Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products
Abstract
:1. Introduction
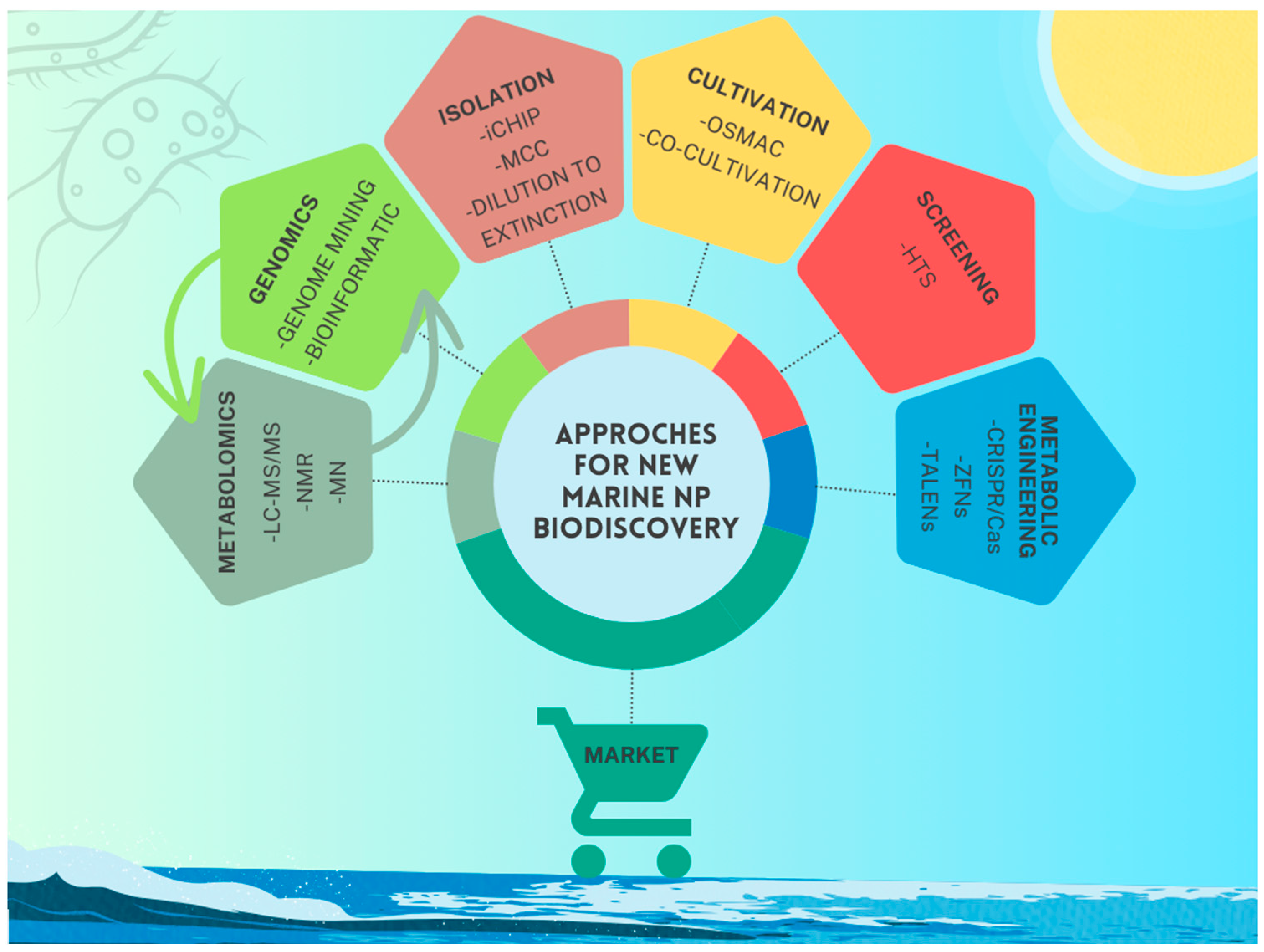
2. Microbial Isolation and Innovative Cultivation Strategies
2.1. New Techniques to Overcome the Problem of Uncultivability in Marine Microorganisms: A Special Focus on Marine Bacteria
2.2. Microbial Cultivation Approaches to Harness Untapped Biosynthetic Potential
| Microorganism | Origin | Place | Class | Compounds | Innovative Strategies | Bioactivity | Assay | Activity Value | References |
|---|---|---|---|---|---|---|---|---|---|
| BACTERIA | |||||||||
| Alteromonas sp. RKMC-009 | Sponge (Xestospongia muta) | San Salvador, The Bahamas | N-acyltyrosine | N-palmitoyl-α,O-dimethyl-l-tyrosine | iChip | Antibacterial | Microdilution broth | IC50 < 8 μM vs. Enterococcus strains | [87] |
| Gallaecimonas mangrovi HK-28 | Mangrove sediment | Hainan province, China | Diketopiperazines | Gallaecimonamides A–C | iChip | Antibacterial (Gallaecimonamides B) | Microdilution broth | MIC 50 μM vs. V. harveyi | [42] |
| Mooreia alkaloidigena CNX-216T Catalinimonas alkaloidigena CNU-914T | Marine sediment | Palmyra Atoll, USA | Alkaloids | Marinoazepinone A–B; Marinoaziridine A–B; Marinoquinolines G–I, Marinopyrazinones A | Low-nutrient media and extended incubation times | Antibacterial | Agar diffusion disk | Inhibiton diameter ranging from 9 to 18 mm vs. Pontibacillus sp. | [48] |
| Marinopyrazinones B | 14 mm of inhibiton diameter vs. Pontibacillus sp., 9 mm vs. Vibrio shiloi | ||||||||
| Nocardiopsis sp. SCA21 | Marine sediment | Havelock Andaman Nicobar Islands | Not reported | 4-bromophenol (1), Bis(2-ethylhexyl) phthalate (2) | Addition of inhibitors into the media | Antibacterial, antioxidant, and metal chelating activity | Microdilution broth, DPPH, ABTS Ferrozine assay | MIC ranging from 7.81 to 250 μg/mL vs. Bacillus subtilis ATCC 6633, Klebsiella pneumoniae ATCC 13883, Listeria cytogens ATCC 13932, Salmonella typhi ATCC 25241, Staphylococcus aureus ATCC 12600, MRSA ATCC NR-46071, and MRSA ATCC NR-46171; DPPH IC50 values 187.31 μg/mL (1), 96.57 μg/mL (2); ABTS 102.22 μg/mL (1), 151.60 μg/mL (2) Ferrozine assay 19.41% at 31.25 μg/mL and 62.89% at 1 mg/mL (1) 56.10% at 1 mg/mL (2) | [61] |
| Saccharothrix xinjiangensis Act24Zk | Marine sediment | Caspian Sea, Iran | PKS | caerulomycin M; saccharopyrone; saccharonic acid | Isolation method for myxobacteria | Antiproliferative and antibacterial | MTT and microdilution broth | MTT IC50 ranging from 0.1 to 50.7 µM vs. L920 and KB3.1 MIC ranging from 4.2 to 66.7 µg/mL vs. Staphylococcus aureus Newman, Micrococcus luteus DSM1790, Escherichia coli DSM1116, Mucor hiemalis DSM2656, and Candida albicans DSM1665 | [62] |
| Streptomyces pratensis NA-zhous1 | Marine sediment | Zhoushan Coast, China | Angucycline (PKS) | Stremycin A–B | OSMAC | Antibacterial | Microdilution broth | MIC 16 µg/mL vs. MRSA, K. pneumoniae, E. coli (Stremycin A-B) and vs. B. subtilis (8 µg/mL Stremycin A and 16 µg/mL Stremycin B) | [88] |
| Janthinobacterium spp. ZZ145 Janthinobacterium spp. ZZ148 | Marine sediment | Sindh, Karachi, Pakistan | PKS | Janthinopolyenemycins A–B | Co-cultivation | Antifungal | Microdilution broth | MIC 15.6 μg/mL vs. C. albicans | [89] |
| Verrucosispora sp. MS100137 | Deep-sea sediment | South China Sea | PKS | Abyssomicin Y | OSMAC | Antiviral | Cell viability | 98% of influenza A virus inhibition at 10 μM | [59] |
| Saccharothrix sp. D09 | Intertidal zone sediment | Fushan Bay, Qingdao, China | PKS | Saccharotrins D–I | OSMAC | Antibacterial (F–G); antioxidant (F) | Microdilution broth | MIC 32 μg/mL vs. H. pylori 159; IC50 28 μM | [64] |
| Glycosylated saccharotrixines J–M | Antibacterial (K) | MIC 16 μg/mL vs. H. pylori G27, H. pylori 159, and S. aureus ATCC25923 | |||||||
| Streptomyces sp. GET02.ST Achromobacter sp. GET02.AC | Marine Wharf Roach (Ligia exotica) | Seocheon, Chungcheongnam-do, Korea | PKS-NRPS | Ligiamycins A–B | Co-cultivation | Antibacterial | Microdilution broth | (A) MIC 16 μg/mL vs. S. aureus ATCC25923 and S. enterica ATCC14028 (B) MIC 64 μg/mL vs. S. aureus ATCC25923 | [90] |
| Streptomyces griseorubiginosus | Cnidarian | Réunion Island, France | Aromatic PKS | Five new aromatic PKS (19–23) differing in novel anthrone backbones, a naphtho[2,3-c]furan-4(9H)-one chromophore group and novel tetralone structure | OSMAC | Antibacterial; cytotoxic | Not reported | No activity | [91] |
| Microbacterium sp. V1 | Marine sponge (Petrosia ficiformis) | Ischia Island, Italy | Peptide | Two proline-rich peptides | OSMAC | Antioxidant | FRAP | FRAP from 51 to 121 μM | [92] |
| FUNGI | |||||||||
| Thrichoderma sp. TPU199 | Red alga | Palau | Diketopiperazine | Chlorotrithiobrevamide | OSMAC | Antiproliferative | MTT | IC50 16 μM vs. T-cell leukemia Jurkat cells | [93] |
| Aspergillus sclerotiorum SCSGAF 0053 Penicillium citrinum SCSGAF 0052 | Gorgonian (Muricella flexuosa) | Hainan, South China Sea | Furanone and oxadiazin derivatives | Sclerotiorumins A–C 4-benzyl-1H-pyrrol-3-yl Aluminiumneohydroxyaspergillin Ferrineohydroxyaspergillin | Co-cultivation | Cytotoxic (Aluminiumneohydroxyaspergillin) | MTT | IC50 4.2 μM vs. U937 | [94] |
| Aspergillus carneus | Sponge (Agelas oroides,) | Aegean Sea coast, Turkey | Quinazolinone and anthraquinone derivatives | Isopropylchaetominine, Isoterrelumamide A, 5′-Epi-Averufanin | OSMAC | Cytotoxic (Isopropylchaetominine) | MTT | IC50 0.4 μM vs. L5178Y | [95] |
| Antibacterial (5′-Epi-Averufanin) | Microdilution broth | MIC 4.63 μg/mL vs. S. aureus ATCC 700699 and 9.3 μg/mL vs. E. faecium ATCC 35667 | |||||||
| Asteromyces cruciatus | Sea foam | Point Prim, Prince Edward Island, Canada | PKS | Primarolides A–B | OSMAC | Antimicrobial | Microdilution broth | No activity | [96] |
| Fusarium equiseti D39 | Plant | Intertidal zone of the Yellow Sea, Qingdao, China | 3-decalinoyltetramic acid derivatives | Fusarisetins B–D | OSMAC | Phytotoxicities | Phytotoxicity | 200 μg/mL vs. growth of amranth | [97] |
| Penicillium restrictum MMS417. | Blue mussel (Mytilus edulis) | Loire estuary, France | Pyranone derivatives | Five pyranone derivatives | OSMAC | Antimicrobial | Paper disk diffusion | No activity | [98] |
| Cytotoxic | MTT | Not cytotoxic Vs. KB at 50 μg/mL | |||||||
| Antileishmanial activity | Luminescence | No activity | |||||||
| Aspergillus sp. SCSIO 41501 | Gorgonian (Melitodes squamata) | Hainan, South China Sea | Cyclopentenone and cyclohexenones derivatives | Aspergispones A–H | OSMAC | Not reported | Not reported | No activity | [99] |
| Penicillium sp. HLLG-122 | Root of mangrove (Lumnitzera littorea) | Sanya, Hainan Island, China | Meroterpenoids | Peniciacetals A–I | OSMAC | Cytotoxic | Celltiter-glo Kit | (E,F) IC50 < 25 μM vs. HepG2 (B,E,F) IC50 < 26 μM vs. MCF-7 | [100] |
| Phomopsis sp. QYM-13 | Leaves of mangroove (Kandelia candel) | Hainan Province, South China Sea | Cytochalasins | Phomopchalasins D–O | OSMAC | Cytotoxic | MTT | IC50 < 50 μM vs. MDA-MB | [101] |
| Alternaria alternata LW37 | Deep-sea sediment | Southwest Indian Ridge | Dibenzo-α-pyrone derivatives | Alternolides A–C | OSMAC | α-Glucosidase inhibition (C) | α-Glucosidase Inhibition | IC50 of 451.25 μM | [102] |
| Beauveria felina KMM4639 Aspergillus carneus KMM4638 | Brown alga (Laminaria sachalinensis) | Kunashir Island, Russia | Sesquiterpenes | Asperflavinoids B, D, and E | Co-cultivation | Cytotoxic | MTT | IC50 ranging from 51 to 95 μM vs. PC-3, MCF-7, Raji cells, H9c2 | [103] |
| Aspergillus versicolor PS108-62 | Deep-sea sediment | Farm Strait, Arctic Ocean | PKS-NRPS Macrolactone | Versicolide A, isoversicomide A | OSMAC | Not reported | Not reported | Not reported | [104] |
| Phomopsis lithocarpus FS508 | Not reported | Not reported | Benzoic Acid derivative | 4-methoxy-3-[4-(acetyloxy)-3-methyl-2-butenyl]benzoic acid | OSMAC | Not reported | Not reported | Not reported | [105] |
| Emericellopsis maritima BC17 | Marine sediment | Bay of Cádiz, Spain | Eremophilanes | Four new eremophilane-type sesquiterpenes | OSMAC | Antimicrobial antiproliferative | Microdilution broth MTT | Not detected | [106] |
| Cosmospora sp. Magnaporthe oryzae | Windebyer Noor | German Baltic coast | Isochromanones | Soudanones H–I | Co-cultivation | Not reported | Not reported | Not reported | [107] |
| Aspergillus fumigatus KMM 4631 Penicilliumhispanicum KMM 4689 | Coral (Sinularia sp.) | Kunashir Island, north west Pacific Ocean | Not identified | Two unidentified novels compounds | Co-cultivation | Antioxidant cytotoxic urease inhibition | DPPH∙ MTT indophenol method |
53% of scavenging effect and 9% of urease inhibition at 100 µg/mL; 10% of viability reduction at 10 µg/mL vs. HepG2 | [108] |
| Amphichorda KMM4639 Aspergillus carneus KMM4638 | Brown alga (Laminaria sachalinensis) | Kunashir Island, north west Pacific Ocean | Quinazolinone alkaloids Chromene derivative | Felicarnezolines A–E Oxirapentyn M | Co-cultivation | Cytoprotective against CoCl2 (Felicarnezoline B) | MTT | 72.6% and 19% increased viability of SH-SY5Y and H9c2, respective ely | [109] |
| Aspergillus insulicola IMB18-072 Alternaria angustiovoidea IMB20-805 | Unidentified marine sponge | Weizhou Island, China | Cyclic tetrapeptides | Violaceotides B–E | Co-cultivation | Antimicrobial | Microdilution broth | (B–C) MIC values of 64–128 μg/mL vs. aquatic pathogens Edwardsiella tarda ATCC15947, E. tarda QDIO-2, and E. ictaluri ATCC33202 | [110] |
| Phomopsis asparagi DHS-48 Phomopsis sp. DHS-11 | Root of mangrove (Rhizophora mangle) | Dong Zhai Gang-Mangrove Garden on Hainan Island, China | Alkaloids, sterols, polyketides | Phomopyrazine, phomosterol C, phomopyrone E | Co-cultivation | Cytotoxic | MTT | IC50 value 65.97 μM vs. HepG2 and 72.02 vs. Hela | [111] |
| Immunosuppressive | Splenocyte proliferation assay | (Phomopyrazine) IC50 125.1 and 133.87 μM against the proliferation of ConA-induced (T-cell) and LPS-induced (B-Cell), respectively | |||||||
| Acetylcholinesterase (AchE) inhibitory activities | No activity | ||||||||
| Phomopsis asparagi DHS-48 Phomopsis sp. DHS-11 | Root of mangrove (Rhizophora mangle) | Dong Zhai Gang-Mangrove Garden on Hainan Island, China | Dimeric xanthones | Phomoxanthones L–N | Co-cultivation | Cytotoxic | MTT | (L-N) IC50 values 53.72, 50.25 and 67.32 μM vs. HepG2 (L-N) IC50 values 69.53, 67.66 and 87.32 vs. Hela | [112] |
| Immunosuppressive | Splenocyte proliferation assay | (L-N) IC50 55.53, 60.25, and 75.75 μM against the proliferation of ConA-induced (T-cell) (L-N) IC50 89.27, 87.66 and 102.65 against LPS-induced (B-Cell) | |||||||
| Aspergillus alabamensis SYSU-6778 Aspergillus fumigatiaffinis SYSU-6786 | Roots of seagrass (E. acoroides) | Hainan Island, the People’s Republic of China | Chromone, benzoic acid derivative | (10) (2R, 3R)-5,7-dihydroxy-2,3-dimethyl-4-chromanone, (13) 5-hydroxy-3,4-dimethoxy-2-methylbenzoic acid | Co-cultivation | Antifungal | Microdilution broth | (10, 13) MIC 100 μg/mL and >200 μg/mL against A. alabamensis SYSU-6778 | [113] |
| Eutypella sp. D-1 | Marine sediment | London Island of Kongsfjorden in the Ny-Ålesund, Arctic Ocean | Cytosporin polyketides | eutypelleudesmane A, cytosporin Y, cytosporin Z, cytosporin Y1, cytosporin Y2, cytosporin Y3, and cytosporin E1 | OSMAC | Antimicrobial | Microdilution broth | MIC > 128 μg/mL vs. S. aureus, E. coli, B. subtilis | [114] |
| Cytotoxic | CCK-8 | IC50> 50 μM vs. DU145, SW1990, Huh7, and PANC-1 | |||||||
| Immunosuppressive | calcineurin (CN) phosphatase assays | (Z,Y3) 62.9% and 59.5% against ConA-induced T cell proliferation, respectively, at a concentration of 5 μg/mL | |||||||
| Penicillium velutinum ZK-14 | Seagrass (Zostera marina) | Sea of Japan, Japan | 2,5-dibenzylpiperazine | Helvamides B,C | OSMAC | Cytotoxic | MTT | (B) 34.03% and 16.64% (C) 39.41% and 28.42% decreased viability of PC-3 and HEK-293, respectively | [115] |
| Antifungal | Microdilution broth | (B) 7% and (C) 31,54% of inhibition of C albicans | |||||||
| Antioxidant | DPPH | 4.92% and 4.5%, respectively | |||||||
| Hamigera ingelheimensis MSC5 | Anemone | Mariana Trench, Pacific Ocean | NRPS | Avellanins D–O | OSMAC | Antimalarial activity | Not reported | (D, E, F, H, I) IC50 values of >50 μM, 16 μM, 10 μM and 0.19 μM vs. P. falciparum 3D7, respectively. | [116] |
| Cytotoxicity | MTT | No activity vs. A549, MKN-45, HCT-116, HeLa, K562, 786-O, TE-1, 5637, GBC-SD, MCF-7, HepG2, SF126, DU145, GAL-62, PATU8988T, HOS, A-375, A-673, L-02, and 293T | |||||||

2.2.1. One Strain-MAny Compounds (OSMAC) in Marine Microbial Drug Discovery
2.2.2. Co-Cultivation in Marine Microbial Drug Discovery
3. Drug Discovery in the Metabologenomics Era: Recent Advances and Perspectives
3.1. Metabologenomics: Basic Principles and Tools
| Genome Database | URLs |
|---|---|
| GenBank/NCBI | https://www.ncbi.nlm.nih.gov/ |
| RefSeq | https://www.ncbi.nlm.nih.gov/refseq/ |
| In-house genome database | https://ngdc.cncb.ac.cn/gwh/ |
| BGC identification | |
| BLAST | https://blast.ncbi.nlm.nih.gov |
| antiSMASH | https://antismash.secondarymetabolites.org |
| FASTA | https://www.ebi.ac.uk/Tools/sss/fasta/ |
| RIPper | https://github.com/TheRIPper-Fungi/TheRIPper |
| PRISM | https://prism.adapsyn.com/about |
