Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue
Abstract
:1. Introduction
1.1. Factors Influencing Fungal and Mycotoxin Contamination of Agricultural Commodities
1.2. Mycotoxins and Human Health
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Management
2.5. Assessment of Methodological Quality of Included Studies and Data Synthesis
3. Results
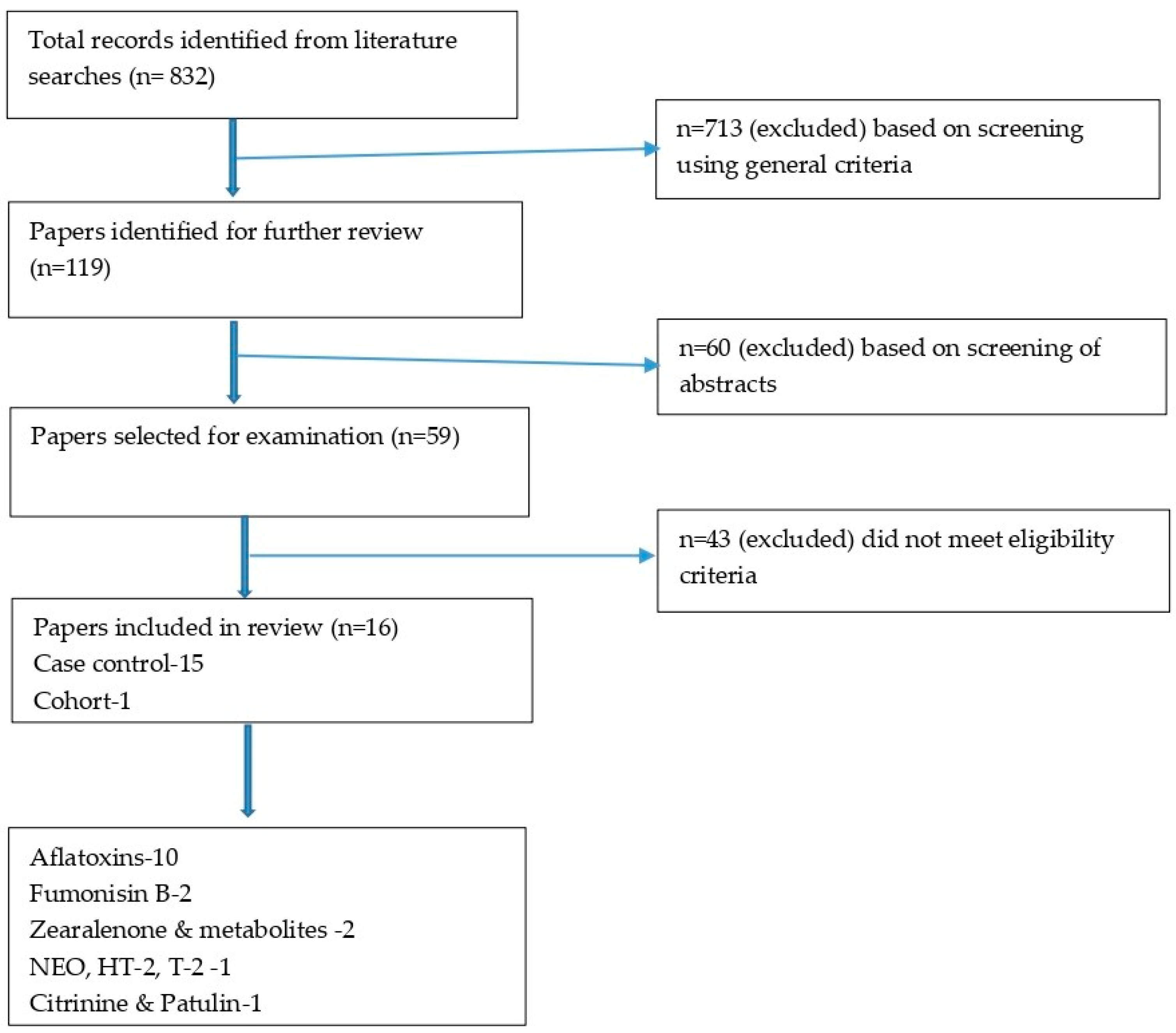
3.1. Results of the Search
3.2. Characteristics of the Included Studies
3.3. Results on the Quality of Studies Using the Modified Newcastle-Ottawa Scale (NOS) for Observational Studies
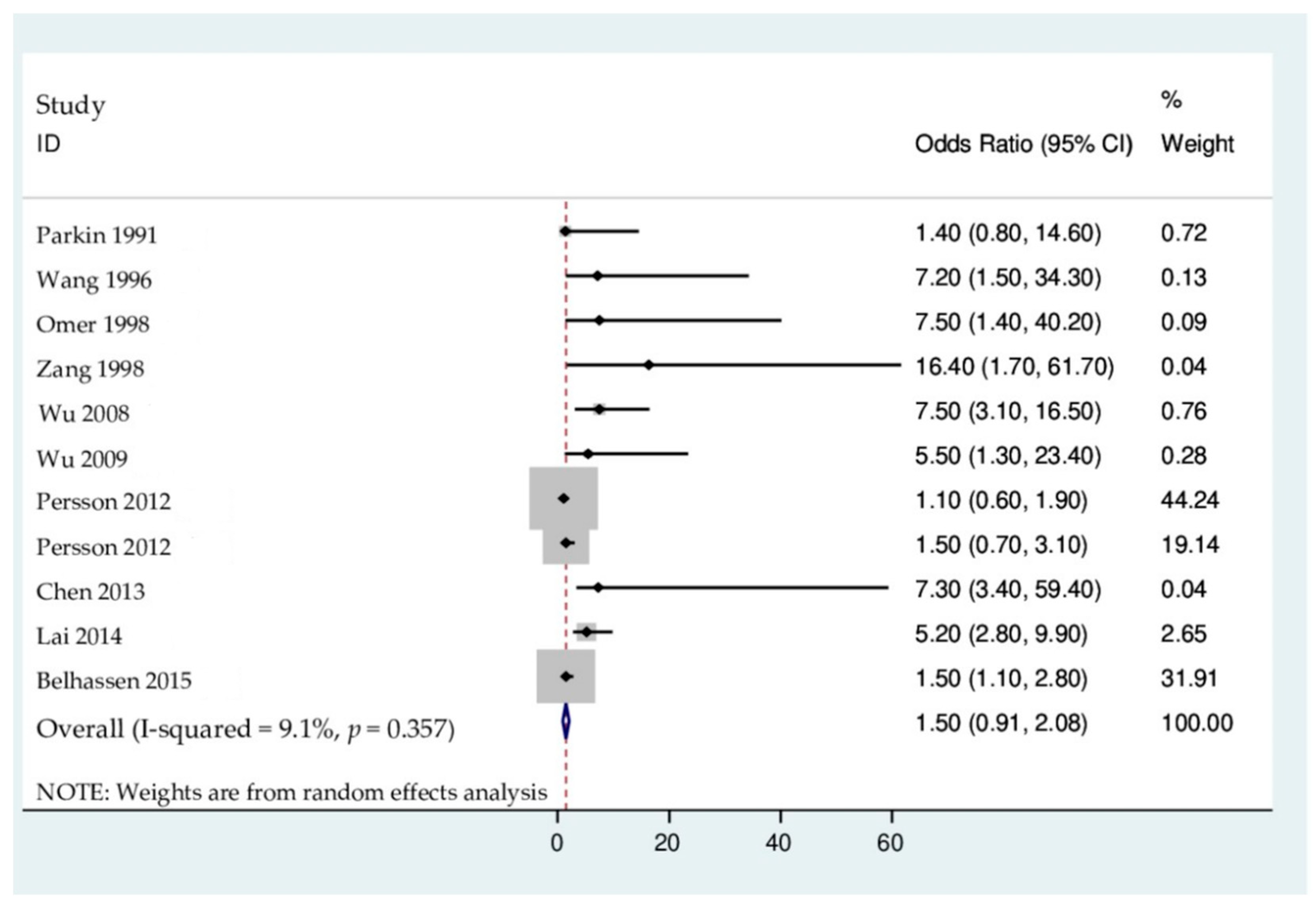
3.4. Results of the Association on Mycotoxin-Linked Mutations and Cancer Risk
4. Discussion
4.1. Mycotoxin-Linked Mutations and Increased Primary Liver Cancer Risks
4.2. Mycotoxin-Linked Mutations and Increased Breast Cancer Risks
4.3. Mycotoxin-Linked Mutations and Increased Cervical Cancer Risks
4.4. Mycotoxin-Linked Mutations and Increased Colorectal Cancer Risks
4.5. Mycotoxin-Linked Mutations and Increased Esophageal Cancer Risks
5. Way Forward and Future Research
5.1. Conclusions
5.2. Limitations of the Systematic Review
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- De Ruyck, K.; Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: Short review. Mutat. Res.-Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, M.A.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of different mycotoxins on humans, cell genome and their involvement in cancer. Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin exposure and human cancer risk: A systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Ekwomadu, T.I. Occurrence and Variation of Fusarium Free and Masked Mycotoxins in Maize from Agriculture Regions of South Africa. Ph.D. Thesis, North-West University, Mmabatho, South Africa, 2019. [Google Scholar]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-Harvest Fungal Ecology: Impact of Fungal Growth and Mycotoxin Accumulation in Stored Grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Magan, N.; Medina-Vaya, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- D’Mello, J.F.; Macdonald, A.M.; Postel, D.; Dijksma, W.T.; Dujardin, A.; Placinta, C.M. Pesticide Use and Mycotoxin Production in Fusarium and Aspergillus Phytopathogens. Eur. J. Plant Pathol. 1998, 104, 741–751. [Google Scholar]
