Effects of Tartrazine on Some Sexual Maturation Parameters in Immature Female Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Housing
2.3. Dose and Concentation Calculation
2.4. Experimental Design
2.5. Measurement of Biochemical Parameters
2.6. Histopathological Evaluation
2.7. Statistical Analysis
3. Results
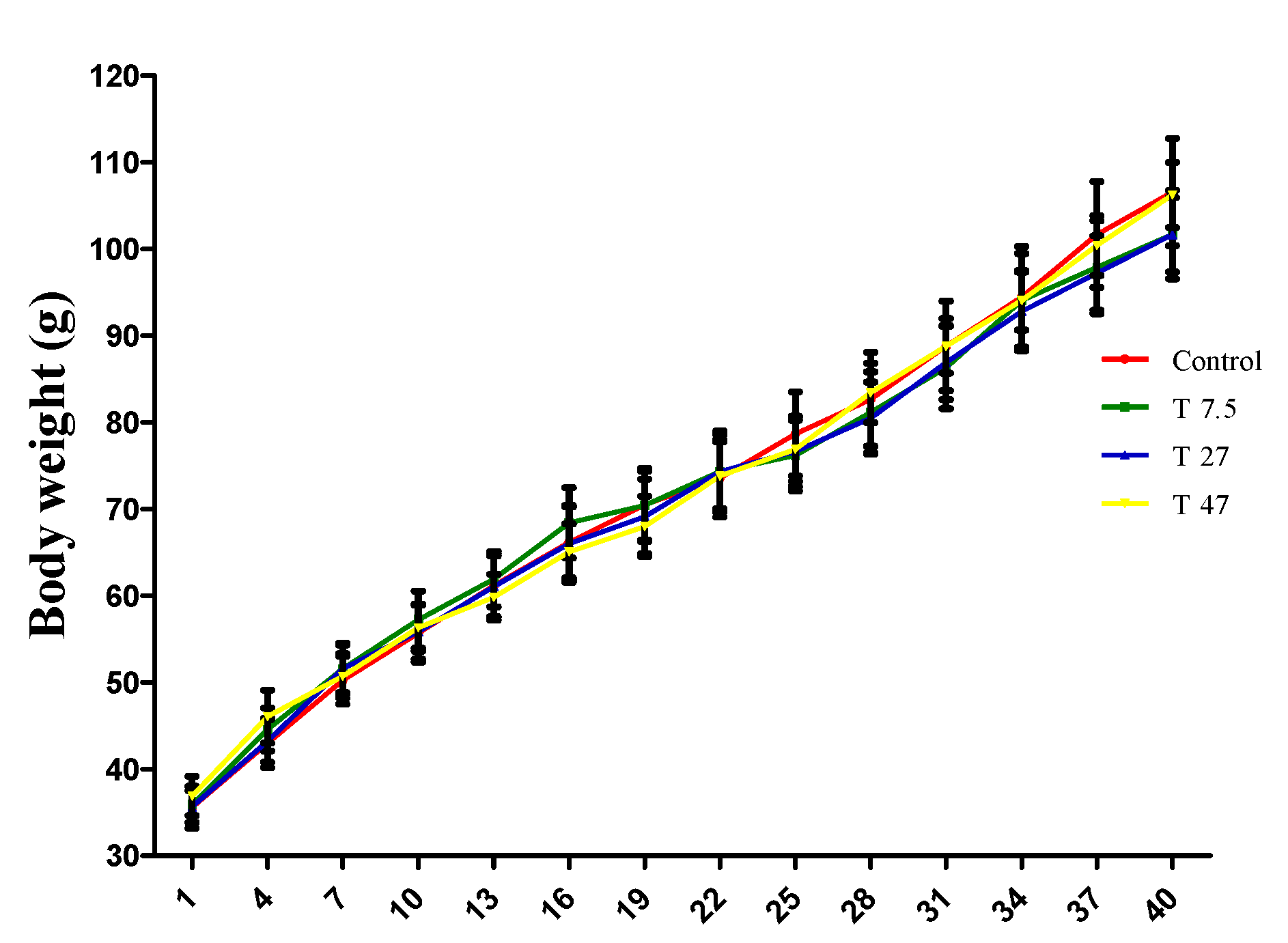
3.1. Bodyweight of Animals
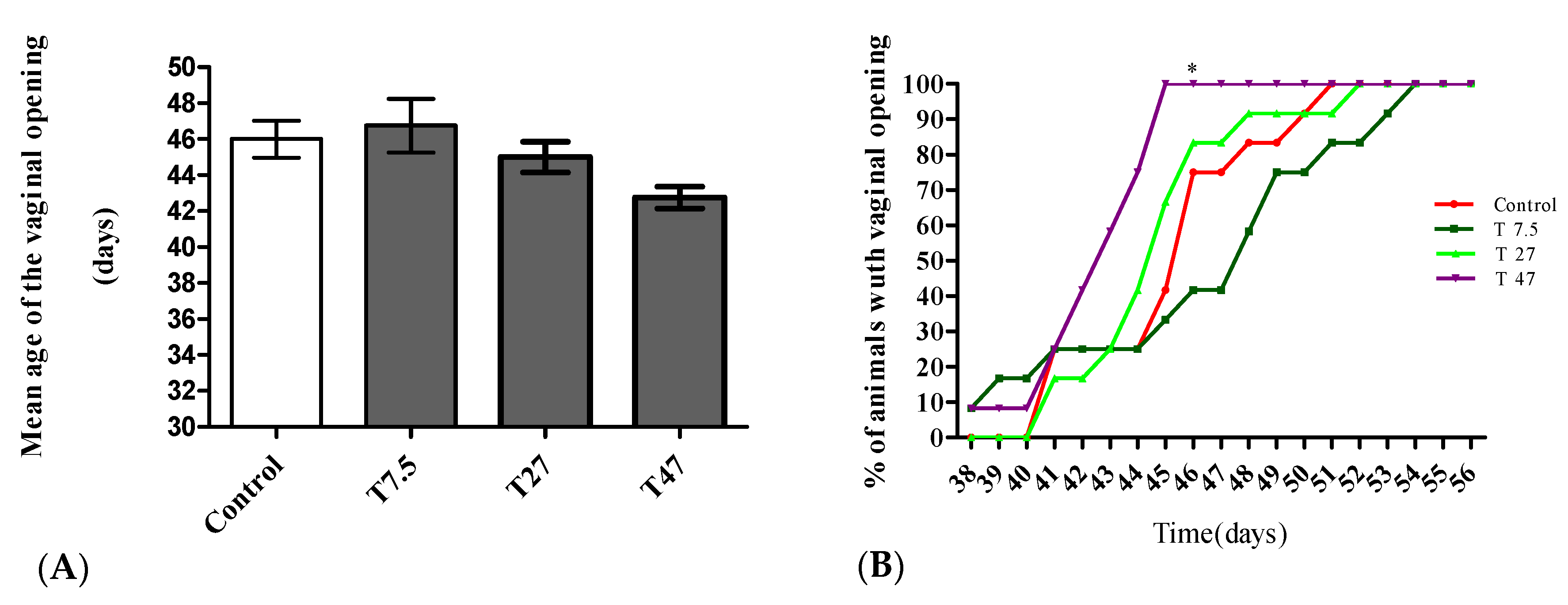
3.2. Vaginal Opening
3.3. Relative Weight of Ovary and Uterus
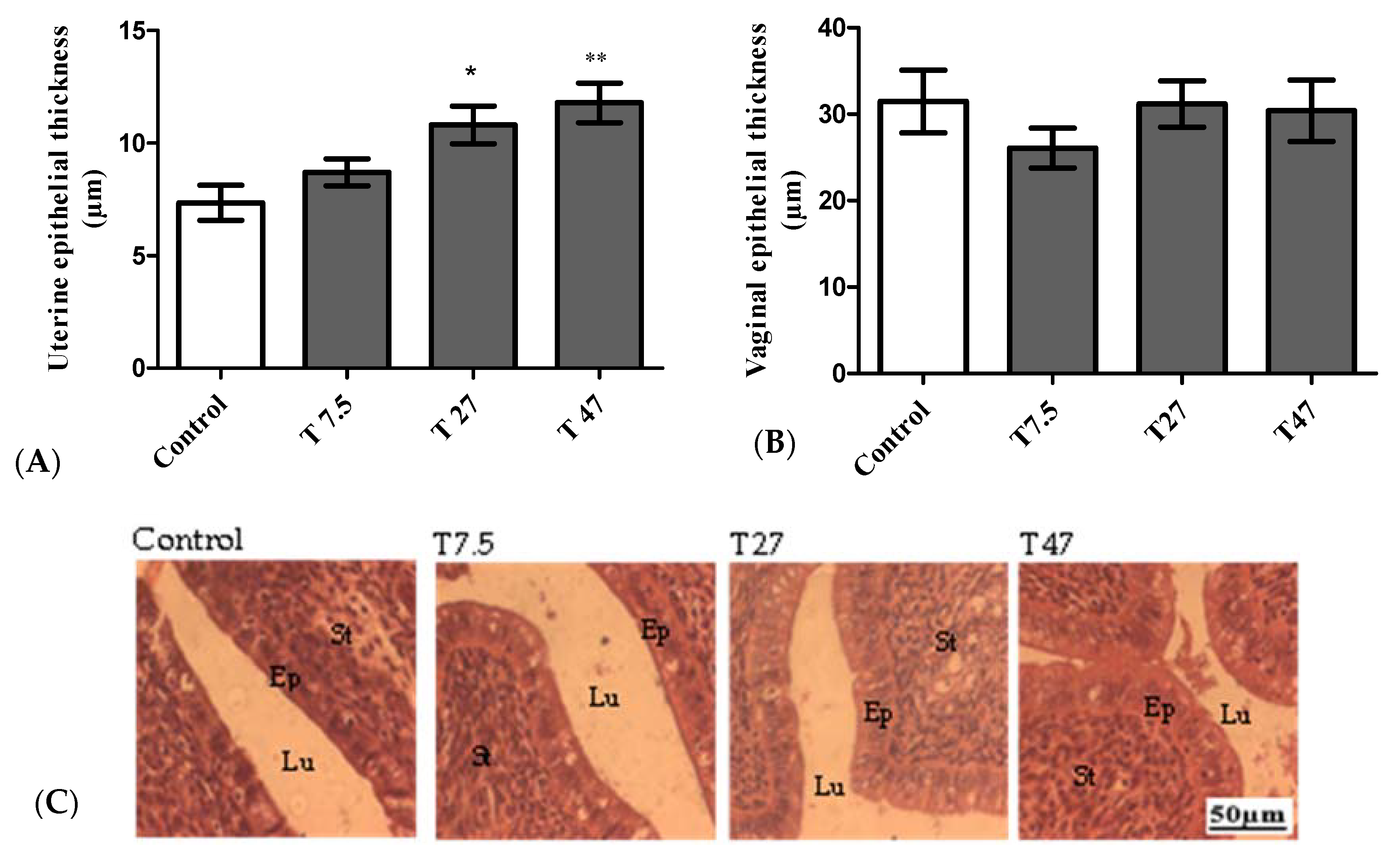
3.4. Epithelial Thickness of the Uterus and Vaginas
3.5. Mammary Glands
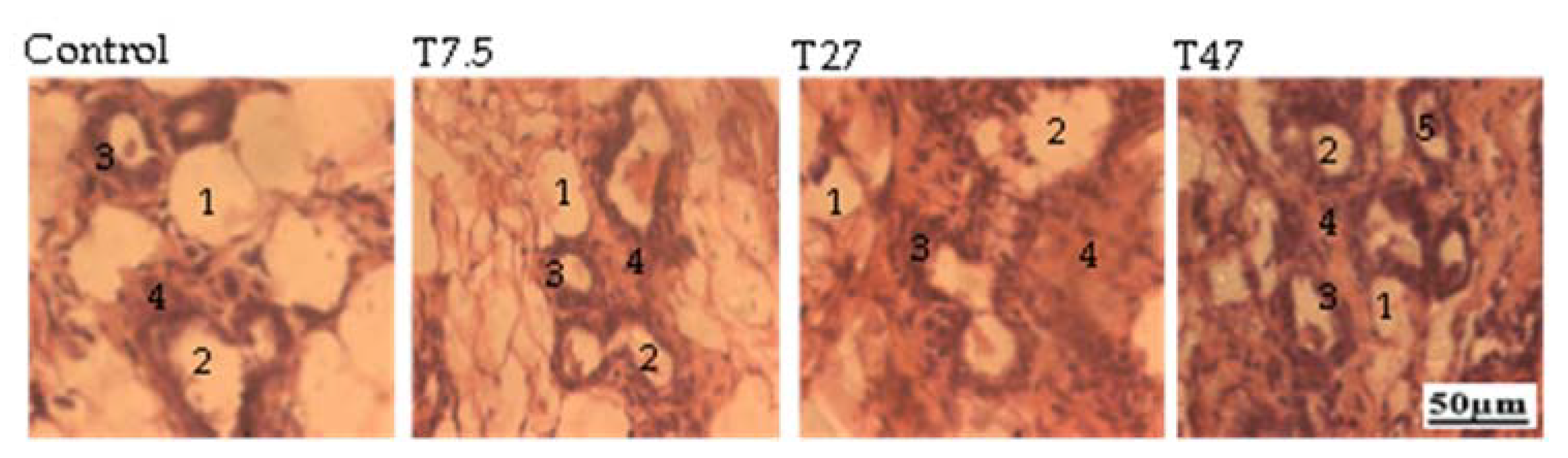
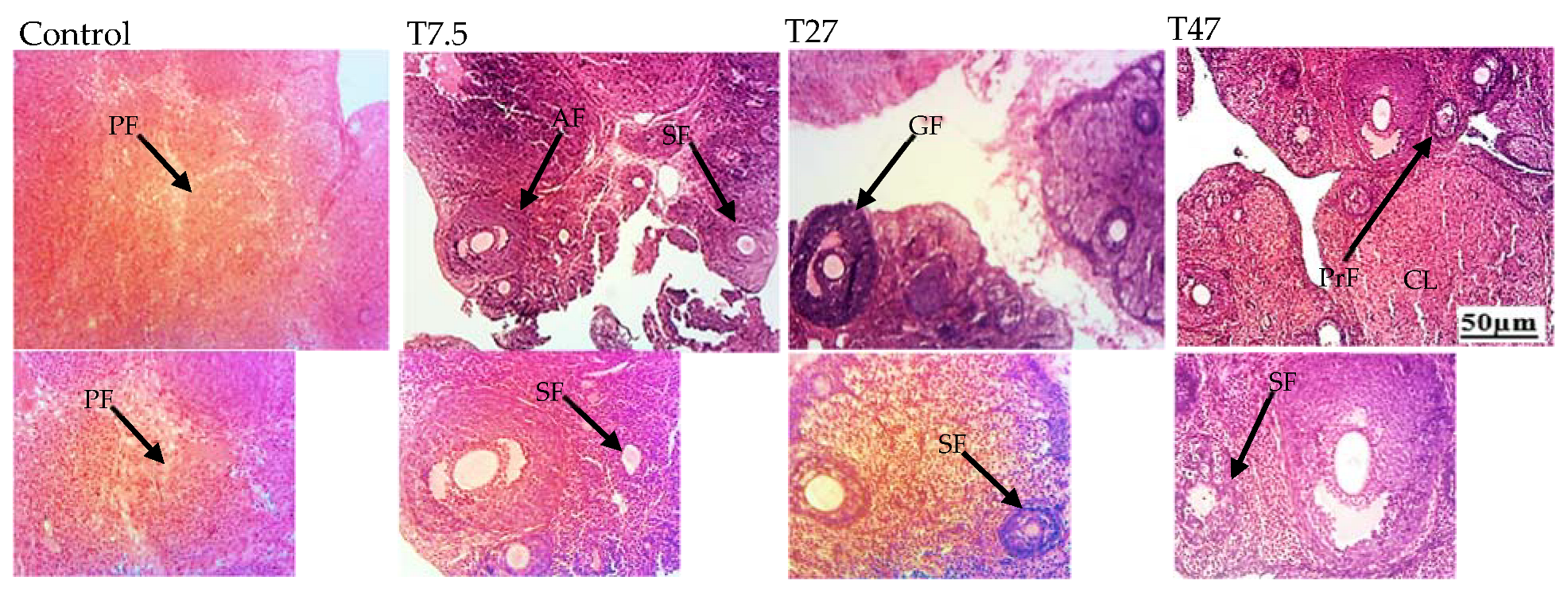
3.6. Ovarian Follicles
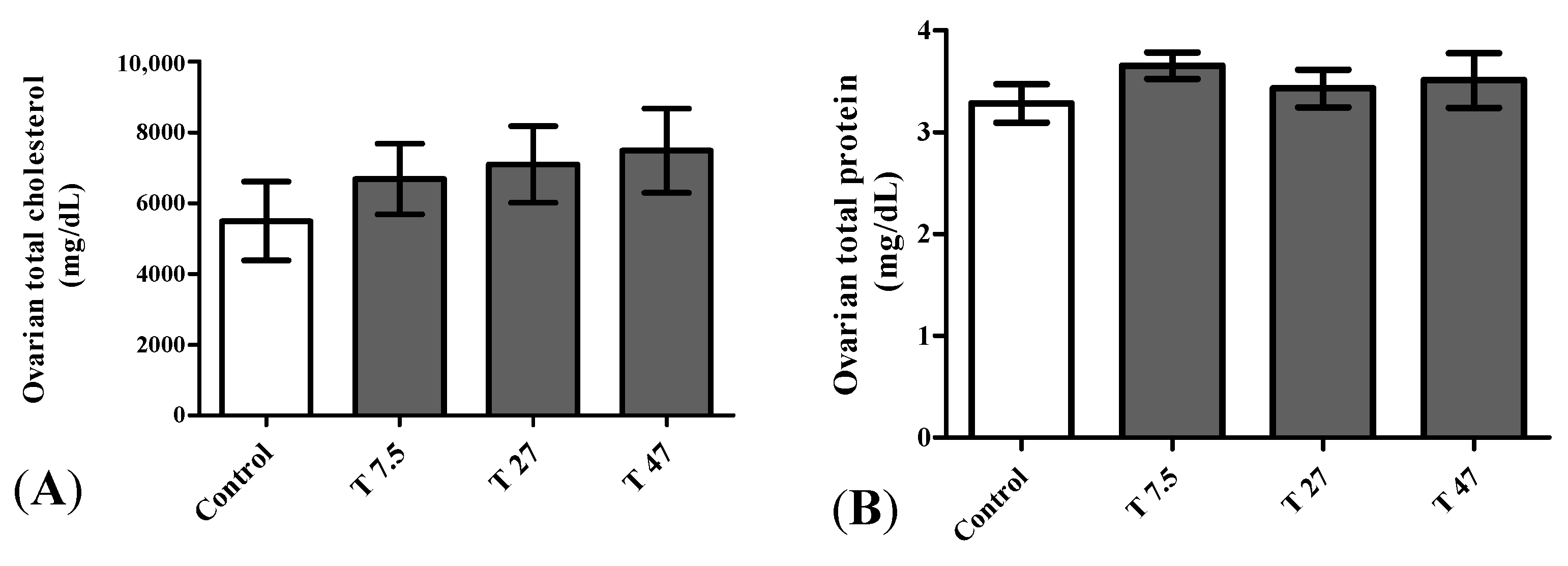
3.7. Ovarian Total Cholesterol and Proteins
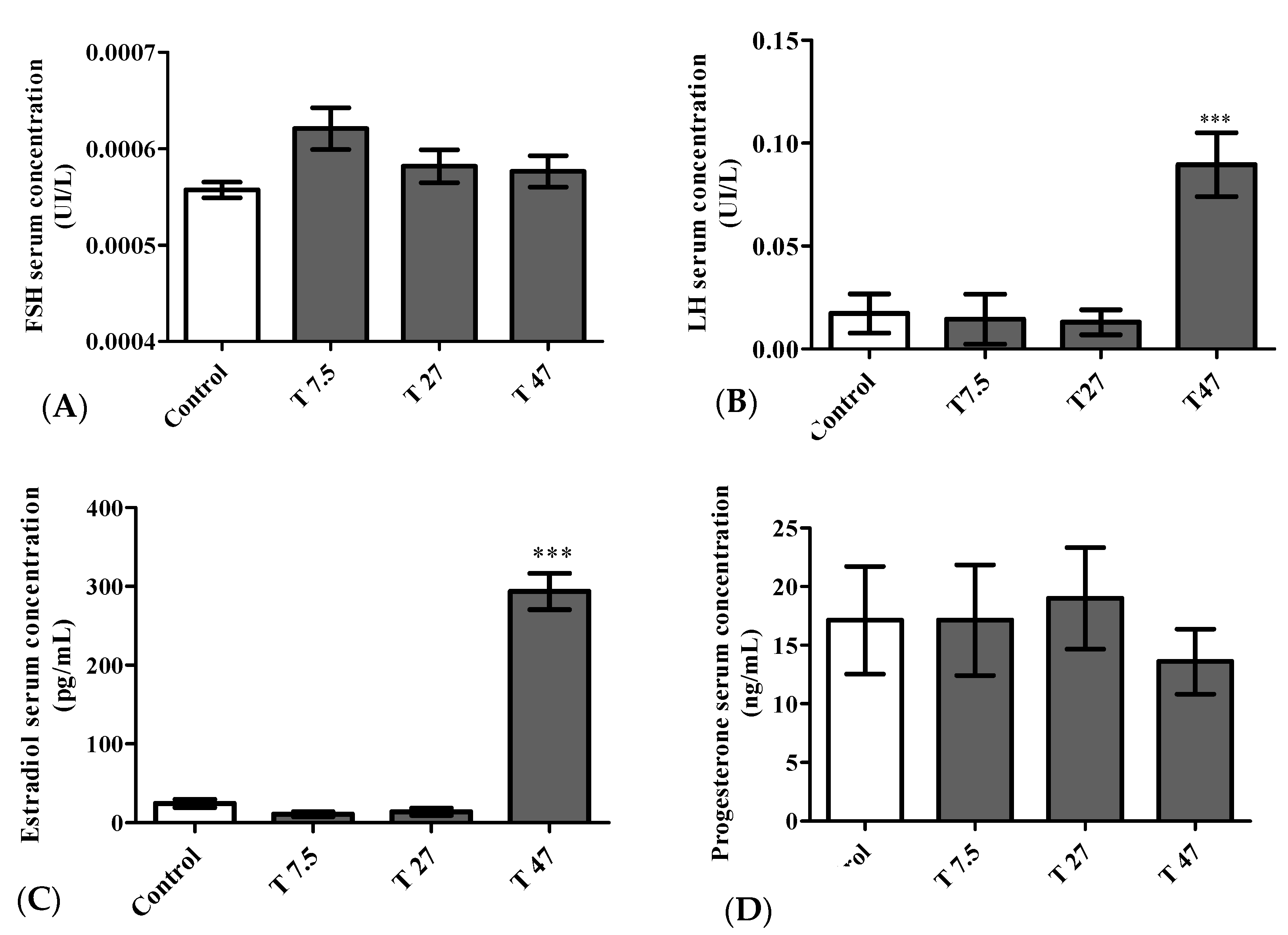
3.8. Hormone Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FSH | Follicle-stimulating hormone |
| GnRH | Gonadotropin-releasing hormone |
| LH | Luteinizing hormone |
| HED | Human equivalent dose |
| AED | Animal equivalent dose |
| BW | Body weight |
| CV | Coefficient of variation |
References
- Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Mogensen, S.S.; Juul, A. Recent Secular Trends in Pubertal Timing: Implications for Evaluation and Diagnosis of Precocious Puberty. Horm. Res. Paediatr. 2012, 77, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Camilla Eckert-Lind, M.B.; Busch, A.S.; Petersen, J.H.; Biro, F.M.; Butler, G.; Bräuner, E.V.; Juul, A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, e195881. [Google Scholar] [CrossRef] [PubMed]
- Bellis, M.A.; Downing, J.; Ashton, J.R. Adults at 12? Trends in puberty and their public health consequences. J. Epidemiol. Community Health 2006, 60, 910–911. [Google Scholar] [CrossRef] [Green Version]
- Nahime Brito, V.; Spinola-Castro, A.M.; Kochi, C.; Kopacek, C.; Alves da Silva, P.C.; Guerra-Júnior, G. Central precocious puberty: Revisiting the diagnosis and therapeutic management. Arch. Endocrinol. Metab. 2016, 60, 2. [Google Scholar]
- Aksglaede, L.; Olsen, L.W.; Sørensen, T.I.A.; Juul, A. Forty Years Trends in Timing of Pubertal Growth Spurt in 157,000 Danish School Children. PLoS ONE 2008, 3, e2728. [Google Scholar] [CrossRef] [Green Version]
