Feasibility and Safety Properties of Metabolic-Flow Anesthesia Driven by Automated Gas Control® in Pediatric Patients: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
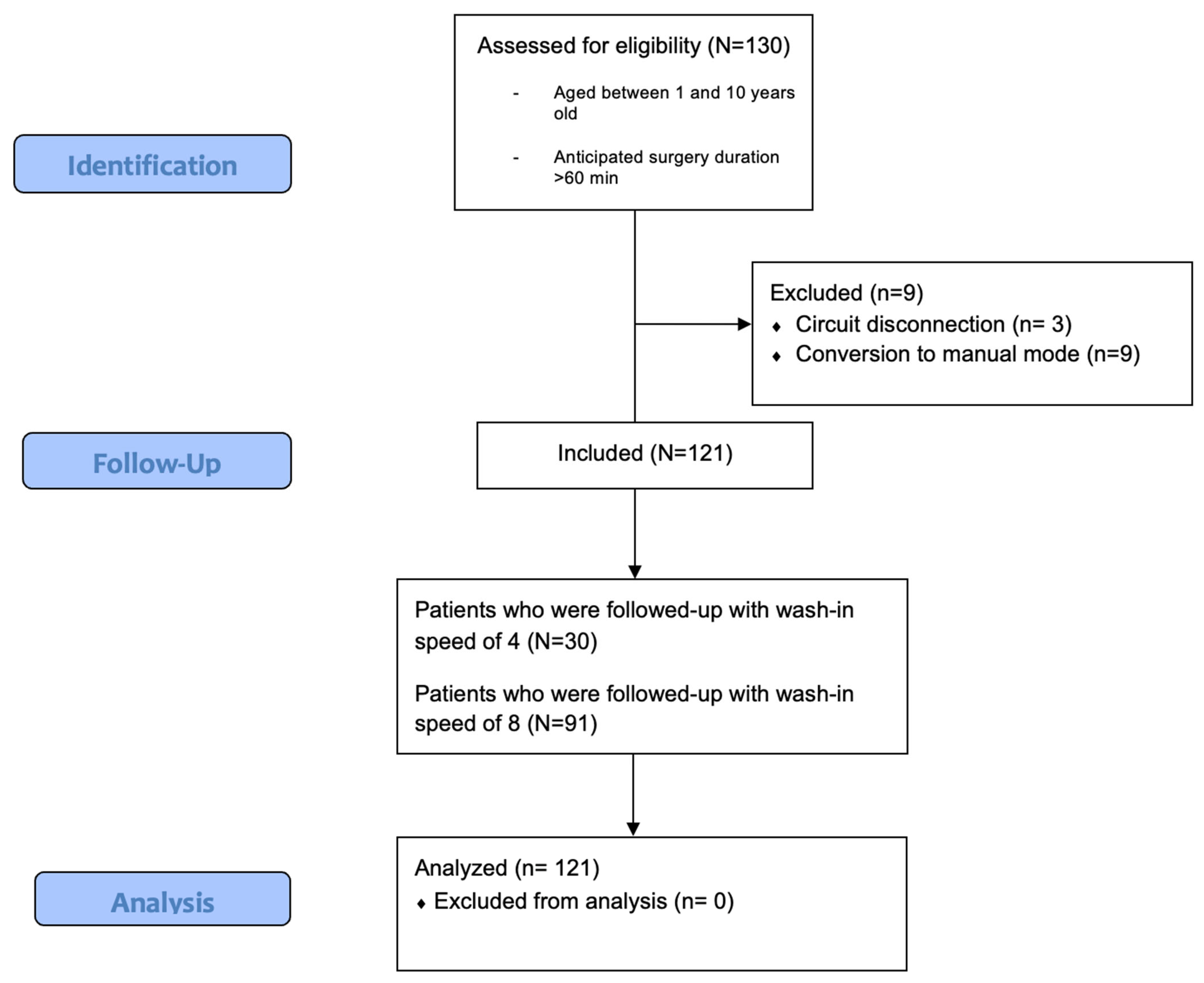
2.1. Study Population and Regulatory Aspects
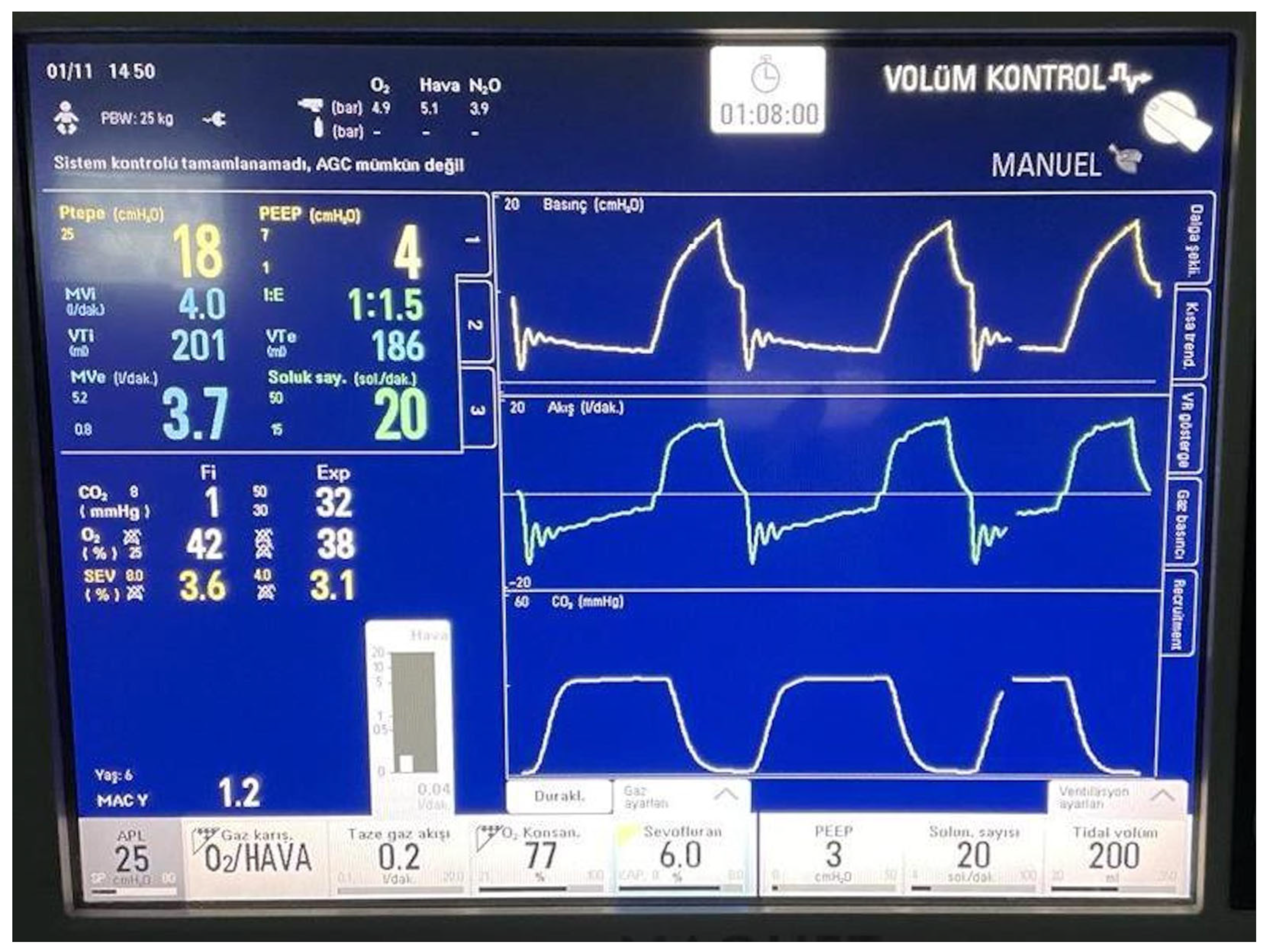
2.2. Anesthetic Management
2.3. Outcome Measurement
2.4. Statistical Analysis
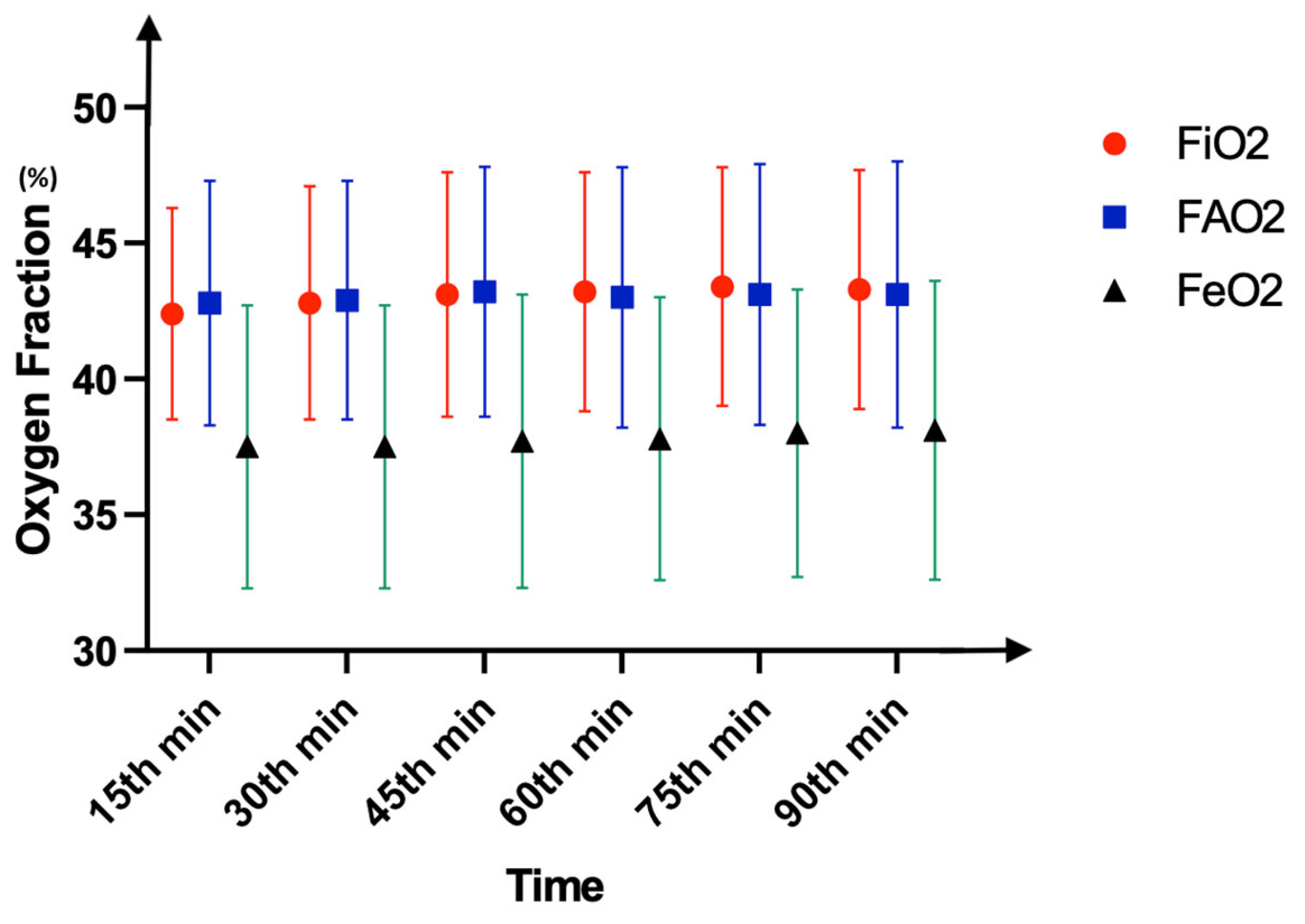
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGC® | Automated gas control |
| EtCO2 | End-tidal carbon dioxide |
| FAO2 | Alveolar fraction of oxygen |
| FEO2 | Expiratory fraction of oxygen |
| FiO2 | Inspiratory fraction of oxygen |
| FASevo | Alveolar fraction of sevoflurane |
| FiSevo | Inspiratory fraction of sevoflurane |
| FGF | Fresh gas flow |
| IV | Intravenous |
| LFA | Low-flow anesthesia |
| MAC | Minimum alveolar concentration |
| PEEP | Positive end-expiratory pressure |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| WI4 | Wash-in speed of four |
| WI8 | Wash-in speed of eight |
References
- Honemann, C.; Hagemann, O.; Doll, D. Inhalational anaesthesia with low fresh gas flow. Indian J. Anaesth. 2013, 57, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.R.; Hendrickx, J.F.; Feldman, J.M. There are no dragons: Low-flow anaesthesia with sevoflurane is safe. Anaesth. Intensive Care 2019, 47, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Glenski, T.A.; Levine, L. The implementation of low-flow anesthesia at a tertiary pediatric center: A quality improvement initiative. Paediatr. Anaesth. 2020, 30, 1139–1145. [Google Scholar] [CrossRef]