- D’Mello, J.P.F.; Macdonald, A.M.C. Mycotoxins. Anim. Feed Sci. Technol. 1997, 69, 155–166. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Bouras, N.; Kim, Y.M.; Strelkov, S.E. Influence of water activity and temperature on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Int. J. Food Microbiol. 2009, 131, 251–255. [Google Scholar] [CrossRef]
- Lemmens, M.; Buerstmayr, H.; Krska, R.; Schuhmacher, R.; Grausgruber, H.; Ruckenbauer, P. The effect of inoculation treatment and long-term application of moisture on Fusarium head blight symptoms and deoxynivalenol contamination in wheat grains. Eur. J. Plant Pathol. 2004, 110, 299–308. [Google Scholar] [CrossRef]
- Sinha, V.; Ranjan, K.; Pandey, T. Mycotoxigenic infestation in samples of cereal straw from Bihar, India. Mycotoxin Res. 2001, 17, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Alinezhad, S.; Tolouee, M.; Kamalzadeh, A.; Motalebi, A.A.; Nazeri, M.; Yasemi, M.; Shams-Ghahfarokhi, M.; Tolouei, R.; Razzaghi-Abyaneh, M. Mycobiota and aflatoxin B1 contamination of rainbow trout (Oncorhinchus mykiss) feed with emphasis to Aspergillus section Flavi. Iran. J. Fish. 2011, 10, 363–374. [Google Scholar]
- Ekwomadu, T.; Mwanza, M. A decade of mycotoxin research in Africa: A review. In Mycotoxins, Occurrence, Toxicology and Management Strategies; Rios, C., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2015; pp. 169–213. [Google Scholar]
- Jarvis, B.B.; Miller, J.D. Mycotoxins as harmful indoor air contaminants. Appl. Microbiol. Biotechnol. 2005, 66, 367–372. [Google Scholar] [CrossRef]
- Serra, R.; Braga, A.; Venâncio, A. Mycotoxin-producing and other fungi isolated from grapes for wine production, with particular emphasis on Ochratoxin A. Res. Microbiol. 2005, 156, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Baertschi, S.W.; Raney, K.D.; Stone, M.P.; Harris, T.M. Preparation of the 8, 9-epoxide of the mycotoxin aflatoxin B1: The ultimate carcinogenic species. J. Am. Chem. Soc. 1988, 110, 7929–7931. [Google Scholar] [CrossRef]