- Bräuner, E.V.; Busch, A.S.; Camilla Eckert-Lind, M.B.; Koch, T.; Hickey, M.; Juul, A. Trends in the Incidence of Central Precocious Puberty and Normal Variant Puberty Among Children in Denmark, 1998 to 2017. Pediatrics 2020, 3, e2015665. [Google Scholar] [CrossRef]
- Stagi, S.; De Masi, S.; Bencin, E.; Losi, S.; Paci, S.; Parpagnoli, M.; Ricci, F.; Ciofi, D.; Azzari, C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital. J. Pediatr. 2020, 46, 165. [Google Scholar] [CrossRef]
- Euling, S.Y.; Herman-Giddens, M.E.; Lee, P.A.; Selevan, S.G.; Juul, A.; Sørensen, T.I.A.; Dunkel, L.; Himes, J.H.; Teilmann, G.; Swan, S.H. Examination of US puberty-timing data from 1940 to 1994 for secular trends: Panel findings. Pediatrics 2008, 121, 172–191. [Google Scholar] [CrossRef] [Green Version]
- Biro, F.M.; Galvez, M.P.; Greenspan, L.C.; Succop, P.A.; Vangeepuram, N.; Pinney, S.M.; Teitelbaum, S.; Windham, G.C.; Kushi, L.H. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatric 2010, 126, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Elks, C.E.; Perry, J.R.; Sulem, P.; Chasman, D.I.; Franceschini, N.; He, C.; Lunetta, K.L.; Visser, J.A.; Byrne, E.M.; Cousminer, D.L.; et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 2010, 42, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Merzenich, H.; Boeing, H.; Wahrendorf, J. Dietary fat and sports activity as determinants for age at menarche. Am. J. Epidemiol. 1993, 138, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Zhang, Y.; Sun, W.; Jiang, Y.; Song, Y.; Zhu, Q.; Mei, H.; Wang, X.; Liu, S.; et al. Association between Dietary Patterns and Precocious Puberty in Children: A Population-Based Study. Int. J. Endocrinol. 2018, 2018, 4528704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, A.; De Sanctis, V.; Elalaily, R. Nutrition and pubertal development. Indian J. Endocrinol. Metab. 2014, 18, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Muir, A. Precocious Puberty. Endocrine 2006, 27, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Marques-Pinto, A.; Carvalho, D. Human infertility: Are endocrine disruptors to blame? Endocr. Connect. 