Exploiting the Waste Biomass of Durian Shell as a Heterogeneous Catalyst for Biodiesel Production at Room Temperature
Abstract
:1. Introduction
2. Experimental
2.1. Preparation and Collection of Catalyst
2.2. Characterization of the Ash Catalyst
2.3. Transesterification Reaction of Palm Oil
2.4. Reusability Analysis
3. Results and Discussion
3.1. Catalyst Characterization
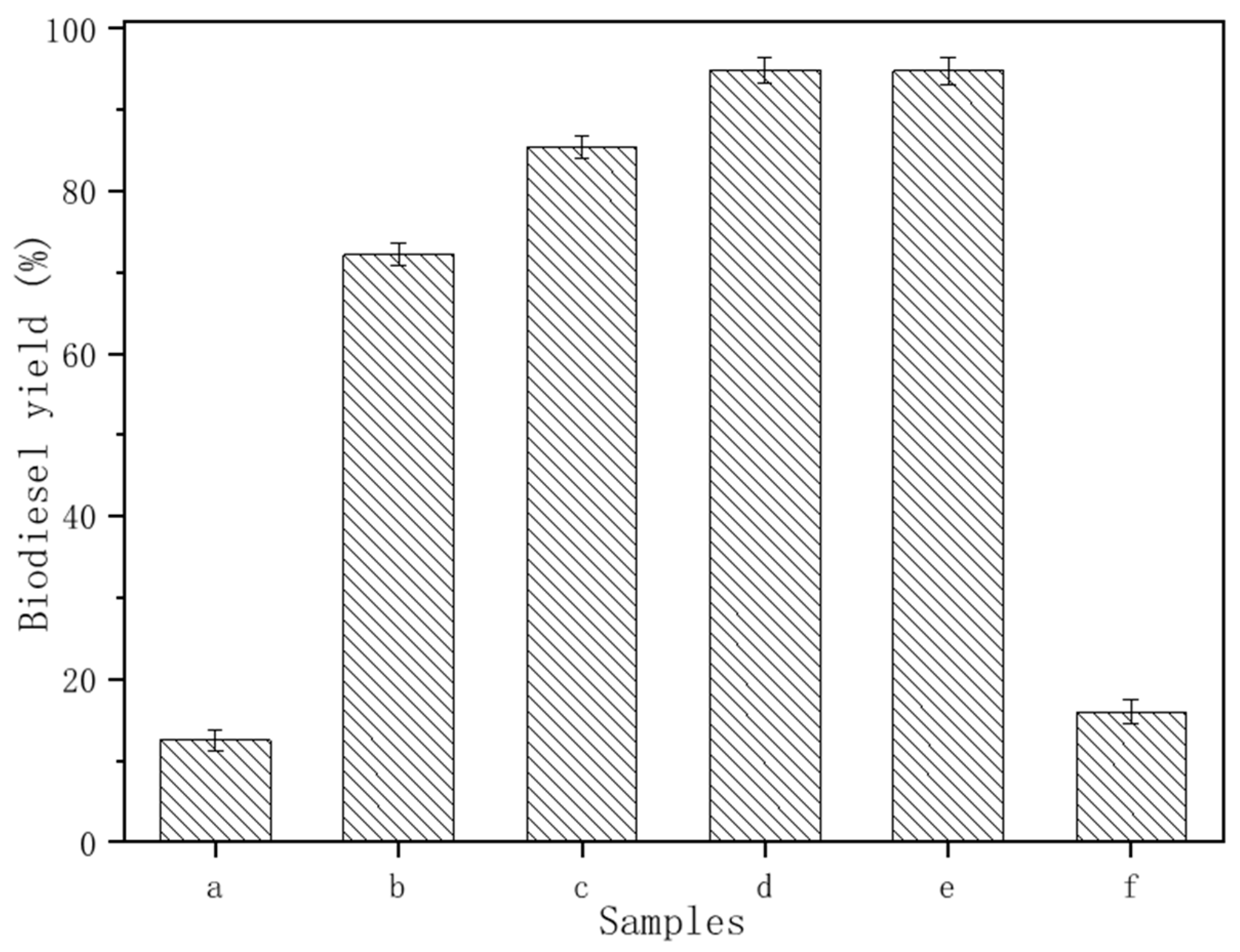
3.2. Influence of Preparation Conditions on the Transesterification Reaction
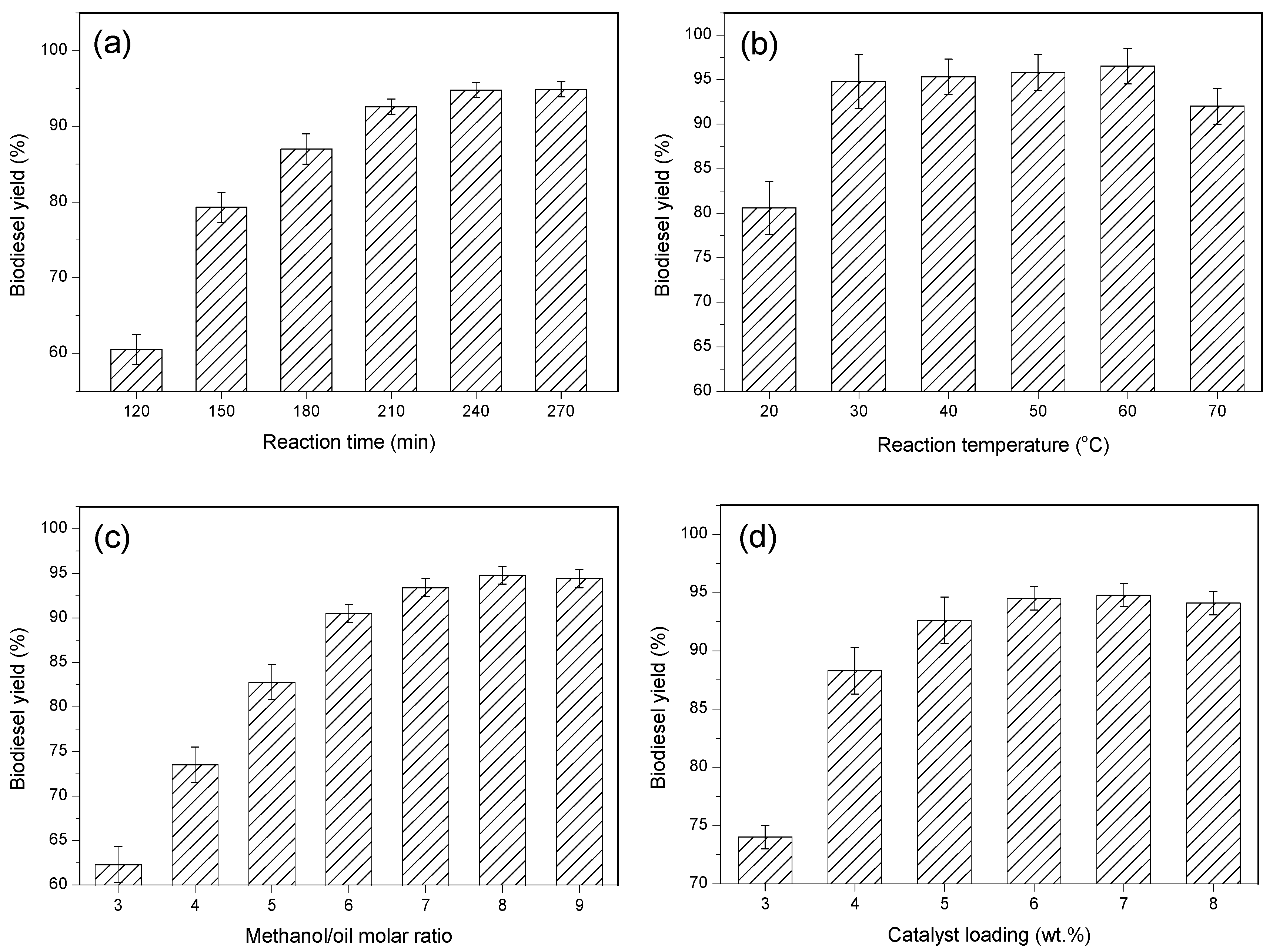
3.3. Influence of Reaction Conditions on the Transesterification Reaction
3.4. Reusability of the Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Wang, R.; Yu, M. An efficient, recoverable solid base catalyst of magnetic bamboo charcoal: Preparation, characterization, and performance in biodiesel production. Renew. Energy 2018, 127, 531–538. [Google Scholar] [CrossRef]
- Alismaeel, Z.T.; Abbas, A.S.; Albayati, T.M.; Doyle, A.M. Biodiesel from batch and continuous oleic acid esterification using zeolite catalysts. Fuel 2018, 234, 170–176. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.D.; Robles-Medina, A.; Jiménez-Callejón, M.J.; Hita-Peña, E.; Estéban-Cerdán, L.; González-Moreno, P.A.; Navarro-López, E.; Molina-Grima, E. Optimization of biodiesel production from wet microalgal biomass by direct transesterification using the surface response methodology. Renew. Energy 2018, 129, 141–149. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Biodiesel production from Calophyllum inophyllum oil a potential non-edible feedstock: An overview. Renew. Energy 2019, 131, 459–471. [Google Scholar] [CrossRef]