- Yates, M.S.; Kwak, M.-K.; Egner, P.A.; Groopman, J.D.; Bodreddigari, S.; Sutter, T.R.; Baumgartner, K.J.; Roebuck, B.D.; Liby, K.T.; Yore, M.M.; et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl] imidazole. Cancer Res. 2006, 66, 2488–2494. [Google Scholar] [CrossRef] [Green Version]
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas–Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef]
- Dragan, Y.P.; Bidlack, W.R.; Cohen, S.M.; Goldsworthy, T.L.; Hard, G.C.; Howard, P.C.; Riley, R.T.; Voss, K.A. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: Fumonisin B as an example. Toxicol. Sci. 2001, 61, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Voss, K.A.; Howard, P.C.; Riley, R.; Sharma, R.P.; Bucci, T.J.; Lorentzen, R.J. Carcinogenicity and mechanism of action of fumonisin B1, A mycotoxin produced by Fusarium moniliforme (F. verticillioides). Cancer Detect Prev. 2002, 26, 1–9. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsyn-Ellis, F.E. Aflatoxins and ochratoxins in serum samples of school children. J. Nutr. Environ. Med. 2007, 16, 52–58. [Google Scholar] [CrossRef]
- Marquardt, R.R.; Frohlich, A.A. A review of recent advances in understanding ochratoxicosis. J. Anim. Sci. 1992, 70, 3968–3988. [Google Scholar] [CrossRef]
- Rahimtula, A.; Béréziat, J.-C.; Bussacchini-Griot, V.; Bartsch, H. Lipid peroxidation as a possible cause of ochratoxin a toxicity. Biochem. Pharmacol. 1988, 37, 4469–4477. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone (ZEA) in Food; EFSA European Food Safety Authority: Parma, Italy, 2011. [Google Scholar]
- Schothorst, R.C.; van Egmond, H.P. Schothorst and van Egmond Report from SCOOP task 3.2.10 ‘collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states’. Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef]
- Moss, M.O. Mycotoxin review-2. Fusarium Mycol. 2002, 16, 158–161. [Google Scholar]