2013, 2, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Frade Costa1, E.L.; Spritzer, P.M.; Hohl, A.; Tânia, A.S.S.B. Effects of endocrine disruptors in the development of the female reproductive tract. Arq. Bras. Endocrinol. Metabol. 2014, 5, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Mínguez-Alarcon, L.; Christou, G.; Messerlian, C.; Williams, P.L.; Carignan, C.C.; Souter, I.; Ford, J.B.; Calafat, A.M.; Hauser, R. Urinary triclosan concentrations and diminished ovarian reserve among women undergoing treatment in a fertility clinic. Fertil. Steril. 2017, 108, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Colborn, T.; Clement, C. Statement from the work session on chemically-induced alterations in sexual development. In Proceedings of the Wildlife/Human Connection in Wingspread Conference Center, Racine, WI, USA, 26–28 July 1991. [Google Scholar]
- Ricard, L.-E. Les Perturbateurs Endocriniens dans L’environnement de L’enfant et de L’adolescent et le Risqué Pour la Santé, L’exemple des Phtalates et du Bisphénol A. Ph.D. Thesis, Université Henri Poincaré, Nancy, Paris, 18 May 2011. [Google Scholar]
- Tassinari, R.; Tait, S.; Busani, L.; Martinelli, A.; Valeri, M.; Gastaldelli, A.; Deodati, A.; La Rocca, C.; Maranghi, F.; LIFE PERSUADED Project Group. Toxicological Assessment of Oral Co-Exposure to Bisphenol A (BPA) and Bis(2-ethylhexyl) Phthalate (DEHP) in Juvenile Rats at Environmentally Relevant Dose Levels: Evaluation of the Synergic, Additive or Antagonistic Effects. Int. J. Environ. Res. Public Health 2021, 18, 4584. [Google Scholar] [CrossRef]
- Zingue, S.; Ndjengue Mindang, E.L.; Awounfack, C.F.; Kalgonbe Yanfou, A.; Kada Mohamet, M.; Njamen, D.; Ndinteh Tantoh, D. Oral administration of tartrazine (E102) accelerates the incidence and the development of 7,12-dimethylbenz(a) anthracene (DMBA)- induced breast cancer in rats. BMC Complement. Med. Ther. 2021, 21, 303. [Google Scholar] [CrossRef]
- Scippo, M.I.; Maghuin-Rogiste, G. Les perturbateurs endocriniens dans l’alimentation humaine: Impact potential sur la santé. Ann. Méd. Vét. 2007, 151, 44–54. [Google Scholar]
