to the Mediterranean Diet Improved Clinical Scores and Inflammatory Markers in Children with Active Inflammatory Bowel Disease: A Randomized Trial. J. Inflamm. Res. 2022, 15, 2075–2086. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Assa, A.; Lev-Tzion, R.; Matar, M.; Shouval, D.; Shubeli, C.; Tsadok Perets, T.; Chodick, G.; Shamir, R. Adherence to the Mediterranean Diet Is Associated with Decreased Fecal Calprotectin Levels in Children with Crohn’s Disease in Clinical Remission under Biological Therapy. Dig. Dis. 2024, 42, 199–210. [Google Scholar] [CrossRef]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults With Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9, Erratum in: Gastroenterology 2022, 163, 1473. [Google Scholar] [CrossRef] [PubMed]
- Maharshak, N.; Cohen, N.A.; Reshef, L.; Tulchinsky, H.; Gophna, U.; Dotan, I. Alterations of Enteric Microbiota in Patients with a Normal Ileal Pouch Are Predictive of Pouchitis. J. Crohns Colitis 2017, 11, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Dong, M.; Sasson, G.; Raygoza Garay, J.A.; Espin-Garcia, O.; Lee, S.H.; Neustaeter, A.; Smith, M.I.; Leibovitzh, H.; Guttman, D.S.; et al. Mediterranean-Like Dietary Pattern Associations With Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Tomic, I.J.; Krnic, M.; Ticinovic Kurir, T.; Bozic, J. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients 2022, 14, 757. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Dotan, I. Is the Mediterranean Diet in Inflammatory Bowel Diseases Ready for Prime Time? J. Can. Assoc. Gastroenterol. 2023, 7, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Hansen, T.; Duerksen, D.R. Enteral Nutrition in the Management of Pediatric and Adult Crohn’s Disease. Nutrients 2018, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Zachos, M.; Tondeur, M.; Griffiths, A.M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007, 24, CD000542. [Google Scholar] [CrossRef] [PubMed]
- Critch, J.; Day, A.S.; Otley, A.; King-Moore, C.; Teitelbaum, J.E.; Shashidhar, H.; NASPGHAN IBD Committee. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 298–305. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Brotherton, C.S.; Martin, C.A.; Long, M.D.; Kappelman, M.D.; Sandler, R.S. Avoidance of Fiber Is Associated With Greater Risk of Crohn’s Disease Flare in a 6-Month Period. Clin. Gastroenterol. Hepatol. 