- Yazar, S.; Omurtag, G.Z. Fumonisins, trichothecenes and zearalenone in cereals. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, D.K. A Review on the Statistical Methods and Implementation to Homogeneity Assessment of Certified Reference Materials in Relation to Uncertainty. MAPAN 2020, 35, 457–470. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; McKenzie, J.E. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Wells, G.A.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp (accessed on 15 January 2021).
- Bulatao-Jayme, J.; Almero, E.M.; Castro, M.C.; Jardeleza, M.T.; Salamat, L.A. A case-control dietary study of primary liver cancer risk from aflatoxin exposure. Int. J. Epidemiol. 1982, 11, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Srivatanakul, P.; Khlat, M.; Chenvidhya, D.; Chotiwan, P.; Insiripong, S.; Wild, C.P. Liver cancer in Thailand. I. A case–control study of cholangiocarcinoma. Int. J. Cancer 1991, 48, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.C.; Lo, D.; Bloodworth, B.C.; Gunasegaram, R.; Koh, T.H.; Ng, H.S. Aflatoxin Exposure in Singapore: Blood Aflatoxin Levels in Normal Subjects, Hepatitis B Virus Carriers and Primary Hepatocellular Carcinoma Patients. Med. Sci. Law 1994, 34, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Hatch, M.; Chen, C.J.; Levin, B.; You, S.L.; Lu, S.N.; Santella, R.M. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int. J. Cancer. 1996, 67, 620–625. [Google Scholar] [CrossRef]
- Omer, R.E.; Bakker, M.I.; Veer, P.V.; Hoogenboom, R.L.A.P.; Polman, T.H.G.; Alink, G.M.; Idris, M.O.; Kadaru, A.M.Y.; Kok, F.J. Aflatoxin and liver cancer in Sudan. Nutr. Cancer 1998, 32, 174–180. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Han, S.G.; Wang, X.; Zhuang, H. A case-control study of risk factors for hepatocellular carcinoma in Henan, China. Am. J. Trop. Med. Hyg. 1998, 59, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Pillay, D.; Chuturgoon, A.A.; Nevines, E.; Manickum, T.; Deppe, W.; Dutton, M.F. The Quantitative Analysis of Zearalenone and Its Derivatives in Plasma of Patients with Breast and Cervical Cancer. Clin. Chem. Lab. Med. 2002, 40, 946–951. [Google Scholar] [CrossRef]
