The Involvement and Manifestations of SARS-CoV-2 Virus in Cardiovascular Pathology
Abstract
:1. Introduction
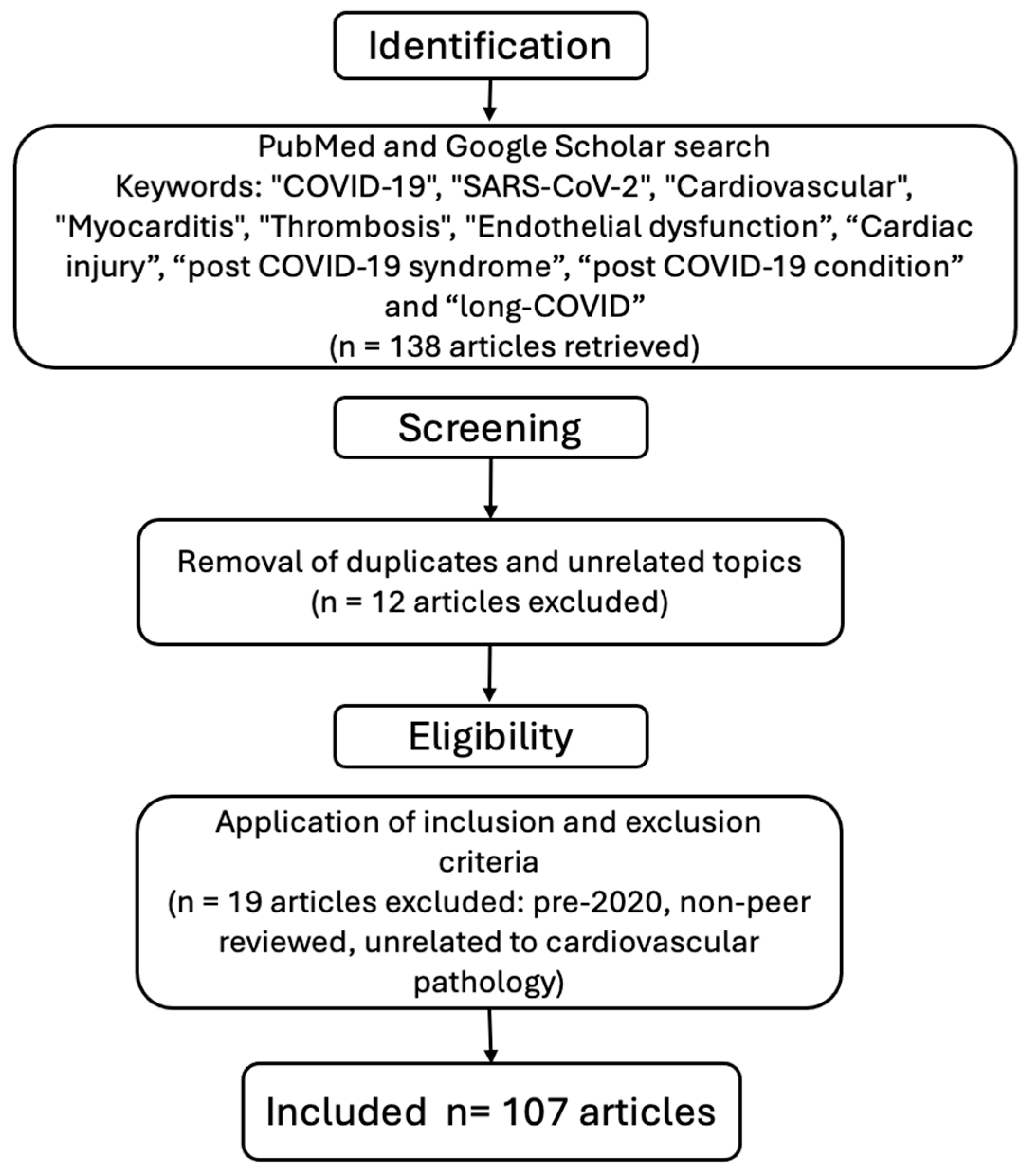
2. Materials and Methods
- Articles published between January 2020 and March 2025;
- Studies published in English;
- Original research, systematic reviews, meta-analyses, and narrative reviews focused on cardiovascular manifestations or complications related to COVID-19.
- Articles published prior to 2020 to avoid outdated pre-pandemic data;
- Studies not directly addressing cardiovascular involvement in COVID-19.
- Non-peer-reviewed articles, conference abstracts, letters to the editor, and case reports with insufficient data.