- Sandouqa, A.; Al-Hamamre, Z.; Asfar, J. Preparation and performance investigation of a lignin-based solid acid catalyst manufactured from olive cake for biodiesel production. Renew. Energy 2019, 132, 667–682. [Google Scholar] [CrossRef]
- Sánchez-Cantú, M.; Morales Téllez, M.; Pérez-Díaz, L.M.; Zeferino-Díaz, R.; Hilario-Martínez, J.C.; Sandoval-Ramírez, J. Biodiesel production under mild reaction conditions assisted by high shear mixing. Renew. Energy 2019, 130, 174–181. [Google Scholar] [CrossRef]
- Eldiehy, K.S.H.; Bardhan, P.; Borah, D.; Gohain, M.; Ahmad, R.M.; Deka, D.; Mandal, M. A comprehensive review on microalgal biomass production and processing for biodiesel production. Fuel 2022, 324, 124773. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.T.; Yusaf, T. Characterization of biodiesel production (ultrasonic-assisted) from evening-primroses (Oenothera lamarckiana) as novel feedstock and its effect on CI engine parameters. Renew. Energy 2019, 130, 50–60. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sowmya, V.; Sridharan, S.; Yuvaraj, D.; Jayamuthunagai, J.; Praveenkumar, R. Biodiesel production from microbial oil derived from wood isolate Trichoderma reesei. Bioresour. Technol. 2017, 239, 538–541. [Google Scholar] [CrossRef]
- Mancini, M.; Lanza, V.M.; Gatti, B.; Malik, Y.; Moreno, A.C.; Leskovar, D.; Cravero, V. Characterization of cardoon accessions as feedstock for biodiesel production. Fuel 2019, 235, 1287–1293. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production. Bioresour. Technol. 2018, 248, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Generation of biodiesel from edible waste oil using ZIF-67-KOH modified Luffa cylindrica biomass catalyst. Fuel 2022, 322, 124181. [Google Scholar] [CrossRef]
