Can We Reduce the Diagnostic Burden of Sleep Disorders? A Single-Centre Study of Subjective and Objective Sleep-Related Diagnostic Parameters
Abstract
:1. Introduction
1.1. Obstructive Sleep Apnoea
1.2. Insomnia
1.3. Hypersomnia
1.4. Parasomnias
1.5. REM Sleep Behaviour Disorder (RBD)
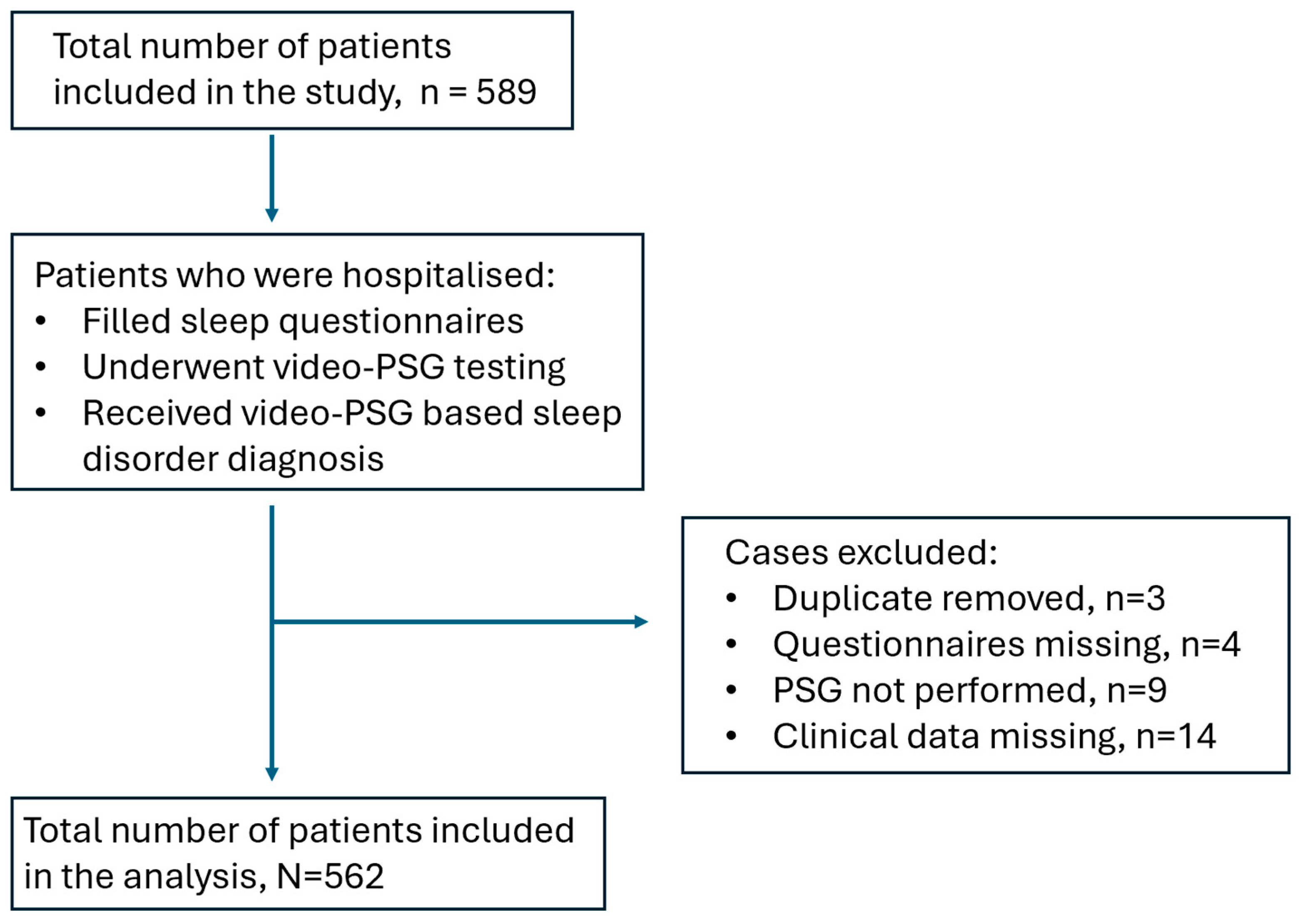
2. Materials and Methods
3. Results
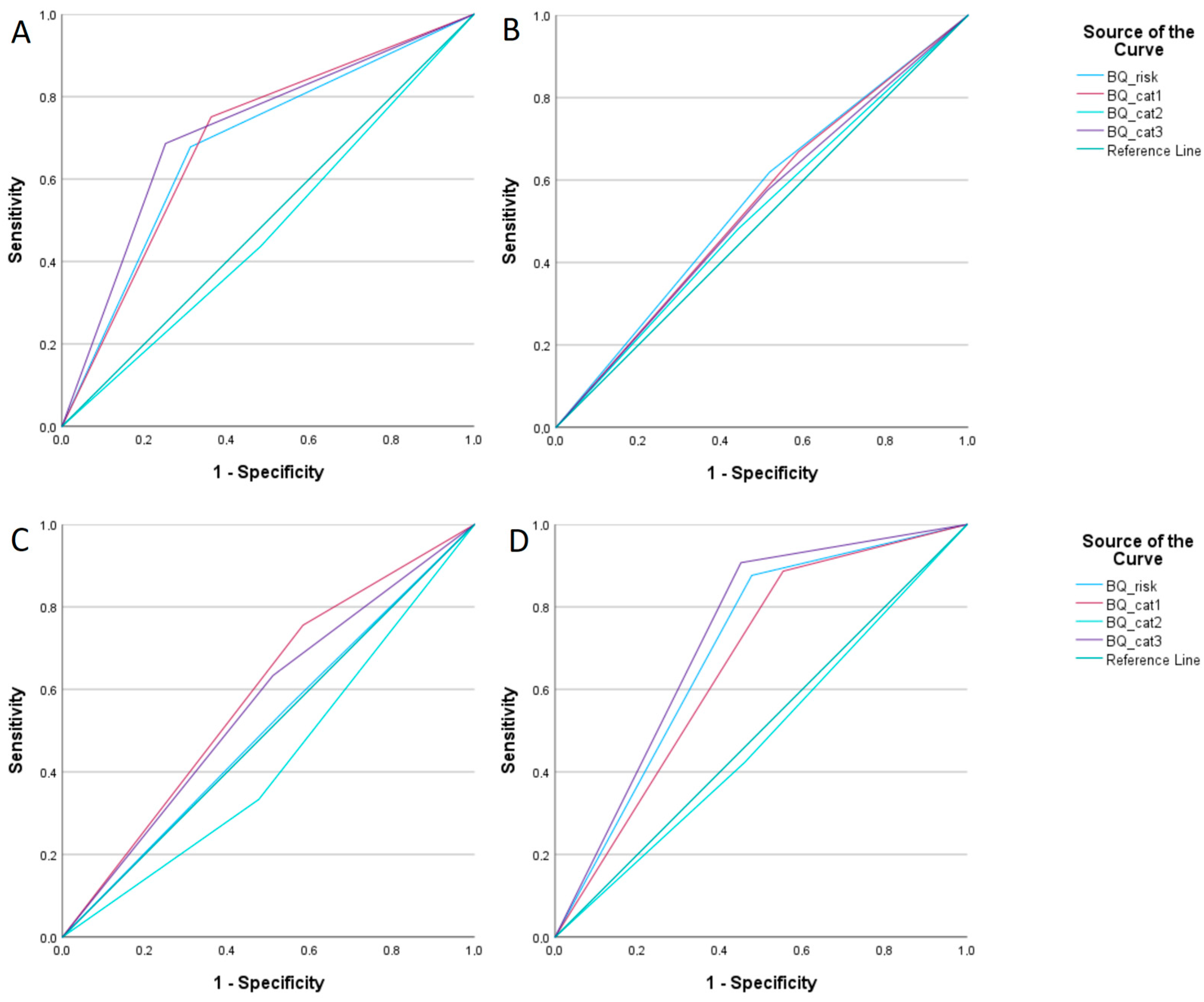
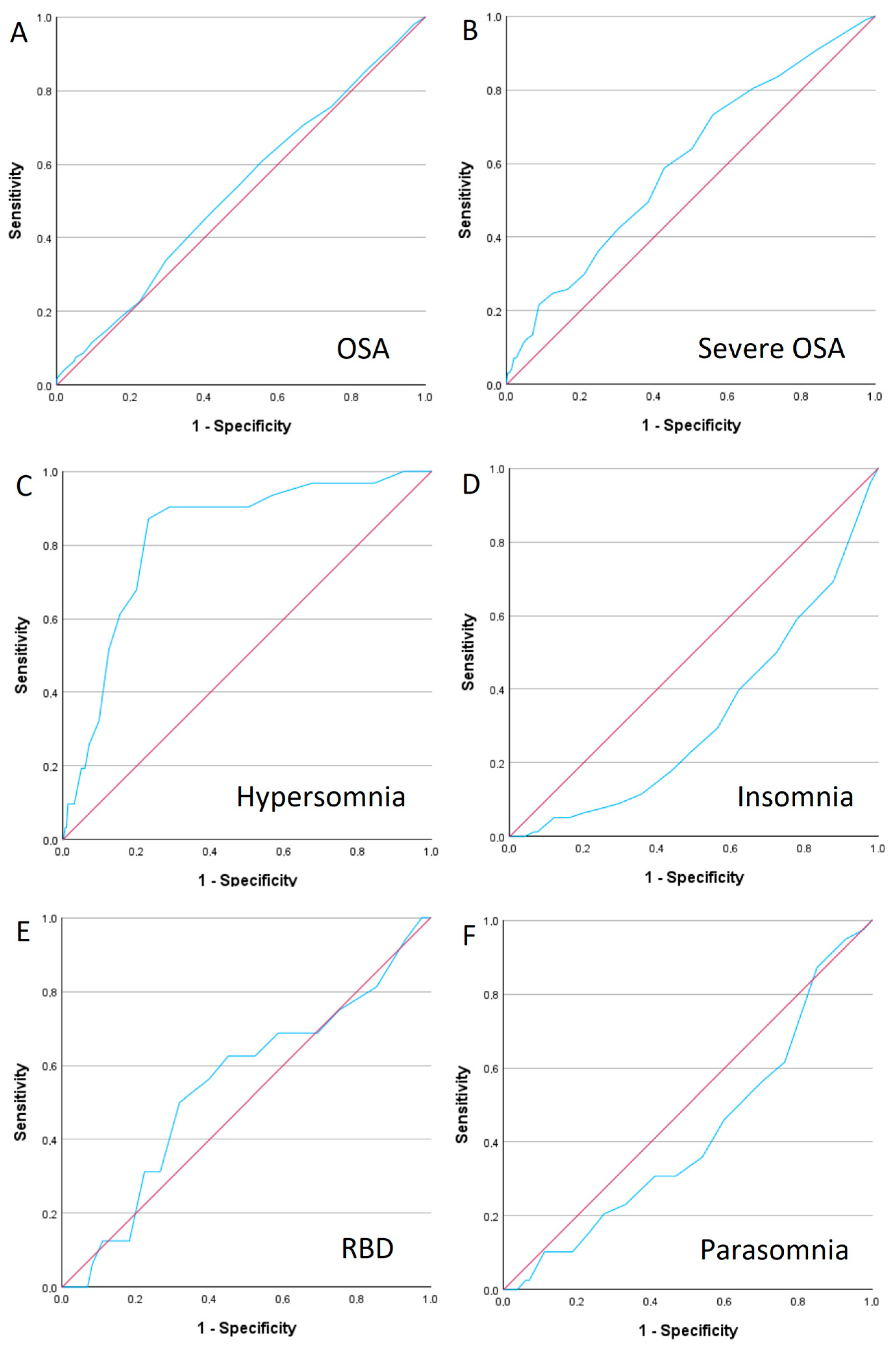
3.1. Obstructive Sleep Apnoea (OSA)
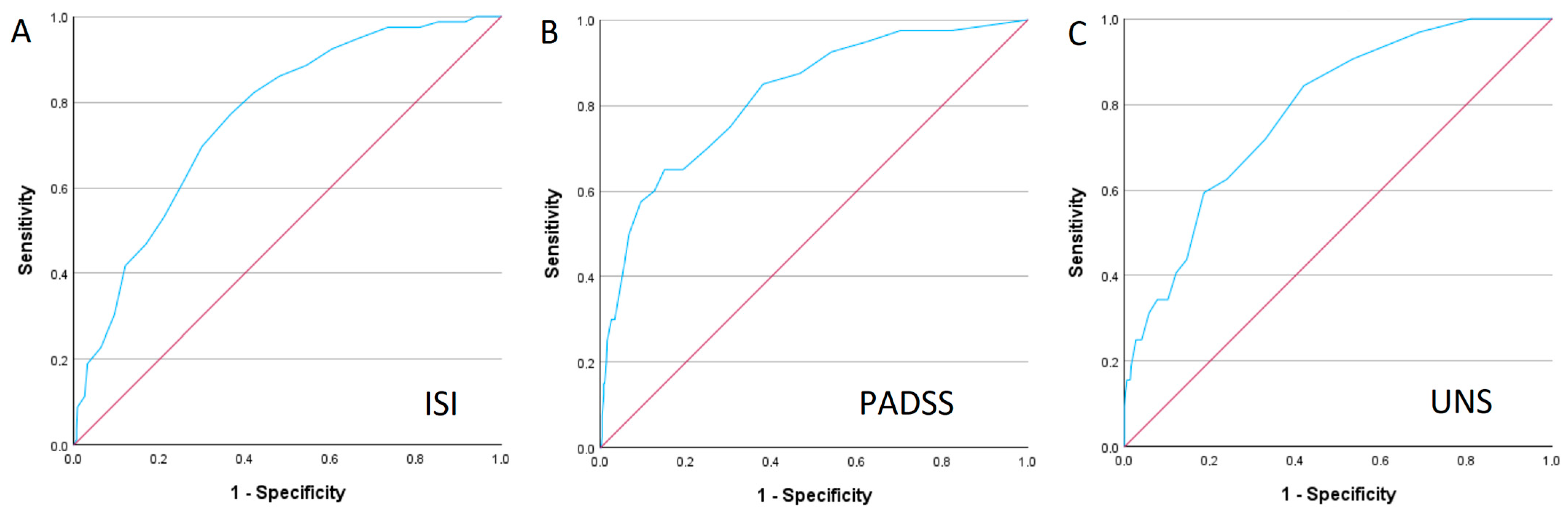
3.2. Insomnia
3.3. Parasomnia
3.4. Hypersomnia
3.5. REM Sleep Behaviour Disorder (RBD)
3.6. Epworth Sleepiness Scale (ESS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, M.; Liu, M.; Wang, Y.; Qin, C.; Liu, J. Global burden of sleep disturbances among older adults and the disparities by geographical regions and pandemic periods. SSM Popul. Health 2023, 25, 101588. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Wheaton, A.-W.A.; Johnson, D.A. Sleep Deprivation, Sleep Disorders, and Chronic Disease. Prev. Chronic Dis. 2023, 20, E77. [Google Scholar] [CrossRef] [PubMed]
- Sankri-Tarbichi, A.G. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna J. Med. 2012, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mehrtash, M.; Bakker, J.P.; Ayas, N. Predictors of Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnea. Lung 2019, 197, 115–121. [Google Scholar] [CrossRef]