2016, 14, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, K.; Morhardt, T.L.; Kamada, N. The Role of Dietary Nutrients in Inflammatory Bowel Disease. Front. Immunol. 2019, 9, 3183. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Kalfa, N.; Beckers, G.M.A.; Kaefer, M.; Nieuwhof-Leppink, A.J.; Fossum, M.; Herbst, K.W.; Bagli, D.; ESPU Research Committee. The impact of COVID-19 on research. J. Pediatr. Urol. 2020, 16, 715–716. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number of Participants (%)/Mean ± SD |
|---|---|
| Gender Males Females | 22 (36.7%) 38 (63.3%) |
| Weight (kg) | 80.4 ± 15.5 |
| BMI (kg/ m2) <18.5 (kg/ m2) 18.5–25 (kg/ m2) 25–30 (kg/ m2) >30 (kg/ m2) | 25.1 ± 5.7 4 (6.7%) 33 (55%) 13 (21.7%) 10 (16.7%) |
| Age (years) | 37.6 ± 12.5 |
| Smoking status ex-smoker smoker at present never smoked | 25 (41.7%) 16 (26.7%) 35 (58.3%) |
| Pharmacological therapy aminosalicylates antibiotics corticosteroids immunosuppressants biologics surgical therapy | 22 (36.7%) 3 (5%) 2 (3.3%) 11 (18.3%) 22 (36.7%) 16 (26.7%) |
| Physical activity level Never Rarely Often High | 16 (26.7%) 29 (48.3%) 7 (11.7%) 8 (13.3%) |
| Appetite poor moderate good very good | 1 (1.7%) 15 (25%) 23 (38.3%) 21 (35%) |
| Number of meals per day one per day two per day three per day more than three per day | 5 (8.3%) 13 (21.7%) 22 (36.7%) 20 (33.3%) |
| Harvey-Bradshaw Index (HBI) | 5.65 ± 5.2 |
| CUCQ-8 | 9.3 ± 5.8 |
| MedDiet Score | 30 ± 3.6 |
| Variables | Active (n = 32) (%/Mean ± SD) | Inactive (n = 28) (%/Mean ± SD) | p Value |
|---|---|---|---|
| Gender Male Female | 9 (28.1%) 23 (71.9%) | 13 (46.4%) 15 (53.6%) | 0.142 |
| Age years | 41 ± 11 | 33 ± 12 | 0.020 |
| BMI (kg/m2) | 26.4 ± 6 | 23.6 ± 4 | 0.063 |
| Smoking status smoker at present nonsmoker | 16 (34.4%) 21 (65.6%) | 8 (17.9%) 23 (82.1%) | 0.149 |
| Physical activity level Never Rarely Often High | 9 (28.1%) 19 (59.4%) 2 (6.2) 2 (6.2%) | 7 (25%) 10 (35.7%) 5 (17.9%) 6 (21.4%) | 0.107 |
| CUCQ-8 | 12.6 ± 5 | 5.5 ± 3 | 0.001 |
| MedDiet Score | 29 ± 3 | 31.2 ± 3 | 0.019 |
| Appetite poor moderate good very good | 1 (3.1%) 9 (28.1%) 12 (37.5%) 10 (31.2%) | 0 (0%) 6 (21.4%) 11 (39.3%) 11 (39.3%) | 0.698 |
| Reduced dietary intake (last week) No Yes | 25 (78.1%) 7 (21.9%) | 26 (92.9%) 2 (7.1%) | 0.047 |
| Active (n = 32) (mean ± SD) | Inactive (n = 28) (mean ± SD) | p Value | |
|---|---|---|---|
| Hemoglobin (gr/dL) | 12.8 ± 1.7 | 12.9 ± 1.3 | 0.817 |
