Gastroenteritis Outbreaks after Contamination of Water Supply Systems: Public Health Response Gaps and Challenges, Greece, 2004–2023
Abstract
:1. Introduction
2. Methods
2.1. Definitions
2.2. Data Source
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Notification of WGDOs, 2004–2023
3.2. Timeliness of Reporting
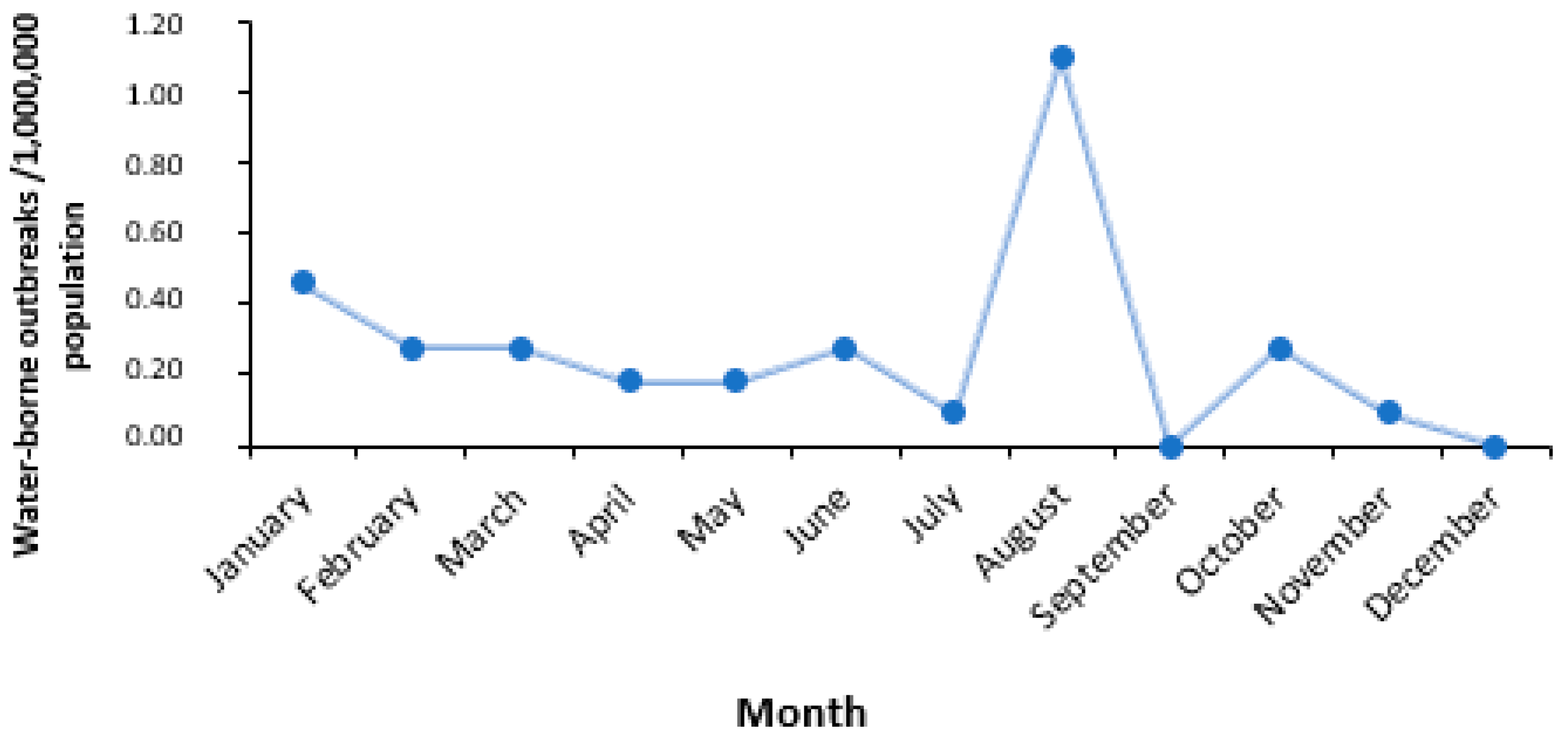
3.3. Time and Place of the Outbreaks
3.4. Size of the Outbreaks
3.5. Demographics and Severity of Disease
3.6. Analytical Epidemiology
3.7. Laboratory Investigation of Clinical Samples
3.8. Environmental Investigation
3.9. Classification of WGDOs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, N.A.; Bondelind, M. Safe drinking water and waterborne outbreaks. J. Water Health 2017, 15, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Ashbolt, N.J. Microbial Contamination of Drinking Water and Human Health from Community Water Systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Widerström, M.; Schönning, C.; Lilja, M.; Lebbad, M.; Ljung, T.; Allestam, G.; Ferm, M.; Björkholm, B.; Hansen, A.; Hiltula, J.; et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg. Infect. Dis. 2014, 20, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Mouly, D.; Van Cauteren, D.; Vincent, N.; Vaissière, E.; Beaudeau, P.; Ducrot, C.; Gallay, A. Description of two waterborne disease outbreaks in France: A comparative study with data from cohort studies and from health administrative databases. Epidemiol. Infect. 2016, 144, 591–601. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Drinking Water—Key Facts. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 27 March 2024).