- Carneiro-Barrera, A.; Diaz-Roman, A.; Guillen-Riquelme, A.; Buela-Casal, G. Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: Systematic review and meta-analysis. Obes. Rev. 2019, 20, 750–762. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Chiu, H.; Chen, P.; Chuang, L.; Chen, N.; Tu, Y.; Hsieh, Y.; Wang, Y.-C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Goncalves, M.T.; Malafaia, S.; Moutinho Dos Santos, J.; Roth, T.; Marques, D.R. Epworth sleepiness scale: A meta-analytic study on the internal consistency. Sleep Med. 2023, 109, 261–269. [Google Scholar] [CrossRef]
- Rosenberg, R.; Babson, K.; Menno, D.; Morris, S.; Baladi, M.; Hyman, D.; Black, J. Test-retest reliability of the Epworth Sleepiness Scale in clinical trial settings. J. Sleep Res. 2022, 31, e13476. [Google Scholar] [CrossRef]
- Tan, A.; Yin, J.D.C.; Tan, L.W.L.; van Dam, R.M.; Cheung, Y.Y.; Lee, C. Using the Berlin Questionnaire to Predict Obstructive Sleep Apnea in the General Population. J. Clin. Sleep Med. 2017, 13, 427–432. [Google Scholar] [CrossRef]
- Ahmadi, N.; Chung, S.A.; Gibbs, A.; Shapiro, C.M. The Berlin questionnaire for sleep apnea in a sleep clinic population: Relationship to polysomnographic measurement of respiratory disturbance. Sleep Breath 2008, 12, 39–45. [Google Scholar] [CrossRef]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Spurling, B.C.; Bollu, P.C. Chronic Insomnia. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Morin, C.M.; Belleville, G.; Belanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Slowik, J.M.; Collen, J.F.; Yow, A.G. Narcolepsy. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hublin, C.; Kaprio, J.; Partinen, M.; Koskenvuo, M.; Heikkila, K. The Ullanlinna Narcolepsy Scale: Validation of a measure of symptoms in the narcoleptic syndrome. J Sleep Res 1994, 3, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Mathias, S.D.; Crosby, R.D.; Bron, M.; Bujanover, S.; Menno, D.; Villa, K.F.; Drake, C. Relationship between sleep efficacy endpoints and measures of functional status and health-related quality of life in participants with narcolepsy or obstructive sleep apnea treated for excessive daytime sleepiness. J. Sleep Res. 2021, 30, e13210. [Google Scholar] [CrossRef]
- Kuts, A.; Poluektov, M.; Zakharov, A.; Govzman, V.; Ponomareva, I.; Yakupov, E.; Zavalko, I.; Tikhomirova, O.; Sviryaev, Y.; Yakovlev, A.; et al. Clinical and neurophysiological characteristics of 89 patients with narcolepsy and cataplexy from the Russian Narcolepsy Network. J. Clin. Sleep Med. 2023, 19, 355–359. [Google Scholar] [CrossRef]
- Sarkanen, T.; Alakuijala, A.; Partinen, M. Ullanlinna Narcolepsy Scale in diagnosis of narcolepsy. Sleep 2019, 42, zsy238. [Google Scholar] [CrossRef]
- Sturzenegger, C.; Baumann, C.R.; Lammers, G.J.; Kallweit, U.; van der Zande, W.L.; Bassetti, C.L. Swiss Narcolepsy Scale: A Simple Screening Tool for Hypocretin-Deficient Narcolepsy with Cataplexy. Clin. Transl. Neurosci. 2018, 2, 2514183X18794175. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, H.; Singh, S.; Khawaja, I. Parasomnias: A Comprehensive Review. Cureus 2018, 10, e3807. [Google Scholar] [CrossRef]
- Irfan, M.; Schenck, C.H.; Howell, M.J. NonREM Disorders of Arousal and Related Parasomnias: An Updated Review. Neurotherapeutics 2021, 18, 124–139. [Google Scholar] [CrossRef]