- Carette, R.; De Wolf, A.M.; Hendrickx, J.F. Automated gas control with the Maquet FLOW-i. J. Clin. Monit. Comput. 2016, 30, 341–346. [Google Scholar] [CrossRef]
- Avidan, M.S.; Jacobsohn, E.; Glick, D.; Burnside, B.A.; Zhang, L.; Villafranca, A.; Karl, L.; Kamal, S.; Torres, B.; O’Connor, M.; et al. Prevention of intraoperative awareness in a high-risk surgical population. N. Engl. J. Med. 2011, 365, 591–600. [Google Scholar] [CrossRef]
- Egbuta, C.; Evans, F. Extubation of children in the operating theatre. BJA Educ. 2022, 22, 75–81. [Google Scholar] [CrossRef]
- Brattwall, M.; Warren-Stomberg, M.; Hesselvik, F.; Jakobsson, J. Brief review: Theory and practice of minimal fresh gas flow anesthesia. Can. J. Anaesth. 2012, 59, 785–797. [Google Scholar] [CrossRef]
- Ekbom, K.; Assareh, H.; Anderson, R.E.; Jakobsson, J.G. The effects of fresh gas flow on the amount of sevoflurane vaporized during 1 minimum alveolar concentration anaesthesia for day surgery: A clinical study. Acta Anaesthesiol. Scand. 2007, 51, 290–293. [Google Scholar] [CrossRef]
- Simsek, T.; Derman, S.; Kordi, R.G.M.; Saracoglu, A.; Saracoglu, K.T. The effect of different flow levels and concentrations of sevoflurane during the wash-in phase on volatile agent consumption: A randomized controlled trial. J. Clin. Monit. Comput. 2022, 36, 1257–1262. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Stewart, L.J. Fresh gas flow is not the only determinant of volatile agent consumption: A multi-centre study of low-flow anaesthesia. Br. J. Anaesth. 2002, 88, 46–55. [Google Scholar] [CrossRef]