- Marieb, E.N.; Hoehn, K. Anatomie et Physiologie Humaines, Adaptation de la 8e Edition Américaine; Nouv. Hor.-ARS: Paris, France, 2010; pp. 683–729. [Google Scholar]
- Kriszt, R.; Winkler, Z.; Polyák, Á.; Kuti, D.; Molnár, C.; Hrabovszky, E.; Kalló, I.; Szoke, Z.; Ferenczi, S.; Kovács, K.J. Xenoestrogens Ethinyl Estradiol and Zearalenone Cause Precocious Puberty in Female Rats via Central Kisspeptin Signaling. Endocrinology 2015, 156, 3996–4007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awounfack, C.F.; Mvondo, M.A.; Zingue, S.; Ateba, S.B.; Djiogue, S.; Megnekou, R.; Ndinteh, D.T.; Njamen, D. Myrianthus arboreus P. Beauv (Cecropiaceae) Extracts Accelerates Sexual Maturation, and Increases Fertility Index and Gestational Rate in Female Wistar Rats. Medicines 2018, 5, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubscher, C.H.; Brooks, D.L.; Johnson, J.R. A quantitative method for assessing stages of the rat estrous cycle. Biotech. Histochem. 2005, 80, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E. The neuroendocrine control of the ovarian cycle of the rat. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 613–658. [Google Scholar]
- Paccola, C.C.; Resende, C.G.; Stumpp, T.; Miraglia, S.M.; Cipriano, I. The rat estrous cycle revisited: A quantitative and qualitative analysis. Anim. Reprod. 2013, 10, 677–683. [Google Scholar]
- Johnson, M.H. Essential Reproduction, 6th ed.; Wiley-Blackwell: New York, NY, USA, 2007; p. 316. [Google Scholar]
- Gore, A.C.; Crews, D.; Doan, L.L.; La Merrill, M.; Heather Patisaul, M.P.H.; Zota, A. Introduction to endocrine disrupting chemicals (EDCs) a guide for public interest organizations and policy-makers. Endocr. Soc. J. 2014, 99, 21–22. [Google Scholar]
- Williamson-Hughes, P.S.; Flickinger, B.D.; Messina, M.J.; Empie, M.W. Isoflavone supplements containing predominantly genistein reduce hot flash symptoms: A critical review of published studies. Menopause 2006, 13, 831–839. [Google Scholar] [CrossRef]
- Wopara, I.C.; Modo, E.U.; Mobisson, S.K.; Olusegun, G.A.; Umoren, E.B.; Orji, B.O.; Mounmbegna, P.E.; Ujunwa, S.O. Synthetic Food dyes cause testicular damage via up-regulation of pro-inflammatory cytokines and down-regulation of FSH-R and TESK-1 gene expression. JBRA Assist. Reprod. 2021, 25, 341–348. [Google Scholar] [CrossRef]