- Fariba, K.A.; Tadi, P. Parasomnias. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Loddo, G.; Lopez, R.; Cilea, R.; Dauvilliers, Y.; Provini, F. Disorders of Arousal in adults: New diagnostic tools for clinical practice. Sleep Sci. Pract. 2019, 3, 5. [Google Scholar] [CrossRef]
- Hrozanova, M.; Morrison, I.; Riha, R.L. Adult NREM Parasomnias: An Update. Clocks Sleep 2018, 1, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Dauvilliers, Y. Challenges in diagnosing NREM parasomnias: Implications for future diagnostic classifications. Sleep Med. Rev. 2024, 73, 101888. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, I.; Zhang, B.; Uguccioni, G.; Flamand, M.; Noel de Fontreaux, A.; Leu-Semenescu, S.; Brion, A. A scale for assessing the severity of arousal disorders. Sleep 2014, 37, 127–136. [Google Scholar] [CrossRef]
- van Mierlo, P.; Hermans, L.W.A.; Arnulf, I.; Pijpers, A.; Overeem, S.; van Gilst, M.M. Validation of the Dutch translation of the Paris Arousal Disorders Severity Scale for non-REM parasomnias in a 1-year and 1-month version. J. Clin. Sleep Med. 2022, 18, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Loddo, G.; La Fauci, G.; Vignatelli, L.; Zenesini, C.; Cilea, R.; Mignani, F.; Cecere, A.; Mondini, S.; Baldelli, L.; Bisulli, F.; et al. The Arousal Disorders Questionnaire: A new and effective screening tool for confusional arousals, Sleepwalking and Sleep Terrors in epilepsy and sleep disorders units. Sleep Med. 2021, 80, 279–285. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Schenck, C.H.; Postuma, R.B.; Iranzo, A.; Luppi, P.; Plazzi, G.; Montplaisir, J.; Boeve, B. REM sleep behaviour disorder. Nat. Rev. Dis. Primers. 2018, 4, 19–25. [Google Scholar] [CrossRef]