| MetaBGC | https://github.com/donia-lab/MetaBGC |
| RODEO | https://bio.tools/rodeo |
| pHMMs | https://arxiv.org/abs/2207.09765 |
| BGC database | |
| MiBIG | https://mibig.secondarymetabolites.org |
| IMG-ABC | https://img.jgi.doe.gov/cgi-bin/abc/main.cgi |
| BIG-FAM | https://bigfam.bioinformatics.nl/home |
| antiSMASH-DB | https://antismashdb.secondarymetabolites.org |
| ARTS-DB | https://arts-db.ziemertlab.com/ |
| BGC analysis | |
| Big-SCAPE | https://github.com/medema-group/BiG-SCAPE |
| BLAST | https://blast.ncbi.nlm.nih.gov |
| EFI-EST | https://efi.igb.illinois.edu/efi-est/ |
| ARTS | https://arts.ziemertlab.com |
| EvoMining | https://github.com/nselem/EvoMining/wiki |
| FastTree | https://bio.tools/fasttree#! |
| MS Data Pre-Processing | URLs | Access Type |
|---|---|---|
| MzMine | https://mzmine.github.io/ | Open Access |
| MN generation | ||
| GNPS | https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp | Open Access |
| MetGem | https://metgem.github.io/ | Open Access |
| MN visualization | ||
| Cytoscape | https://cytoscape.org/ | Open Access |
| Natural Product database | ||
| GNPS | https://gnps.ucsd.edu/ProteoSAFe/static/gnps-splash.jsp | Open Access |
| Dictionary of Natural Products | https://dnp.chemnetbase.com/chemical/ChemicalSearch.xhtml?dswid=9675 | Paid Access |
| Natural Product Atlas | https://www.npatlas.org/ | Open Access |
| COCONUT | https://coconut.naturalproducts.net/ | Open Access |
| MarinLit | https://marinlit.rsc.org/ | Paid Access |
| Metlin | https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage | Partially Paid |
3.2. Marine Natural Products Discovered Through Metabologenomics
| Microorganism | Origin | Place | Class | Compound | Bioactivity | Test | Activity Value | References |
|---|---|---|---|---|---|---|---|---|
| BACTERIA | ||||||||
| Saccharomonospora sp. CNQ-490 | Marine sediment | Submarine Canyon, California, USA | NRPS | Taromycin A | Antibacterial | Microdilution broth | MIC ranging from 12.5 to 50.0 μg/mL vs. different strains of methicillin-resistant S. aureus (MRSA) and vancomycin-resistant E. faecium (VRE) | [192] |
| Salinispora arenicola CNS-205 | Deep-sea sediments | Palau | Terpenoids/isoprenoids | Isopimara-8,15-diene-19-ol | Not reported | Not reported | Not reported | [193] |
| Vibrio harveyi | Sponge (Tectitethya crypta) | Long Key/Big Pine, Florida, USA | Nucleoside | Spongosine | Anti-inflammatory | NO production inhibition | 3 µg/mL | [194] |
| Analgesic vasodilation | Not reported | Not reported | ||||||
| Salinispora arenicola CNT-005 | Anellida | Fiji | NRPS | Retimycin A | Antiproliferative | Not reported | IC50 <0.076 μg/mL on HCT116 | [195] |
| Salinispora tropica CNB-440 | Marine sediment | Bahamas | Terpenoids/isoprenoids | Sioxanthin | Not reported | Not reported | Not reported | [196] |
| Vibrio sp. QWI-06 | Marine sediment | Pingtung County, Taiwan | PKS | Vitroprocines A–J | Antibacterial (Vitroprocines A2, B1, B4) | Microdilution broth | MIC ≤ 8, 32, and 16 μg/mL against A. baumannii ATCC 19606L | [183] |
| Salinispora pacifica CNS-237 | Deep-sea sediments | Palau | PKS type I |
18-dihydro-14-hydroxyrosamicin, 18-dihydro-21-hydroxyrosamicin, 14-hydroxyrosamicin | Antibacterial | Not reported | MIC 50–100 μg/mL vs. A. baumannii ATCC17978 and E. coli CFT073 MIC 25–100 μg/mL vs. P. aeruginosa PA01 MIC 6.125–100 μg/mL vs. S. pyogenes NZ131 1.56–25 μg/mL vs. S. aureus USA300 | [197,198] |
| Pseudoalteromonas luteoviolacea Strain 2ta16 | Coral (M. annularis) | Florida Keys, Florida, USA | NRPS-PKS hybrid | Thiomarinol | Not reported | Not reported | Not reported | [182] |
| Pseudoalteromonas luteoviolacea NCIMB1944 | Surface water | Nice, France, Mediterranean | ||||||
| Streptomyces sp. JAMM992 | Deep-sea sediment | Kinko Bay, Kagoshima, Japan | NRPS | Surugamide F | Antiproteolytic | Cathepsin B inhibition | No activity | [199] |
| Streptosporangium sp. CGMCC 4.7309 | Marine sediment | Lijiao Bay, Huanghai Sea, China | PKS Type II | Hexaricins A–H |
Antioxidant (A,C–H) | DPPH∙ Scavenging | EC50 < 45 μM (E-F < 3 μM) | [200,201] |
| OH− Scavenging | EC50 < 1530 μM (E-F < 620 μM) | |||||||
| O2− Scavenging | EC50 < 1110 μM (E-F < 140 μM) | |||||||
| Streptomyces pactum SCSIO 02999 | Deep-sea sediment | South China Sea | PKS–NRPS hybrid | Totopotensamide TPMC | Antibacterial | Microdilution broth | No activity | [202] |
| Streptomyces sp. MP131-18 | Deep-sea sediment | Trondheim fjord, Norway | PKS | Lagunapyrone D and E | Antibacterial | Disk diffusion | No activity | [203] |
| Marinactinospora thermotolerans SCSIO 00652 | Deep-sea sediment | South China Sea | RiPPs | Mathermycin | Antibacterial | Agar diffusion | 1 μM vs. B. subtilis LH45 | [204] |
| Streptomyces sp. PKU-MA00045 |
Sponge (Sinularia sp.) | Not Reported | PKS type II | Fluostatins M–Q | Antibacterial; anti-inflammatory; cytotoxic | Not reported; NO inhibition; not reported | No activity | [205] |
| Fulvivirga sp. W222 | Marine sediment | Aoshan Bay, Qingdao, China | NIS | Fulvivirgamides A2, B2, B3, and B4 | Cytotoxic | MTT | A2, B2, B3 IC50 from 9 to 19 μM on A549, SMMC-7721 and HCT-116 | [206] |
| Streptomyces pactum SCSIO 02999 | Deep-sea sediment | South China Sea | Hybrid PKS–NRPS | Pactamides A–F | Cytotoxic | SRB | IC50 from 0.5 to 26 μM on SF-268, MCF-7, NCI-H460, HepG2 | [207] |
| Thermoactinomyces vulgaris | Coastal hydrothermal vents | Iceland | NRPS | Thermoactinoamides A–K | Antiproliferative (A) | Real-time proliferation monitoring | 5 μM on BxPC-3 | [208] |
| Antibacterial (A) | Microdilution broth | MIC 35 μM vs. S. aureus ATCC 6538 | ||||||
| Saccharothrix sp. D09 | Intertidal zone sediment | Fushan Bay, Qingdao, China | NRPS | Saccharochelins A–H | Cytotoxic | MTT | IC50 from 1 to 20 μM on A549, MCF-7, HepG2, and HCT-116 | [64] |
| Lacinutrix shetlandiensis sp. nov. WUR7 | Deep-sea sediment | South Shetland Trough, Antarctica | Tryptophan decarboxylase | Indole alkaloids | Antibacterial (8,9-dihydrocoscinamide B) | Microdilution broth | IC50 14.0 µg/mL vs. S. aureus and 39.1 µg/mL vs. MRSA | [92] |
| Streptomyces sp. S063 | Marine sediment | Xinghai Bay, China | NRPS | Lenziamide D and B1 | Cytotoxic | MTT | IC50 from 8 to 24 μM on HEL, H1975, H1299, A549–taxol. | [188] |
| Streptomyces sp. CNY-716 | Marine sediment | Not reported | PKS | Indanopyrrole A and B | Antibacterial | Microdilution broth | (A) MIC 4 μg/mL, 2 μg/mL, 4 μg/mL, 2 μg/mL 4 μg/mL and 1–2 μg/mL against E. coli lptD4213, MRSA TCH1516, Streptococcus M1T1, VR-Enterococcus faecium DAPS, MR-Staphylococcus epidermis and Haemophilus influenzae, respectively | [209] |
| Cytotoxic | MTT | after 24 h at 16 μg/mL vs. A549 indicating an antibiotic therapeutic index of 4–8 | ||||||
| CYANOBACTERIA | ||||||||
| Moorea bouillonii PNG | Coral (Stylophora pistillata) | Pigeon Island, Papua New Guinea | NRPS/PKS hybrid |
Columbamides A Columbamides B Columbamides C | Cannabinomimetic | Displacements | Potent ligands of cannabinoid receptors CB1 and CB2 | [185] |
| Moorea producens JHB | Shallow water | Hector’s Bay, Jamaica | NRPS | Hectoramide | Not reported | Not reported | Not reported | [210] |
| Neolyngbya sp | Intertidal zone | Bangtang Bay, Hainan, China | NRPS/PKS hybrid | Wenchangamide A Wenchangamide B | Antiproliferative | XTT | 60% of cell viability reduction at 25 µg/mL on HCT116 | [184] |
| FUNGI | ||||||||
| Engyodontium album DFFSCS021 | Marine sediment | South China Sea | PKS | Engyodontiumones A–J 2-methoxyl cordyol C | Cytotoxic; antibacterial (Engyodontiumones H) | MTT | IC50 4.9 μM on U937 | [211] |
| Microdilution broth | MIC ≤ 64 μg/mL vs. E. coli and B. subtilis | |||||||
| Aspergillus sp. 16-02-1 | Deep-sea sediment | Lau Basin hydrothermal vent, Southwest Pacific | PKS | Aspiketolactonol Aspilactonols A–F Aspyronol Epiaspinonediol | Cytotoxic (Aspyronol, Epiaspinonediol) | MTT | Aspyronol, IC50 of 241.2 μM on HL-60 Epiaspinonediol, IC50 260.6 μM on K562 and 192.9 μM on HL-60 | [212] |
| Aspergillus ochraceus Jcma1F1 | Marine alga (Coelarthrum sp.) | Paracel Islands, South China Sea | Terpenoid | 6b,9a-dihydroxy-14-p-nitrobenzoylcinnamolide (1) Insulicolide A | Antiviral | Cytopathic effect protection | IC50 17.0 μM vs. H3N2 and 9.4 μM vs. EV71 | [213] |
| Cytotoxic | CCK8 | IC50 ranging from 1.95 μM to 6.35 μM against 10 human cancer lines | ||||||
| Lindgomycetaceae KF970 Lindgomycetaceae LF327 | - | Arctic | PKS | Lindgomycin Ascosetin | Antimicrobial | Microdilution broth | IC50 ranging from 2.0 to 18.0 µM vs. B. subtilis DSM 347, X. campestris DSM 2405, S. epidermidis DSM 20044, S. aureus DSM 18827, MRSA, C. albicans DSM 1386, S. tritici, P. acnes DSM 1897 | [214] [215] |
| Sponge (Halichondria panicea) | Kiel Fjord, Baltic Sea, Germany | |||||||
| Penicillium sp. Y-50-10 | Hydrothermal vent sediment | Kueishantao, Taiwan | PKS | Methyl-isoverrucosidinol | Antimicrobial | Microdilution broth | MIC 32 μg/mL vs. B. subtilis CMCC (B) 6350 | [216] |
| Aspergillus wentii SD-310 | Deep-sea sediment | South China Sea | Terpenoid | Aspewentin A and D–M | Antimicrobial (I-M) | Microdilution broth | MIC ranging from 4 to 32 μg/mL vs. E. coli QDIO-1, A. hydrophilia QDIO-3, E. tarda QDIO-4, P. aeruginosa QDIO-6, V. anguillarum QDIO-8, V. harveyi QDIO-9, V. parahaemolyticus QDIO-10, F. graminearum QDIO-13 | [217,218,219] |
| Asperethers A–E | Cytotoxic | MTT | IC50 ranging from 10 to 48 μM on A549, HEK293, MCF-7, SMMC-7721, and T-47D | |||||
| Asperolides D and E | Antibacterial; cytotoxic (E) | Microdilution broth, MTT | MIC 16 µg/mL vs. E. tarda IC50 ranging from 10.0 to 16.0 µM on HeLa, MCF-7, and NCI-H446 | |||||
| Stachybotrys longispora FG216 | Not reported | Not reported | Hybrid PKS–NRPS | FGFC4–7 | Fibrinolytic activities (FGFC6-7) | Nitroaniline formation | 25 µg/mL | [220] |
| Penicillium granulatum MCCC 3A00475 | Deep-sea sediment | Prydz Bay, Antarctica | Terpenoid | Spirograterpene A Conidiogenone C and I | Antiallergic (Spirograterpene A) | Antiallergy assay | 18% inhibition on E (IgE)-mediated rat mast RBL-2H3 at 20 µg/mL | [221] |
| Trichoderma sp. FM652 | Marine sediment | Hanauma bay, Hawaii, USA | PKS | Sorbicillinoid 1–2 | Antiproliferative (Sorbicillinoid 1) | Inhibition of NF-κB | IC50 13.83 µM | [222] |
| Trichoderma endophyticum MMSRG85 | Ascidian (Botrylloides giganteus) | São Paulo, Brazil | PKS-NRPS hybrid | Endophytins A1–A13 Endophytins B1–B8 Hypomuricina Harzianin HC | Not reported | Not reported | Not reported | [186] |
| Beauveria felina SYSU-MS7908 | Ascidian | Xisha Islands, South China Sea | NRPS | Isaridin I-N | Antifungal (Isaridin J) | Agar dilution | EC50 50 µg/mL vs. G. citriaurantii | [223] |
| Exophiala xenobiotica SDU 039 | Deep-sea sediment | Not reported | HR-PKS and NR-PKS | Exopxenmycins A–G | Not reported | Not reported | Not reported | [224] |

4. High-Throughput Screening Approach in Marine Drug Discovery
| Microorganism | Origin | Place | Class | Compounds | Bioactivity | Assay | Activity Value | References |
|---|---|---|---|---|---|---|---|---|
| BACTERIA | ||||||||
| Streptomyces sp. 1675 | Marine sediment | Westport Jetty, WA, USA | Depsipeptides | Skyllamycins B–C | Biofilm inhibitor and dispersant | Biofilm inhibition model biofilm dispersion model | P. aeruginosa biofilm inhibition EC50 30 μM (B) and 60 μM (C) | [232] |
| Bacillus sp. BCP32 | Marine sediment | Dohrn Canyon, Gulf of Naples, Italy | Depsipeptides | Nobilamides T1–S1 | Antimicrobial (T1) | Microdilution broth | No activity | [233] |
| Antimicrobial (S1) | MIC 15.6 μg/mL vs. S. aureus 6538p, 7.8 μg/mL vs. S. aureus 6538, 31.5 μg/mL vs. S. xilosus MB5209, 3.9 μg/mL vs. Listeria monocytogenes 677 | |||||||
| FUNGI | ||||||||
| Penicillium antarcticum | Tunicate (Aplidium pallidum) | Corranroo Bay, Galway, Ireland | Itaconate derivatives | ethyl 8-hydroxyhexylitaconate, ethyl 9-hydroxyhexylitaconate, methyl 8-hydroxyhexylitaconate methyl hexylitaconate, 9-hydroxyhexylitaconate | Osteogenic and chondrogenic differentiation inhibition | Osteogenic and chondrogenic assays | Inhibition of hMSCs from 1 to 10 µM | [237] |
| Penicillium sp. Y-5-2 | Hydrothermal vent sediment | Kueishantao Island, Taiwan | Isocumarins Austin derivatives | Austinone Penicillisocoumarin A–C Aspergillumarins A | Antimicrobial | Combined microdilution broth and agar diffusion assays | MIC 32 μg/mL vs. E. coli | [235] |

5. Metabolic Engineering
| Microorganism | Class | Metabolite Type | Yield Increment | References |
|---|---|---|---|---|
| BACTERIA | ||||
| Paracoccus sp. N-81106 | Terpenoids | Astaxanthin | 1700% | [259] |
| Sphingomonas sp. ATCC 55669 | Terpenoids | Astaxanthin | 540% | [279] |
| CYANOBACTERIA | ||||
| Synechocystis sp. PCC 6803 | Terpenoids | Squalene | 300% | [280] |
| MICROALGAE | ||||
| Aurantiochytrium sp. | Terpenoids | Astaxanthin | 900% | [264] |
| Dunaliella salina | Terpenoids | Zeaxanthin | 200% | [266] |
| Phaeodactylum tricornutum | PKS | DHA | 800% | [272] |
| Aurantiochytrium sp. | PKS | DHA | 57.34% | [277] |
| Nannochloropsis oceanica | PKS | EPA | 8%; 5%; 3% | [275] |
6. Linking the Biotechnological Potential of Marine Microorganisms with the Blue Economy Sector