- Shakoor, S.; Ismail, A.; Zia-Ur-Rahman; Sabran, M.R.; Bekhit, A.E.D.A.; Roohinejad, S. Impact of tartrazine and curcumin on mineral status, and thyroid and reproductive hormones disruption in vivo. Int. Food Res. J. 2022, 29, 186–199. [Google Scholar] [CrossRef]
- Khera, K.S.; Munro, I.C. A review of the specifications and toxicity of synthetic food colors permitted in Canada. CRC Crit. Rev. Toxicol. 1979, 6, 81–133. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives [JECFA]. Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) 1956–1995 (First through 44th Meetings); International Life Sciences Institute (ILSI) Press: Washington, DC, USA, 1996. [Google Scholar]
- Tanaka, T. Reproductive and neurobehavioral toxicity study of tartrazine administered to mice in the diet. Food Chem. Toxicol. 2006, 44, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Rowe, K.S.; Rowe, K.J. Synthetic food coloring and behavior: A dose response effect in a double-blind, placebo controlled, repeated-measures study. J. Paediatr. 1994, 125, 691–698. [Google Scholar] [CrossRef]
- Dawodu, M.; Akpomie, K. Evaluating the potential of a Nigerian soil as an adsorbent for tartrazine dye: Isotherm, kinetic and thermodynamic studies. Alex. Eng. J. 2016, 55, 3211–3218. [Google Scholar] [CrossRef] [Green Version]
- Khayyat, L.; Essawy, A.; Sorour, J.; Soffar, A. Tartrazine induces structural and functional aberrations and genotoxic effects in vivo. PeerJ 2017, 5, e3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moutinho, I.L.D.; Bertges, L.C.; Assis, R.V.C. Prolonged use of the food dye tartrazine (FD and C yellow n°5) and its effects on the gastric mucosa of Wistar rats. Braz. J. Biol. 2007, 67, 141–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussada, M.; Lamine, J.A.; Bini, I.; Abidi, N.; Lasrem, M.; El-Fazaa, S.; El-Golli, N. Assessment of a sub-chronic consumption of tartrazine (E102) on sperm and oxidative stress features in Wistar rat. Int. Food Res. J. 2017, 24, 1473–1481. [Google Scholar]