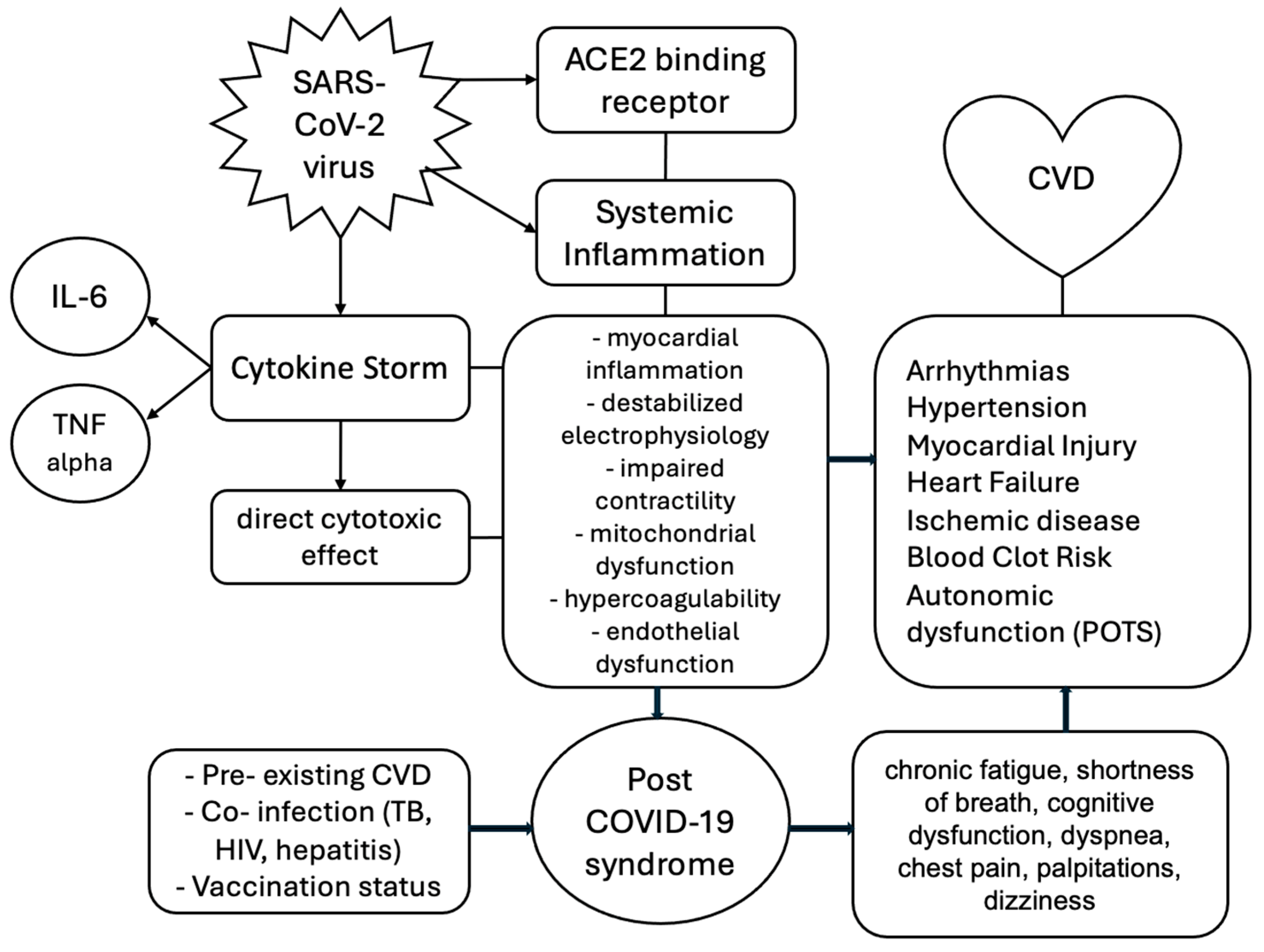
3. Pathogenesis of the SARS-CoV-2 Virus
4. Pathophysiology of Cardiovascular Involvement
5. Pre-Existing Cardiovascular Disease
5.1. Atherosclerosis
5.2. Hypertension
5.3. Diabetes
5.4. Atrial Fibrillation
6. Acute Complications
6.1. Acute Myocardial Injury
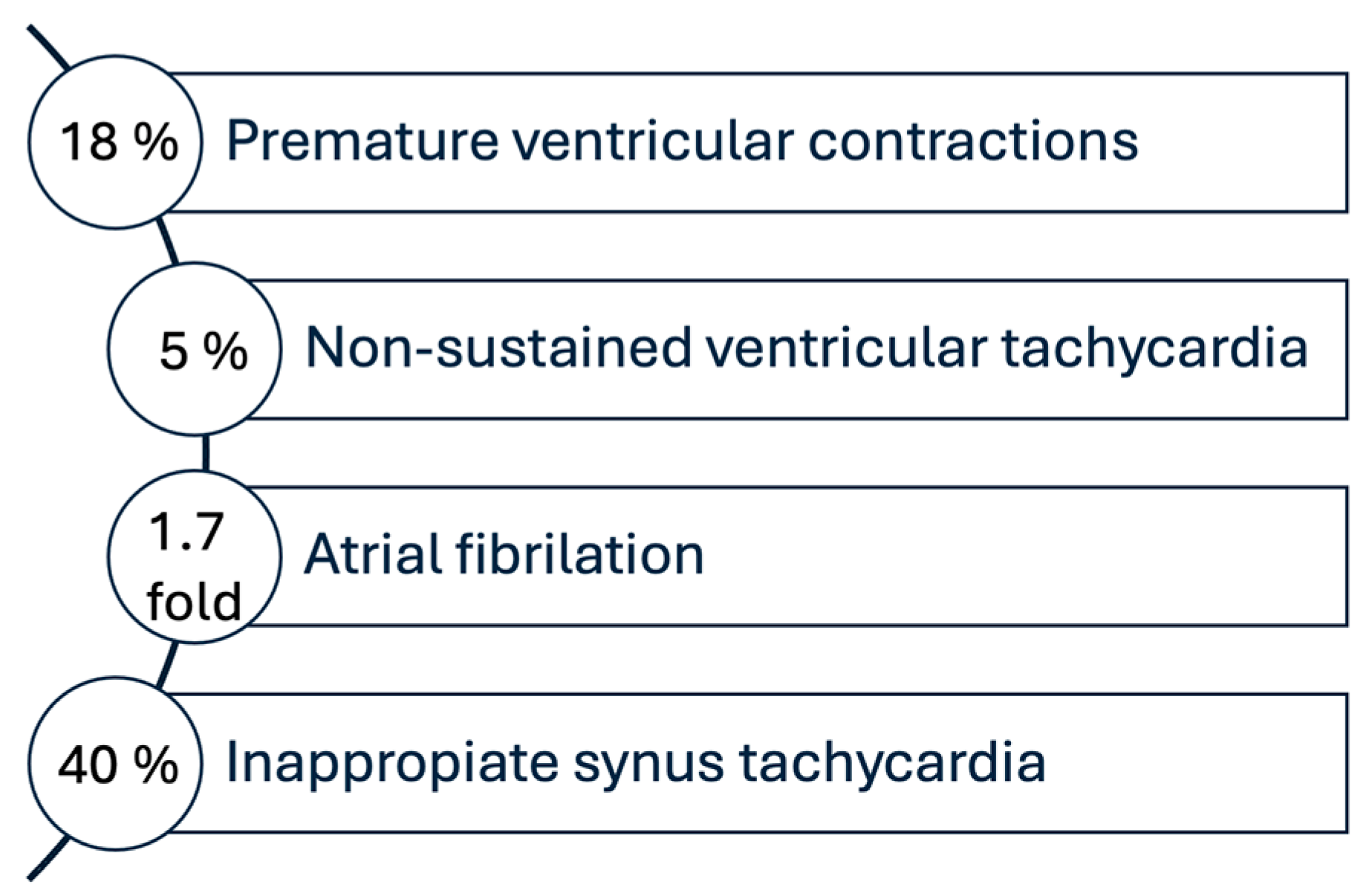
6.2. Cardiac Arrhythmias
6.3. Acute Myocarditis
6.4. Thromboembolism
7. Long-Term Cardiovascular Effects
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Available online: www.who.int (accessed on 30 March 2025).
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: www.cdc.gov (accessed on 30 March 2025).
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Udoakang, A.J.; Dike, E.I.; Adebisi, Y.A.; Bolarinwa, O.A.; Lucero-Prisno, D.E. The COVID-19, Tuberculosis and HIV/AIDS: Ménage à Trois. Front. Immunol. 2023, 14, 1104828. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.K.; Adebisi, Y.A.; Muhammad, A.; Ekpenyong, A.; Lucero-Prisno, D.E. Viral Hepatitis Amidst COVID-19 in Africa: Implications and Recommendations. J. Med. Virol. 2022, 94, 7–10. [Google Scholar] [CrossRef]
- Worldometer. Available online: www.worldometers.info/coronavirus (accessed on 30 March 2025).
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H.; Martin-Sancho, L.; Kao, K.S.; Solis, L.E.; Kemp, M.G.; Prasanth, S.G.; Strand, S.S.; et al. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Steiner, S.; Kratzel, A.; Tuba, B.G.; Lang, R.M.; Moreira, E.G.; Thomann, L.; Kelly, J.N.; Thiel, V. SARS-CoV-2 biology and host interactions. Nat. Rev. Microbiol. 2024, 22, 206–225. [Google Scholar] [CrossRef]
- Alcock, J.; Masters, A. Cytokine storms, evolution and COVID-19. EMed. Public Health 2021, 9, 83–92. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Zhang, X.; Zhao, H.; Chen, Y.; Zhang, Y.; Liu, H. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Wang, H.; Luo, S.; Xie, M.; Chen, Z.; Zhang, Y.; Xie, Z.; Zhang, Y.; Zhang, Y.; Yang, L.; Wu, F.; et al. ACE2 Receptor-Targeted Inhaled Nanoemulsions Inhibit SARS-CoV-2 and Attenuate Inflammatory Responses. Adv. Mater. 2024, 36, e2311537. [Google Scholar] [CrossRef]
- Nguyen, L.; McCord, K.A.; Bui, D.T.; Bouwman, K.M.; Kitova, E.N.; Elaish, M.; Kumawat, D.; Daskhan, G.C.; Tomris, I.; Han, L.; et al. Sialic Acid-Containing Glycolipids Mediate Binding and Viral Entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. [Google Scholar] [CrossRef]
- Suryadevara, N.; Subramanian, R.; Ghoneim, H.E.; Tang, J.; Adhikari, U.; Prabakaran, P.; Zheng, J.; Sandoval, D.; Moffat, E.; Sambhara, S.; et al. Structural characterization of human monoclonal antibodies targeting uncommon antigenic sites on spike glycoprotein of SARS-CoV. J. Clin. Investig. 2025, 135, 3. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H. Peripheral helper T cells, mavericks of peripheral immune responses. Int. Immunol. 2024, 36, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, A.; De Angelis, M.; Benvenuto, D.; Marra, M.; d’Ettorre, G.; Ciccozzi, M.; Ciccozzi, A.; Martini, M.; Angeletti, S. Immune signature in vaccinated versus non-vaccinated aged people with COVID-19 pneumonia. J. Transl. Med. 2024, 22, 755. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus Biology and Replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Y.; Liu, S.; Chen, X.; Wang, J.; Li, H.; Zhao, Y. The possible mechanism and research progress of ACE2 involved in cardiovascular injury caused by COVID-19: A review. Front. Cardiovasc. Med. 2024, 11, 1409723. [Google Scholar] [CrossRef]