- Wu, H.C.; Wang, Q.; Yang, H.I.; Ahsan, H.; Tsai, W.Y.; Wang, L.Y.; Santella, R.M. Urinary 15-F2t-isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis 2008, 29, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Wang, Q.; Yang, H.-I.; Ahsan, H.; Tsai, W.-Y.; Wang, L.-Y.; Chen, S.-Y.; Chen, C.-J.; Santella, R.M. Aflatoxin B1 Exposure, Hepatitis B Virus Infection, and Hepatocellular Carcinoma in Taiwan. Cancer Epidemiol. Biomark. Prev. 2009, 18, 846–853. [Google Scholar] [CrossRef] [Green Version]
- Persson, E.C.; Sewram, V.; Evans, A.A.; London, W.T.; Volkwyn, Y.; Shen, Y.-J.; Van Zyl, J.A.; Chen, G.; Lin, W.; Shephard, G.S.; et al. Fumonisin B1 and risk of hepatocellular carcinoma in two Chinese cohorts. Food Chem. Toxicol. 2012, 50, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.; Mo, X.; Yang, Y.; He, K.; Xiao, J.; Liu, C.; Chen, J.; Lin, Y. Association between aflatoxin B1 occupational airway exposure and risk of hepatocellular carcinoma: A case-control study. Tumor Biol. 2014, 35, 9577–9584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belhassen, H.; Jiménez-Díaz, I.; Arrebola, J.; Ghali, R.; Ghorbel, H.; Olea, N.; Hedili, A. Zearalenone and its metabolites in urine and breast cancer risk: A case-control study in Tunisia. Chemosphere 2015, 128, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ouhibi, S.; Vidal, A.; Martins, C.; Gali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. LC-MS/MS methodology for simultaneous determination of patulin and citrinin in urine and plasma applied to a pilot study in colorectal cancer patients. Food Chem. Toxicol. 2020, 136, 110994. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, F.; Escrivá, L.; Adel Rad, K.B.; Khoshnia, M.; Barba, F.J.; Berrada, H. Biomonitoring of Multiple Mycotoxins in Urine by GC–MS/MS: A Pilot Study on Patients with Esophageal Cancer in Golestan Province, Northeastern Iran. Toxins 2021, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Egner, P.A.; Ng, D.; Jacobson, L.P.; Munoz, A.; Zhu, Y.R.; Kensler, T.W. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev. Res. 2013, 6, 1038–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forner, A.; Llovet, J.; Bruix, J. Diet, Nutrition, Physical Activity and Liver Cancer. Diet Nutr. Phys. Act. Liver Cancer 2015, 49. Available online: https://www.wcrf.org/sites/default/files/Liver-cancer-report.pdf (accessed on 14 January 2021).