- Kulinkina, A.V.; Shinee, E.; Guzmán Herrador, B.R.; Nygård, K.; Schmoll, O. The Situation of Water-Related Infectious Diseases in the Pan-European Region; World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2016; Available online: https://iris.who.int/handle/10665/329534 (accessed on 27 March 2024).
- European Food Safety Authority (EFSA) EFS; European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Gallay, A.; De Valk, H.; Cournot, M.; Ladeuil, B.; Hemery, C.; Castor, C.; Bon, F.; Mégraud, F.; Le Cann, P.; Desenclos, J.C.; et al. A large multi-pathogen waterborne community outbreak linked to faecal contamination of a groundwater system, France, 2000. Clin. Microbiol. Infect. 2006, 12, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Herrador, B.; Carlander, A.; Ethelberg, S.; de Blasio, B.F.; Kuusi, M.; Lund, V.; Löfdahl, M.; MacDonald, E.; Nichols, G.; Schönning, C.; et al. Waterborne outbreaks in the Nordic countries, 1998 to 2012. Eurosurveillance 2015, 20, 21160. Available online: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES2015.20.24.21160 (accessed on 27 March 2024). [CrossRef]
- O’Reilly, C.E.; Bowen, A.B.; Perez, N.E.; Sarisky, J.P.; Shepherd, C.A.; Miller, M.D.; Hubbard, B.C.; Herring, M.; Buchanan, S.D.; Fitzgerald, C.C.; et al. A Waterborne Outbreak of Gastroenteritis with Multiple Etiologies among Resort Island Visitors and Residents: Ohio, 2004. Clin. Infect. Dis. 2007, 44, 506–512. [Google Scholar] [CrossRef]
- Tzani, M.; Mellou, K.; Kyritsi, M.; Kolokythopoulou, F.; Vontas, A.; Sideroglou, T.; Chrysostomou, A.; Mandilara, G.D.; Tryfinopoulou, K.; Georgakopoulou, T.; et al. Evidence for waterborne origin of an extended mixed gastroenteritis outbreak in a town in Northern Greece, 2019. Epidemiol. Infect. 2020, 149, e83. [Google Scholar] [CrossRef] [PubMed]
- Duchenne-Moutien, R.A.; Neetoo, H. Climate Change and Emerging Food Safety Issues: A Review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef]
- Edelson, P.J.; Harold, R.; Ackelsberg, J.; Duchin, J.S.; Lawrence, S.J.; Manabe, Y.C.; Zahn, M.; LaRocque, R.C. Climate Change and the Epidemiology of Infectious Diseases in the United States. Clin. Infect. Dis. 2023, 76, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Cissé, G. Food-borne and water-borne diseases under climate change in low- and middle-income countries: Further efforts needed for reducing environmental health exposure risks. Acta Trop. 2019, 194, 181–188. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Waterborne Disease Outbreak 2010 Case Definition. Available online: https://ndc.services.cdc.gov/case-definitions/waterborne-disease-outbreak-2010/ (accessed on 27 March 2024).
- Barrett, C.E.; Pape, B.J.; Benedict, K.M.; Foster, M.A.; Roberts, V.A.; Rotert, K.; Mattioli, M.C.; Yoder, J.S. Impact of Public Health Interventions on Drinking Water–Associated Outbreaks of Hepatitis A—United States, 1971–2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 766–770. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Reporting and Classification|Water-Related Topics|Healthy Water|CDC. 2019. Available online: https://www.cdc.gov/healthywater/surveillance/reporting-classification.html (accessed on 27 March 2024).