- Fox, S.E.; Vander Heide, R.S. COVID-19: The Heart of the Matter—Pathological Changes and a Proposed Mechanism. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 217–224. [Google Scholar] [CrossRef]
- Xu, S.W.; Ilyas, I.; Weng, J.P. Endothelial Dysfunction in COVID-19: An Overview of Evidence, Biomarkers, Mechanisms and Potential Therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Mackman, N. Tissue Factor and COVID-19 Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 523–529. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Pignatelli, P.; Cangemi, R.; Loffredo, L. COVID-19 and Long-COVID Thrombosis: From Clinical and Basic Science to Therapeutics. Thromb. Haemost. 2024, 124, 286–296. [Google Scholar] [CrossRef]
- Page, E.M.; Ariens, R.A.S. Mechanisms of Thrombosis and Cardiovascular Complications in COVID-19. Thromb. Res. 2021, 200, 1–8. [Google Scholar] [CrossRef]
- Karakasis, P.; Kapelouzou, A.; Apostolopoulos, V.; Tsironis, L.D. Vascular Alterations Following COVID-19 Infection: A Comprehensive Literature Review. Life 2024, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, J.W.; Loftus, R.M.; Polson, A.P.; de Almeida, J.P.; Saucier, N.; Deffit, S.N.; McCann, M.H.; Wright, K.E.; Kim, K.W.; Rauch, I.; et al. Mitochondrial antioxidants abate SARS-COV-2 pathology in mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2321972121. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xie, Y.; Zhang, Z.; Su, J.; Zhang, Y.; Zhang, J.; Wang, Y.; Sun, J.; Jiang, H.; Xu, L.; et al. Damage to endothelial barriers and its contribution to long COVID. Angiogenesis 2024, 27, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Caricchio, R.; Casanova, J.-L.; Combes, A.J.; Diamond, B.; D’Cruz, D.; Elkon, K.B.; Greenbaum, B.; Gruber, C.N.; Karp, D.R.; et al. The Intersection of COVID-19 and autoimmunity. J. Clin. Investig. 2021, 131, e154886. [Google Scholar] [CrossRef]
- Shu, H.; Zhao, C.; Wang, D.W. Understanding COVID-19-Related Myocarditis: Pathophysiology, Diagnosis, and Treatment Strategies. Cardiol. Plus 2023, 8, 72–81. [Google Scholar] [CrossRef]
- Gupta, P.; Jain, S.; Gupta, S.; Shah, N.; Shah, S.; Verma, K.; Gupta, V. Cardiac troponin in hospitalized COVID-19 patients: Incidence, predictors, and outcomes. Ann. Clin. Biochem. 2024, 61, 255–264. [Google Scholar] [CrossRef]
- McGonagle, D.; Giryes, S. An immunology model for accelerated coronary atherosclerosis and unexplained sudden death in the COVID-19 era. Autoimmun. Rev. 2024, 23, 103642. [Google Scholar] [CrossRef]
- Blagov, A.V.; Kalmykov, V.A.; Rakitin, A.L.; Grechko, A.V.; Orekhov, A.N. Cytokines are the Basis of the Development and Suppression of Inflammation in Atherosclerosis. Rev. Cardiovasc. Med. 2025, 26, 26421. [Google Scholar] [CrossRef]
- Chidambaram, V.; Tun, N.L.; Cheung, M.M.; Garg, A.; Jain, N.B.; Ghimire, S.; Kumar, A.; Soliman, A.; Saeed, M.A.; Ghosh, M.; et al. COVID-19 in the Initiation and Progression of Atherosclerosis: Pathophysiology During and Beyond the Acute Phase. JACC Adv. 2024, 3, 101107. [Google Scholar] [CrossRef]