- Baertschi, S.W.; Raney, K.D.; Shimada, T.; Harris, T.M.; Guengerich, F.P. Comparison of rates of enzymatic oxidation of aflatoxin B1, aflatoxin G1, and sterigmatocystin and activities of the epoxides in forming guanyl-N7 adducts and inducing different genetic responses. Chem. Res. Toxicol. 1989, 2, 112–114. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2016, 33, 65–73. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- IARC. Aflatoxins. Chemical Agents and Related Occupations. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F, pp. 225–248. [Google Scholar]
- IARC. Agents Classified by the IARC Monographs; IARC: Lyon, France, 2019; Volumes 1–123. [Google Scholar]
- IARC. Mycotoxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; No. 56; IARC: Lyon, France, 1993; p. 599. [Google Scholar]
- McGlynn, K.A.; London, W.T. Epidemiology and natural history of hepatocellular carcinoma. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 3–23. [Google Scholar] [CrossRef]
- Long, X.D.; Yao, J.G.; Huang, Y.Z.; Huang, X.Y.; Ban, F.Z.; Yao, L.M.; Fan, L.D. DNA repair gene XRCC7 polymorphisms (rs# 7003908 and rs# 10109984) and hepatocellular carcinoma related to AFB1 exposure among Guangxi population, China. Hepatol. Res. 2011, 41, 1085–1093. [Google Scholar] [PubMed]
- Huang, M.; Yu, W.; Teoh, W.W.; Ardin, M.; Jusakul, A.; Ng, A.W.T.; Boot, A.; Abedi-Ardekani, B.; Villar, S.; Myint, S.S.; et al. Genome-scale mutational signatures of aflatoxin in cells, mice, and human tumors. Genome Res. 2017, 27, 1475–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCullough, A.K.; Lloyd, R.S. Mechanisms underlying aflatoxin-associated mutagenesis—Implications in carcinogenesis. DNA Repair 2019, 77, 76–86. [Google Scholar] [CrossRef]
- Eaton, D.L.; Groopman, J.D. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- He, X.Y.; Tang, L.; Wang, S.L.; Cai, Q.S.; Wang, J.S.; Hong, J.Y. Efficient activation of aflatoxin B1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. Int. J. Cancer 2006, 118, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Bbosa, G.S.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.W.; Kyegombe, D.B. Review of the biological and health effects of aflatoxins on body organs and body systems. Aflatoxins-Recent Adv. Future Prospect. 2015, 12, 239–265. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Petersen, A. Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA J. 2018, 16, e05175. [Google Scholar]
- Turner, P.C.; Sylla, A.; Diallo, M.S.; Castegnaro, J.J.; Hall, A.J.; Wild, C.P. The role of aflatoxins and hepatitis viruses in the etiopathogenesis of hepatocellular carcinoma: A basis for primary prevention in Guinea-Conakry, West Africa. J. Gastroenterol. Hepatol. 2002, 17, S441–S448. [Google Scholar] [CrossRef]
- IARC. Mycotoxin control in low- and middle-income countries. In IARC Working Group Report; Wild, C.P., Miller, J.D., Groopman, J.D., Eds.; IARC: Lyon, France, 2015. [Google Scholar]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by Fusarium Species. Appl. Environ. Microbiol. 2002, 68, 2101–2105. [Google Scholar] [CrossRef] [Green Version]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2002; Volume 82, p. 338. [Google Scholar]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef]
- Riley, R.T.; Torres, O.; Matute, J.; Gregory, S.; Ashley-Koch, A.; Showker, J.L.; Mitchell, T.R.; Voss, K.A.; Maddox, J.R.; Waes, J.B.G.-V. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize-based foods and living in high exposure communities in Guatemala. Mol. Nutr. Food Res. 2015, 59, 2209–2224. [Google Scholar] [CrossRef] [Green Version]