- Lee, W.; Baek, S.; Im, H.; Lee, S.; Yoon, J.; Thomas, R.J.; Wing, Y.-K.; Shin, C.; Yun, C.-H. REM Sleep Behavior Disorder and Its Possible Prodromes in General Population: Prevalence, Polysomnography Findings, and Associated Factors. Neurology 2023, 101, e2364–e2375. [Google Scholar] [CrossRef]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Hogl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef]
- Sobreira-Neto, M.A.; Stelzer, F.G.; Gitai, L.L.G.; Alves, R.C.; Eckeli, A.L.; Schenck, C.H. REM sleep behavior disorder: Update on diagnosis and management. Arq. Neuropsiquiatr. 2023, 81, 1179–1194. [Google Scholar] [CrossRef]
- St Louis, E.K.; Boeve, B.F. REM Sleep Behavior Disorder: Diagnosis, Clinical Implications, and Future Directions. Mayo Clin. Proc. 2017, 92, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Arnulf, I.; Hogl, B.; Iranzo, A.; Miyamoto, T.; Dauvilliers, Y.; Oertel, W.; Ju, Y.; Puligheddu, M.; Jennum, P.; et al. A single-question screen for rapid eye movement sleep behavior disorder: A multicenter validation study. Mov. Disord. 2012, 27, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, B.; Ehrmann, L.; Zamarian, L.; Auer, F.; Mitterling, T.; Gabelia, D.; Brandauer, E.; Delazer, M.; Poewe, W.; Högl, B. Validation of the Innsbruck REM sleep behavior disorder inventory. Mov. Disord. 2012, 27, 1673–1678. [Google Scholar] [CrossRef]