- Ryu, H.G.; Lee, J.H.; Lee, K.K.; Gil, N.S.; Kim, C.S.; Sim, S.E.; Lee, S.C.; Min, S.-W. The effect of low fresh gas flow rate on sevoflurane consumption. Korean J. Anesthesiol. 2011, 60, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sinha, R.; Aravindan, A.; Kumar, K.R.; Datta, P.K. Comparison of low-fresh gas flow technique to standard technique of sevoflurane induction in children-A randomized controlled trial. Paediatr. Anaesth. 2019, 29, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.; Weinberg, L.; Peyton, P.; Story, D.; Briedis, J. Financial and environmental costs of manual versus automated control of end-tidal gas concentrations. Anaesth. Intensive Care 2013, 41, 95–101. [Google Scholar] [CrossRef]
- Singaravelu, S.; Barclay, P. Automated control of end-tidal inhalation anaesthetic concentration using the GE Aisys Carestation. Br. J. Anaesth. 2013, 110, 561–566. [Google Scholar] [CrossRef]
- Kalmar, A.F.; Van Der Vekens, N.; De Rydt, F.; Allaert, S.; Van De Velde, M.; Mulier, J. Minimizing sevoflurane wastage by sensible use of automated gas control technology in the flow-i workstation: An economic and ecological assessment. J. Clin. Monit. Comput. 2022, 36, 1601–1610. [Google Scholar] [CrossRef]
- De Medts, R.; Carette, R.; De Wolf, A.M.; Hendrickx, J.F.A. Desflurane usage during anesthesia with and without N(2)O using FLOW-i Automatic Gas Control with three different wash-in speeds. J. Clin. Monit. Comput. 2018, 32, 763–769. [Google Scholar] [CrossRef]
- Stevanovic, P.D.; Petrova, G.; Miljkovic, B.; Scepanovic, R.; Perunovic, R.; Stojanovic, D.; Dobrasinovic, J. Low fresh gas flow balanced anesthesia versus target controlled intravenous infusion anesthesia in laparoscopic cholecystectomy: A cost-minimization analysis. Clin. Ther. 2008, 30, 1714–1725. [Google Scholar] [CrossRef]