| Hematocrit (%) | 38.7 ± 4 | 39.4 ± 4 | 0.577 |
| CRP (mg/L) | 3.5 ± 4 | 2 ± 3 | 0.265 |
| Albumin (g/dL) | 4.3 ± 0.4 | 4.5 ± 0.6 | 0.481 |
| Glucose (mg/dL) | 91 ± 11 | 90 ± 8 | 0.875 |
| Alkaline phosphatase (IU/L) | 70 ± 46 | 85 ± 41 | 0.347 |
| SGOT (IU/L) | 19.7 ± 10 | 18.7 ± 8 | 0.775 |
| SGPT (IU/L) | 24.5 ± 22 | 18.3 ± 11 | 0.315 |
| γ-GT (IU/L) | 34 ± 42 | 18 ± 11 | 0.140 |
| Amylase (IU/L) | 64 ± 20 | 70 ± 28 | 0.645 |
| Lactate dehydrogenase (U/L) | 241 ± 155 | 174 ± 71 | 0.327 |
| B12 (pg/mL) | 482 ± 304 | 419 ± 203 | 0.505 |
| Triglycerides (mg/dL) | 127 ± 66 | 90 ± 55 | 0.276 |
| Folic acid (ng/mL) | 10.6 ± 6 | 11.9 ± 6 | 0.661 |
| White Blood Cells (K/μL) | 7.6 ± 2 | 7.1 ± 2 | 0.466 |
| Potassium (mmol/l) | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.761 |
| r/rho | p Value | |
|---|---|---|
| HBI | −0.267 | 0.039 |
| CUCQ-8 | −0.197 | 0.046 |
| Food Groups | Frequency | Active (n = 32) (Number of Participants/%) | Inactive (n = 28) (Number of Participants/%) | p Value |
|---|---|---|---|---|
| Nonrefined cereals | Never 1–6 portions/week 7–12 portions/week 13–18 portions/week 19–31 portions/week ≥32 portions/week | 12 (37.5%) 15 (46.9%) 5 (15.6%) 0 (0%) 0 (0%) 0 (0%) | 9 (32.1%) 11 (39.3%) 7 (25%) 0 (0%) 1 (3.6%) 0 (0%) | 0.548 |
| Potatoes | Never <1 portion/week 1–2 portions/week 3 portions/week 4 portions/week >4 portions/week | 0 (0%) 9 (28.1%) 11 (34.4%) 6 (18.8%) 4 (12.5%) 2 (6.2%) | 1 (3.6%) 1 (3.6%) 15 (53.6%) 5 (17.9%) 4 (14.3%) 2 (7.1%) | 0.163 |
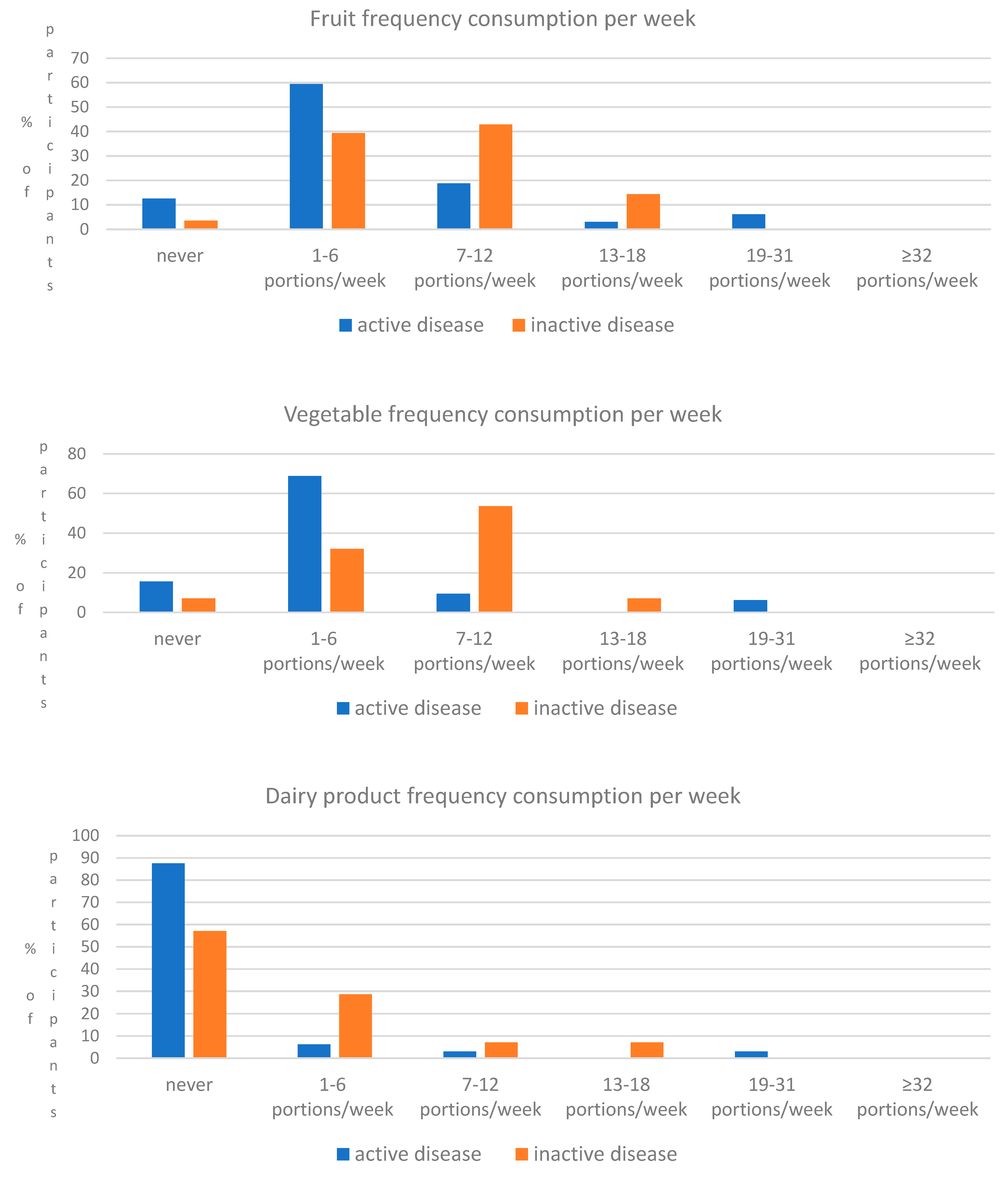
| Fruits | Never 1–4 portions/week 5–8 portions/week 9–15 portions/week 16–21 portions/week ≥22 portions/week | 4 (12.5%) 19 (59.4%) 6 (18.8%) 1 (3.1%) 2 (6.2%) 0 (0%) | 1 (3.6%) 11 (39.3%) 12 (42.9%) 4 (14.3%) 0 (0%) 0 (0%) | 0.046 |
| Vegetables | Never 1–6 portions/week 7–12 portions/week 13–20 portions/week 21–32 portions/week ≥33 portions/week | 5 (15.6%) 22 (68.8%) 3 (9.4%) 0 (0%) 2 (6.2%) 0 (0%) | 2 (7.1%) 9 (32.1%) 15 (53.6%) 2 (7.1%) 0 (0%) 0 (0%) | 0.001 |
| Legumes | Never <1 portions/week 1–2 portions/week 3–4 portions/week 5–6 portions/week ≥6 portions/week | 13 (40.6%) 11 (34.4%) 8 (25%) 0 (0%) 0 (0%) 0 (0%) | 8 (28.6%) 4 (14.3%) 15 (53.6%) 1 (3.6%) 0 (0%) 0 (0%) | 0.061 |
| Fish | Never <1 portions/week 1–2 portions/week 3–4 portions/week 5–6 portions/week ≥6 portions/week | 1 (3.1%) 12 (37.5%) 14 (43.8%) 3 (9.4%) 1 (3.1%) 1 (3.1%) | 2 (7.1%) 8 (28.6%) 17 (60.7%) 1 (3.6%) 0 (0%) 0 (0%) | 0.524 |
