The Contribution of Legionella anisa to Legionella Contamination of Water in the Built Environment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Overview of Data Sets Provided for Analysis and Limitations of the Data Obtained
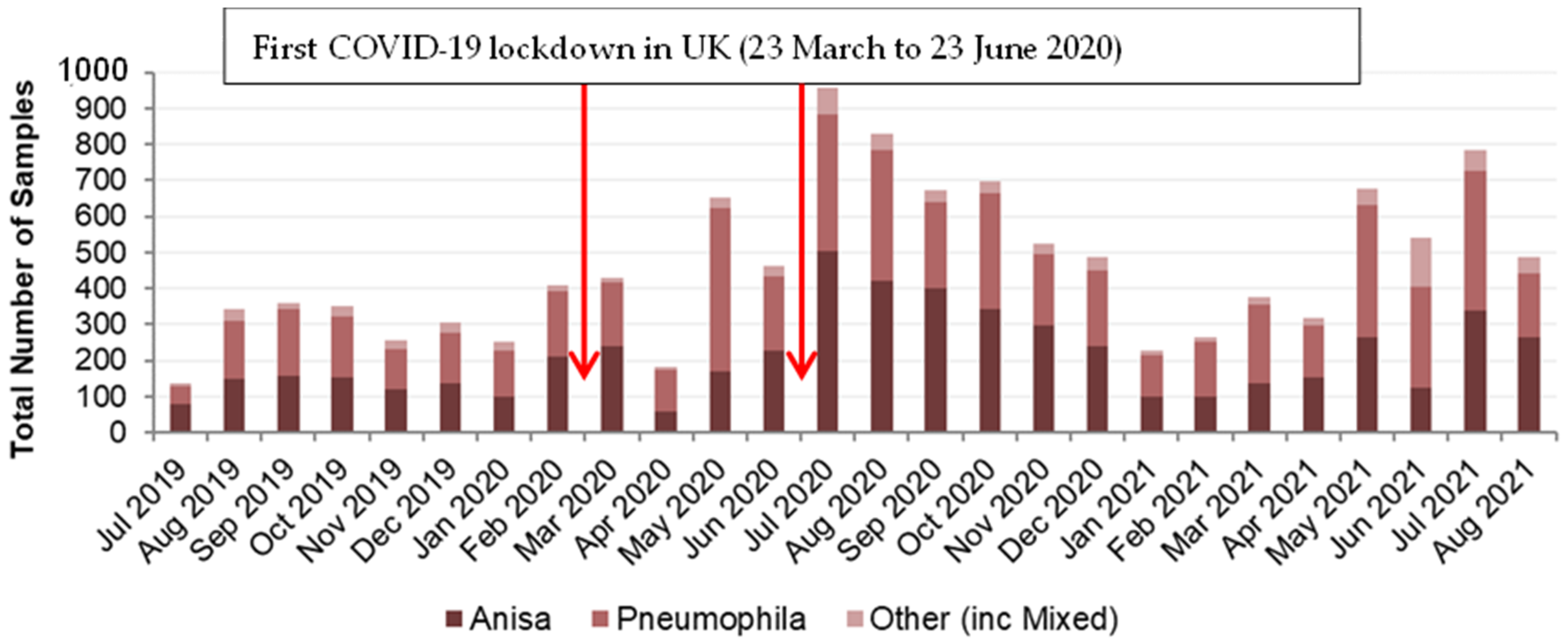
3.2. Legionella Data Set 1
3.3. Legionella Data Set 2
3.4. Legionella Data Set 3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, J.T.; McDermott, P.J. Confirming the presence of Legionella pneumophila in your water system: A review of current Legionella Testing Methods. J. AOAC Int. 2021, 104, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Muder, R.R.; Yu, V.L. Infection Due to Legionella Species Other Than L. pneumophila. Clin. Infect. Dis. 2002, 35, 990–998. [Google Scholar] [CrossRef]
- Kozak-Muiznieks, N.A.; Lucas, C.E.; Brown, E.; Pondo, T.; Taylor Jr, T.H.; Frace, M.; Miskowski, D.; Winchell, J.M. Prevalence of sequence types among clinical and environmental isolates of Legionella pneumophila serogroup 1 in the United States from 1982 to 2012. J. Clin. Microbiol. 2014, 52, 201–211. [Google Scholar] [CrossRef]
- ECDC. Legionnaires’ Disease Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/legionnaires-disease-annual-epidemiological-report-2021 (accessed on 21 May 2024).