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine Bacterial Secondary Metabolites: A Treasure House for Structurally Unique and Effective Antimicrobial Compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef]
- Giugliano, R.; Della Sala, G.; Buonocore, C.; Zannella, C.; Tedesco, P.; Palma Esposito, F.; Ragozzino, C.; Chianese, A.; Morone, M.V.; Mazzella, V.; et al. New Imidazolium Alkaloids with Broad Spectrum of Action from the Marine Bacterium Shewanella aquimarina. Pharmaceutics 2023, 15, 2139. [Google Scholar] [CrossRef]
- Lauritano, C.; Coppola, D. Biotechnological Applications of Products Released by Marine Microorganisms for Cold Adaptation Strategies: Polyunsaturated Fatty Acids, Antioxidants, and Antifreeze Proteins. J. Mar. Sci. Eng. 2023, 11, 1399. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Chapter Five—Biotechnological Applications of Bioactive Peptides From Marine Sources. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 73, pp. 171–220. [Google Scholar]
- Giordano, D.; Coppola, D.; Russo, R.; Denaro, R.; Giuliano, L.; Lauro, F.M.; Di Prisco, G.; Verde, C. Marine Microbial Secondary Metabolites. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 66, pp. 357–428. ISBN 978-0-12-803299-2. [Google Scholar]
- Medema, M.H.; Fischbach, M.A. Computational Approaches to Natural Product Discovery. Nat. Chem. Biol. 2015, 11, 639–648. [Google Scholar] [CrossRef]
- Smanski, M.J.; Zhou, H.; Claesen, J.; Shen, B.; Fischbach, M.A.; Voigt, C.A. Synthetic Biology to Access and Expand Nature’s Chemical Diversity. Nat. Rev. Microbiol. 2016, 14, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Goering, A.W.; McClure, R.A.; Doroghazi, J.R.; Albright, J.C.; Haverland, N.A.; Zhang, Y.; Ju, K.-S.; Thomson, R.J.; Metcalf, W.W.; Kelleher, N.L. Metabologenomics: Correlation of Microbial Gene Clusters with Metabolites Drives Discovery of a Nonribosomal Peptide with an Unusual Amino Acid Monomer. ACS Cent. Sci. 2016, 2, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Bayona, L.M.; de Voogd, N.J.; Choi, Y.H. Metabolomics on the Study of Marine Organisms. Metabolomics 2022, 18, 17. [Google Scholar] [CrossRef]
- Perez de Souza, L.; Fernie, A.R. Computational Methods for Processing and Interpreting Mass Spectrometry-Based Metabolomics. Essays Biochem. 2023, 68, 5–13. [Google Scholar] [CrossRef]
- Mattoli, L.; Gianni, M.; Burico, M. Mass Spectrometry-Based Metabolomic Analysis as a Tool for Quality Control of Natural Complex Products. Mass. Spectrom. Rev. 2023, 42, 1358–1396. [Google Scholar] [CrossRef]
- Huang, Z.; Bi, T.; Jiang, H.; Liu, H. Review on NMR as a Tool to Analyse Natural Products Extract Directly: Molecular Structure Elucidation and Biological Activity Analysis. Phytochem. Anal. 2024, 35, 5–16. [Google Scholar] [CrossRef]
- Chen, G.; Seukep, A.J.; Guo, M. Recent Advances in Molecular Docking for the Research and Discovery of Potential Marine Drugs. Mar. Drugs 2020, 18, 545. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; Mangoni, A.; Della Sala, G.; Taglialatela-Scafati, O. Challenges in the Configuration Assignment of Natural Products. A Case-Sel. Perspect. Nat. Prod. Rep. 2019, 36, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Gaudêncio, S.P.; Bayram, E.; Lukić Bilela, L.; Cueto, M.; Díaz-Marrero, A.R.; Haznedaroglu, B.Z.; Jimenez, C.; Mandalakis, M.; Pereira, F.; Reyes, F.; et al. Advanced Methods for Natural Products Discovery: Bioactivity Screening, Dereplication, Metabolomics Profiling, Genomic Sequencing, Databases and Informatic Tools, and Structure Elucidation. Mar. Drugs 2023, 21, 308. [Google Scholar] [CrossRef]
- Fuhrman, J.A.; Cram, J.A.; Needham, D.M. Marine Microbial Community Dynamics and Their Ecological Interpretation. Nat. Rev. Microbiol. 2015, 13, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.S.; Abdelhamid, S.A.; Ali, R.H. Isolation and Identification of Marine Microbial Products. J. Genet. Eng. Biotechnol. 2021, 19, 162. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, S.K.; Guldhe, A.; Rawat, I.; Bux, F. Chapter 4—Microalgae Isolation and Basic Culturing Techniques. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 43–54. ISBN 978-0-12-800776-1. [Google Scholar]
- Pachiappan, P.; Prasath, B.B.; Perumal, S.; Ananth, S.; Shenbaga Devi, A.; Kumar, S.D.; Jeyanthi, S. Isolation and Culture of Microalgae. In Advances in Marine and Brackishwater Aquaculture; Perumal, S., Thirunavukkarasu, A.R., Pachiappan, P., Eds.; Springer: New Delhi, India, 2015; pp. 1–15. ISBN 978-81-322-2271-2. [Google Scholar]
- Pereira, H.; Schulze, P.S.C.; Schüler, L.M.; Santos, T.; Barreira, L.; Varela, J. Fluorescence Activated Cell-Sorting Principles and Applications in Microalgal Biotechnology. Algal Res. 2018, 30, 113–120. [Google Scholar] [CrossRef]
- Fernandez-Valenzuela, S.; Ruvalcaba, F.; Beltrán-Rocha, J.; Claudio, P.; Reyna, G. Isolation and Culturing Axenic Microalgae: Mini–Review. Open Microbiol. J. 2021, 15, 111–119. [Google Scholar] [CrossRef]
- Reich, M.; Labes, A. How to Boost Marine Fungal Research: A First Step towards a Multidisciplinary Approach by Combining Molecular Fungal Ecology and Natural Products Chemistry. Mar. Genom. 2017, 36, 57–75. [Google Scholar] [CrossRef]
- Jones, E.B.G.; Pang, K.-L.; Abdel-Wahab, M.A.; Scholz, B.; Hyde, K.D.; Boekhout, T.; Ebel, R.; Rateb, M.E.; Henderson, L.; Sakayaroj, J.; et al. An Online Resource for Marine Fungi. Fungal Divers. 2019, 96, 347–433. [Google Scholar] [CrossRef]
- Rodrigues, C.J.C.; de Carvalho, C.C.C.R. Cultivating Marine Bacteria under Laboratory Conditions: Overcoming the “Unculturable” Dogma. Front. Bioeng. Biotechnol. 2022, 10, 964589. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, M.; Huang, L.; Zhang, X.-H. Cultivation of Uncultured Marine Microorganisms. Mar. Life Sci. Technol. 2021, 3, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Rygaard, A.M.; Thøgersen, M.S.; Nielsen, K.F.; Gram, L.; Bentzon-Tilia, M. Effects of Gelling Agent and Extracellular Signaling Molecules on the Culturability of Marine Bacteria. Appl. Environ. Microbiol. 2017, 83, e00243-17. [Google Scholar] [CrossRef]
- Kato, S.; Yamagishi, A.; Daimon, S.; Kawasaki, K.; Tamaki, H.; Kitagawa, W.; Abe, A.; Tanaka, M.; Sone, T.; Asano, K.; et al. Isolation of Previously Uncultured Slow-Growing Bacteria by Using a Simple Modification in the Preparation of Agar Media. Appl. Environ. Microbiol. 2018, 84, e00807-18. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kamagata, Y. Phosphate-Catalyzed Hydrogen Peroxide Formation from Agar, Gellan, and κ-Carrageenan and Recovery of Microbial Cultivability via Catalase and Pyruvate. Appl. Environ. Microbiol. 2017, 83, e01366-17. [Google Scholar] [CrossRef]
- Yang, S.-J.; Kang, I.; Cho, J.-C. Expansion of Cultured Bacterial Diversity by Large-Scale Dilution-to-Extinction Culturing from a Single Seawater Sample. Microb. Ecol. 2016, 71, 29–43. [Google Scholar] [CrossRef]
- Connon, S.A.; Giovannoni, S.J. High-Throughput Methods for Culturing Microorganisms in Very-Low-Nutrient Media Yield Diverse New Marine Isolates. Appl. Environ. Microbiol. 2002, 68, 3878–3885. [Google Scholar] [CrossRef]
- Rappé, M.S.; Connon, S.A.; Vergin, K.L.; Giovannoni, S.J. Cultivation of the Ubiquitous SAR11 Marine Bacterioplankton Clade. Nature 2002, 418, 630–633. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Ruiz-Fresneda, M.A.; Bakkali, M.; Kämpfer, P.; Glaeser, S.P.; Busse, H.J.; López-Fernández, M.; Martínez-Rodríguez, P.; Merroun, M.L. Stenotrophomonas bentonitica sp. nov., Isolated from Bentonite Formations. Int. J. Syst. Evol. Microbiol. 2017, 67, 2779–2786. [Google Scholar] [CrossRef]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating “Uncultivable” Microorganisms in Pure Culture in a Simulated Natural Environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.S.; Lewis, K.; Nichols, D.; Gavrish, E. New Approaches to Microbial Isolation. In Manual of Industrial Microbiology and Biotechnology; Baltz, R.H., Davies, J.E., Demain, A.L., Bull, A.T., Junker, B., Katz, L., Lynd, L.R., Masurekar, P., Reeves, C.D., Zhao, H., Eds.; ASM Press: Washington, DC, USA, 2014; pp. 3–12. ISBN 978-1-68367-128-2. [Google Scholar]
- Piddock, L.J.V. Teixobactin, the First of a New Class of Antibiotics Discovered by iChip Technology? J. Antimicrob. Chemother. 2015, 70, 2679–2680. [Google Scholar] [CrossRef]
- Sherpa, R.T.; Reese, C.J.; Montazeri Aliabadi, H. Application of iChip to Grow “Uncultivable” Microorganisms and Its Impact on Antibiotic Discovery. J. Pharm. Pharm. Sci. 2015, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Berdy, B.; Spoering, A.L.; Ling, L.L.; Epstein, S.S. In Situ Cultivation of Previously Uncultivable Microorganisms Using the Ichip. Nat. Protoc. 2017, 12, 2232–2242. [Google Scholar] [CrossRef]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of Ichip for High-Throughput In Situ Cultivation of “Uncultivable” Microbial Species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Ding, L.; Xu, P.; Zhang, W.; Yuan, Y.; He, X.; Su, D.; Shi, Y.; Naman, C.B.; Yan, X.; Wu, B.; et al. Three New Diketopiperazines from the Previously Uncultivable Marine Bacterium Gallaecimonas mangrovi HK-28 Cultivated by iChip. Chem. Biodivers. 2020, 17, e2000221. [Google Scholar] [CrossRef]
- Palma Esposito, F.; Ingham, C.J.; Hurtado-Ortiz, R.; Bizet, C.; Tasdemir, D.; de Pascale, D. Isolation by Miniaturized Culture Chip of an Antarctic Bacterium Aequorivita sp. with Antimicrob. Anthelmintic Activity. Biotechnol. Rep. 2018, 20, e00281. [Google Scholar] [CrossRef]
- Chianese, G.; Esposito, F.P.; Parrot, D.; Ingham, C.; De Pascale, D.; Tasdemir, D. Linear Aminolipids with Moderate Antimicrobial Activity from the Antarctic Gram-Negative Bacterium Aequorivita sp. Mar. Drugs 2018, 16, 187. [Google Scholar] [CrossRef]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Strategies for Culture of ‘Unculturable’ Bacteria: Culturing the Unculturable. FEMS Microbiol. Lett. 2010, 309, 1–7. [Google Scholar] [CrossRef]
- Choi, E.J.; Beatty, D.S.; Paul, L.A.; Fenical, W.; Jensen, P.R. Mooreia alkaloidigena Gen. Nov., Sp. Nov. and Catalinimonas alkaloidigena Gen. Nov., Sp. Nov., Alkaloid-Producing Marine Bacteria in the Proposed Families Mooreiaceae Fam. Nov. Catalimonadaceae Fam. Nov. Phylum Bacteroidetes. Int. J. Syst. Evol. Microbiol. 2013, 63, 1219–1228. [Google Scholar] [CrossRef]
- Jensen, P.R.; Gontang, E.; Mafnas, C.; Mincer, T.J.; Fenical, W. Culturable Marine Actinomycete Diversity from Tropical Pacific Ocean Sediments. Environ. Microbiol. 2005, 7, 1039–1048. [Google Scholar] [CrossRef]
- Choi, E.J.; Nam, S.-J.; Paul, L.; Beatty, D.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Previously Uncultured Marine Bacteria Linked to Novel Alkaloid Production. Chem. Biol. 2015, 22, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Seo, E.-Y.; Epstein, S.S.; Joung, Y.; Han, J.; Parfenova, V.V.; Belykh, O.I.; Gladkikh, A.S.; Ahn, T.S. Application of a New Cultivation Technology, I-Tip, for Studying Microbial Diversity in Freshwater Sponges of Lake Baikal, Russia. FEMS Microbiol. Ecol. 2014, 90, 417–423. [Google Scholar] [CrossRef]
- Jung, D.; Machida, K.; Nakao, Y.; Kindaichi, T.; Ohashi, A.; Aoi, Y. Triggering Growth via Growth Initiation Factors in Nature: A Putative Mechanism for in Situ Cultivation of Previously Uncultivated Microorganisms. Front. Microbiol. 2021, 12, 537194. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Gao, A.-H.; Xie, Q.-Y.; Gao, H.G.; Zhuang, L.; Lin, H.-P.; Yu, H.-P.; Li, J.; Yao, X.-S.; Goodfellow, M.; et al. Actinomycetes for Marine Drug Discovery Isolated from Mangrove Soils and Plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically Active Secondary Metabolites of Marine Actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Q.; Chen, X.; Jiang, C.; Jiang, Y.; Li, Q.; Chen, X.; Jiang, C. Isolation and Cultivation Methods of Actinobacteria. In Actinobacteria—Basics and Biotechnological Applications; IntechOpen: London, UK, 2016; ISBN 978-953-51-2248-7. [Google Scholar]
- El Samak, M.; Solyman, S.M.; Hanora, A. Antimicrobial Activity of Bacteria Isolated from Red Sea Marine Invertebrates. Biotechnol. Rep. 2018, 19, e00275. [Google Scholar] [CrossRef]
- Subramani, R.; Sipkema, D. Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 1–43. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Marinomycins A−D, Antitumor-Antibiotics of a New Structure Class from a Marine Actinomycete of the Recently Discovered Genus “Marinispora”. J. Am. Chem. Soc. 2006, 128, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.J.; Navarro, G.; Ebert, D.; DeRisi, J.; Linington, R.G. Salinipostins A–K, Long-Chain Bicyclic Phosphotriesters as a Potent and Selective Antimalarial Chemotype. J. Org. Chem. 2015, 80, 1312–1320. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Qin, Y.; Karthik, L.; Zhu, G.; Hou, C.; Jiang, L.; Liu, M.; Ye, X.; Liu, M.; et al. A New Abyssomicin Polyketide with Anti-Influenza A Virus Activity from a Marine-Derived Verrucosispora sp. MS100137. Appl. Microbiol. Biotechnol. 2020, 104, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Bennur, T.; Kumar, A.R.; Zinjarde, S.; Javdekar, V. Nocardiopsis Species: Incidence, Ecological Roles and Adaptations. Microbiol. Res. 2015, 174, 33–47. [Google Scholar] [CrossRef]