- Goli, J.; Sahu, O. Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew. Energy 2018, 128, 142–154. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, H.; Fan, M.; Sun, W.; Jiang, P.; Dong, Y. Direct and postsynthesis of tin-incorporated SBA-15 functionalized with sulfonic acid for efficient biodiesel production. Fuel 2019, 235, 426–432. [Google Scholar] [CrossRef]
- Nazloo, E.K.; Moheimani, N.R.; Ennaceri, H. Graphene-based catalysts for biodiesel production: Characteristics and performance. Sci. Total Environ. 2023, 859, 160000. [Google Scholar] [CrossRef]
- Jeon, K.-W.; Shim, J.-O.; Jang, W.-J.; Lee, D.-W.; Na, H.-S.; Kim, H.-M.; Lee, Y.-L.; Yoo, S.-Y.; Roh, H.-S.; Jeon, B.-H.; et al. Effect of calcination temperature on the association between free NiO species and catalytic activity of Ni-Ce0.6Zr0.4O2 deoxygenation catalysts for biodiesel production. Renew. Energy 2019, 131, 144–151. [Google Scholar] [CrossRef]
- Sandouqa, A.; Al-Hamamre, Z. Energy analysis of biodiesel production from jojoba seed oil. Renew. Energy 2019, 130, 831–842. [Google Scholar] [CrossRef]
- Anderson, E.; Addy, M.; Chen, P.; Ruan, R. Development and operation of innovative scum to biodiesel pilot-system for the treatment of floatable wastewater scum. Bioresour. Technol. 2018, 249, 1066–1068. [Google Scholar] [CrossRef]
- Sun, H.; Ma, M.; Fan, M.; Sun, K.; Xu, W.; Wang, K.; Li, B.; Jiang, J. Controllable preparation of biomass derived mesoporous activated carbon supported nano-CaO catalysts for biodiesel production. Energy 2022, 261, 125369. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Syazwani, O.N.; Rashid, U.; Mastuli, M.S.; Taufiq-Yap, Y.H. Esterification of palm fatty acid distillate (PFAD) to biodiesel using Bi-functional catalyst synthesized from waste angel wing shell (Cyrtopleura costata). Renew. Energy 2019, 131, 187–196. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Klai, N.; Kaur, R.; Tyagi, R.D.; Surampalli, R.Y. Oleaginous yeast biomass flocculation using bioflocculant produced in wastewater sludge and transesterification using petroleum diesel as a co-solvent. Renew. Energy 2019, 131, 217–228. [Google Scholar] [CrossRef]
- Sathiyamoorthi, R.; Sankaranarayanan, G.; Adhith, S.B.; Chiranjeevi, T.; Dilip Kumar, D. Experimental investigation on performance, combustion and emission characteristics of a single cylinder diesel engine fuelled by biodiesel derived from Cymbopogon Martinii. Renew. Energy 2019, 132, 394–415. [Google Scholar]
- Bolonio, D.; García-Martínez, M.-J.; Ortega, M.F.; Lapuerta, M.; Rodríguez-Fernández, J.; Canoira, L. Fatty acid ethyl esters (FAEEs) obtained from grapeseed oil: A fully renewable biofuel. Renew. Energy 2019, 132, 278–283. [Google Scholar] [CrossRef]
- Mirhashemi, M.S.; Mohseni, S.; Hasanzadeh, M.; Pishvaee, M.S. Moringa oleifera biomass-to-biodiesel supply chain design: An opportunity to combat desertification in Iran. J. Clean. Prod. 2018, 203, 313–327. [Google Scholar] [CrossRef]
- Son, J.; Kim, B.; Park, J.; Yang, J.; Lee, J.W. Wet in situ transesterification of spent coffee grounds with supercritical methanol for the production of biodiesel. Bioresour. Technol. 2018, 259, 465–468. [Google Scholar]
- Laskar, I.B.; Deshmukhya, T.; Bhanja, P.; Paul, B.; Gupta, R.; Chatterjee, S. Transesterification of soybean oil at room temperature using biowaste as catalyst; an experimental investigation on the effect of co-solvent on biodiesel yield. Renew. Energy 2020, 162, 98–111. [Google Scholar] [CrossRef]