- Mehedi, N.; Ainad-Tabet, S.; Mokrane, N.; Addou, S.; Zaoui, C.; Kheroua, O.; Saidi, D. Reproductive Toxicology of Tartrazine (FD and C Yellow No. 5) in Swiss Albino Mice. Am. J. Pharmacol. Toxicol. 2009, 4, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Axon, A.; May, E.B.F.; Gaughan, L.E.; Williams, F.M.; Blain, P.G.; Wright, M.C. Tartrazine and sunset yellow are xenoestrogens in a new screening assay to identify modulators of human estrogen receptor transcriptional activity. Toxicology 2012, 298, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Nasri, A.; Pohjanvirta, R. In vitro estrogenic, cytotoxic, and genotoxic profiles of the xenoestrogens 8-prenylnaringenine, genistein and tartrazine. Environ. Sci. Pollut. Res. 2021, 28, 27988–27997. [Google Scholar] [CrossRef]
- Datta, P.; Lundin-Schiller, S. Estrogenicity of the synthetic food colorants tartrazine, erythrosin B, and sudan I in an estrogenresponsive human breast cancer cell line. Tennessee Acad. Sci. J. 2008, 83, 45–51. [Google Scholar]
- Elhkim, M.; Heraud, F.; Bemrah, N.; Gauchard, F.; Torino, T.; Lambré, C.; Fremy, J.; Poul, J. New considerations regarding the risk assessment on tartrazine an update toxicological assessment, in intolerance reactions and maximum theoretical daily intake in France. Regul. Toxicol. Pharmacol. 2007, 47, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives [JECFA]. Specifications for identity and purity and toxicological evaluation of food colours. In FAO Nutrition Meetings Report Series No. 38B; WHO: Geneva, Switzerland, 1964. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry: Estimating the Maximum Safe Starting Dose in Adult Healthy Volunteer; Food and Drug Administration (FDA): Washington, DC, USA, 2005. [Google Scholar]
- Ramirez, V.D.; Sawywe, C.H. Advancement of puberty in the female rat by estrogen. Endocrinology 1965, 6, 1158–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forcatoa, S.; Montagninia, B.G.; Marino de Góesa, M.L.; Barrionuevo da Silva Novia, D.R.; Inhasz Kissb, A.C.; Ceravoloa, G.S.; Ceccatto Gerardina, D.C. Reproductive evaluations in female rat offspring exposed to metformin during intrauterine and intrauterine/lactational periods. Reprod. Toxicol. 2019, 87, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Faghani, M.; Saedi, S.; Khanaki, K.; Ghasemi, F.M. Ginseng alleviates folliculogenesis disorders via induction of cell proliferation and downregulation of apoptotic markers in nicotine-treated mice. J. Ovarian Res. 2022, 151, 4. [Google Scholar] [CrossRef]