- Siddharth, S.; Rai, V.R. Isolation and Characterization of Bioactive Compounds with Antibacterial, Antioxidant and Enzyme Inhibitory Activities from Marine-Derived Rare Actinobacteria, Nocardiopsis sp. SCA21. Microb. Pathog. 2019, 137, 103775. [Google Scholar] [CrossRef]
- Babadi, Z.K.; Sudarman, E.; Ebrahimipour, G.H.; Primahana, G.; Stadler, M.; Wink, J. Structurally Diverse Metabolites from the Rare Actinobacterium Saccharothrix xinjiangensis. J. Antibiot. 2020, 73, 48–55. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Wray, V.; Lai, D.; Chaidir, C. Induced Production of Depsipeptides by Co-Culturing Fusarium tricinctum and Fusarium begoniae. Tetrahedron Lett. 2013, 54, 2492–2496. [Google Scholar] [CrossRef]
- Shen, Q.; Dai, G.; Ravichandran, V.; Liu, Y.; Zhong, L.; Sui, H.; Ren, X.; Jiao, N.; Zhang, Y.; Zhou, H.; et al. Saccharochelins A–H, Cytotoxic Amphiphilic Siderophores from the Rare Marine Actinomycete Saccharothrix sp. D09. J. Nat. Prod. 2021, 84, 2149–2156. [Google Scholar] [CrossRef]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Production of Induced Secondary Metabolites by a Co-Culture of Sponge-Associated Actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vázquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vílchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 478. [Google Scholar] [CrossRef]
- Palma Esposito, F.; Giugliano, R.; Della Sala, G.; Vitale, G.A.; Buonocore, C.; Ausuri, J.; Galasso, C.; Coppola, D.; Franci, G.; Galdiero, M.; et al. Combining OSMAC Approach and Untargeted Metabolomics for the Identification of New Glycolipids with Potent Antiviral Activity Produced by a Marine Rhodococcus. Int. J. Mol. Sci. 2021, 22, 9055. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef]
- English, A.L.; Boufridi, A.; Quinn, R.J.; Kurtböke, D.I. Evaluation of Fermentation Conditions Triggering Increased Antibacterial Activity from a Near-Shore Marine Intertidal Environment-Associated Streptomyces species. Synth. Syst. Biotechnol. 2017, 2, 28–38. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.-L. Metabolite Induction via Microorganism Co-Culture: A Potential Way to Enhance Chemical Diversity for Drug Discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A Communal Catalogue Reveals Earth’s Multiscale Microbial Diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Caudal, F.; Tapissier-Bontemps, N.; Edrada-Ebel, R.A. Impact of Co-Culture on the Metabolism of Marine Microorganisms. Mar. Drugs 2022, 20, 153. [Google Scholar] [CrossRef]
- Müller, C.; Caspers, B.A.; Gadau, J.; Kaiser, S. The Power of Infochemicals in Mediating Individualized Niches. Trends Ecol. Evol. 2020, 35, 981–989. [Google Scholar] [CrossRef]
- Lewis, W.H.; Tahon, G.; Geesink, P.; Sousa, D.Z.; Ettema, T.J.G. Innovations to Culturing the Uncultured Microbial Majority. Nat. Rev. Microbiol. 2021, 19, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Kitcha, S.; Cheirsilp, B. Enhanced Lipid Production by Co-Cultivation and Co-Encapsulation of Oleaginous Yeast Trichosporonoides spathulata with Microalgae in Alginate Gel Beads. Appl. Biochem. Biotechnol. 2014, 173, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Byun, C.K.; Hwang, H.; Choi, W.S.; Yaguchi, T.; Park, J.; Kim, D.; Mitchell, R.J.; Kim, T.; Cho, Y.-K.; Takayama, S. Productive Chemical Interaction between a Bacterial Microcolony Couple Is Enhanced by Periodic Relocation. J. Am. Chem. Soc. 2013, 135, 2242–2247. [Google Scholar] [CrossRef]
- Paul, C.; Mausz, M.; Pohnert, G. A Co-Culturing/Metabolomics Approach to Investigate Chemically Mediated Interactions of Planktonic Organisms Reveals Influence of Bacteria on Diatom Metabolism. Metabolomics 2013, 9, 349–359. [Google Scholar] [CrossRef]
- Santos, C.A.; Caldeira, M.L.; Lopes da Silva, T.; Novais, J.M.; Reis, A. Enhanced Lipidic Algae Biomass Production Using Gas Transfer from a Fermentative Rhodosporidium toruloides Culture to an Autotrophic Chlorella protothecoides Culture. Bioresour. Technol. 2013, 138, 48–54. [Google Scholar] [CrossRef]
- Serrano, R.; González-Menéndez, V.; Rodríguez, L.; Martín, J.; Tormo, J.R.; Genilloud, O. Co-Culturing of Fungal Strains Against Botrytis Cinerea as a Model for the Induction of Chemical Diversity and Therapeutic Agents. Front. Microbiol. 2017, 8, 649. [Google Scholar] [CrossRef]
- Padmaperuma, G.; Kapoore, R.V.; Gilmour, D.J.; Vaidyanathan, S. Microbial Consortia: A Critical Look at Microalgae Co-Cultures for Enhanced Biomanufacturing. Crit. Rev. Biotechnol. 2018, 38, 690–703. [Google Scholar] [CrossRef]
- Rashid, N.; Ryu, A.J.; Jeong, K.; Lee, B.; Chang, Y.-K. Co-Cultivation of Two Freshwater Microalgae Species to Improve Biomass Productivity and Biodiesel Production. Energy Convers. Manag. 2019, 196, 640–648. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- González-González, L.M.; de-Bashan, L.E. Toward the Enhancement of Microalgal Metabolite Production through Microalgae-Bacteria Consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef]
- MacIntyre, L.W.; Haltli, B.A.; Kerr, R.G. Draft Genome Sequence of Alteromonas sp. Strain RKMC-009, Isolated from Xestospongia muta via In Situ Culturing Using an Isolation Chip Diffusion Chamber. Microbiol. Resour. Announc. 2019, 8, e00508-19. [Google Scholar] [CrossRef]
- Akhter, N.; Liu, Y.; Auckloo, B.N.; Shi, Y.; Wang, K.; Chen, J.; Wu, X.; Wu, B. Stress-Driven Discovery of New Angucycline-Type Antibiotics from a Marine Streptomyces pratensis NA-ZhouS1. Mar. Drugs 2018, 16, 331. [Google Scholar] [CrossRef]
- Anjum, K.; Sadiq, I.; Chen, L.; Kaleem, S.; Li, X.-C.; Zhang, Z.; Lian, X.-Y. Novel Antifungal Janthinopolyenemycins A and B from a Co-Culture of Marine-Associated Janthinobacterium spp. ZZ145 and ZZ148. Tetrahedron Lett. 2018, 59, 3490–3494. [Google Scholar] [CrossRef]
- Lim, H.-J.; An, J.S.; Bae, E.S.; Cho, E.; Hwang, S.; Nam, S.-J.; Oh, K.-B.; Lee, S.K.; Oh, D.-C. Ligiamycins A and B, Decalin-Amino-Maleimides from the Co-Culture of Streptomyces sp. and Achromobacter sp. Isolated from the Marine Wharf Roach, Ligia exotica. Mar. Drugs 2022, 20, 83. [Google Scholar] [CrossRef]
- Martín-Aragón, V.R.; Millán, F.R.; Cuadrado, C.; Daranas, A.H.; Medarde, A.F.; López, J.M.S. Induction of New Aromatic Polyketides from the Marine Actinobacterium Streptomyces griseorubiginosus through an OSMAC Approach. Mar. Drugs 2023, 21, 526. [Google Scholar] [CrossRef]
- Vitale, G.A.; January, G.G.; Oppong-Danquah, E.; Della Sala, G.; Palma Esposito, F.; Tasdemir, D.; de Pascale, D. A Metabologenomics Approach to Unlock the Metabolome of the Novel Antarctic Deep-Sea Isolate Lacinutrix shetlandiensis sp. Nov. WUR7. PNAS Nexus 2023, 2, pgad221. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takahashi, O.; Murakami, K.; Namikoshi, M. Induced Production of a New Unprecedented Epitrithiodiketopiperazine, Chlorotrithiobrevamide, by a Culture of the Marine-Derived Trichoderma cf. brevicompactum with Dimethyl Sulfoxide. Tetrahedron Lett. 2015, 56, 6262–6265. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.-Y.; Nong, X.-H.; Qi, S.-H. New Furanone Derivatives and Alkaloids from the Co-Culture of Marine-Derived Fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [Google Scholar] [CrossRef]
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tansel Tanrıkul, T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.; Zou, K.; Liu, Z.; Proksch, P. Induction of New Metabolites from Sponge-Associated Fungus Aspergillus carneus by OSMAC Approach. Fitoterapia 2018, 131, 9–14. [Google Scholar] [CrossRef]
- Igboeli, H.A.; Marchbank, D.H.; Correa, H.; Overy, D.; Kerr, R.G. Discovery of Primarolides A and B from Marine Fungus Asteromyces cruciatus Using Osmotic Stress and Treatment with Suberoylanilide Hydroxamic Acid. Mar. Drugs 2019, 17, 435. [Google Scholar] [CrossRef]
- Zhao, D.; Han, X.; Wang, D.; Liu, M.; Gou, J.; Peng, Y.; Liu, J.; Li, Y.; Cao, F.; Zhang, C. Bioactive 3-Decalinoyltetramic Acids Derivatives from a Marine-Derived Strain of the Fungus Fusarium equiseti D39. Front. Microbiol. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Le, V.-T.; Bertrand, S.; Robiou du Pont, T.; Fleury, F.; Caroff, N.; Bourgeade-Delmas, S.; Gentil, E.; Logé, C.; Genta-Jouve, G.; Grovel, O. Untargeted Metabolomics Approach for the Discovery of Environment-Related Pyran-2-Ones Chemodiversity in a Marine-Sourced Penicillium restrictum. Mar. Drugs 2021, 19, 378. [Google Scholar] [CrossRef]
- Yao, F.-H.; Liang, X.; Qi, S.-H. Eight New Cyclopentenone and Cyclohexenone Derivatives from the Marine-Derived Fungus Aspergillus sp. SCSIO 41501 by OSMAC Strategy. Nat. Prod. Res. 2021, 35, 3810–3819. [Google Scholar] [CrossRef]
- Qin, Y.; Zou, L.; Lei, X.; Su, J.; Yang, R.; Xie, W.; Li, W.; Chen, G. OSMAC Strategy Integrated with Molecular Networking Discovery Peniciacetals A–I, Nine New Meroterpenoids from the Mangrove-Derived Fungus Penicillium sp. HLLG-122. Bioorganic Chem. 2023, 130, 106271. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Zou, G.; Wang, G.; Kang, W.; Yuan, J.; She, Z. Cytotoxic Bromine- and Iodine-Containing Cytochalasins Produced by the Mangrove Endophytic Fungus Phomopsis sp. QYM-13 Using the OSMAC Approach. J. Nat. Prod. 2022, 85, 1229–1238. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Cai, L.; Liu, L. New Dibenzo-α-Pyrone Derivatives with α-Glucosidase Inhibitory Activities from the Marine-Derived Fungus Alternaria alternata. Mar. Drugs 2022, 20, 778. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Belousova, E.B.; Oleinikova, G.K.; Antonov, A.S.; Khudyakova, Y.V.; Rasin, A.B.; Popov, R.S.; Menchinskaya, E.S.; Trinh, P.T.H.; Yurchenko, A.N.; et al. Cytotoxic Drimane-Type Sesquiterpenes from Co-Culture of the Marine-Derived Fungi Aspergillus carneus KMM 4638 and Beauveria felina (=Isaria felina) KMM 4639. Mar. Drugs 2022, 20, 584. [Google Scholar] [CrossRef]
- Magot, F.; Van Soen, G.; Buedenbender, L.; Li, F.; Soltwedel, T.; Grauso, L.; Mangoni, A.; Blümel, M.; Tasdemir, D. Bioactivity and Metabolome Mining of Deep-Sea Sediment-Derived Microorganisms Reveal New Hybrid PKS-NRPS Macrolactone from Aspergillus versicolor PS108-62. Mar. Drugs 2023, 21, 95. [Google Scholar] [CrossRef]
- Tan, Z.-L.; Chen, Y.-C.; Zhang, J.-P.; Liu, H.-X.; Zhang, W.-M.; Yan, H.-J. A New Secondary Metabolite from the Marine-Derived Fungus Phomopsis lithocarpus FS508. J. Asian Nat. Prod. Res. 2023, 26, 534–540. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Millán, C.; Pinedo, C.; González-Rodríguez, V.E.; Papaspyrou, S.; Zorrilla, D.; Mackenzie, T.A.; Ramos, M.C.; de la Cruz, M.; Aleu, J.; et al. New Eremophilane-Type Sesquiterpenes from the Marine Sediment-Derived Fungus Emericellopsis maritima BC17 and Their Cytotoxic and Antimicrobial Activities. Mar. Drugs 2023, 21, 634. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Blümel, M.; Scarpato, S.; Mangoni, A.; Tasdemir, D. Induction of Isochromanones by Co-Cultivation of the Marine Fungus Cosmospora sp. and the Phytopathogen Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 782. [Google Scholar] [CrossRef]
- Yurchenko, A.N.; Nesterenko, L.E.; Popov, R.S.; Kirichuk, N.N.; Chausova, V.E.; Chingizova, E.A.; Isaeva, M.P.; Yurchenko, E.A. The Metabolite Profiling of Aspergillus fumigatus KMM4631 and Its Co-Cultures with Other Marine Fungi. Metabolites 2023, 13, 1138. [Google Scholar] [CrossRef]
- Belousova, E.B.; Zhuravleva, O.I.; Yurchenko, E.A.; Oleynikova, G.K.; Antonov, A.S.; Kirichuk, N.N.; Chausova, V.E.; Khudyakova, Y.V.; Menshov, A.S.; Popov, R.S.; et al. New Anti-Hypoxic Metabolites from Co-Culture of Marine-Derived Fungi Aspergillus carneus KMM 4638 and Amphichorda sp. KMM 4639. Biomolecules 2023, 13, 741. [Google Scholar] [CrossRef]
- Li, Q.; Li, S.; Li, S.; Hao, X.; Wang, A.; Si, S.; Xu, Y.; Shu, J.; Gan, M. Antimicrobial and Anti-Inflammatory Cyclic Tetrapeptides from the Co-Cultures of Two Marine-Derived Fungi. J. Nat. Prod. 2024, 87, 365–370. [Google Scholar] [CrossRef]
- Wu, J.; Ye, J.; Cen, J.; Chen, Y.; Xu, J. Induction of Three New Secondary Metabolites by the Co-Culture of Endophytic Fungi Phomopsis asparagi DHS-48 and Phomopsis sp. DHS-11 Isolated from the Chinese Mangrove Plant Rhizophora mangle. Mar. Drugs 2024, 22, 332. [Google Scholar] [CrossRef]
- Wu, J.; Chen, D.; Li, Q.; Feng, T.; Xu, J. Metabolomics-Guided Discovery of New Dimeric Xanthones from Co-Cultures of Mangrove Endophytic Fungi Phomopsis asparagi DHS-48 and Phomopsis sp. DHS-11. Mar. Drugs 2024, 22, 102. [Google Scholar] [CrossRef]
- Hu, Z.; Cui, H.; Wang, Q.; Li, C.; Chen, S.; Gao, Z.; Liu, L.; Peng, B.; Li, J. Induced Production of Defensive Secondary Metabolites from Aspergillus fumigatiaffinis by Co-Culture with Aspergillus alabamensis. Phytochemistry 2024, 225, 114187. [Google Scholar] [CrossRef]
- Yu, H.-B.; Ning, Z.; Hu, B.; Zhu, Y.-P.; Lu, X.-L.; He, Y.; Jiao, B.-H.; Liu, X.-Y. Cytosporin Derivatives from Arctic-Derived Fungus Eutypella sp. D-1 via the OSMAC Approach. Mar. Drugs 2023, 21, 382. [Google Scholar] [CrossRef]