- Torres, O.; Matute, J.; Waes, J.G.-V.; Maddox, J.; Gregory, S.; Ashley-Koch, A.; Showker, J.; Voss, K.; Riley, R. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin J. 2015, 8, 143–159. [Google Scholar] [CrossRef]
- Sewram, V.; Nair, J.J.; Nieuwoudt, T.W.; Gelderblom, W.C.; Marasas, W.F.; Shephard, G.S. Assessing chronic exposure to fumonisin mycotoxins: The use of hair as a suitable non-invasive matrix. J. Anal. Toxicol. 2001, 25, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewram, V.; Mshicileli, N.; Shephard, G.S.; Marasas, W.F.O. Fumonisin mycotoxins in human hair. Biomarkers 2003, 8, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Delongchamp, R.R.; Young, J.F. Tissue sphinganine as a biomarker of fumonisin-induced apoptosis. Food Addit. Contam. 2001, 18, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Van Der Westhuizen, L.; Sewram, V. Biomarkers of exposure to fumonisin mycotoxins: A review. Food Addit. Contam. 2007, 24, 1196–1201. [Google Scholar] [CrossRef]
- Riley, R.T.; Torres, O.; Showker, J.L.; Zitomer, N.C.; Matute, J.; Voss, K.A.; Waes, J.G.-V.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol. Nutr. Food Res. 2012, 56, 1445–1455. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.; Mengelers, M.; Yang, S.; De Saeger, S.; De Boevre, M. Mycotoxin Biomarkers of Exposure: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1127–1155. [Google Scholar] [CrossRef] [Green Version]
- Pfohl-Leszkowicz, A.; Chekir-Ghedira, L.; Bacha, H. Genotoxicity of zearalenone, an estrogenic mycotoxin: DNA adduct formation in female mouse tissues. Carcinogenesis 1995, 16, 2315–2320. [Google Scholar] [CrossRef]
- Eriksen, G.S.; Alexander, J. Fusarium Toxins in Cereals—A Risk Assessment; Tema Nord 502; Nordic Council of Ministers: Copenhagen, Denmark, 1998. [Google Scholar]
- EFSA European Food Safety Authority. Scientific Opinion on the Risks for Public and Animal Health Related to the Presence of Citrinin in Food and Feed; EFSA European Food Safety Authority: Parma, Italy, 2012. [Google Scholar]
- JECFA. Evaluations of Certain Food Additives and Contaminants; WHO Technical Report Series 859; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and Toxicological Effects of Patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Ref. | First Author | Year | Study Population | Study Design | Follow-Up Duration | Exposure | Matrix |
|---|---|---|---|---|---|---|---|
| [35] | Bulatao-Jayme | 1982 | Philippines | Case control | N/R | Dietary | Urine |
| [36] | Parkin | 1991 | Thailand | Case control | 1 year | Dietary | Blood and faeces |
| [37] | Chao | 1994 | Singapore | Case control | 2 years | N/R | Blood and liver |
| [38] | Wang | 1996 | Taiwan | Case control | 4 years 5 months | Environmental | Blood and urine |
| [39] | Omer | 1998 | Sudan | Case control | 7 months | N/R | Food |
| [40] | Zang | 1998 | China | Case control | 1 year 10 months | Dietary | Food |
| [41] | Pillay | 2002 | South Africa | Case control | N/R | N/R | Plasma |
| [42] | Wu | 2008 | Taiwan | Case control | 10 years 5 months | Environmental | Urine |
| [43] | Wu | 2009 | Taiwan | Case control | 13 years 5 months | Environmental | Urine |
| [44]a | Persson | 2012 | China | Case control | 7 years 8 months | N/R | Toenails |
| [44]b | Persson | 2012 | China | Case control | 10 years | N/R | Toenails |
| [49] | Chen | 2013 | China | Cohort | 30 years | Dietary | Serum |
| [45] | Lai | 2014 | China | Case control | 6 months | Environmental | Dust and serum |