- Sitepu, E.K.; Sembiring, Y.; Supeno, M.; Tarigan, K.; Ginting, J.; Karo-karo, J.A.; Tarigan, J.B. Homogenizer-intensified room temperature biodiesel production using heterogeneous palm bunch ash catalyst. S. Afr. J. Chem. Eng. 2022, 40, 240–245. [Google Scholar] [CrossRef]
- Gutiérrez-López, A.N.; Mena-Cervantes, V.Y.; González-Espinosa, M.A.; Sosa-Rodríguez, F.S.; Vazquez-Arenas, J.; Rodríguez-Ramírez, R.; Hernández-Altamirano, R. Green and fast biodiesel production at room temperature using soybean and Jatropha curcas L. oils catalyzed by potassium ferrate. J. Clean. Prod. 2022, 372, 133739. [Google Scholar] [CrossRef]
- Vadery, V.; Narayanan, B.N.; Ramakrishnan, R.M.; Cherikkallinmel, S.K.; Sugunan, S.; Narayanan, D.P.; Sasidharan, S. Room temperature production of jatropha biodiesel over coconut husk ash. Energy 2014, 70, 588–594. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rajkumari, K.; Rokhum, L. Exploiting waste: Towards a sustainable production of biodiesel using Musa acuminata peel ash as a heterogeneous catalyst. Green Chem. 2018, 20, 2365–2373. [Google Scholar] [CrossRef]


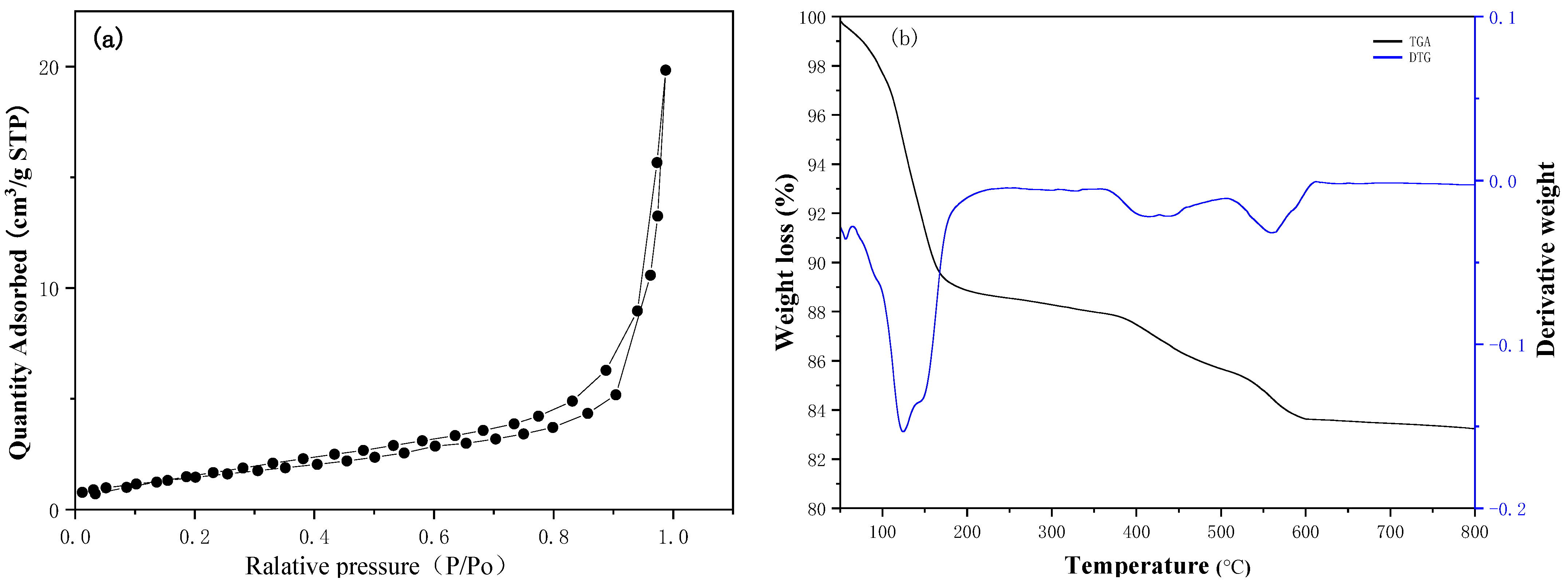
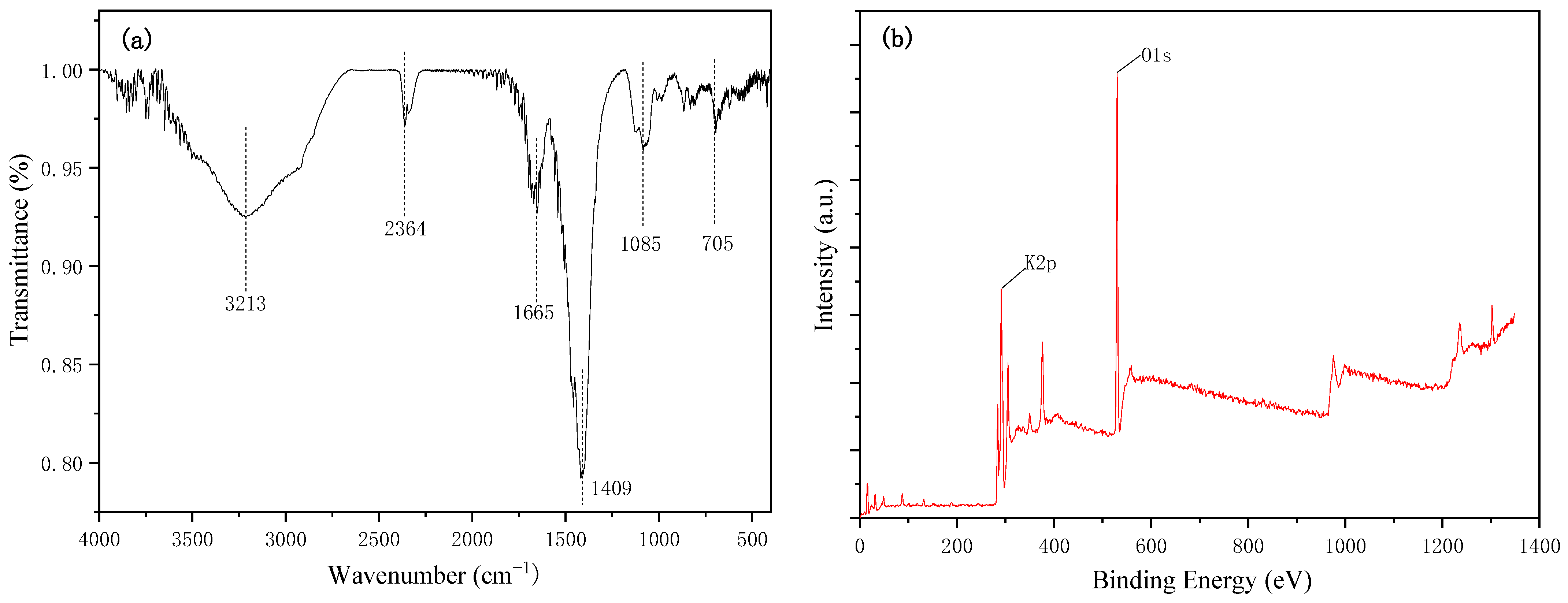

| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Basic Strength (H_) | Total Basicity (mmol/g) |
|---|---|---|---|---|
| DN | 20.25 | 0.057 | H_ < 7.2 | 0.8 |
| DN-250 | 23.35 | 0.065 | 9.8 < H_ < 15.0 | 6.6 |
| DN-300 | 28.30 | 0.076 | 15.0 < H_ < 18.4 | 9.4 |
| DN-350 | 24.36 | 0.068 | 15.0 < H_ < 18.4 | 11.4 |
| DN-400 | 15.91 | 0.045 | 15.0 < H_ < 18.4 | 11.6 |
| PDN-350 | 44.71 | 0.114 | 7.2 < H_ < 9.8 | 1.6 |