- Kim, J.; Kang, D.; Lee, H.; Ryu, S.; Ryu, S.; Kim, D. Change of inspired oxygen concentration in low flow anesthesia. Anesth. Pain Med. 2020, 15, 434–440. [Google Scholar] [CrossRef]
- Upadya, M.; Saneesh, P.J. Low-flow anaesthesia—Underused mode towards “sustainable anaesthesia”. Indian J. Anaesth. 2018, 62, 166–172. [Google Scholar] [CrossRef]
- Datta, P.K.; Sinha, R.; Ray, B.R.; Jambunathan, V.; Kundu, R. Anesthesia maintenance with ‘induction dose only’ sevoflurane during pediatric ophthalmic examination: Comparison with standard low-flow technique through a randomized controlled trial. Paediatr. Anaesth. 2017, 27, 162–169. [Google Scholar] [CrossRef]
- Kennedy, R.R.; French, R.A. A ten-year audit of fresh gas flows in a New Zealand hospital: The influence of the introduction of automated agent delivery and comparisons with other hospitals. Anaesth. Intensive Care 2014, 42, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Varughese, S.; Ahmed, R. Environmental and Occupational Considerations of Anesthesia: A Narrative Review and Update. Anesth. Analg. 2021, 133, 826–835. [Google Scholar] [CrossRef]


| Total (N = 121) | |||
|---|---|---|---|
| Age (months) | 60 (30–120) | ||
| Weight (kg) | 24.7 ± 16.1 | ||
| Female/Male (n(%)) | 34 (28)/87 (72) | ||
| Surgery Type (n) | |||
| Orchiopexy ± hernia repair | 35 | ||
| Bilateral inguinal hernia repair | 14 | ||
| Pyeloplasty | 10 | ||
| Hypospadias repair | 31 | ||
| Major oncologic surgeries | 10 | ||
| Laparoscopic appendectomy | 14 | ||
| Cyst hydatic extirpation | 4 | ||
| Intussusception repair | 3 | ||
| Surgery duration (min) | 84 (81–101) | ||
| WI4 (N = 30) | WI8 (N = 91) | p * | |
| Age (months) | 60 (30–110) | 63 (28–120) | 0.94 |