- Borkunov, G.V.; Leshchenko, E.V.; Berdyshev, D.V.; Popov, R.S.; Chingizova, E.A.; Shlyk, N.P.; Gerasimenko, A.V.; Kirichuk, N.N.; Khudyakova, Y.V.; Chausova, V.E.; et al. New Piperazine Derivatives Helvamides B–C from the Marine-Derived Fungus Penicillium velutinum ZK-14 Uncovered by OSMAC (One Strain Many Compounds) Strategy. Nat. Prod. Bioprospect. 2024, 14, 32. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Tang, B.; Liu, Z.; Peng, B.; Li, J.; Gao, H.; Wang, S.; Li, Z. Cyclopeptide Avellanins D–O with Antimalarial Activity from the Mariana Trench Anemone-Derived Hamigera ingelheimensis MSC5. J. Nat. Prod. 2024, 87, 2695–2708. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring Structural Diversity of Microbe Secondary Metabolites Using OSMAC Strategy: A Literature Review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Pinedo-Rivilla, C.; Aleu, J.; Durán-Patrón, R. Cryptic Metabolites from Marine-Derived Microorganisms Using OSMAC and Epigenetic Approaches. Mar. Drugs 2022, 20, 84. [Google Scholar] [CrossRef]
- Rathinam, A.J.; Santhaseelan, H.; Dahms, H.-U.; Dinakaran, V.T.; Murugaiah, S.G. Bioprospecting of Unexplored Halophilic Actinobacteria against Human Infectious Pathogens. 3 Biotech 2023, 13, 398. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and Temperature Effects on Bioactivity in Diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef]
- Osvik, R.D.; Ingebrigtsen, R.A.; Norrbin, M.F.; Andersen, J.H.; Eilertsen, H.C.; Hansen, E.H. Adding Zooplankton to the OSMAC Toolkit: Effect of Grazing Stress on the Metabolic Profile and Bioactivity of a Diatom. Mar. Drugs 2021, 19, 87. [Google Scholar] [CrossRef]
- Sproule, A.; Correa, H.; Decken, A.; Haltli, B.; Berrué, F.; Overy, D.P.; Kerr, R.G. Terrosamycins A and B, Bioactive Polyether Ionophores from Streptomyces sp. RKND004 from Prince Edward Island Sediment. Mar. Drugs 2019, 17, 347. [Google Scholar] [CrossRef]
- Shen, Q.; Dai, G.; Li, A.; Liu, Y.; Zhong, G.; Li, X.; Ren, X.; Sui, H.; Fu, J.; Jiao, N.; et al. Genome-Guided Discovery of Highly Oxygenated Aromatic Polyketides, Saccharothrixins D–M, from the Rare Marine Actinomycete Saccharothrix sp. D09. J. Nat. Prod. 2021, 84, 2875–2884. [Google Scholar] [CrossRef]
- Yan, S.; Zeng, M.; Wang, H.; Zhang, H. Micromonospora: A Prolific Source of Bioactive Secondary Metabolites with Therapeutic Potential. J. Med. Chem. 2022, 65, 8735–8771. [Google Scholar] [CrossRef]
- Kokkini, M.; González Heredia, C.; Oves-Costales, D.; De La Cruz, M.; Sánchez, P.; Martín, J.; Vicente, F.; Genilloud, O.; Reyes, F. Exploring Micromonospora as Phocoenamicins Producers. Mar. Drugs 2022, 20, 769. [Google Scholar] [CrossRef]
- Le Loarer, A.; Dufossé, L.; Bignon, J.; Frédérich, M.; Ledoux, A.; Fouillaud, M.; Gauvin-Bialecki, A. OSMAC Method to Assess Impact of Culture Parameters on Metabolomic Diversity and Biological Activity of Marine-Derived Actinobacteria. Mar. Drugs 2023, 22, 23. [Google Scholar] [CrossRef]
- Gamaleldin, N.M.; Bahr, H.S.; Millán-Aguiñaga, N.; Danesh, M.; Othman, E.M.; Dandekar, T.; Hassan, H.M.; Abdelmohsen, U.R. Targeting Antimalarial Metabolites from the Actinomycetes Associated with the Red Sea Sponge Callyspongia siphonella Using a Metabolomic Method. BMC Microbiol. 2023, 23, 396. [Google Scholar] [CrossRef]
- Abdelaleem, E.R.; Samy, M.N.; Rateb, M.E.; Hendawy, O.M.; Abdelmohsen, U.R.; Yehia Desoukey, S. Metabolomic Profiling and Biological Evaluations of Spongia irregularis—Associated Actinomycetes Supported by Multivariate Statistical Analysis. J. Appl. Microbiol. 2023, 134, lxad120. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Miranda, M.; Blümel, M.; Tasdemir, D. Bioactivity Profiling and Untargeted Metabolomics of Microbiota Associated with Mesopelagic Jellyfish Periphylla periphylla. Mar. Drugs 2023, 21, 129. [Google Scholar] [CrossRef]
- Soldatou, S.; Eldjárn, G.H.; Ramsay, A.; Van Der Hooft, J.J.J.; Hughes, A.H.; Rogers, S.; Duncan, K.R. Comparative Metabologenomics Analysis of Polar Actinomycetes. Mar. Drugs 2021, 19, 103. [Google Scholar] [CrossRef]
- de Felício, R.; Ballone, P.; Bazzano, C.F.; Alves, L.F.G.; Sigrist, R.; Infante, G.P.; Niero, H.; Rodrigues-Costa, F.; Fernandes, A.Z.N.; Tonon, L.A.C.; et al. Chemical Elicitors Induce Rare Bioactive Secondary Metabolites in Deep-Sea Bacteria under Laboratory Conditions. Metabolites 2021, 11, 107. [Google Scholar] [CrossRef]
- Lindig, A.; Schwarz, J.; Hubmann, G.; Rosenthal, K.; Lütz, S. Bivariate One Strain Many Compounds Designs Expand the Secondary Metabolite Production Space in Corallococcus coralloides. Microorganisms 2023, 11, 2592. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Zhao, X.-N.; Zhang, P.-X.; Wang, C.-F.; Li, J.; Wei, X.-Y.; Shi, J.-Q.; Dai, W.; Zhang, Q.; Liu, J.-Q. Rapid Discovery of Substances with Anticancer Potential from Marine Fungi Based on a One Strain–Many Compounds Strategy and UPLC-QTOF-MS. Mar. Drugs 2023, 21, 646. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, B.; Sun, C.; Li, Y.; Yang, G.; Zhao, Y.; Pan, K. UDP-Glucose Pyrophosphorylase as a Target for Regulating Carbon Flux Distribution and Antioxidant Capacity in Phaeodactylum tricornutum. Commun. Biol. 2023, 6, 750. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Muñoz-Mira, S.; Durán-Patrón, R.; Aleu, J. Marine-Derived Fungi as Biocatalysts. Front. Microbiol. 2023, 14, 1125639. [Google Scholar] [CrossRef]
- Bovio, E.; Garzoli, L.; Poli, A.; Luganini, A.; Villa, P.; Musumeci, R.; McCormack, G.P.; Cocuzza, C.E.; Gribaudo, G.; Mehiri, M.; et al. Marine Fungi from the Sponge Grantia compressa: Biodiversity, Chemodiversity, and Biotechnological Potential. Mar. Drugs 2019, 17, 220. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Ye, X.; Wei, B.; Emam, M.; Zhang, H.; Wang, H. The Structural Diversity of Marine Microbial Secondary Metabolites Based on Co-Culture Strategy: 2009–2019. Mar. Drugs 2020, 18, 449. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Li, Y.; Liao, S.; Liu, Y. Exploring Diverse Bioactive Secondary Metabolites from Marine Microorganisms Using Co-Culture Strategy. Molecules 2023, 28, 6371. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Hu, Z.; Shao, Y.; Ying, J.; Zhang, H. The Potential Use of Fungal Co-Culture Strategy for Discovery of New Secondary Metabolites. Microorganisms 2023, 11, 464. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From Axenic to Mixed Cultures: Technological Advances Accelerating a Paradigm Shift in Microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in Microbial Culturing Conditions to Activate Silent Biosynthetic Gene Clusters for Novel Metabolite Production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1381–1400. [Google Scholar] [CrossRef]
- Naseema Rasheed, R.; Pourbakhtiar, A.; Mehdizadeh Allaf, M.; Baharlooeian, M.; Rafiei, N.; Alishah Aratboni, H.; Morones-Ramirez, J.R.; Winck, F.V. Microalgal Co-Cultivation—Recent Methods, Trends in Omic-Studies, Applications, and Future Challenges. Front. Bioeng. Biotechnol. 2023, 11, 1193424. [Google Scholar] [CrossRef]
- Hoshino, S.; Wong, C.P.; Ozeki, M.; Zhang, H.; Hayashi, F.; Awakawa, T.; Asamizu, S.; Onaka, H.; Abe, I. Umezawamides, New Bioactive Polycyclic Tetramate Macrolactams Isolated from a Combined-Culture of Umezawaea sp. and Mycolic Acid-Containing Bacterium. J. Antibiot. 2018, 71, 653–657. [Google Scholar] [CrossRef]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.-W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate Bacterial-Fungal Interaction Triggers Biosynthesis of Archetypal Polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Berthold, D.E.; Shetty, K.G.; Jayachandran, K.; Laughinghouse, H.D.; Gantar, M. Enhancing Algal Biomass and Lipid Production through Bacterial Co-Culture. Biomass Bioenergy 2019, 122, 280–289. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Removal of Nitrogen from Wastewater Using Microalgae and Microalgae–Bacteria Consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Goswami, G.; Makut, B.B.; Das, D. Sustainable Production of Bio-Crude Oil via Hydrothermal Liquefaction of Symbiotically Grown Biomass of Microalgae-Bacteria Coupled with Effective Wastewater Treatment. Sci. Rep. 2019, 9, 15016. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial Co-Culturing Strategies for the Production High Value Compounds, a Reliable Framework towards Sustainable Biorefinery Implementation—An Overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Barati, B.; Zafar, F.F.; Rupani, P.F.; Wang, S. Bacterial Pretreatment of Microalgae and the Potential of Novel Nature Hydrolytic Sources. Environ. Technol. Innov. 2021, 21, 101362. [Google Scholar] [CrossRef]
- Das, P.K.; Rani, J.; Rawat, S.; Kumar, S. Microalgal Co-Cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. Bioenerg. Res. 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Kadalag, N.L.; Pawar, P.R.; Prakash, G. Co-Cultivation of Phaeodactylum tricornutum and Aurantiochytrium limacinum for Polyunsaturated Omega-3 Fatty Acids Production. Bioresour. Technol. 2022, 346, 126544. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Avalon, N.E.; Murray, A.E.; Baker, B.J. Integrated Metabolomic–Genomic Workflows Accelerate Microbial Natural Product Discovery. Anal. Chem. 2022, 94, 11959–11966. [Google Scholar] [CrossRef]
- Malit, J.J.L.; Leung, H.Y.C.; Qian, P.-Y. Targeted Large-Scale Genome Mining and Candidate Prioritization for Natural Product Discovery. Mar. Drugs 2022, 20, 398. [Google Scholar] [CrossRef]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. antiSMASH 3.0-a Comprehensive Resource for the Genome Mining of Biosynthetic Gene Clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef]
- Wohlleben, W.; Mast, Y.; Stegmann, E.; Ziemert, N. Antibiotic Drug Discovery. Microb. Biotechnol. 2016, 9, 541–548. [Google Scholar] [CrossRef]
- Ziemert, N.; Alanjary, M.; Weber, T. The Evolution of Genome Mining in Microbes—A Review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef]
- Bachmann, B.O.; Van Lanen, S.G.; Baltz, R.H. Microbial Genome Mining for Accelerated Natural Products Discovery: Is a Renaissance in the Making? J. Ind. Microbiol. Biotechnol. 2014, 41, 175–184. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and Applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Merwin, N.J.; Johnston, C.W.; Magarvey, N.A. PRISM 3: Expanded Prediction of Natural Product Chemical Structures from Microbial Genomes. Nucleic Acids Res. 2017, 45, W49–W54. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Johnston, C.W.; Gunabalasingam, M.; Merwin, N.J.; Kieliszek, A.M.; MacLellan, R.J.; Li, H.; Ranieri, M.R.M.; Webster, A.L.H.; Cao, M.P.T.; et al. Comprehensive Prediction of Secondary Metabolite Structure and Biological Activity from Microbial Genome Sequences. Nat. Commun. 2020, 11, 6058. [Google Scholar] [CrossRef]
- Blin, K.; Wolf, T.; Chevrette, M.G.; Lu, X.; Schwalen, C.J.; Kautsar, S.A.; Suarez Duran, H.G.; de los Santos, E.L.C.; Kim, H.U.; Nave, M.; et al. antiSMASH 4.0—Improvements in Chemistry Prediction and Gene Cluster Boundary Identification. Nucleic Acids Res. 2017, 45, W36–W41. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Weber, T. In Silico Tools for the Analysis of Antibiotic Biosynthetic Pathways. Int. J. Med. Microbiol. 2014, 304, 230–235. [Google Scholar] [CrossRef]
- Zdouc, M.M.; Blin, K.; Louwen, N.L.L.; Navarro, J.; Loureiro, C.; Bader, C.D.; Bailey, C.B.; Barra, L.; Booth, T.J.; Bozhüyük, K.A.J.; et al. MIBiG 4.0: Advancing Biosynthetic Gene Cluster Curation through Global Collaboration. Nucleic Acids Res. 2025, 53, D678–D690. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; Mohimani, H.; Bauermeister, A.; Dorrestein, P.C.; Duncan, K.R.; Medema, M.H. Linking Genomics and Metabolomics to Chart Specialized Metabolic Diversity. Chem. Soc. Rev. 2020, 49, 3297–3314. [Google Scholar] [CrossRef]
- Teta, R.; Della Sala, G.; Mangoni, A.; Lega, M.; Costantino, V. Tracing Cyanobacterial Blooms to Assess the Impact of Wastewaters Discharges on Coastal Areas and Lakes. Int. J. Sustain. Dev. Plan. 2016, 11, 804–811. [Google Scholar] [CrossRef]
- Della Sala, G.; Coppola, D.; Virgili, R.; Vitale, G.; Tanduo, V.; Teta, R.; Crocetta, F.; Pascale, D. Untargeted Metabolomics Yields Insights Into the Lipidome of Botrylloides niger Herdman, 1886, An Ascidian Invading the Mediterranean Sea. Front. Mar. Sci. 2022, 9, 865751. [Google Scholar] [CrossRef]
- Quinn, R.A.; Nothias, L.-F.; Vining, O.; Meehan, M.; Esquenazi, E.; Dorrestein, P.C. Molecular Networking As a Drug Discovery, Drug Metabolism, and Precision Medicine Strategy. Trends Pharmacol. Sci. 2017, 38, 143–154. [Google Scholar] [CrossRef]
- Scarpato, S.; Teta, R.; De Cicco, P.; Borrelli, F.; Pawlik, J.R.; Costantino, V.; Mangoni, A. Molecular Networking Revealed Unique UV-Absorbing Phospholipids: Favilipids from the Marine Sponge Clathria faviformis. Mar. Drugs 2023, 21, 58. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Scarpato, S.; Teta, R.; Della Sala, G.; Pawlik, J.R.; Costantino, V.; Mangoni, A. New Tricks with an Old Sponge: Feature-Based Molecular Networking Led to Fast Identification of New Stylissamide L from Stylissa caribica. Mar. Drugs 2020, 18, 443. [Google Scholar] [CrossRef]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.-F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of Microbial Metabolites through Database Search of Mass Spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. NMR-Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar] [CrossRef]