- Logan-Jackson, A.; Rose, J.B. Co-occurrence of Five Pathogenic Legionella spp. and Two Free-Living Amoebae Species in a Complete Drinking Water System and Cooling Towers. Pathogens 2021, 10, 1407. [Google Scholar] [CrossRef]
- Gorman, G.W.; Feeley, J.C.; Steigerwalt, A.; Edelstein, P.H.; Moss, W.; Brenner, D.J. Legionella anisa: A new species of Legionella isolated from potable waters and a cooling tower. Appl. Environ. Microbiol. 1985, 49, 305–309. [Google Scholar] [CrossRef]
- Cross, K.E.; Mercante, J.W.; Benitez, A.J.; Brown, E.W.; Diaz, M.H.; Winchell, J.M. Simultaneous detection of Legionella species and L. anisa, L. bozemanii, L. longbeachae and L. micdadei using conserved primers and multiple probes in a multiplex real-time PCR. Diagn. Microbiol. Infect. Dis. 2016, 85, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, L.; Izquierdo, F.; Magnet, A.; Hurtado, C.; Salinas, M.A.; Gomes, T.S. First Case of Legionnaire’s Disease Caused by Legionella anisa in Spain and the Limitations on the Diagnosis of Legionella non-pneumophila Infections. PLoS ONE 2016, 11, e0159726. [Google Scholar] [CrossRef]
- Chambers, S.T.; Slow, S.; Scott-Thomas, A.; Murdoch, D.R. Legionellosis Caused by Non-Legionella pneumophila Species, with a Focus on Legionella longbeachae. Microorganisms 2021, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Fallon, R.J.; Stack, B.H.R. Legionnaires’ disease due to Legionella anisa. J. Infect. 1990, 20, 227–229. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Sebti, R.; Hassoun, P.; Mannion, C.; Goy, A.H.; Feldman, T.; Mato, A.; Hong, T. Osteomyelitis of the Patella Caused by Legionella anisa. J. Clin. Microbiol. 2013, 51, 2791–2793. [Google Scholar] [CrossRef]
- Thacker, L.; Benson, R.F.; Hawes, L.; Mayberry, W.R.; Brenner, D.J. Characterization of a Legionella anisa strain isolated from a patient with pneumonia. J. Clin. Microbiol. 1990, 28, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Logan-Jackson, A.R.; Flood, M.; Rose, J.B. Enumeration and characterization of five pathogenic Legionella species from large research and educational buildings. Environ. Sci. Water Res. Technol. 2021, 7, 321. [Google Scholar] [CrossRef]
- Mazzotta, M.; Salaris, S.; Pascale, M.R.; Girolamini, L.; Cristino, S. Occurrence of Legionella spp. In Man-Made Water Sources: Isolates Distribution and Phylogenetic Characterization in the Emilia-Romagna Region. Pathogens 2021, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Pascale, M.R.; Mazzotta, M.; Salaris, S.; Girolamini, L.; Grottola, A.; Simone, M.L.; Cordovana, M.; Bisognin, F.; Dal Monte, P.; Bucci Sabattini, M.A.; et al. Evaluation of MALDI–TOF Mass Spectrometry in Diagnostic and Environmental Surveillance of Legionella Species: A Comparison with Culture and Mip-Gene Sequencing Technique. Front. Microbiol. 2020, 11, 589369. [Google Scholar] [CrossRef]
- Crook, B.; Willerton, L.; Smith, D.; Wilson, L.; Poran, V.; Helps, J.; McDermott, P. Legionella risk in evaporative cooling systems and underlying causes of associated breaches in health and safety compliance. Int. J. Hyg. Environ. Health 2020, 224, 113425. [Google Scholar] [CrossRef]
- Yu, V.L.; Plouffe, J.F.; Castellani Pastoris, M.; Stout, J.E.; Schousboe, M.; Widmer, A.; Summersgill, J.; File, T.; Heath, C.M.; Paterson, D.L.; et al. Distribution of Legionella Species and Serogroups Isolated by Culture in Patients with Sporadic Community-Acquired Legionellosis: An International Collaborative Survey. J. Infect. Dis. 2002, 186, 127–128. [Google Scholar] [CrossRef]
- Collins, S.; Jorgensen, F.; Willis, C.; Walker, J. Real-time PCR to supplement gold-standard culture-based detection of Legionella in environmental samples. J. Appl. Microbiol. 2015, 119, 1158–1169. [Google Scholar] [CrossRef]
- Lee, T.C.; Vickers, R.M.; Yu, V.L.; Wagener, M.M. Growth of 28 Legionella species on selective culture media: A comparative study. J. Clin. Microbiol. 1993, 31, 2764–2768. [Google Scholar] [CrossRef]