- Buss, S.N.; Leber, A.; Chapin, K.; Fey, P.D.; Bankowski, M.J.; Jones, M.K.; Rogatcheva, M.; Kanack, K.J.; Bourzac, K.M. Multicenter Evaluation of the BioFire FilmArray Gastrointestinal Panel for Etiologic Diagnosis of Infectious Gastroenteritis. J. Clin. Microbiol. 2015, 53, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Politi, L.; Mellou, K.; Chrysostomou, A.; Mandilara, G.; Spiliopoulou, I.; Theofilou, A.; Polemis, M.; Tryfinopoulou, K.; Sideroglou, T. A Community Waterborne Salmonella Bovismorbificans Outbreak in Greece. Int. J. Environ. Res. Public Health 2024, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Queensland Government. Queensland Health. The State of Queensland. Superchlorination. 2017. Available online: https://www.health.qld.gov.au/public-health/industry-environment/environment-land-water/water/risk-management/plan/manage/superchlorination (accessed on 27 March 2024).
- Centers for Diseases Control and Prevention (CDC). Interpreting Waterborne Disease Outbreak Data|Water-Related Topics|Healthy Water|CDC. 2019. Available online: https://www.cdc.gov/healthywater/surveillance/interpreting-data.html (accessed on 27 March 2024).
- Craun, G.F.; Brunkard, J.M.; Yoder, J.S.; Roberts, V.A.; Carpenter, J.; Wade, T.; Calderon, R.L.; Roberts, J.M.; Beach, M.J.; Roy, S.L. Causes of Outbreaks Associated with Drinking Water in the United States from 1971 to 2006. Clin. Microbiol. Rev. 2010, 23, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Stathatou, P.; Manoli, E.; Assimacopoulos, D. Sustainability of Water & Sanitation Services in Greece. Available online: https://f-origin.hypotheses.org/wp-content/blogs.dir/146/files/2012/01/ATHENS_11_Assimacopoulos.pdf (accessed on 27 March 2024).
- Hellenic Statistical Authority. Available online: https://www.statistics.gr/en/2021-census-res-pop-results (accessed on 27 March 2024).
- Guzman Herrador, B.R.; de Blasio, B.F.; MacDonald, E.; Nichols, G.; Sudre, B.; Vold, L.; Semenza, J.C.; Nygård, K. Analytical studies assessing the association between extreme precipitation or temperature and drinking water-related waterborne infections: A review. Environ. Health 2015, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Bjelkmar, P.; Hulth, A.; Lindh, J.; Stenmark, S.; Widerström, M. Syndromic surveillance for local outbreak detection and awareness: Evaluating outbreak signals of acute gastroenteritis in telephone triage, web-based queries and over-the-counter pharmacy sales. Epidemiol. Infect. 2014, 142, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Coly, S.; Vincent, N.; Vaissiere, E.; Charras-Garrido, M.; Gallay, A.; Ducrot, C.; Mouly, D. Waterborne disease outbreak detection: An integrated approach using health administrative databases. J. Water Health 2017, 15, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Swaan, C.; van den Broek, A.; Kretzschmar, M.; Richardus, J.H. Timeliness of notification systems for infectious diseases: A systematic literature review. PLoS ONE 2018, 13, e0198845. [Google Scholar] [CrossRef]
- Hyllestad, S.; Iversen, A.; MacDonald, E.; Amato, E.; Borge, B.Å.S.; Bøe, A.; Sandvin, A.; Brandal, L.T.; Lyngstad, T.M.; Naseer, U.; et al. Large waterborne Campylobacter out-break: Use of multiple approaches to investigate contamination of the drinking water supply system, Norway, June 2019. Eurosurveillance 2020, 25, 2000011. [Google Scholar] [CrossRef] [PubMed]
- Mellou, K.; Sideroglou, T.; Kefaloudi, C.; Tryfinopoulou, K.; Chrysostomou, A.; Mandilara, G.; Pavlaki, M.; Maltezou, H.C. Waterborne outbreak in a rural area in Greece during the COVID-19 pandemic: Contribution of community pharmacies. Rural Remote Health 2021, 21, 1–5. Available online: https://www.rrh.org.au/journal/article/6630/ (accessed on 27 March 2024). [CrossRef] [PubMed]
- Mellou, K.; Katsioulis, A.; Potamiti-Komi, M.; Pournaras, S.; Kyritsi, M.; Katsiaflaka, A.; Kallimani, A.; Kokkinos, P.; Petinaki, E.; Sideroglou, T.; et al. A large waterborne gastroenteritis outbreak in central Greece, March 2012: Challenges for the investigation and management. Epidemiol. Infect. 2014, 142, 40–50. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Water Association. Water Safety Plan Manual: Step-by-Step Risk Management for Drinking-Water Suppliers, 2nd ed.; World Health Organization: Geneva, Switzerland, 2009. Available online: https://www.who.int/publications/i/item/9789240067691 (accessed on 28 March 2024).