- Brinkmann, M.; Brämer, D.; Katschinski, D.M.; Burek, K. Autoantibody development is associated with clinical severity of COVID-19: A cohort study. Clin. Immunol. 2025, 274, 110471. [Google Scholar] [CrossRef]
- Rus, M.; Mihai, C.; Popescu, A.; Ionescu, D.; Petrescu, R.; Enache, R.; Radu, C.M.; Luca, C.T.; Stan, M.; Dumitrescu, A.; et al. Acute Myocardial Infarction during the COVID-19 Pandemic: Long-Term Outcomes and Prognosis—A Systematic Review. Life 2024, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.; Marshall, J.; Patel, R.K.; Thompson, D.; Nguyen, P.; Li, H.; Scott, L.; Cameron, J.I.; Richards, J.; Fraser, G.; et al. Cardiovascular Outcomes in Nova Scotia During the Early Phase of the COVID-19 Pandemic. CJC Open 2022, 4, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Wright, L.; Khandkar, C.; Feridooni, H.A.; Briffa, T.; Moran, A.E.; Nichols, M.; Miranda, J.J. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana-Carnerero, C.; Giner-Soriano, M.; Dominguez, À.; Morros, R.; Pericas, C.; Álamo-Junquera, D.; Toledo, D.; Gallego, C.; Redondo, A.; Grau, M. Atherosclerosis, Cardiovascular Disease, and COVID-19: A Narrative Review. Biomedicines 2023, 11, 1206. [Google Scholar] [CrossRef]
- Makarova, Y.A.; Ivanov, A.V.; Petrova, M.Y.; Sokolov, A.V.; Kuznetsova, I.A.; Nikitina, E.A.; Korneva, V.A.; Lebedeva, N.S.; Sidorov, D.A.; Baranova, A.V.; et al. Atherosclerosis, Cardiovascular Disorders and COVID-19: Comorbid Pathogenesis. Diagnostics 2023, 13, 478. [Google Scholar] [CrossRef]
- Arif, Y.A.; Johnson, K.J.; Martin, C.A.; Liu, Y.; Bell, A.C.; Chen, X.; Tanaka, A.; Reynolds, D.A.; Patel, M.K.; Williams, M.T.; et al. Estimated Atherosclerotic Cardiovascular Disease Risk: Disparities and Severe COVID-19 Outcomes (from the National COVID Cohort Collaborative). Am. J. Cardiol. 2022, 183, 16–23. [Google Scholar] [CrossRef]
- Peng, M.; Chen, Z.; Liu, C.; Zhang, J.; Wang, Y.; Zhou, X.; Hu, J.; Li, N.; Guo, L.; Huang, F.; et al. Role of Hypertension on the Severity of COVID-19: A Review. J. Cardiovasc. Pharmacol. 2021, 78, e648–e655. [Google Scholar] [CrossRef]
- Gallo, G.; Calvez, V.; Savoia, C. Hypertension and COVID-19: Current Evidence and Perspectives. High Blood Press. Cardiovasc. Prev. 2022, 29, 115–123. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Strategies for the Prevention and Management of Coronavirus Disease 2019. Eur. Respir. J. 2020, 55, 2000597. [Google Scholar] [CrossRef]
- Savoia, C.; Volpe, M.; Kreutz, R. Hypertension, a Moving Target in COVID-19: Current Views and Perspectives. Circ. Res. 2021, 128, 1062–1079. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Mancia, G.; Dell’Oro, R.; Grassi, G.; Ivanovic, B. Hypertension and COVID-19: Ongoing Controversies. Front. Cardiovasc. Med. 2021, 8, 639222. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P.; Nardone, P.; Murata, T.; El-Hack, M.E.A.; Taha, A.E.; Al-Sagheer, A.A.; et al. Hypertension and Its Management in COVID-19 Patients: The Assorted View. Int. J. Cardiol. Cardiovasc. Risk Prev. 2021, 11, 200121. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Cipponeri, E.; Roden, M. Diabetes Mellitus, Energy Metabolism, and COVID-19. Endocr. Rev. 2024, 45, 281–308. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, M.; Kim, Y.H.; Kim, S.; Lee, J.H.; Cho, E.; Park, J.H.; Kim, S.Y.; Kwon, S.H.; Song, S.; et al. Relationship Between Natural Killer Cell Activity and Glucose Control in Patients with Type 2 Diabetes and Prediabetes. J. Diabetes Investig. 2019, 10, 1223–1228. [Google Scholar] [CrossRef]