- Moco, S. Studying Metabolism by NMR-Based Metabolomics. Front. Mol. Biosci. 2022, 9, 882487. [Google Scholar] [CrossRef]
- Wishart, D.S.; Sayeeda, Z.; Budinski, Z.; Guo, A.; Lee, B.L.; Berjanskii, M.; Rout, M.; Peters, H.; Dizon, R.; Mah, R.; et al. NP-MRD: The Natural Products Magnetic Resonance Database. Nucleic Acids Res. 2022, 50, D665–D677. [Google Scholar] [CrossRef]
- Zhang, C.; Idelbayev, Y.; Roberts, N.; Tao, Y.; Nannapaneni, Y.; Duggan, B.M.; Min, J.; Lin, E.C.; Gerwick, E.C.; Cottrell, G.W.; et al. Small Molecule Accurate Recognition Technology (SMART) to Enhance Natural Products Research. Sci. Rep. 2017, 7, 14243. [Google Scholar] [CrossRef]
- Caesar, L.K.; Montaser, R.; Keller, N.P.; Kelleher, N.L. Metabolomics and Genomics in Natural Products Research: Complementary Tools for Targeting New Chemical Entities. Nat. Prod. Rep. 2021, 38, 2041–2065. [Google Scholar] [CrossRef]
- Rai, A.; Saito, K.; Yamazaki, M. Integrated Omics Analysis of Specialized Metabolism in Medicinal Plants. Plant J. 2017, 90, 764–787. [Google Scholar] [CrossRef]
- Maansson, M.; Vynne, N.G.; Klitgaard, A.; Nybo, J.L.; Melchiorsen, J.; Nguyen, D.D.; Sanchez, L.M.; Ziemert, N.; Dorrestein, P.C.; Andersen, M.R.; et al. An Integrated Metabolomic and Genomic Mining Workflow To Uncover the Biosynthetic Potential of Bacteria. mSystems 2016, 1, e00028-15. [Google Scholar] [CrossRef]
- Liaw, C.-C.; Chen, P.-C.; Shih, C.-J.; Tseng, S.-P.; Lai, Y.-M.; Hsu, C.-H.; Dorrestein, P.C.; Yang, Y.-L. Vitroprocines, New Antibiotics against Acinetobacter baumannii, Discovered from Marine Vibrio Sp. QWI-06 Using Mass-Spectrometry-Based Metabolomics Approach. Sci. Rep. 2015, 5, 12856. [Google Scholar] [CrossRef]
- Ding, L.; Bar-Shalom, R.; Aharonovich, D.; Kurisawa, N.; Patial, G.; Li, S.; He, S.; Yan, X.; Iwasaki, A.; Suenaga, K.; et al. Metabolomic Characterization of a cf. Neolyngbya Cyanobacterium from the South China Sea Reveals Wenchangamide A, a Lipopeptide with In Vitro Apoptotic Potential in Colon Cancer Cells. Mar. Drugs 2021, 19, 397. [Google Scholar] [CrossRef]
- Kleigrewe, K.; Almaliti, J.; Tian, I.Y.; Kinnel, R.B.; Korobeynikov, A.; Monroe, E.A.; Duggan, B.M.; Di Marzo, V.; Sherman, D.H.; Dorrestein, P.C.; et al. Combining Mass Spectrometric Metabolic Profiling with Genomic Analysis: A Powerful Approach for Discovering Natural Products from Cyanobacteria. J. Nat. Prod. 2015, 78, 1671–1682. [Google Scholar] [CrossRef]
- Castro, G.S.; Sousa, T.F.; da Silva, G.F.; Pedroso, R.C.N.; Menezes, K.S.; Soares, M.A.; Dias, G.M.; Santos, A.O.; Yamagishi, M.E.B.; Faria, J.V.; et al. Characterization of Peptaibols Produced by a Marine Strain of the Fungus Trichoderma endophyticum via Mass Spectrometry, Genome Mining and Phylogeny-Based Prediction. Metabolites 2023, 13, 221. [Google Scholar] [CrossRef]
- Buedenbender, L.; Kumar, A.; Blümel, M.; Kempken, F.; Tasdemir, D. Genomics- and Metabolomics-Based Investigation of the Deep-Sea Sediment-Derived Yeast, Rhodotorula mucilaginosa 50-3-19/20B. Mar. Drugs 2021, 19, 14. [Google Scholar] [CrossRef]
- Huang, H.; Yue, L.; Deng, F.; Wang, X.; Wang, N.; Chen, H.; Li, H. NMR-Metabolomic Profiling and Genome Mining Drive the Discovery of Cyclic Decapeptides from a Marine Streptomyces. J. Nat. Prod. 2023, 86, 2122–2130. [Google Scholar] [CrossRef]
- Mohimani, H.; Liu, W.-T.; Kersten, R.D.; Moore, B.S.; Dorrestein, P.C.; Pevzner, P.A. NRPquest: Coupling Mass Spectrometry and Genome Mining for Nonribosomal Peptide Discovery. J. Nat. Prod. 2014, 77, 1902–1909. [Google Scholar] [CrossRef]
- Cao, L.; Gurevich, A.; Alexander, K.L.; Naman, C.B.; Leão, T.; Glukhov, E.; Luzzatto-Knaan, T.; Vargas, F.; Quinn, R.; Bouslimani, A.; et al. MetaMiner: A Scalable Peptidogenomics Approach for Discovery of Ribosomal Peptide Natural Products with Blind Modifications from Microbial Communities. Cell Syst. 2019, 9, 600–608.e4. [Google Scholar] [CrossRef]
- Merwin, N.J.; Mousa, W.K.; Dejong, C.A.; Skinnider, M.A.; Cannon, M.J.; Li, H.; Dial, K.; Gunabalasingam, M.; Johnston, C.; Magarvey, N.A. DeepRiPP Integrates Multiomics Data to Automate Discovery of Novel Ribosomally Synthesized Natural Products. Proc. Natl. Acad. Sci. USA 2020, 117, 371–380. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Luhavaya, H.; Li, J.; Dahesh, S.; Nizet, V.; Yamanaka, K.; Moore, B.S. Isolation and Structure Elucidation of Lipopeptide Antibiotic Taromycin B from the Activated Taromycin Biosynthetic Gene Cluster. J. Antibiot. 2018, 71, 333–338. [Google Scholar] [CrossRef]
- Jensen, P.R.; Moore, B.S.; Fenical, W. The Marine Actinomycete Genus Salinispora: A Model Organism for Secondary Metabolite Discovery. Nat. Prod. Rep. 2015, 32, 738–751. [Google Scholar] [CrossRef]
- Bertin, M.J.; Schwartz, S.L.; Lee, J.; Korobeynikov, A.; Dorrestein, P.C.; Gerwick, L.; Gerwick, W.H. Spongosine Production by a Vibrio Harveyi Strain Associated with the Sponge Tectitethya crypta. J. Nat. Prod. 2015, 78, 493–499. [Google Scholar] [CrossRef]
- Duncan, K.R.; Crüsemann, M.; Lechner, A.; Sarkar, A.; Li, J.; Ziemert, N.; Wang, M.; Bandeira, N.; Moore, B.S.; Dorrestein, P.C.; et al. Molecular Networking and Pattern-Based Genome Mining Improves Discovery of Biosynthetic Gene Clusters and Their Products from Salinispora species. Chem. Biol. 2015, 22, 460–471. [Google Scholar] [CrossRef]
- Richter, T.K.S.; Hughes, C.C.; Moore, B.S. Sioxanthin, a Novel Glycosylated Carotenoid Reveals an Unusual Subclustered Biosynthetic Pathway. Env. Microbiol. 2015, 17, 2158–2171. [Google Scholar] [CrossRef]
- Crüsemann, M. Coupling Mass Spectral and Genomic Information to Improve Bacterial Natural Product Discovery Workflows. Mar. Drugs 2021, 19, 142. [Google Scholar] [CrossRef]
- Awakawa, T.; Crüsemann, M.; Munguia, J.; Ziemert, N.; Nizet, V.; Fenical, W.; Moore, B.S. Salinipyrone and Pacificanone Are Biosynthetic By-Products of the Rosamicin Polyketide Synthase. ChemBioChem 2015, 16, 1443–1447. [Google Scholar] [CrossRef]
- Ninomiya, A.; Katsuyama, Y.; Kuranaga, T.; Miyazaki, M.; Nogi, Y.; Okada, S.; Wakimoto, T.; Ohnishi, Y.; Matsunaga, S.; Takada, K. Biosynthetic Gene Cluster for Surugamide A Encompasses an Unrelated Decapeptide, Surugamide F. ChemBioChem 2016, 17, 1709–1712. [Google Scholar] [CrossRef]
- Tian, J.; Chen, H.; Guo, Z.; Liu, N.; Li, J.; Huang, Y.; Xiang, W.; Chen, Y. Discovery of Pentangular Polyphenols Hexaricins A–C from Marine Streptosporangium sp. CGMCC 4.7309 by Genome Mining. Appl. Microbiol. Biotechnol. 2016, 100, 4189–4199. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Lu, X.; Chen, H.; Liu, L.; Yu, Z.; Chen, Y. Hexaricins, Pradimicin-like Polyketides from a Marine Sediment-Derived Streptosporangium sp. and Their Antioxidant Effects. J. Nat. Prod. 2018, 81, 2069–2074. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Q.; Tan, B.; Zheng, L.; Li, H.; Zhu, Y.; Zhang, C. Genome Mining and Activation of a Silent PKS/NRPS Gene Cluster Direct the Production of Totopotensamides. Org. Lett. 2017, 19, 5697–5700. [Google Scholar] [CrossRef]
- Paulus, C.; Rebets, Y.; Tokovenko, B.; Nadmid, S.; Terekhova, L.P.; Myronovskyi, M.; Zotchev, S.B.; Rückert, C.; Braig, S.; Zahler, S.; et al. New Natural Products Identified by Combined Genomics-Metabolomics Profiling of Marine Streptomyces sp. MP131-18. Sci. Rep. 2017, 7, 42382. [Google Scholar] [CrossRef]
- Chen, E.; Chen, Q.; Chen, S.; Xu, B.; Ju, J.; Wang, H. Mathermycin, a Lantibiotic from the Marine Actinomycete Marinactinospora thermotolerans SCSIO 00652. Appl. Environ. Microbiol. 2017, 83, e00926. [Google Scholar] [CrossRef]
- Jin, J.; Yang, X.; Liu, T.; Xiao, H.; Wang, G.; Zhou, M.; Liu, F.; Zhang, Y.; Liu, D.; Chen, M.; et al. Fluostatins M–Q Featuring a 6-5-6-6 Ring Skeleton and High Oxidized A-Rings from Marine Streptomyces sp. PKU-MA00045. Mar. Drugs 2018, 16, 87. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Zhou, H.; Zhong, G.; Huo, L.; Tang, Y.-J.; Zhang, Y.; Bian, X. Genome Mining and Biosynthesis of Primary Amine-Acylated Desferrioxamines in a Marine Gliding bacterium. Org. Lett. 2020, 22, 939–943. [Google Scholar] [CrossRef]
- Saha, S.; Wenjun, Z.; Zhang, G.; Zhu, Y.; Chen, Y.; Liu, W.; Yuan, C.-S.; Zhang, Q.; Zhang, H.; Zhang, L.; et al. Activation and Characterization of a Cryptic Gene Cluster Reveal a Cyclization Cascade for Polycyclic Tetramate Macrolactams. Chem. Sci. 2016, 8, 1607–1612. [Google Scholar] [CrossRef]
- Della Sala, G.; Mangoni, A.; Costantino, V.; Teta, R. Identification of the Biosynthetic Gene Cluster of Thermoactinoamides and Discovery of New Congeners by Integrated Genome Mining and MS-Based Molecular Networking. Front. Chem. 2020, 8, 397. [Google Scholar] [CrossRef]
- Sweeney, D.; Bogdanov, A.; Chase, A.B.; Castro-Falcón, G.; Trinidad-Javier, A.; Dahesh, S.; Nizet, V.; Jensen, P.R. Pattern-Based Genome Mining Guides Discovery of the Antibiotic Indanopyrrole A from a Marine Streptomycete. J. Nat. Prod. 2024, 87, 2768–2778. [Google Scholar] [CrossRef]
- Boudreau, P.D.; Monroe, E.A.; Mehrotra, S.; Desfor, S.; Korobeynikov, A.; Sherman, D.H.; Murray, T.F.; Gerwick, L.; Dorrestein, P.C.; Gerwick, W.H. Expanding the Described Metabolome of the Marine Cyanobacterium Moorea producens JHB through Orthogonal Natural Products Workflows. PLoS ONE 2015, 10, e0133297. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, J.; Zhang, X.; Nong, X.; Xu, X.; Qi, S. Cytotoxic Polyketides from the Deep-Sea-Derived Fungus Engyodontium album DFFSCS021. Mar. Drugs 2014, 12, 5902–5915. [Google Scholar] [CrossRef]
- Chen, X.-W.; Li, C.-W.; Cui, C.-B.; Hua, W.; Zhu, T.-J.; Gu, Q.-Q. Nine New and Five Known Polyketides Derived from a Deep Sea-Sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef]
- Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and Antiviral Nitrobenzoyl Sesquiterpenoids from the Marine-Derived Fungus Aspergillus ochraceus Jcma1F17. Med. Chem. Commun. 2014, 5, 701–705. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, X.; Liu, J.; Kaliyaperumal, K.; Ai, W.; Ju, Z.; Yang, B.; Wang, J.; Yang, X.; Liu, Y. Ascomycotin A, a New Citromycetin Analogue Produced by Ascomycota sp. Ind19F07 Isolated from Deep Sea Sediment. Nat. Prod. Res. 2015, 29, 820–826. [Google Scholar] [CrossRef]
- Wu, B.; Wiese, J.; Labes, A.; Kramer, A.; Schmaljohann, R.; Imhoff, J.F. Lindgomycin, an Unusual Antibiotic Polyketide from a Marine Fungus of the Lindgomycetaceae. Mar. Drugs 2015, 13, 4617–4632. [Google Scholar] [CrossRef]
- Pan, C.; Shi, Y.; Auckloo, B.N.; Chen, X.; Chen, C.-T.A.; Tao, X.; Wu, B. An Unusual Conformational Isomer of Verrucosidin Backbone from a Hydrothermal Vent Fungus, Penicillium sp. Y-50-10. Mar. Drugs 2016, 14, 156. [Google Scholar] [CrossRef]
- Li, X.; Li, X.-M.; Li, X.-D.; Xu, G.-M.; Liu, Y.; Wang, B.-G. 20-Nor-Isopimarane Cycloethers from the Deep-Sea Sediment-Derived Fungus Aspergillus wentii SD-310. RSC Adv. 2016, 6, 75981–75987. [Google Scholar] [CrossRef]
- Li, X.-D.; Li, X.; Li, X.-M.; Xu, G.-M.; Zhang, P.; Meng, L.-H.; Wang, B.-G. Tetranorlabdane Diterpenoids from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. Planta Med. 2016, 82, 877–881. [Google Scholar] [CrossRef]
- Li, X.-D.; Li, X.-M.; Li, X.; Xu, G.-M.; Liu, Y.; Wang, B.-G. Aspewentins D–H, 20-Nor-Isopimarane Derivatives from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. J. Nat. Prod. 2016, 79, 1347–1353. [Google Scholar] [CrossRef]
- Yin, Y.; Fu, Q.; Wu, W.; Cai, M.; Zhou, X.; Zhang, Y. Producing Novel Fibrinolytic Isoindolinone Derivatives in Marine Fungus Stachybotrys longispora FG216 by the Rational Supply of Amino Compounds According to Its Biosynthesis Pathway. Mar. Drugs 2017, 15, 214. [Google Scholar] [CrossRef]
- Niu, S.; Fan, Z.-W.; Xie, C.-L.; Liu, Q.; Luo, Z.-H.; Liu, G.; Yang, X.-W. Spirograterpene A, a Tetracyclic Spiro-Diterpene with a Fused 5/5/5/5 Ring System from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475. J. Nat. Prod. 2017, 80, 2174–2177. [Google Scholar] [CrossRef]
- Zaman, K.A.U.; Wu, X.; Sarotti, A.M.; Cao, S. New and Bioactive Polyketides from Hawaiian Marine-Derived Fungus Trichoderma sp. FM652. Nat. Prod. Res. 2022, 36, 5984–5990. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, S.; Lu, X.; Guo, H.; Chen, S.; Yin, X.; Li, H.; Dai, G.; Liu, L. Integrating Genomics and Metabolomics for the Targeted Discovery of New Cyclopeptides with Antifungal Activity from a Marine-Derived Fungus Beauveria felina. J. Agric. Food Chem. 2023, 71, 9782–9795. [Google Scholar] [CrossRef]
- Yang, H.; Shang, Z.; Chen, Y.; Li, F.; Li, K.; Zhu, H.; Peng, M.; Yang, J.; Cai, C.; Ju, J. Metabologenomics-Inspired Discovery and Combinatorial Biosynthesis-Based Diversification of Fungal O-Glycosylated Depsides. Org. Lett. 2024, 26, 8317–8322. [Google Scholar] [CrossRef]
- Szymański, P.; Markowicz, M.; Mikiciuk-Olasik, E. Adaptation of High-Throughput Screening in Drug Discovery—Toxicological Screening Tests. Int. J. Mol. Sci. 2011, 13, 427–452. [Google Scholar] [CrossRef]
- Mayr, L.M.; Fuerst, P. The Future of High-Throughput Screening. J. Biomol. Screen. 2008, 13, 443–448. [Google Scholar] [CrossRef]
- Brandish, P.E.; Chiu, C.-S.; Schneeweis, J.; Brandon, N.J.; Leech, C.L.; Kornienko, O.; Scolnick, E.M.; Strulovici, B.; Zheng, W. A Cell-Based Ultra-High-Throughput Screening Assay for Identifying Inhibitors of D-Amino Acid Oxidase. J. Biomol. Screen. 2006, 11, 481–487. [Google Scholar] [CrossRef]