| Red meat | ≤1 portion/week 2–3 portions/week 4–5 portions/week 6–7 portions/week 8–10 portions/week >10 portions/week | 9 (28.1%) 12 (37.5%) 9 (28.1%) 1 (3.1%) 1 (3.1%) 0 (0%) | 9 (32.1%) 12 (42.9%) 2 (7.1%) 4 (14.3%) 1 (3.6%) 0 (0%) | 0.198 |
| Poultry | ≤3 portions/week 4–5 portions/week 5–6 portions/week 7–8 portions/week 9–10 portions/week >10 portions/week | 18 (56.2%) 10 (31.2%) 3 (9.4%) 1 (3.1%) 0 (0%) 0 (0%) | 16 (57.1%) 6 (21.4%) 5 (17.9%) 1 (3.6%) 0 (0%) 0 (0%) | 0.716 |
| Dairy products | ≤10 portions/week 11–15 portions/week 16–20 portions/week 21–28 portions/week 29–30 portions/week >30 portions/week | 28 (87.5%) 2 (6.2%) 1 (3.1%) 0 (0%) 1 (3.1%) 0 (0%) | 16 (57.1%) 8 (28.6%) 2 (7.1%) 2 (7.1%) 0 (0%) 0 (0%) | 0.041 |
| Olive oil | Never Rarely <1 portions/week 1–3 portions/week 3–5 portions/week everyday | 0 (0%) 1 (3.1%) 2 (6.2%) 2 (6.2%) 2 (6.2%) 25 (78.1%) | 0 (0%) 1 (3.6%) 0 (0%) 5 (17.9%) 0 (0%) 22 (78.6%) | 0.264 |
| Alcohol | <300 mL/day 300 mL/day 400 mL/day 500 mL/day 600 mL/day >700 or 0 mL/day | 17 (53.1%) 3 (9.4%) 2 (6.2%) 1 (3.1%) 0 (0%) 9 (28.1%) | 19 (67.9%) 5 (17.9%) 2 (7.1%) 0 (0%) 0 (0%) 2 (7.1%) | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migdanis, A.; Migdanis, I.; Gkogkou, N.D.; Papadopoulou, S.K.; Giaginis, C.; Manouras, A.; Polyzou Konsta, M.A.; Kosti, R.I.; Oikonomou, K.A.; Argyriou, K.; et al. The Relationship of Adherence to the Mediterranean Diet with Disease Activity and Quality of Life in Crohn’s Disease Patients. Medicina 2024, 60, 1106. https://doi.org/10.3390/medicina60071106
Migdanis A, Migdanis I, Gkogkou ND, Papadopoulou SK, Giaginis C, Manouras A, Polyzou Konsta MA, Kosti RI, Oikonomou KA, Argyriou K, et al. The Relationship of Adherence to the Mediterranean Diet with Disease Activity and Quality of Life in Crohn’s Disease Patients. Medicina. 2024; 60(7):1106. https://doi.org/10.3390/medicina60071106
Chicago/Turabian StyleMigdanis, Athanasios, Ioannis Migdanis, Nikoleta D. Gkogkou, Sousana K. Papadopoulou, Constantinos Giaginis, Athanasios Manouras, Maria Anna Polyzou Konsta, Rena I. Kosti, Konstantinos A. Oikonomou, Konstantinos Argyriou, and et al. 2024. "The Relationship of Adherence to the Mediterranean Diet with Disease Activity and Quality of Life in Crohn’s Disease Patients" Medicina 60, no. 7: 1106. https://doi.org/10.3390/medicina60071106