| [46] | Belhassen | 2015 | Tunisia | Case control | 6 months | N/R | Urine |
| [47] | Ouhibi | 2020 | Tunisia | Case control | N/R | N/R | Blood and urine |
| [48] | Niknejad | 2021 | Iran | Case control | N/R | N/R | Urine |
| Study | Selection | Comparability | Exposure/Outcome | Total Score |
|---|---|---|---|---|
| Bulatao-Jayme et al., 1982 [35] | *** | * | ** | 6 |
| Parkin et al., 1991 [36] | *** | ** | * | 6 |
| Chao et al., 1994 [37] | * | - | - | 1 |
| Wang et al., 1996 [38] | *** | ** | * | 6 |
| Omer et al., 1998 [39] | *** | ** | * | 6 |
| Zang et al., 1998 [40] | *** | ** | * | 6 |
| Pillay et al., 2002 [41] | * | * | - | 2 |
| Wu et al., 2008 [42] | **** | ** | * | 7 |
| Wu et al., 2008 [43] | **** | ** | * | 7 |
| Persson et al., 2012 [44]a | **** | ** | * | 7 |
| Persson et al., 2012 [44]b | **** | ** | * | 7 |
| Chen et al., 2013 [49] | - | * | - | 1 |
| Lai et al., 2014 [45] | **** | ** | * | 7 |
| Belhassen et al., 2015 [46] | ** | * | *** | 6 |
| Ouhibi et al., 2020 [47] | *** | * | * | 5 |
| Niknejad et al., 2021 [48] | ** | * | * | 4 |
| Ref. | Author | Year | Sample Size | Mycotoxin | Technique | LOD:LOQ | Cancer Type (s) | RRs | ORs | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| [35] | Bulatao-Jayme | 1982 | 180 | Aflatoxins | N/R | N/R | PLC | 1/3.9/17.5/35.0 | N/R | N/R |
| [36] | Parkin | 1991 | 206 | Aflatoxins | ELISA | N/R | PLC | N/R | 1.4 | 0.8–14.6 |
| [37] | Chao | 1994 | 481 | Aflatoxins | N/R | PLC:HCC | N/R | N/R | N/R | |
| [38] | Wang | 1996 | 276 | Aflatoxins | ELISA | 0.1 fm/ug | PLC:HCC | N/R | 7.22 | 1.5–34.3 |
| [39] | Omer | 1998 | 58 | Aflatoxins | HPLC | N/R | PLC:HCC | N/R | 7.5 | 1.4–40.2 |
| [40] | Zang | 1998 | 267 | Aflatoxins | N/R | PLC:HCC | N/R | 16.44 | 1.67–61.65 | |
| [41] | Pillay | 2002 | 106 | Zearalenone α-zearalanol b-zearalenol | HPLC GC-MS | 25 ng/mL | Breast: cervix | N/R | N/R | N/R |
| [42] | Wu | 2008 | 364 | Aflatoxin B1 | ELISA | 0.2 ng/mL | PLC:HCC | N/R | 7.5 | 3.14–16.46 |
| [43] | Wu | 2009 | 1102 | Aflatoxin B1 | ELISA | N/R | PLC:HCC | N/R | 5.5 | 1.3–23.4 |
| [44]a | Persson | 2012 | 551 | FumonisinB1 | HPLC-MS-MS | 6 pg/L:20 pg/L | PLC:HCC | N/R | 1.1 | 0.64–1.89 |
| [44]b | Persson | 2012 | 219 | FumonisinB1 | HPLC-MS- MS | 6 pg/L:20 pg/L | PLC:HCC | N/R | 1.47 | 0.70–3.07 |
| [49] | Chen | 2013 | 652 | Aflatoxins | N/R | N/R | PLC | 7.3, 3.4, 59.4 | N/R | N/R |
| [45] | Lai | 2014 | 218 | Aflatoxins | ELISA | N/R | PLC:HCC | N/R | 5.24 | 2.77–9.88 |
| [46] | Belhassen | 2015 | 110 | α-zearalanol | UHPLC-MS/MS | 0.2 ng/mL:0.7 ng/mL | Breast | N/R | 1.54 | 1.10–2.77 |
| [47] | Ouhibi | 2020 | 100 | Citrinin and Patulin | LC-MS/MS | 1 ng/mL:2.88 ng/mL | Colorectal | N/R | N/R | N/R |
| [48] | Niknejad | 2021 | 27 | NEO, HT-2, T-2 | GC-MS/MS | 0.25:0.5 ug/L 1:2 ug/L 0.5:1 ug/L | Esophageal | N/R | N/R | N/R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekwomadu, T.; Mwanza, M.; Musekiwa, A. Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue. Int. J. Environ. Res. Public Health 2022, 19, 7754. https://doi.org/10.3390/ijerph19137754
Ekwomadu T, Mwanza M, Musekiwa A. Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue. International Journal of Environmental Research and Public Health. 2022; 19(13):7754. https://doi.org/10.3390/ijerph19137754
Chicago/Turabian StyleEkwomadu, Theodora, Mulunda Mwanza, and Alfred Musekiwa. 2022. "Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue" International Journal of Environmental Research and Public Health 19, no. 13: 7754. https://doi.org/10.3390/ijerph19137754