- Laubscher, W.E.; Rautenbach, M. Direct Detection of Antibacterial-Producing Soil Isolates Utilizing a Novel High-Throughput Screening Assay. Microorganisms 2022, 10, 2235. [Google Scholar] [CrossRef]
- Teschler, J.K.; Nadell, C.D.; Drescher, K.; Yildiz, F.H. Mechanisms Underlying Vibrio cholerae Biofilm Formation and Dispersion. Annu. Rev. Microbiol. 2022, 76, 503–532. [Google Scholar] [CrossRef]
- Coppola, D.; Buonocore, C.; Palisse, M.; Tedesco, P.; De Pascale, D. Exploring Oceans for Curative Compounds: Potential New Antimicrobial and Anti-Virulence Molecules against Pseudomonas aeruginosa. Mar. Drugs 2022, 21, 9. [Google Scholar] [CrossRef]
- Peach, K.C.; Bray, W.M.; Shikuma, N.J.; Gassner, N.C.; Lokey, R.S.; Yildiz, F.H.; Linington, R.G. An Image-Based 384-Well High-Throughput Screening Method for the Discovery of Biofilm Inhibitors in Vibrio cholerae. Mol. BioSyst. 2011, 7, 1176. [Google Scholar] [CrossRef]
- Navarro, G.; Cheng, A.T.; Peach, K.C.; Bray, W.M.; Bernan, V.S.; Yildiz, F.H.; Linington, R.G. Image-Based 384-Well High-Throughput Screening Method for the Discovery of Skyllamycins A to C as Biofilm Inhibitors and Inducers of Biofilm Detachment in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 1092–1099. [Google Scholar] [CrossRef]
- Casella, V.; Della Sala, G.; Scarpato, S.; Buonocore, C.; Ragozzino, C.; Tedesco, P.; Coppola, D.; Vitale, G.A.; De Pascale, D.; Palma Esposito, F. Novel Insights into the Nobilamide Family from a Deep-Sea Bacillus: Chemical Diversity, Biosynthesis and Antimicrobial Activity Towards Multidrug-Resistant Bacteria. Mar. Drugs 2025, 23, 41. [Google Scholar] [CrossRef]
- Asker, D. High Throughput Screening and Profiling of High-Value Carotenoids from a Wide Diversity of Bacteria in Surface Seawater. Food Chem. 2018, 261, 103–111. [Google Scholar] [CrossRef]
- Pan, C.; Shi, Y.; Auckloo, B.N.; Hassan, S.S.U.; Akhter, N.; Wang, K.; Ye, Y.; Arthur Chen, C.-T.; Tao, X.; Wu, B. Isolation and Antibiotic Screening of Fungi from a Hydrothermal Vent Site and Characterization of Secondary Metabolites from a Penicillium Isolate. Mar. Biotechnol. 2017, 19, 469–479. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Lai, Q.; Lin, Y.; Zhang, Q.; Chen, J. Screening of Anti-Vibrio Activity of Marine Fungal Methanolic Fractions to Control Vibriosis in White Shrimp (Litopenaeus vannamei). Aquac. Res. 2021, 52, 5517–5526. [Google Scholar] [CrossRef]
- Marchese, P.; Mahajan, N.; O’Connell, E.; Fearnhead, H.; Tuohy, M.; Krawczyk, J.; Thomas, O.P.; Barry, F.; Murphy, M.J. A Novel High-Throughput Screening Platform Identifies Itaconate Derivatives from Marine Penicillium antarcticum as Inhibitors of Mesenchymal Stem Cell Differentiation. Mar. Drugs 2020, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- Satpati, G.G.; Pal, R. Rapid Detection of Neutral Lipid in Green Microalgae by Flow Cytometry in Combination with Nile Red Staining—An Improved Technique. Ann. Microbiol. 2015, 65, 937–949. [Google Scholar] [CrossRef]
- Doan, T.-T.Y.; Obbard, J.P. Improved Nile Red Staining of Nannochloropsis sp. J. Appl. Phycol. 2011, 23, 895–901. [Google Scholar] [CrossRef]
- Südfeld, C.; Hubáček, M.; D’Adamo, S.; Wijffels, R.H.; Barbosa, M.J. Optimization of High-Throughput Lipid Screening of the Microalga Nannochloropsis oceanica Using BODIPY 505/515. Algal Res. 2021, 53, 102138. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.S. Isolation and Characterization of a Novel Lutein-Producing Marine Microalga Using High Throughput Screening. Food Res. Int. 2019, 116, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Su, Y.; Xu, M.; Bergmann, A.; Ingthorsson, S.; Rolfsson, O.; Salehi-Ashtiani, K.; Brynjolfsson, S.; Fu, W. Chemical Mutagenesis and Fluorescence-Based High-Throughput Screening for Enhanced Accumulation of Carotenoids in a Model Marine Diatom Phaeodactylum tricornutum. Mar. Drugs 2018, 16, 272. [Google Scholar] [CrossRef]
- Argyle, P.A.; Hinners, J.; Walworth, N.G.; Collins, S.; Levine, N.M.; Doblin, M.A. A High-Throughput Assay for Quantifying Phenotypic Traits of Microalgae. Front. Microbiol. 2021, 12, 706235. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.; Kourtchenko, O.; Rajala, T.; Andersson, B.; Fernandez, L.; Blomberg, A.; Godhe, A. Optimization of a High-throughput Phenotyping Method for Chain-forming Phytoplankton Species. Limnol. Ocean. Methods 2018, 16, 57–67. [Google Scholar] [CrossRef]
- Lessard, P. Metabolic Engineering: The Concept Coalesces. Nat. Biotechnol. 1996, 14, 1654–1655. [Google Scholar] [CrossRef]
- Yang, D.; Eun, H.; Prabowo, C.P.S.; Cho, S.; Lee, S.Y. Metabolic and Cellular Engineering for the Production of Natural Products. Curr. Opin. Biotechnol. 2022, 77, 102760. [Google Scholar] [CrossRef]
- Lu, L.; Shen, X.; Sun, X.; Yan, Y.; Wang, J.; Yuan, Q. CRISPR-Based Metabolic Engineering in Non-Model Microorganisms. Curr. Opin. Biotechnol. 2022, 75, 102698. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Pruett-Miller, S.M.; Huang, Y.; Gjoka, M.; Duda, K.; Taunton, J.; Collingwood, T.N.; Frodin, M.; Davis, G.D. High-Frequency Genome Editing Using ssDNA Oligonucleotides with Zinc-Finger Nucleases. Nat. Methods 2011, 8, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Reyon, D.; Tsai, S.Q.; Khayter, C.; Foden, J.A.; Sander, J.D.; Joung, J.K. FLASH Assembly of TALENs for High-Throughput Genome Editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Jakočiūnas, T.; Bonde, I.; Herrgård, M.; Harrison, S.J.; Kristensen, M.; Pedersen, L.E.; Jensen, M.K.; Keasling, J.D. Multiplex Metabolic Pathway Engineering Using CRISPR/Cas9 in Saccharomyces cerevisiae. Metab. Eng. 2015, 28, 213–222. [Google Scholar] [CrossRef]
- Moutinho Cabral, I.; Gonçalves, C.; Grosso, A.R.; Costa, P.M. Bioprospecting and Marine ‘Omics’: Surfing the Deep Blue Sea for Novel Bioactive Proteins and Peptides. Front. Mar. Sci. 2024, 11, 1362697. [Google Scholar] [CrossRef]
- Cho, J.S.; Kim, G.B.; Eun, H.; Moon, C.W.; Lee, S.Y. Designing Microbial Cell Factories for the Production of Chemicals. JACS Au 2022, 2, 1781–1799. [Google Scholar] [CrossRef]
- Kadjo, A.E.; Eustáquio, A.S. Bacterial Natural Product Discovery by Heterologous Expression. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad044. [Google Scholar] [CrossRef]
- Huo, L.; Hug, J.J.; Fu, C.; Bian, X.; Zhang, Y.; Müller, R. Heterologous Expression of Bacterial Natural Product Biosynthetic Pathways. Nat. Prod. Rep. 2019, 36, 1412–1436. [Google Scholar] [CrossRef]
- Giuffrida, D.; Sutthiwong, N.; Dugo, P.; Donato, P.; Cacciola, F.; Girard-Valenciennes, E.; Le Mao, Y.; Monnet, C.; Fouillaud, M.; Caro, Y.; et al. Characterisation of the C50 Carotenoids Produced by Strains of the Cheese-Ripening Bacterium Arthrobacter arilaitensis. Int. Dairy J. 2016, 55, 10–16. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef]
- Ide, T.; Hoya, M.; Tanaka, T.; Harayama, S. Enhanced Production of Astaxanthin in Paracoccus Sp. Strain N-81106 by Using Random Mutagenesis and Genetic Engineering. Biochem. Eng. J. 2012, 65, 37–43. [Google Scholar] [CrossRef]
- Henke, N.; Heider, S.; Peters-Wendisch, P.; Wendisch, V. Production of the Marine Carotenoid Astaxanthin by Metabolically Engineered Corynebacterium glutamicum. Mar. Drugs 2016, 14, 124. [Google Scholar] [CrossRef]
- Miura, Y.; Kondo, K.; Saito, T.; Shimada, H.; Fraser, P.D.; Misawa, N. Production of the Carotenoids Lycopene, β-Carotene, and Astaxanthin in the Food Yeast Candida utilis. Appl. Environ. Microbiol. 1998, 64, 1226–1229. [Google Scholar] [CrossRef]
- Lemoine, Y.; Schoefs, B. Secondary Ketocarotenoid Astaxanthin Biosynthesis in Algae: A Multifunctional Response to Stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Suen, Y.L.; Tang, H.; Huang, J.; Chen, F. Enhanced Production of Fatty Acids and Astaxanthin in Aurantiochytrium sp. by the Expression of Vitreoscilla Hemoglobin. J. Agric. Food Chem. 2014, 62, 12392–12398. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, I.D.; Udenigwe, C.C.; Aluko, R.E. Lutein and Zeaxanthin: Production Technology, Bioavailability, Mechanisms of Action, Visual Function, and Health Claim Status. Trends Food Sci. Technol. 2016, 49, 74–84. [Google Scholar] [CrossRef]
- Simon, D.P.; Anila, N.; Gayathri, K.; Sarada, R. Heterologous Expression of β-Carotene Hydroxylase in Dunaliella salina by Agrobacterium—Mediated Genetic Transformation. Algal Res. 2016, 18, 257–265. [Google Scholar] [CrossRef]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in Microalgae: Health Benefits, Biosynthesis, and Metabolic Engineering Advances. J. Am. Oil Chem. Soc. 2023, 100, 831–842. [Google Scholar] [CrossRef]
- Demaison, L.; Moreau, D. Dietary N-3 Polyunsaturated Fatty Acids and Coronary Heart Disease-Related Mortality: A Possible Mechanism of Action. Cell. Mol. Life Sci. 2002, 59, 463–477. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.-J.; Li, J.-J.; Liang, Z.-Z.; Zhao, C.-Q. Novel Natural Products from Extremophilic Fungi. Mar. Drugs 2018, 16, 194. [Google Scholar] [CrossRef]
- Lupette, J.; Benning, C. Human Health Benefits of Very-Long-Chain Polyunsaturated Fatty Acids from Microalgae. Biochimie 2020, 178, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The Effect of Omega-3 Fatty Acid Supplementation on Clinical and Biochemical Parameters of Critically Ill Patients with COVID-19: A Randomized Clinical Trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Metabolic Engineering of Phaeodactylum tricornutum for the Enhanced Accumulation of Omega-3 Long Chain Polyunsaturated Fatty Acids. Metab. Eng. 2014, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, X.; Yu, X.; Jiang, Q.; Zheng, K.; Xu, J.; Wang, P. Recent Advances in the Heterologous Expression of Biosynthetic Gene Clusters for Marine Natural Products. Mar. Drugs 2022, 20, 341. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Hu, D.; Wang, X.; Balamurugan, S.; Alimujiang, A.; Yang, W.; Liu, J.; Li, H. Identification of a Malonyl CoA-acyl Carrier Protein Transacylase and Its Regulatory Role in Fatty Acid Biosynthesis in Oleaginous Microalga Nannochloropsis oceanica. Biotech. App Biochem. 2017, 64, 620–626. [Google Scholar] [CrossRef]
- Poliner, E.; Pulman, J.A.; Zienkiewicz, K.; Childs, K.; Benning, C.; Farré, E.M. A Toolkit for Nannochloropsis oceanica CCMP 1779 Enables Gene Stacking and Genetic Engineering of the Eicosapentaenoic Acid Pathway for Enhanced Long-chain Polyunsaturated Fatty Acid Production. Plant Biotechnol. J. 2018, 16, 298–309. [Google Scholar] [CrossRef]
- Xin, Y.; Shen, C.; She, Y.; Chen, H.; Wang, C.; Wei, L.; Yoon, K.; Han, D.; Hu, Q.; Xu, J. Biosynthesis of Triacylglycerol Molecules with a Tailored PUFA Profile in Industrial Microalgae. Mol. Plant 2019, 12, 474–488. [Google Scholar] [CrossRef]
- Liang, S.; Yang, X.; Zhu, X.; Ibrar, M.; Liu, L.; Li, S.; Li, X.; Tian, T.; Li, S. Metabolic Engineering to Improve Docosahexaenoic Acid Production in Marine Protist Aurantiochytrium sp. by Disrupting 2,4-Dienoyl-CoA Reductase. Front. Mar. Sci. 2022, 9, 939716. [Google Scholar] [CrossRef]
- Choi, K.R.; Lee, S.Y. CRISPR Technologies for Bacterial Systems: Current Achievements and Future Directions. Biotechnol. Adv. 2016, 34, 1180–1209. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhou, Y.; Li, X.; Zhu, F.; Cheng, Y.; Liu, Y.; Deng, Z.; Liu, T. Genome Mining of Astaxanthin Biosynthetic Genes from Sphingomonas sp. ATCC 55669 for Heterologous Overproduction in Escherichia coli. Biotechnol. J. 2016, 11, 228–237. [Google Scholar] [CrossRef]
- Pattanaik, B.; Englund, E.; Nolte, N.; Lindberg, P. Introduction of a Green Algal Squalene Synthase Enhances Squalene Accumulation in a Strain of Synechocystis sp. PCC 6803. Metab. Eng. Commun. 2020, 10, e00125. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk Dalay, M.; et al. The Essentials of Marine Biotechnology. Front. Mar. Sci. 2021, 8, 629629. [Google Scholar]
- Buonocore, C.; Giugliano, R.; Della Sala, G.; Palma Esposito, F.; Tedesco, P.; Folliero, V.; Galdiero, M.; Franci, G.; de Pascale, D. Evaluation of Antimicrobial Properties and Potential Applications of Pseudomonas gessardii M15 Rhamnolipids towards Multiresistant Staphylococcus aureus. Pharmaceutics 2023, 15, 700. [Google Scholar] [CrossRef]
- Giugliano, R.; Buonocore, C.; Zannella, C.; Chianese, A.; Palma Esposito, F.; Tedesco, P.; De Filippis, A.; Galdiero, M.; Franci, G.; de Pascale, D. Antiviral Activity of the Rhamnolipids Mixture from the Antarctic Bacterium Pseudomonas gessardii M15 against Herpes Simplex Viruses and Coronaviruses. Pharmaceutics 2021, 13, 2121. [Google Scholar] [CrossRef]
- Andreolli, M.; Villanova, V.; Zanzoni, S.; D’Onofrio, M.; Vallini, G.; Secchi, N.; Lampis, S. Characterization of Trehalolipid Biosurfactant Produced by the Novel Marine Strain Rhodococcus sp. SP1d and Its Potential for Environmental Applications. Microb. Cell Factories 2023, 22, 126. [Google Scholar] [CrossRef]
- Gopikrishnan, V.; Radhakrishnan, M.; Shanmugasundaram, T.; Ramakodi, M.P.; Balagurunathan, R. Isolation, Characterization and Identification of Antibiofouling Metabolite from Mangrove Derived Streptomyces sampsonii PM33. Sci. Rep. 2019, 9, 12975. [Google Scholar] [CrossRef]