- Yu, V.L.; Stout, J.E. Legionella anisa and hospital water systems. J. Infect. Chemother. 2004, 10, 133. [Google Scholar] [CrossRef]
- Doleans, A.; Aurell, H.; Reyrolle, M.; Lina, G.; Freney, J.; Vandenesch, F.; Etienne, J.; Jarraud, S. Clinical and Environmental Distributions of Legionella Strains in France Are Different. J. Clin. Microbiol. 2004, 42, 458–460. [Google Scholar] [CrossRef]
- Svarrer, C.W.; Uldum, S.A. The occurrence of Legionella species other than Legionella pneumophila in clinical and environmental samples in Denmark identified by mip gene sequencing and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 2012, 18, 1004–1009. [Google Scholar] [CrossRef]
- van der Mee-Marquet, N.; Domelier, A.-S.; Arnault, L.; Bloc, D.; Laudat, P.; Hartemann, P.; Quentin, R. Legionella anisa, a Possible Indicator of Water Contamination by Legionella pneumophila. J. Clin. Microbiol. 2006, 44, 56–59. [Google Scholar] [CrossRef]
- Wilkinson, I.J.; Sangster, N.; Ratcliff, R.M.; Mugg, P.A.; Davos, D.E.; Lanser, J.E. Problems Associated with Identification of Legionella Species from the Environment and Isolation of Six Possible New Species. Appl. Environ. Microbiol. 1990, 56, 796–802. [Google Scholar] [CrossRef]
- Edagawa, A.; Kimura, A.; Doi, H.; Tanaka, H.; Tomioka, K.; Sakabe, K.; Nakajima, C.; Suzuki, Y. Detection of culturable and nonculturable Legionella species from hot water systems of public buildings in Japan. J. Appl. Microbiol. 2008, 105, 2104–2114. [Google Scholar] [CrossRef]
- Lee, H.K.; Shim, J.I.; Kim, H.E.; Yu, J.Y.; Kang, Y.H. Distribution of Legionella Species from Environmental Water Sources of Public Facilities and Genetic Diversity of L. pneumophila Serogroup 1 in South Korea. Appl. Environ. Microbiol. 2010, 76, 6547–6554. [Google Scholar] [CrossRef]
- Fleres, G.; Couto, N.; Lokate, M.; van der Sluis, L.W.M.; Ginevra, C.; Jarraud, S.; Deurenberg, R.H.; Rossen, J.W.; García-Cobos, S.; Friedrich, A.W. Detection of Legionella anisa in Water from Hospital Dental Chair Units and Molecular Characterization by Whole-Genome Sequencing. Microorganisms 2018, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Girolamini, L.; Salaris, S.; Pascale, M.R.; Mazzotta, M.; Cristino, S. Dynamics of Legionella Community Interactions in Response to Temperature and Disinfection Treatment: 7 Years of Investigation. Microb. Ecol. 2021, 83, 353–362. [Google Scholar] [CrossRef]
- Salinas, M.B.; Fenoy, S.; Magnet, A.; Vaccaro, L.; Gomes, T.D.S.; Angulo, S.; Hurtado, C.; Ollero, D.; Valdivieso, E.; del Águila, D.; et al. Are pathogenic Legionella non- pneumophila a common bacteria in Water Distribution Networks? Water Res. 2021, 196, 117013. [Google Scholar] [CrossRef]
- Fenstersheib, M.D.; Miller, M.; Diggins, C.; Liska, S.; Detwiler, L.; Werner, S.B.; Lindquist, D.; Thacker, W.L.; Benson, R.F. Outbreak of Pontiac fever due to Legionella anisa. Lancet 1990, 336, 35–37. [Google Scholar] [CrossRef]
- Jones, T.F.; Benson, R.F.; Brown, E.W.; Rowland, J.R.; Crosier, S.C.; Schaffner, W. Epidemiologic Investigation of a Restaurant- Associated Outbreak of Pontiac Fever. Clin. Infect. Dis. 2003, 37, 1292–1297. [Google Scholar] [CrossRef]
- Fields, B.S.; Barbaree, J.M.; Sanden, G.N.; Morrill, W.E. Virulence of a Legionella anisa Strain Associated with Pontiac Fever: An Evaluation Using Protozoan, Cell Culture, and Guinea Pig Models. Infect. Immun. 1990, 58, 3139–3142. [Google Scholar] [CrossRef]
- Stout, J.E.; Muder, R.R.; Mietzner, S.; Wagener, M.M.; Perri, M.B.; DeRoos, K.; Goodrich, D.; Arnold, W.; Williamson, T.; Ruark, O.; et al. Role of Environmental Surveillance in Determining the Risk of Hospital-Acquired Legionellosis: A National Surveillance Study with Clinical Correlations. Infect. Control. Hosp. Epidemiol. 2007, 28, 818–824. [Google Scholar] [CrossRef]