- Centers for Disease Control and Prevention. Water, Sanitation, & Hygiene (WASH)-Related Emergencies & Outbreaks. Waterborne Disease Outbreak Investigation Toolkit. Available online: https://www.cdc.gov/healthywater/emergency/preparedness-resources/outbreak-response.html (accessed on 27 March 2024).
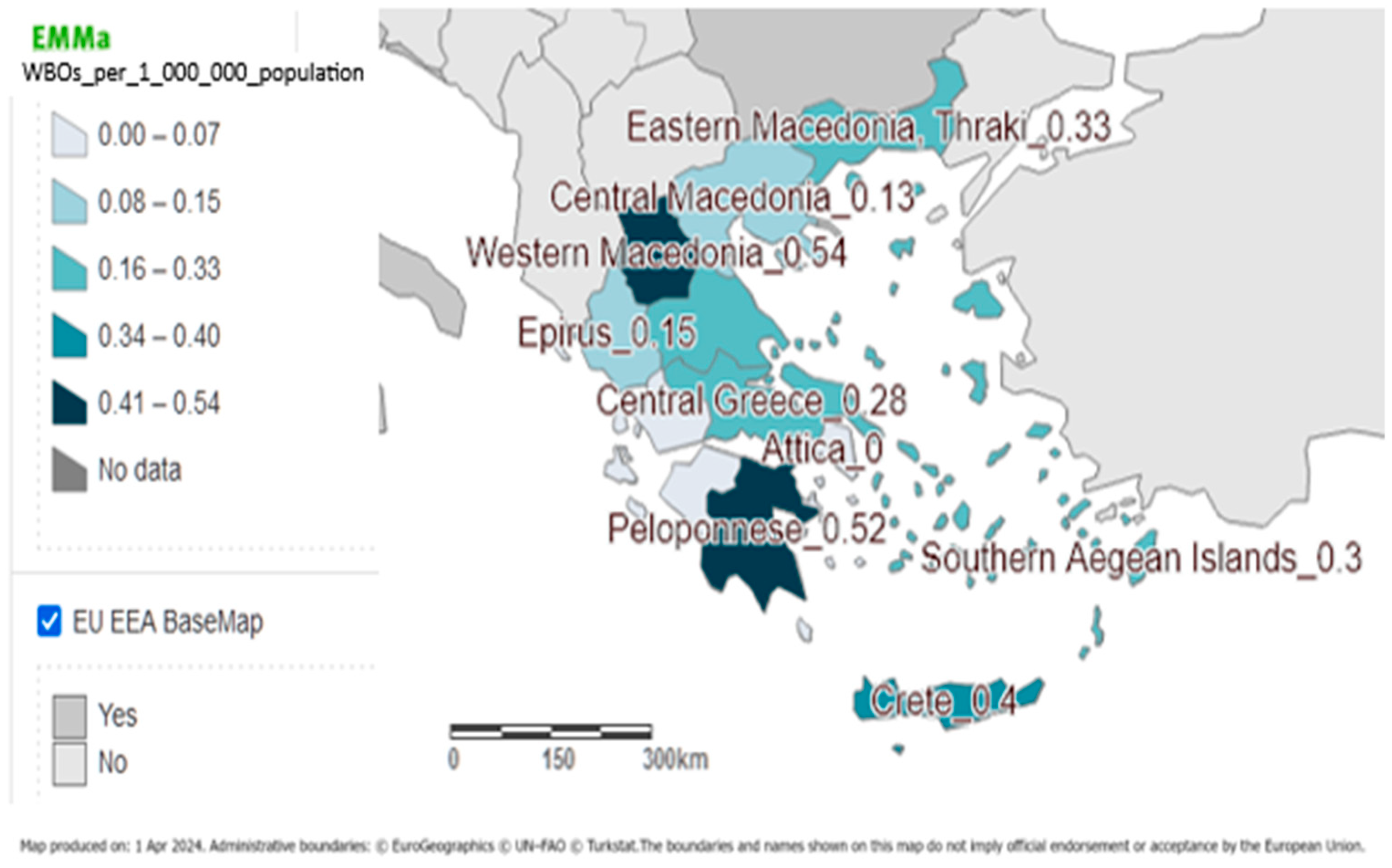
| Time * | Region | Number of Cases | Number of Laboratory-Confirmed Cases | Type of Study | Measure of Association | Pathogen (Clinical Samples) | Water Sampling before or after Chlorination | Contributing Factors |
|---|---|---|---|---|---|---|---|---|
| 15 February 2004–10 March 2004 | Crete | 37 | 35 | CCS | OR = 10.91 (95%CI: 1.86–63.8) | Salmonella typhimurium | Unknown |
|
| 27 May 2009–7 June 2009 | Crete | 54 | 54 | CCS and CCrS | OR = 5.88 (95%CI: 1.75–20.0) OR = 4.39 (95%CI: 1.30–14.8) | Campylobacter jejuni | After | Shortcomings in water sanitation procedures |
| 10 January 2012–16 February 2012 | Central Macedonia | 79 | 7 | CS | RR = 2.34 (95%CI: 1.55–3.53) | Norovirus and Adenovirus | Before |
|
| 6 March 2012–21 March 2012 | Thessaly | 552 | 38 | CCS | OR = 2.18 (95%CI: 1.11–4.28) | Rotavirus | Before |
|
| 9 August 2015–30 August 2015 | Central Macedonia | 230 | 7 | CCS | OR = 36.9 (p = 0.018) | Norovirus | Before |
|
| 25 January 2019–4 February 2019 | Western Macedonia | 638 | 11 | CCS and CS | OR = 10 (95%CI: 2.09–93.4) RR = 2.22 (95%CI: 1.42–3.46) | Mixed aetiology (norovirus, Campylobacter jejuni, EHEC and EPEC) | After | Unknown |
| 29 May 2020–18 June 2020 | Peloponnese | 87 | 6 | CCS | OR = 10.9 (95%CI: 3.1–38.0) | Mixed aetiology (STEC, EPEC, E. coli O157) | Unknown | Shortcomings in water sanitation procedures |