- Pena-Pereira, M.A.; Sobreira-Neto, M.A.; Sobreira, E.; Chagas, M.H.N.; Oliveira DSd Rodrigues, G.R.; de Souza, C.P.; Eckeli, A.L.; Fernandes, R.M.F.; Tumas, V. Validation of the Brazilian Portuguese version of the Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ-BR). Arq. Neuropsiquiatr. 2020, 78, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Stiasny-Kolster, K.; Mayer, G.; Schafer, S.; Moller, J.C.; Heinzel-Gutenbrunner, M.; Oertel, W.H. The REM sleep behavior disorder screening questionnaire—A new diagnostic instrument. Mov. Disord. 2007, 22, 2386–2393. [Google Scholar] [CrossRef]
- Marelli, S.; Rancoita, P.M.V.; Giarrusso, F.; Galbiati, A.; Zucconi, M.; Oldani, A.; Di Serio, C.; Ferini-Strambi, L. National validation and proposed revision of REM sleep behavior disorder screening questionnaire (RBDSQ). J. Neurol. 2016, 263, 2470–2475. [Google Scholar] [CrossRef]

| Sleep Disorder | OSA | Insomnia | Hypersomnia | Parasomnias | RBD |
|---|---|---|---|---|---|
| Prevalence | 362, 64.4% | 80, 14.2% | 32, 5.7% | 40, 7.1% | 16, 2.8% |
| Gender | F 153, 42.3% M 209, 57.7% * p = 0.015 | F 39, 48.8% M 41, 51.2% | F 24, 75.0% M 8, 25.9% * p < 0.001 | F 19, 47.5% M 21, 52.5% | F 7, 43.8% M 9, 56.3% |
| Age, years | (−) 38.9 ± 15.5 (+) 53.03 ± 14.3 * p < 0.001 | (−) 47.2 ± 16.0 (+) 52.6 ± 16.6 * p = 0.006 | (−) 48.5 ± 16.3 (+) 40.2 ± 12.5 * p = 0.004 | (−) 49.02 ± 15.7 (+) 34.5 ± 16.6 * p < 0.001 | (−) 47.6 ± 16.2 (+) 59.2 ± 11.4 * p = 0.003 |
| BMI, kg/m2 | (−) 24.8 ± 4.2 (+) 30.6 ± 6.7 * p < 0.001 | (−) 28.7 ± 6.8 (+) 27.6 ± 4.5 | (−) 28.5 ± 6.3 (+) 29.1 ± 10.2 | (−) 28.7 ± 6.4 (+) 26.9 ± 8.6 | (−) 28.6 ± 6.5 (+) 26.5 ± 9.1 |