- Lecube, A.; García-Manzanares, A.; Martín, P.; Vendrell, J.; Fernández-Real, J.M.; Simo, R.; Soler, J.; Ríos, A.; Calle-Pascual, A.; González-Arribas, E.; et al. Phagocytic Activity is Impaired in Type 2 Diabetes Mellitus and Increases After Metabolic Improvement. PLoS ONE 2011, 6, e23366. [Google Scholar] [CrossRef]
- Lim, S.; Kim, M.K.; Kim, M.; Lee, S.H.; Lee, S.; Yang, S.; Lee, J.; Cho, J.; Kim, M.; Jeong, H.; et al. COVID-19 and Diabetes Mellitus: From Pathophysiology to Clinical Management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Holman, N.; Knight, R.; Hussain, A.; Lomax, J.; Boyle, G.; Thomas, S.; Hughes, K.; Anderson, G.; Shaw, J.; Wierzbicki, A.; et al. Risk Factors for COVID-19-Related Mortality in People with Type 1 and Type 2 Diabetes in England: A Population-Based Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Guo, L.; Wei, D.; Zhang, X.; Chen, J.; Hu, L.; Li, S.; Lei, Y.; Zhang, Q.; Zhang, C.; Li, J.; et al. Comorbid Diabetes and the Risk of Disease Severity or Death Among 8807 COVID-19 Patients in China: A Meta-Analysis. Diabetes Res. Clin. Pract. 2020, 166, 108346. [Google Scholar] [CrossRef]
- Arulanandam, B.; Beladi, H.; Chakrabarti, A. Obesity and COVID-19 Mortality Are Correlated. Sci. Rep. 2023, 13, 5895. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.J.; Cho, Y.J.; Lee, H.W.; Park, M.; Kim, S.; Shin, H.; Kim, D.S.; Ryu, H.; Kwon, J.; et al. New-Onset Diabetes After COVID-19. J. Clin. Endocrinol. Metab. 2023, 108, 1164–1174. [Google Scholar] [CrossRef]
- Harding, J.L.; Li, L.; Yu, H.; Wang, X.; Chen, J.; Zhang, H.; Zhao, X.; Li, Z.; Liu, J.; Xu, L.; et al. The Bidirectional Association Between Diabetes and Long-COVID-19: A Systematic Review. Diabetes Res. Clin. Pract. 2023, 195, 110202. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W.; Zhang, L.; Guo, H.; Li, J.; Wang, S.; Zhang, Q.; Liang, Z.; Xu, Y.; Song, L.; et al. Prevalence of Atrial Fibrillation and Associated Mortality Among Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 720129. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Meneguzzo, A.; Ochsner, L.; Muratore, M.; Saba, S.; Barbieri, G.; Rizzardo, S.; Caffo, M.; Tondi, L.; et al. Pre-Existing Atrial Fibrillation is Associated with Increased Mortality in COVID-19 Patients. J. Interv. Card. Electrophysiol. 2021, 62, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Verdecchia, P.; Angeli, F.; Cavallari, I.; De Luca, N.; Tontodonati, M.; Martella, L.; Ferrante, G.; Santoro, C.; Mazzone, A.; et al. Pre-Admission Atrial Fibrillation in COVID-19 Patients: Prevalence and Clinical Impact. Eur. J. Intern. Med. 2021, 88, 133. [Google Scholar] [CrossRef]
- Terzic, C.M.; Medina-Inojosa, B.J. Cardiovascular Complications of Coronavirus Disease-2019. Phys. Med. Rehabil. Clin. 2023, 34, 551–561. [Google Scholar] [CrossRef]