- Milad, M.R.; Igoe, S.A.; Lebron-Milad, K.; Novales, J.E. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 2009, 164, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Chaitra, R.S.; Vani, V.B.; Jayamma, Y.C.; Laxmi, S. Inamdar (Doddamani). Estrous Cycle in Rodents: Phases, Characteristics and Neuroendocrine regulation. J. Sci. 2020, 51, 40–53. [Google Scholar]
- Peters, H.A.; Byskov, G.; Grinsted, J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clin. Endocrinol. Metab. 1978, 7, 469–485. [Google Scholar] [CrossRef]
- Picut, C.A.; Remick, A.K.; Asakawa, M.G.; Simons, M.L.; Parker, G.A. Histologic features of prepubertal and pubertal reproductive development in female Sprague-Dawley rats. Toxicol. Pathol. 2014, 42, 403–413. [Google Scholar] [CrossRef] [Green Version]
- te Velde, E.R. Ovarian ageing and postponement of childbearing. Maturitas 1998, 30, 103–104. [Google Scholar]
- Wilkosz, P.; Greggains, G.D.; Tanbo, T.G.; Fedorcsak, P. Female Reproductive Decline Is Determined by Remaining Ovarian Reserve and Age. PLoS ONE 2014, 9, e108343. [Google Scholar] [CrossRef] [Green Version]
- Cruz, G.; Fernandois, D.; Paredes, A.H. Ovarian function and reproductive senescence in the rat: Role of ovarian sympathetic innervation. Reproduction 2017, 153, R59–R68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njamen, D.; Mvomdo, M.A.; Nanbo, N.T.; Zingue, S.; Tanee, F.S.; Wandji, J. Erythrina lysistemon-derived flavonoids account only in part for the plant’s specific effects on rat uterus and vagina. J. Basic Clin. Physiol. Pramacol. 2015, 26, 287–294. [Google Scholar] [CrossRef]
- Mvondo, M.A.; Njamen, D.; Tanee, F.S.; Wandji, J. Effects of alpinumisoflavone and abyssinone V-4’-methyl ether derived from Erythrina lysistemon (Fabaceae) on the genital tract of ovariectomized female Wistar rat. Phytother. Res. 2012, 26, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Njamen, D.; Djiogue, S.; Zingue, S.; Mvondo, M.A.; Nkeh-Chungag, B.N. In vitro and in vivo estrogenic activity of extracts from Erythrina poeppigiana (Fabaceae). J. Complement. Int. Med. 2013, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Organs | Control | Tartrazine (mg/kg BW) | ||