| Comorbidities PAH | (−) 23, 13.7% (+) 145, 49.2% * p < 0.001 | 27, 43.5% | (−) 21, 84.0% (+) 4, 16.0% * p = 0.023 | 6, 20.7% | 4, 30.8% |
| AF | (−) 3, 1.9% (+) 24, 8.2% * p = 0.008 | 5, 8.1 | 1, 4.0% | 1, 3.4% | 1, 7.7% |
| Diabetes | (−) 7, 4.6% (+) 32, 10.9% * p = 0.025 | 8, 12.9% | 1, 4.2% | 1, 3.4% | 0 |
| Dyslipidaemia | (−) 11, 7.0% (+) 59, 18.6% * p < 0.001 | 12, 17.4% | 1, 3.8% | 1, 4.3% | 1, 7.1% |
| PD | 6, 2.0% | 0 | 0 | 1, 2.5% | 8, 50.0% |
| Epilepsy | 20, 6.8% | 0 | 1, 4.0% | 1, 3.4% | 0 |
| MS | 1, 0.3% | 0 | 0 | 0 | 0 |
| OSA | BQ | AUC | 95% CI | Sensitivity | Specificity | p-Value |
|---|---|---|---|---|---|---|
| Mild | Overall | 0.551 | 0.498–0.603 | 61.9% | 48.2% | 0.062 |
| Intermediate | Cat. 1 | 0.586 | 0.524–0.648 | 75.6% | 41.6% | 0.01 |
| Cat. 2 | 0.428 | 0.365–0.492 | 33.3% | 52.4% | 0.032 | |
| Overall | 0.505 | 0.440–0.570 | 55.6% | 45.5% | 0.875 | |
| Severe | Cat. 1 | 0.667 | 0.614–0.719 | 88.7% | 44.7% | <0.001 |
| Cat. 3 | 0.728 | 0.680–0.776 | 90.7% | 54.9% | <0.001 | |
| Overall | 0.700 | 0.648–0.751 | 87.6% | 52.3% | <0.001 | |
| Overall | Cat. 1 | 0.694 | 0.648–0.741 | 75.1% | 63.8% | <0.001 |
| Cat. 3 | 0.718 | 0.427–0.527 | 68.6% | 74.9% | <0.001 | |
| Overall | 0.683 | 0.637–0.730 | 67.8% | 68.8% | <0.001 |
| RBD-I-9 Questions | AUC | 95% CI | p-Value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Question 1 | 0.665 | 0.538–0.793 | 0.024 | 75.0% | 58.1% |
| Question 2 | 0.796 | 0.671–0.921 | <0.001 | 75.0% | 84.2% |
| Question 3 | 0.481 | 0.342–0.621 | 0.799 | - | - |
| Question 4 | 0.487 | 0.346–0.628 | 0.863 | - | - |
| Question 5 | 0.613 | 0.462–0.764 | 0.124 | - | - |