- Sandoval, Y.; Januzzi, J.L., Jr.; Jaffe, A.S. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1244–1258. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Goyal, P.; Ray, A.; Soffer, J.; Ingraham, C.; Lipsitz, S.; Sager, J.; Wolk, D.; Bertoni, A.; Patel, R.; et al. Myocardial Injury in Adults Hospitalized with COVID-19. Circulation 2020, 142, 2393–2395. [Google Scholar] [CrossRef]
- Bonow, R.O.; Fonarow, G.C.; O’Connor, C.M.; Gale, C.P.; Givertz, M.M.; Kloner, R.A.; McManus, D.D.; Phelan, D.; Solomon, S.D.; Vaduganathan, M.; et al. Association of Coronavirus Disease 2019 (COVID-19) with Myocardial Injury and Mortality. JAMA Cardiol. 2020, 5, 751–753. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Smeeth, L.; Linder, J.; Akhavan, P.; Nitsch, D.; Edwards, R.; Nielsen, S.F.; Weiskopf, D.; et al. Risk of Acute Myocardial Infarction and Ischaemic Stroke Following COVID-19 in Sweden: A Self-Controlled Case Series and Matched Cohort Study. Lancet 2021, 398, 599–607. [Google Scholar] [CrossRef]
- Moayed, M.S.; Rahimi-Bashar, F.; Vahedian-Azimi, A.; Sathyapalan , T.; Guest, P.C.; Jamialahmadi, T.; Sahebkar, A. Cardiac Injury in COVID-19: A Systematic Review. In Clinical, Biological and Molecular Aspects of COVID-19; Springer: Cham, Switzerland, 2021; pp. 325–333. [Google Scholar] [CrossRef]
- Lala, A.; van Gaal, W.; Mitchell, S.; Kwiatkowski, M.; Randhawa, P.; Safford, M.; Raikhelkar, J.; Gohil, A.; Vaidya, A.; Sharma, P.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhao, L.; Yang, X.C.; Wang, P.; Li, M.; Zhang, W.; Sun, X.; Wang, X.; Liu, H.; Li, J.; et al. Cardiovascular Complications of SARS-CoV-2 Infection (COVID-19): A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2021, 22, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, M.; Akhavan Rezayat, A.; Zamiri Bidary, M.; Shakibazadeh, E.; Mirzaei, H.; Ghasemifard, F.; Karami, M.; Parvizi, S.; Mousavi, M.; Zarei, M.; et al. Cardiac Complications in COVID-19: A Systematic Review and Meta-Analysis. Arch. Iran. Med. 2021, 24, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.A.; Vail, D.; Iannucci, L.; Sharma, S.; Chopra, R.; Srinivasan, A.; Thomas, A.; Farooqui, M.; Coelho, J.; Merchant, S.; et al. COVID-19 and Cardiac Arrhythmias: A Contemporary Review. Curr. Treat. Options Cardiovasc. Med. 2022, 24, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Varney, J.A.; Alqahtani, F.; Chan, K.; Malik, S.; Baig, M.; Cavanaugh, B.; Zakaria, A.; Haris, M.; Sarwar, R.; Sayed, S.; et al. COVID-19 and Arrhythmia: An Overview. J. Cardiol. 2022, 79, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Coromilas, E.J.; Kochav, S.; Goldenthal, I.; Biviano, A.; Garan, H.; Goldbarg, S.; Kim, J.H.; Yeo, I.; Tracy, C.; Ayanian, S.; et al. Worldwide Survey of COVID-19–Associated Arrhythmias. Circ. Arrhythm. Electrophysiol. 2021, 14, e009458. [Google Scholar] [CrossRef]
- Castiello, T.; Mancusi, C.; Caracciolo, A.; Esposito, A.; Cacciapuoti, F.; Bisogno, R.; Migliaro, F.; Palmisano, A.; Ruggiero, F.; Magliocca, F.; et al. COVID-19 and Myocarditis: A Systematic Review and Overview of Current Challenges. Heart Fail. Rev. 2022, 27, 251–261. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef]
- Florek, K.; Sokolski, M. Myocarditis associated with COVID-19 vaccination. Vaccines 2024, 12, 1193. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: www.ema.europa.eu (accessed on 10 April 2025).