| Weight (kg) | 27.1 ± 15.4 | 23.8 ± 16.3 | 0.34 |
| Female/Male (n (%)) | 12 (40)/18 (60) | 22 (24)/69 (76) | 0.18 |
| Surgery Type (n) | 0.14 | ||
| Orchiopexy ± hernia repair | 7 | 28 | |
| Bilateral inguinal hernia repair | 3 | 11 | |
| Pyeloplasty | 2 | 8 | |
| Hypospadias repair | 10 | 21 | |
| Major oncologic surgeries | 2 | 8 | |
| Laparoscopic appendectomy | 4 | 10 | |
| Cyst hydatic extirpation | 1 | 3 | |
| Intussusception repair | 1 | 2 | |
| Surgery duration (min) | 82 (81–85) | 84 (81–110) | 0.13 |
| WI4 (N = 30) | WI8 (N = 91) | Total (N = 121) | p * | |
|---|---|---|---|---|
| Duration of anesthesia (min) | 99 (96–103) | 101 (95–122) | 100 (95–113) | 0.11 |
| Target FeSevo (%) | 2.20 ± 0.30 | 2.53 ± 0.51 | 2.30 ± 0.39 | 0.06 |
| Time to reach targeted FeSevo (min) | 10 (8–12) | 2 (2–2) | 2 (2–4) | <0.001 |
| Total sevoflurane consumption (mL) | 7.79 ± 4.19 | 9.92 ± 5.08 | 9.35 ± 4.93 | 0.04 |
| Volatile closure to extubation (min) | 11 (5–15) | 8 (5–10) | 10 (5–12) | 0.03 |
| WI4 (n = 16) | WI8 (N = 39) | Total (N = 55) | ||
| Volatile closure to obey commands (min) | 13 (9–17) | 9.5 (5–10.5) | 10 (5–14) | <0.01 |
| Heart Rate (beat/min) | Induction | 15 min | 30 min | 45 min | 60 min | 75 min | 90 min | End of Surgery |
|---|---|---|---|---|---|---|---|---|
| WI4 | 121.5 (110–130) | 115 (100–128) | 109 (91–127.5) | 99.5 (90–113) | 98.5 (87–116) | 93 (77–106) | 92.5 (78–105) | 93 (90–108) |
| WI8 | 112 (106–131) | 102 (99–121) | 104 (98–118) | 101 (93–109) | 100.5 (95–110) | 102 (99–109) | 100 (98–108) | 101 (91–108) |
| p * | 0.7 | 0.4 | 0.9 | 0.7 | 0.8 | 0.1 | 0.2 | 0.8 |
| Mean Arterial Pressure (mmHg) | ||||||||