- John, J.A.; Samuel, M.S.; Govarthanan, M.; Selvarajan, E. A Comprehensive Review on Strategic Study of Cellulase Producing Marine Actinobacteria for Biofuel Applications. Environ. Res. 2022, 214, 114018. [Google Scholar] [CrossRef]
- Qeshmi, F.I.; Homaei, A.; Fernandes, P.; Hemmati, R.; Dijkstra, B.W.; Khajeh, K. Xylanases from Marine Microorganisms: A Brief Overview on Scope, Sources, Features and Potential Applications. Biochim. Biophys. Acta-Proteins Proteom. 2020, 1868, 140312. [Google Scholar] [CrossRef] [PubMed]
- Ghattavi, S.; Homaei, A. Marine Enzymes: Classification and Application in Various Industries. Int. J. Biol. Macromol. 2023, 230, 123136. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ni, J.; Zhong, C.; Xu, P.; Dai, J.; Tang, H. Establishment of a Salt-Induced Bioremediation Platform from Marine Vibrio natriegens. Commun. Biol. 2022, 5, 1352. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the Heavy Metal Bioremediation Efficiency of the Novel Marine Lactic Acid Bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef]
- Ausuri, J.; Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; De Pascale, D. Assessment of the Degradation Potential and Genomic Insights towards Phenanthrene by Dietzia psychralcaliphila JI1D. Microorganisms 2021, 9, 1327. [Google Scholar] [CrossRef]
- Ausuri, J.; Dell’Anno, F.; Vitale, G.A.; Palma Esposito, F.; Funari, V.; Franci, G.; Galdiero, M.; Della Sala, G.; Tedesco, P.; Coppola, D.; et al. Bioremediation of Multiple Heavy Metals Mediated by Antarctic Marine Isolated Dietzia psychralcaliphila JI1D. J. Mar. Sci. Eng. 2022, 10, 1669. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Mathew, N.C.; Sujitha, K.; Kirubagaran, R.; Dharani, G. Genome Analysis of Deep Sea Piezotolerant Nesiotobacter exalbescens COD22 and Toluene Degradation Studies under High Pressure Condition. Sci. Rep. 2019, 9, 18724. [Google Scholar] [CrossRef]
- Sakdapetsiri, C.; Kaokhum, N.; Pinyakong, O. Biodegradation of Crude Oil by Immobilized Exiguobacterium sp. AO-11 and Shelf Life Evaluation. Sci. Rep. 2021, 11, 12990. [Google Scholar] [CrossRef]
- Singh, P.; FNU, K.; Encarnação, T. Marine Bacteria for Biofertilizers. In Marine Organisms: A Solution to Environmental Pollution? Uses in Bioremediation and in Biorefinery; Encarnação, T., Canelas Pais, A., Eds.; Environmental Challenges and Solutions; Springer International Publishing: Cham, Switzerland, 2023; pp. 189–203. ISBN 978-3-031-17226-7. [Google Scholar]
- AlNadhari, S.; Al-Enazi, N.M.; Alshehrei, F.; Ameen, F. A Review on Biogenic Synthesis of Metal Nanoparticles Using Marine Algae and Its Applications. Environ. Res. 2021, 194, 110672. [Google Scholar] [CrossRef]
- Samalens, F.; Thomas, M.; Claverie, M.; Castejon, N.; Zhang, Y.; Pigot, T.; Blanc, S.; Fernandes, S.C.M. Progresses and Future Prospects in Biodegradation of Marine Biopolymers and Emerging Biopolymer-Based Materials for Sustainable Marine Ecosystems. Green Chem. 2022, 24, 1762–1779. [Google Scholar] [CrossRef]
- Esposito, F.P.; Vecchiato, V.; Buonocore, C.; Tedesco, P.; Noble, B.; Basnett, P.; de Pascale, D. Enhanced Production of Biobased, Biodegradable, Poly(3-Hydroxybutyrate) Using an Unexplored Marine Bacterium Pseudohalocynthiibacter aestuariivivens, Isolated from Highly Polluted Coastal Environment. Bioresour. Technol. 2023, 368, 128287. [Google Scholar] [CrossRef]
- Jeong, G.-J.; Khan, S.; Tabassum, N.; Khan, F.; Kim, Y.-M. Marine-Bioinspired Nanoparticles as Potential Drugs for Multiple Biological Roles. Mar. Drugs 2022, 20, 527. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Joshi, P.B.; Chavali, M.S. Updates on Biogenic Metallic and Metal Oxide Nanoparticles: Therapy, Drug Delivery and Cytotoxicity. Pharmaceutics 2023, 15, 1650. [Google Scholar] [CrossRef]
- Abouelkheir, S.S.; Elsherbiny, B.A.; Moffit, S.M.; Mahmoud, N.H.; Mohamed, J.H.; Abdella, B.; El-Sheekh, M.M. Nanobiotechnology: A Sustainable Approach for Marine Environment Bioremediation. In Industrial Wastewater Reuse: Applications, Prospects and Challenges; Shah, M.P., Ed.; Springer Nature: Singapore, 2023; pp. 165–187. ISBN 978-981-99-2489-9. [Google Scholar]
- Funari, V.; Toller, S.; Vitale, L.; Santos, R.M.; Gomes, H.I. Urban Mining of Municipal Solid Waste Incineration (MSWI) Residues with Emphasis on Bioleaching Technologies: A Critical Review. Env. Sci. Pollut. Res. 2023, 30, 59128–59150. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Venil, C.K.; Veera Ravi, A.; Dufossé, L. Marine Bacteria Is the Cell Factory to Produce Bioactive Pigments: A Prospective Pigment Source in the Ocean. Front. Sustain. Food Syst. 2020, 4, 589655. [Google Scholar] [CrossRef]
- Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant Molecules from Marine Fungi: Methodologies and Perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Marine Fungi: An Untapped Bioresource for Future Cosmeceuticals. Phytochem. Lett. 2018, 23, 15–20. [Google Scholar] [CrossRef]
- El-Shall, H.; Abu-Serie, M.; Abu-Elreesh, G.; Eltarahony, M. Unveiling the Anticancer Potentiality of Single Cell Oils Produced by Marine Oleaginous Paradendryphiella sp. under Optimized Economic Growth Conditions. Sci. Rep. 2023, 13, 20773. [Google Scholar] [CrossRef]
- Salgado, C.L.; Muñoz, R.; Blanco, A.; Lienqueo, M.E. Valorization and Upgrading of the Nutritional Value of Seaweed and Seaweed Waste Using the Marine Fungi Paradendryphiella salina to Produce Mycoprotein. Algal Res. 2021, 53, 102135. [Google Scholar] [CrossRef]
- Ekasari, J.; Achmadi, F.F.; Isti’anah, I.; Tarman, K. Growth Performance and Blood Figures of African Catfish (Clarias Sp.) Fed with Marine Fungal Fermented Feed. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2023; Volume 1137, p. 012041. [Google Scholar] [CrossRef]
- Dudhagara, D.R.; Javia, B.M.; Vala, A.K. Exploiting Marine Fungi in the Removal of Hazardous Pollutants and Biomass Valorisation. In Marine Organisms: A Solution to Environmental Pollution? Uses in Bioremediation and in Biorefinery; Encarnação, T., Canelas Pais, A., Eds.; Environmental Challenges and Solutions; Springer International Publishing: Cham, Switzerland, 2023; pp. 117–146. ISBN 978-3-031-17226-7. [Google Scholar]
- Behera, A.D.; Das, S. Ecological Insights and Potential Application of Marine Filamentous Fungi in Environmental Restoration. Rev. Env. Sci. Biotechnol. 2023, 22, 281–318. [Google Scholar] [CrossRef]
- Maamar, A.; Lucchesi, M.-E.; Debaets, S.; Nguyen van Long, N.; Quemener, M.; Coton, E.; Bouderbala, M.; Burgaud, G.; Matallah-Boutiba, A. Highlighting the Crude Oil Bioremediation Potential of Marine Fungi Isolated from the Port of Oran (Algeria). Diversity 2020, 12, 196. [Google Scholar] [CrossRef]
- Balabanova, L.; Slepchenko, L.; Son, O.; Tekutyeva, L. Biotechnology Potential of Marine Fungi Degrading Plant and Algae Polymeric Substrates. Front. Microbiol. 2018, 9, 1527. [Google Scholar] [CrossRef]
- Galasso, C.; Lekube, X.; Cancio, I.; Dell’Anno, A.; Brunet, C.; Sansone, C.; Tangherlini, M. Marine Fungi as Potential Eco-Sustainable Resource for Precious Metals Recovery from Electronic Waste. Waste Biomass Valor. 2022, 13, 967–976. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the Production of Bulk Chemicals and Biofuels. Biofuels Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef]
- Kim, H.; Weiss, T.; Thapa, H.; Devarenne, T.; Han, A. A Microfluidic Photobioreactor Array Demonstrating High-Throughput Screening for Microalgal Oil Production. Lab. A Chip 2014, 14, 1415–1425. [Google Scholar] [CrossRef]
- Heo, J.; Cho, D.-H.; Ramanan, R.; Oh, H.-M.; Kim, H.-S. PhotoBiobox: A Tablet Sized, Low-Cost, High Throughput Photobioreactor for Microalgal Screening and Culture Optimization for Growth, Lipid Content and CO2 Sequestration. Biochem. Eng. J. 2015, 103, 193–197. [Google Scholar] [CrossRef]
- Ahmed, N.; Sheikh, M.A.; Ubaid, M.; Chauhan, P.; Kumar, K.; Choudhary, S. Comprehensive Exploration of Marine Algae Diversity, Bioactive Compounds, Health Benefits, Regulatory Issues, and Food and Drug Applications. Meas. Food 2024, 14, 100163. [Google Scholar] [CrossRef]
- Elagoz, A.M.; Ambrosino, L.; Lauritano, C. De Novo Transcriptome of the Diatom Cylindrotheca closterium Identifies Genes Involved in the Metabolism of Anti-Inflammatory Compounds. Sci. Rep. 2020, 10, 4138. [Google Scholar] [CrossRef]
- Hong, S.-C.; Yu, H.S.; Kim, J.-W.; Lee, E.H.; Pan, C.-H.; Hong, K.W.; Kim, J.-C. Protective Effect of Tisochrysis lutea on Dry Eye Syndrome via NF-κB Inhibition. Sci. Rep. 2022, 12, 19576. [Google Scholar] [CrossRef]
- Zhuang, D.; He, N.; Khoo, K.S.; Ng, E.-P.; Chew, K.W.; Ling, T.C. Application Progress of Bioactive Compounds in Microalgae on Pharmaceutical and Cosmetics. Chemosphere 2022, 291, 132932. [Google Scholar] [CrossRef]
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Park, W.-K.; Suh, W.I.; Chang, Y.K.; Lee, B. Biological Carbon Recovery from Sugar Refinery Washing Water into Microalgal DHA: Medium Optimization and Stress Induction. Sci. Rep. 2019, 9, 19959. [Google Scholar] [CrossRef] [PubMed]
- Nazir, Y.; Phabakaran, P.; Halim, H.; Mohamed, H.; Naz, T.; Abdul Hamid, A.; Song, Y. Strategic Development of Aurantiochytrium sp. Mutants With Superior Oxidative Stress Tolerance and Glucose-6-Phosphate Dehydrogenase Activity for Enhanced DHA Production Through Plasma Mutagenesis Coupled With Chemical Screening. Front. Nutr. 2022, 9, 876649. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Saharia, M.; Srivstava, R.; Kumar, S.; Sahoo, L. Tailoring Microalgae for Efficient Biofuel Production. Front. Mar. Sci. 2018, 5, 382. [Google Scholar] [CrossRef]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. Flocculation: An Effective Way to Harvest Microalgae for Biodiesel Production. J. Environ. Chem. Eng. 2019, 7, 103221. [Google Scholar] [CrossRef]
- Li, D.-W.; Balamurugan, S.; Yang, Y.-F.; Zheng, J.-W.; Huang, D.; Zou, L.-G.; Yang, W.-D.; Liu, J.-S.; Guan, Y.; Li, H.-Y. Transcriptional Regulation of Microalgae for Concurrent Lipid Overproduction and Secretion. Sci. Adv. 2019, 5, eaau3795. [Google Scholar] [CrossRef]
- Meril, D.; Piliyan, R.; Perumal, S.; Sundarraj, D.K.; Binesh, A. Efficacy of Alginate Immobilized Microalgae in the Bioremediation of Shrimp Aquaculture Wastewater. Process Biochem. 2022, 122, 196–202. [Google Scholar] [CrossRef]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.-H.; Linne, U.; Erb, T.; Maier, U.G. Using a Marine Microalga as a Chassis for Polyethylene Terephthalate (PET) Degradation. Microb. Cell Factories 2019, 18, 171. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef]
- Du, S.; Cui, J.; Meng, F.; Li, H.; Cui, H.; Xia, Y. Bioremediation of Propylbenzenes by a Novel Marine Microalga Rhinomonas reticulata S6A Isolated from Daya Bay: Acute Toxicity, Growth Kinetics and Biodegradation Performance. Front. Mar. Sci. 2023, 10, 1171944. [Google Scholar] [CrossRef]
- Hurst, D.; Børresen, T.; Almesjö, L.; Raedemaecker, F.; Bergseth, S. Marine Biotechnology Strategic Research and Innovation Roadmap—Insights to the Future Direction of European Marine Biotechnology; ERA-NET Marine Biotechnology: Oostende, Belgium, 2016; ISBN 978-94-92043-27-6. [Google Scholar]
- Doussineau, M.; Gnamus, A.; Gomez, J.; Haarich, S.; Holstein, F. Smart Specialisation and Blue Biotechnology in Europe; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: Updates 2022. Mar. Drugs 2022, 20, 759. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Honecker, F. Marine Compounds and Cancer: Updates 2020. Mar. Drugs 2020, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life 2021, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Jeewon, R.; Luckhun, A.B.; Bhoyroo, V.; Sadeer, N.B.; Mahomoodally, M.F.; Rampadarath, S.; Puchooa, D.; Sarma, V.V.; Durairajan, S.S.K.; Hyde, K.D. Pharmaceutical Potential of Marine Fungal Endophytes. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–23. ISBN 978-3-319-76900-4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragozzino, C.; Casella, V.; Coppola, A.; Scarpato, S.; Buonocore, C.; Consiglio, A.; Palma Esposito, F.; Galasso, C.; Tedesco, P.; Della Sala, G.; et al. Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products. Mar. Drugs 2025, 23, 116. https://doi.org/10.3390/md23030116
Ragozzino C, Casella V, Coppola A, Scarpato S, Buonocore C, Consiglio A, Palma Esposito F, Galasso C, Tedesco P, Della Sala G, et al. Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products. Marine Drugs. 2025; 23(3):116. https://doi.org/10.3390/md23030116
Chicago/Turabian StyleRagozzino, Costanza, Vincenza Casella, Alessandro Coppola, Silvia Scarpato, Carmine Buonocore, Antonella Consiglio, Fortunato Palma Esposito, Christian Galasso, Pietro Tedesco, Gerardo Della Sala, and et al. 2025. "Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products" Marine Drugs 23, no. 3: 116. https://doi.org/10.3390/md23030116
APA StyleRagozzino, C., Casella, V., Coppola, A., Scarpato, S., Buonocore, C., Consiglio, A., Palma Esposito, F., Galasso, C., Tedesco, P., Della Sala, G., de Pascale, D., Vitale, L., & Coppola, D. (2025). Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products. Marine Drugs, 23(3), 116. https://doi.org/10.3390/md23030116