- Othman, H.Y.; Al-Awadhi, A.; Alhammadi, A.; Al-Khalidi, H. A Systematic Review of Thromboembolic Complications and Outcomes in Hospitalised COVID-19 Patients. BMC Infect. Dis. 2024, 24, 484. [Google Scholar] [CrossRef]
- Xie, J.; Prats-Uribe, A.; Feng, Q.; Yang, D.; Zhang, H.; Chen, Z.; Liu, Y.; Liu, W.; Luo, X.; Wang, Y. Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients with COVID-19. JAMA Intern. Med. 2022, 182, 1063–1070. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Almutairi, F.; Ali, S.H.; Al-Ghamdi, S.; Othman, F.; Al-Haddad, S.; Al-Shehri, M.; Al-Dosary, M.; Al-Jabri, Z.; Al-Mazrouei, N. Thromboprophylaxis and Clinical Outcomes in Moderate COVID-19 Patients: A Comparative Study. Res. Soc. Adm. Pharm. 2022, 18, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.B.; Neupane, K.; Sedhai, Y.R.; Devkota, A.; Lamichhane, J.; Paudel, S.; Aryal, B.B.; Shrestha, R.; Gautam, N.; Ghimire, S.; et al. Long COVID Syndrome and Cardiovascular Manifestations: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Romero-Duarte, Á.; Gómez, A.; López, A.; Fernández, D.; Herrera, R.; Soto, C.; Fernández-Lafuente, R.; Molina, M.; García, J.; González, E. Sequelae, persistent symptomatology and outcomes after COVID-19 hospitalization: The ANCOHVID multicentre 6-month follow-up study. BMC Med. 2021, 19, 129. [Google Scholar] [CrossRef]
- Tereshchenko, L.G.; Maruyama, S.; Nikitin, N.; Chikwe, J.; Shaw, M.; Brown, L.; Wang, H.; Sulaiman, K.; Xu, Y.; Liu, W. Risk of Cardiovascular Events After COVID-19. Am. J. Cardiol. 2022, 179, 102–109. [Google Scholar] [CrossRef]
- Ramadan, M.S.; Fatima, S.; Ahmed, S.; Ali, A.; Khan, R.; Saeed, A.; Fatima, N.; Hussain, A.; Rizwan, M.; Zaman, S. Cardiac Sequelae After Coronavirus Disease 2019 Recovery: A Systematic Review. Clin. Microbiol. Infect. 2021, 27, 1250–1261. [Google Scholar] [CrossRef]
- Cha, C.; Baek, G.; Han, K.; Oh, H.; Kim, Y.; Park, S.; Choi, J.; Kim, M.; Lee, K.; Lee, W. Symptoms and management of long COVID: A scoping review. J. Clin. Nurs. 2024, 33, 11–28. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; McMahon, K.; O’Shea, E.; McConkey, S.; Fitzgerald, M.; Curran, P.; Quigley, A.; O’Neill, T. Persistent Fatigue Following SARS-CoV-2 Infection is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Tleyjeh, I.M.; Saddik, B.; AlSwaidan, N.; Alhammadi, A.; Al-Mutairi, F.; Al-Mansour, A.; Al-Dosary, M.; Al-Khaled, A.; Al-Massad, A.; Al-Hassan, A. Prevalence and Predictors of Post-Acute COVID-19 Syndrome (PACS) After Hospital Discharge: A Cohort Study with 4 Months Median Follow-Up. PLoS ONE 2021, 16, e0260568. [Google Scholar] [CrossRef]
- Gameil, M.A.; Marzouk, R.E.; Elsebaie, A.H.; Rozaik, S.E.; Taha, H.; Abdel-Maguid, A.; Farouk, S.; Mohamed, M.; Sayed, M.; Waly, M. Long-Term Clinical and Biochemical Residue After COVID-19 Recovery. Egypt. Liver J. 2021, 11, 74. [Google Scholar] [CrossRef]
- Liao, B.; Liu, Z.; Tang, L.; Hu, M.; Zhang, F.; Li, Y.; Zeng, N.; Fan, Y.; Lu, T.; Peng, X. Longitudinal Clinical and Radiographic Evaluation Reveals Interleukin-6 as an Indicator of Persistent Pulmonary Injury in COVID-19. Int. J. Med. Sci. 2021, 18, 29–41. [Google Scholar] [CrossRef]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.W.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-Term Effects of SARS-CoV-2 Infection on Multiple Vital Organs, Exercise Capacity, Cognition, Quality of Life and Mental Health, Post-Hospital Discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Ingul, C.B.; Grimsmo, J.; Mecinaj, A.; Trebinjac, D.; Naderi, S.; Einvik, G.; Stavem, K. Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. J. Am. Heart Assoc. 2022, 11, e023473. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Roncon, L.; Bilato, C.; Cervellati, C.; Zuliani, G. Risk of incident atrial fibrillation after COVID-19 infection: A systematic review and meta-analysis. Heart Rhythm 2024, 21, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Voruz, P.; Allali, G.; Benzakour, L.; Nuber-Champier, A.; Thomasson, M.; Caraballo, S.M.; Braillard, O.; Nehme, M.; de Andres, A.; Lalive, P.A. Long COVID Neuropsychological Deficits After Severe, Moderate, or Mild Infection. Clin. Transl. Neurosci. 2022, 6, 9. [Google Scholar] [CrossRef]
- Johansson, M.; Svensson, J.; Nilsson, E.; Bäcklund, B.; Lindblom, J.; Olsson, M.; Kalluri, P.; Persson, L.; Stjernström, M. Blood Pressure Regulation in Post-COVID POTS: Beyond Sinus Tachycardia. Hypertension 2024, 81, 2540–2548. [Google Scholar] [CrossRef]
- Nyasulu, P.S.; Mapesi, H.; Pandya, H.; Mwale, S.E.; Adebayo, E.; Amoako, D.G.; Essack, S. Burden, causation, and particularities of Long COVID in African populations: A rapid systematic review. IJID Reg. 2023, 8, 137–144. [Google Scholar] [CrossRef]