| Question 6 | 0.501 | 0.357–0.645 | 0.986 | - | - |
| Question 7 | 0.519 | 0.372–0.666 | 0.797 | - | - |
| Question 8 | 0.605 | 0.449–0.762 | 0.151 | - | - |
| Question 9 | 0.594 | 0.461–0.727 | 0.202 | - | - |
| Overall RBDI9 | 0.758 | 0.665–0.851 | 0.001 | 93.3% | 52.5% |
| RBD1Q | 0.772 | 0.641–0.903 | <0.001 | 73.3% | 81.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanagas, T.; Lipskytė, D.; Tamošiūnaitė, J.; Petrikonis, K.; Pajėdienė, E. Can We Reduce the Diagnostic Burden of Sleep Disorders? A Single-Centre Study of Subjective and Objective Sleep-Related Diagnostic Parameters. Medicina 2025, 61, 780. https://doi.org/10.3390/medicina61050780
Vanagas T, Lipskytė D, Tamošiūnaitė J, Petrikonis K, Pajėdienė E. Can We Reduce the Diagnostic Burden of Sleep Disorders? A Single-Centre Study of Subjective and Objective Sleep-Related Diagnostic Parameters. Medicina. 2025; 61(5):780. https://doi.org/10.3390/medicina61050780
Chicago/Turabian StyleVanagas, Tadas, Domantė Lipskytė, Jovita Tamošiūnaitė, Kęstutis Petrikonis, and Evelina Pajėdienė. 2025. "Can We Reduce the Diagnostic Burden of Sleep Disorders? A Single-Centre Study of Subjective and Objective Sleep-Related Diagnostic Parameters" Medicina 61, no. 5: 780. https://doi.org/10.3390/medicina61050780
APA StyleVanagas, T., Lipskytė, D., Tamošiūnaitė, J., Petrikonis, K., & Pajėdienė, E. (2025). Can We Reduce the Diagnostic Burden of Sleep Disorders? A Single-Centre Study of Subjective and Objective Sleep-Related Diagnostic Parameters. Medicina, 61(5), 780. https://doi.org/10.3390/medicina61050780