| WI4 | 78 (74–84) | 71 (65–75) | 65 (62.5–69) | 66 (62–70) | 67 (64–69) | 64.5 (62–68.5) | 61 (62–67) | 61 (59–62) |
| WI8 | 77 (70–82) | 70 (66–79) | 70 (63–73.5) | 68 (62.5–70) | 63.5 (61–66.5) | 64.5 (60–67) | 63 (62–65) | 61 (60–64) |
| p * | 0.5 | 0.7 | 0.2 | 0.5 | 0.1 | 0.6 | 0.4 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingül, E.S.; Savran Karadeniz, M.; Şentürk, E.; Vuran Yaz, İ.; Atasever, A.G.; Orhan Sungur, M. Feasibility and Safety Properties of Metabolic-Flow Anesthesia Driven by Automated Gas Control® in Pediatric Patients: A Prospective Observational Study. Medicina 2025, 61, 786. https://doi.org/10.3390/medicina61050786
Bingül ES, Savran Karadeniz M, Şentürk E, Vuran Yaz İ, Atasever AG, Orhan Sungur M. Feasibility and Safety Properties of Metabolic-Flow Anesthesia Driven by Automated Gas Control® in Pediatric Patients: A Prospective Observational Study. Medicina. 2025; 61(5):786. https://doi.org/10.3390/medicina61050786
Chicago/Turabian StyleBingül, Emre Sertaç, Meltem Savran Karadeniz, Emre Şentürk, İrem Vuran Yaz, Ayşe Gülşah Atasever, and Mukadder Orhan Sungur. 2025. "Feasibility and Safety Properties of Metabolic-Flow Anesthesia Driven by Automated Gas Control® in Pediatric Patients: A Prospective Observational Study" Medicina 61, no. 5: 786. https://doi.org/10.3390/medicina61050786
APA StyleBingül, E. S., Savran Karadeniz, M., Şentürk, E., Vuran Yaz, İ., Atasever, A. G., & Orhan Sungur, M. (2025). Feasibility and Safety Properties of Metabolic-Flow Anesthesia Driven by Automated Gas Control® in Pediatric Patients: A Prospective Observational Study. Medicina, 61(5), 786. https://doi.org/10.3390/medicina61050786