- Saeed, S.; Rajani, R. The association of pre-existing comorbid conditions with COVID-19 severity and post-COVID complications; insights from South Asia. Pak. J. Med. Sci. 2022, 38, 439–441. [Google Scholar] [CrossRef]
- Ambrosino, P.; Calcaterra, I.; Molino, A.; Moretta, P.; Papa, A.; Carotenuto, M.; Peluso, V.; Strollo, G.; Motta, A.; Di Minno, M.N.D.; et al. Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study. Biomedicines 2021, 9, 957. [Google Scholar] [CrossRef]
- Akpek, M. Does COVID-19 Cause Hypertension? Angiology 2022, 73, 682–687. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Delaney, B.; Evans, R.; Milne, R.; Nicholson, T.; Rosen, R.; Singh, S.; Thornton, H.; Venkatesan, P.; et al. Long COVID: A clinical update. Lancet 2024, 404, 10453. [Google Scholar] [CrossRef]
- Barker, K.K.; Jones, A.; Robinson, C.; Stevens, E.; Thomas, M. The Long Tail of COVID and the Tale of Long COVID: Diagnostic Construction and the Management of Ignorance. Sociol. Health Illn. 2024, 46, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Samy, W.; Ibrahim, M.; Hossam, A.; El-Moselhy, M.; Ahmed, F. Left Ventricular Assessment by 3D-Echocardiography in Post-COVID-19 Syndrome. Egypt. J. Crit. Care Med. 2025, 12, 4. [Google Scholar] [CrossRef]
- Vlase, C.-M.; Popa, D.; Constantin, M.; Bălăceanu, M.; Dumitrescu, L. Echocardiographic Left Ventricular Function in the Third Year After COVID-19 Hospitalization: A Follow-Up Pilot Study in South-East of Romania. Medicina 2025, 61, 333. [Google Scholar] [CrossRef] [PubMed]
- Asarcikli, L.D.; Karapinar, D.; Tuncer, A.; Kucuk, M.; Yilmaz, F. Heart Rate Variability and Cardiac Autonomic Functions in Post-COVID Period. J. Interv. Card. Electrophysiol. 2022, 63, 715–721. [Google Scholar] [CrossRef]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Post–Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Sarfraz, A.; Barrios, A.; Dhuka, I.; Razzack, A.A.; Patel, G.; Michel, G.; Coschignano, C.; Vohra, I.; Bin Waqar, M.; et al. Cardio-Pulmonary Sequelae in Recovered COVID-19 Patients: Considerations for Primary Care. J. Prim. Care Community Health 2021, 12, 21501327211023730. [Google Scholar] [CrossRef]
- Sørensen, L.; Nielsen, M.; Petersen, J.; Rasmussen, H.; Madsen, A.; Jensen, K. Cardiopulmonary Exercise testing in patients with Long COVID: Evaluating Functional Capacity and Exercise limitations. Chest Pulm. 2024, 2, 100036. [Google Scholar] [CrossRef]
- Sanghvi, S.K.; Patel, A.; Agarwal, S.; Verma, R.; Sharma, R.; Desai, P. Cardiac MRI and Myocardial Injury in COVID-19: Diagnosis, Risk Stratification and Prognosis. Diagnostics 2021, 11, 130. [Google Scholar] [CrossRef]
- Seo, J.-W.; Lee, H.; Kim, M.; Choi, K.; Park, J.; Yoon, S.; Lee, W. Updated Clinical Practice Guidelines for the Diagnosis and Management of Long COVID. Infect. Chemother. 2024, 56, 122–157. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, H.; Lee, S.; Park, M.; Jeong, Y.; Kwon, J.; Han, D. Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7375. [Google Scholar] [CrossRef]
- Ceban, F.; Leber, M.; Jawad, M.Y.; Lui, L.M.W.; Subramaniapillai, M.; Di Vincenzo, J.D.; Gill, H.; Ho, R.; Mansur, R.B.; Lin, K.; et al. COVID-19 Vaccination for the Prevention and Treatment of Long COVID: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2023, 111, 211–229. [Google Scholar] [CrossRef]
| Cardiovascular Manifestation | Pooled Prevalence (%) * | 95% Confidence Interval * | Number of Studies Included * |
|---|---|---|---|
| Chest pain | 10.1 | 6.4–15.5 | 9 |
| Palpitations | 9.8 | 5.4–17.2 | 7 |
| Dyspnea | 28.8 | 21.5–37.5 | 10 |
| Hypertension | 20.3 | 13.3–29.7 | 6 |
| Myocardial injury | 13.5 | 8.3–21.2 | 4 |
| Arrythmia | 9.6 | 5.2–17.0 | 5 |
| Heart failure | 5.8 | 2.8–11.6 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hărșan, S.T.; Sin, A.I. The Involvement and Manifestations of SARS-CoV-2 Virus in Cardiovascular Pathology. Medicina 2025, 61, 773. https://doi.org/10.3390/medicina61050773
Hărșan ST, Sin AI. The Involvement and Manifestations of SARS-CoV-2 Virus in Cardiovascular Pathology. Medicina. 2025; 61(5):773. https://doi.org/10.3390/medicina61050773
Chicago/Turabian StyleHărșan, Sofia Teodora, and Anca Ileana Sin. 2025. "The Involvement and Manifestations of SARS-CoV-2 Virus in Cardiovascular Pathology" Medicina 61, no. 5: 773. https://doi.org/10.3390/medicina61050773
APA StyleHărșan, S. T., & Sin, A. I. (2025). The Involvement and Manifestations of SARS-CoV-2 Virus in Cardiovascular Pathology. Medicina, 61(5), 773. https://doi.org/10.3390/medicina61050773