| Sample | Elements Content (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| C | K | O | Si | Ca | Mg | Other | |
| DN | 64.10 | 13.1 | 18.79 | 0.81 | 2.10 | 0.50 | 0.60 |
| DN-250 | 24.23 | 30.21 | 41.5 | 1.22 | 0.88 | 1.43 | 0.53 |
| DN-300 | 16.26 | 37.14 | 40.93 | 1.57 | 1.01 | 2.61 | 0.48 |
| DN-350 | 11.25 | 42.42 | 39.26 | 2.04 | 1.21 | 3.2 | 0.62 |
| DN-400 | 8.25 | 48.30 | 34.35 | 2.87 | 1.51 | 4.06 | 0.66 |
| PDN-350 | 61.76 | 23.22 | 10.12 | 2.64 | 1.11 | 0.53 | 0.62 |
| Catalyst | Element Content (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| C | K | O | Ca | Si | Mg | Others | |
| DN-250 | 40.23 | 25.21 | 30.06 | 1.33 | 0.95 | 1.66 | 0.56 |
| DN-300 | 36.26 | 30.14 | 27.47 | 1.57 | 1.21 | 2.81 | 0.54 |
| DN-350 | 31.95 | 34.42 | 26.26 | 2.04 | 1.21 | 3.44 | 0.68 |
| DN-400 | 35.37 | 29.28 | 25.35 | 3.27 | 1.88 | 4.16 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Chen, H.; Wu, X.; Shan, R. Exploiting the Waste Biomass of Durian Shell as a Heterogeneous Catalyst for Biodiesel Production at Room Temperature. Int. J. Environ. Res. Public Health 2023, 20, 1760. https://doi.org/10.3390/ijerph20031760
Zhao C, Chen H, Wu X, Shan R. Exploiting the Waste Biomass of Durian Shell as a Heterogeneous Catalyst for Biodiesel Production at Room Temperature. International Journal of Environmental Research and Public Health. 2023; 20(3):1760. https://doi.org/10.3390/ijerph20031760
Chicago/Turabian StyleZhao, Che, Hongyuan Chen, Xiao Wu, and Rui Shan. 2023. "Exploiting the Waste Biomass of Durian Shell as a Heterogeneous Catalyst for Biodiesel Production at Room Temperature" International Journal of Environmental Research and Public Health 20, no. 3: 1760. https://doi.org/10.3390/ijerph20031760