|---|---|---|---|---|
| T 7.5 | T 27 | T 47 | ||
| Total follicles | 92.98 ± 11.90 | 99.38 ± 11.80 | 83.49 ± 13.74 | 145.33 ± 2.37 ** |
| Primordial follicles | 35.33± 7.83 | 28.00 ± 5.42 | 21.33 ± 3.59 | 38.00 ± 3.83 |
| Primary follicles | 22.00 ± 2.60 | 20.33 ± 2.93 | 18.66 ± 3.00 | 37.50 ± 2.53 ** |
| Secondary follicles | 16.50 ± 1.20 | 30.40 ± 5.76 | 28.25 ± 8.29 | 44.25 ± 2.37 ** |
| Antral follicles | 4.20 ± 0.96 | 6.25 ± 1.23 | 4.80 ± 1.59 | 10.33± 1.42 * |
| Graafian follicles | 5.00 ± 1.04 | 6.80 ± 0.8 | 4.80 ± 1.01 | 6.50 ± 0.92 |
| Atresia follicles | 4.75 ± 0.19 | 3.80 ± 0.66 | 3.25 ± 0.58 | 3.75 ± 0.91 |
| Corpora lutea | 5.20 ± 1.20 | 3.80 ± 1.11 | 2.40 ± 0.60 | 5.00 ± 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mindang, E.L.N.; Awounfack, C.F.; Ndinteh, D.T.; Krause, R.W.M.; Njamen, D. Effects of Tartrazine on Some Sexual Maturation Parameters in Immature Female Wistar Rats. Int. J. Environ. Res. Public Health 2022, 19, 10410. https://doi.org/10.3390/ijerph191610410
Mindang ELN, Awounfack CF, Ndinteh DT, Krause RWM, Njamen D. Effects of Tartrazine on Some Sexual Maturation Parameters in Immature Female Wistar Rats. International Journal of Environmental Research and Public Health. 2022; 19(16):10410. https://doi.org/10.3390/ijerph191610410
Chicago/Turabian StyleMindang, Elisabeth Louise Ndjengue, Charline Florence Awounfack, Derek Tantoh Ndinteh, Rui W. M. Krause, and Dieudonne Njamen. 2022. "Effects of Tartrazine on Some Sexual Maturation Parameters in Immature Female Wistar Rats" International Journal of Environmental Research and Public Health 19, no. 16: 10410. https://doi.org/10.3390/ijerph191610410
APA StyleMindang, E. L. N., Awounfack, C. F., Ndinteh, D. T., Krause, R. W. M., & Njamen, D. (2022). Effects of Tartrazine on Some Sexual Maturation Parameters in Immature Female Wistar Rats. International Journal of Environmental Research and Public Health, 19(16), 10410. https://doi.org/10.3390/ijerph191610410