| 10 August 2022–24 August 2022 | Peloponnese | 33 | 15 | CCS | OR = 5.46 (95%CI: 1.02–53.95) | Salmonella Bovismorbificans | Unknown | Unknown |
| 2 March 2023–14 March 2023 | Central Greece | 39 | 1 | CCS | OR = 3.02, (95%CI: 0.88–10.82) | Norovirus | Unknown | Unknown |
| Pathogen | n (%) |
|---|---|
| Norovirus | 7 (35.0%) |
| Salmonella spp. | 2 (10.0%) |
| S. Typhimurium | 1 (5.0%) |
| S. Enteritidis | 1 (5.0%) |
| S. Bovismorbificans | 1 (5.0%) |
| Shigella flexneri | 2 (10.0%) |
| Campylobacter jejuni | 2 (10.0%) |
| Rotavirus | 2 (10.0%) |
| Mixed aetiology | 2 (10.0%) |
| Total | 20 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sideroglou, T.; Chrysostomou, A.; Politi, L.; Georgalis, L.; Mellou, K. Gastroenteritis Outbreaks after Contamination of Water Supply Systems: Public Health Response Gaps and Challenges, Greece, 2004–2023. Int. J. Environ. Res. Public Health 2024, 21, 701. https://doi.org/10.3390/ijerph21060701
Sideroglou T, Chrysostomou A, Politi L, Georgalis L, Mellou K. Gastroenteritis Outbreaks after Contamination of Water Supply Systems: Public Health Response Gaps and Challenges, Greece, 2004–2023. International Journal of Environmental Research and Public Health. 2024; 21(6):701. https://doi.org/10.3390/ijerph21060701
Chicago/Turabian StyleSideroglou, Theologia, Anthi Chrysostomou, Lida Politi, Leonidas Georgalis, and Kassiani Mellou. 2024. "Gastroenteritis Outbreaks after Contamination of Water Supply Systems: Public Health Response Gaps and Challenges, Greece, 2004–2023" International Journal of Environmental Research and Public Health 21, no. 6: 701. https://doi.org/10.3390/ijerph21060701