- Syndnor, E.R.M.; Bova, G.; Gimburg, A.; Cosgrove, S.E.; Perl, T.M.; Maragakis, L.L. Electronic-eye faucets; Legionella species contamination in healthcare settings. Infect. Control. Hosp. Epidemiol. 2012, 33, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Kubota, T.; Tateyama, M.; Koide, M.; Nakasone, C.; Tohyama, M.; Shinzato, T.; Higa, F.; Kusano, N.; Kawakami, K.; et al. Isolation of Legionella anisa from multiple sites of a hospital water system: The eradication of Legionella contamination. J. Infect. Chemother. 2003, 9, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Seng, P.; Drancourt, M.; Gouriet, F.; La Scola, B.; Fournier, P.-E.; Rolain, J.M.; Raoult, D. Ongoing Revolution in Bacteriology: Routine Identification of Bacteria by Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Clin. Infect. Dis. 2009, 49, 543–551. [Google Scholar] [CrossRef]
- La Scola, B.; Mezi, L.; Weiller, P.J.; Raoult, D. Isolation of Legionella anisa Using an Amoebic Coculture Procedure. J. Clin. Microbiol. 2001, 39, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Gaiaa, V.; Casatia, S.; Tonolla, M. Rapid identification of Legionella spp. By MALDI-TOF MS based protein mass fingerprinting Syst. Appl. Microbiol. 2011, 34, 40–44. [Google Scholar] [CrossRef]
- Peci, A.; Winter, A.-L.; Gubbay, J.B. Evaluation and Comparison of Multiple Test Methods, Including Real-time PCR, for Legionella Detection in Clinical Specimens. Front. Public Health 2016, 4, 175. [Google Scholar] [CrossRef]
- Kawasaki, T.; Nakagawa, N.; Murata, M.; Yasuo, S.; Yoshida, T.; Ando, K.; Okamori, S.; Okada, Y. Diagnostic accuracy of urinary antigen tests for legionellosis; A systematic review and meta-analysis. Respir. Investig. 2021, 60, 205–214. [Google Scholar] [CrossRef]
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526. [Google Scholar] [CrossRef]
- Uldum, S.A.; Schjoldager, L.G.; Baig, S.; Cassell, K. A Tale of Four Danish Cities: Legionella pneumophila Diversity in Domestic Hot Water and Spatial Variations in Disease Incidence. Int. J. Environ. Res. Public Health 2022, 19, 2530. [Google Scholar] [CrossRef] [PubMed]
- Head, B.M.; Trajtman, A.; Bernard, K.; Burdz, T.; Vélez, L.; Herrera, M.; Rueda, Z.V.; Keynan, Y. Legionella co-infection in HIV-associated pneumonia. Diagn. Microbiol. Infect. Dis. 2019, 95, 71–76. [Google Scholar] [CrossRef]
- Boczek, L.A.; Tang, M.; Formal, C.; Lytle, D.; Ryu, H. Comparison of two culture methods for the enumeration of Legionella pneumophila from potable water samples. J. Water Health 2021, 19, 468–477. [Google Scholar] [CrossRef]
- Health and Safety Executive. Legionnaires’ Disease Technical Guidance HSG 274 Part2: The Control of Legionella Bacteria in Hot and Cold Water Systems. Available online: https://www.hse.gov.uk/pubns/books/hsg274.htm (accessed on 27 July 2024).
- Levin, A.S.S.; Caiaffa Filho, H.H.; Sinto, S.I.; Sabbaga, E.; Barone, A.A.; Mendes, C.M.F. An outbreak of nosocomial Legionnaires’ disease in a renal transplant unit in Sao Paulo, Brazil. J. Hosp. Infect. 1991, 18, 243–248. [Google Scholar] [CrossRef]
- Koide, M.; Owan, T.; Nakasone, C.; Yamamoto, N.; Haranaga, S.; Higa, F.; Tateyama, M.; Yamane, N.; Fujita, J. Prospective Monitoring Study: Isolating Legionella pneumophila in a Hospital Water System Located in the Obstetrics and Gynecology Ward after Eradication of Legionella anisa and Reconstruction of Shower Units. Jpn. J. Infect. Dis. 2007, 60, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Girolamini, L.; Salaris, S.; Lizzadro, J.; Mazzotta, M.; Pascale, M.R.; Pellati, T.; Cristino, S. How Molecular Typing Can Support Legionella Environmental Surveillance in Hot Water Distribution Systems: A Hospital Experience. Int. J. Environ. Res. Public Health 2020, 17, 8662. [Google Scholar] [CrossRef] [PubMed]
- Krøjgaard, L.H.; Krogfelt, K.A.; Albrechtsen, H.J.; Uldum, S.A. Cluster of Legionnaires’ disease in a newly built block of flats, Denmark, December 2008—January 2009. Euro Surveill. 2011, 16, 19759. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.; Moletta-Denat, M.; Frère, J.; Onillon, S.; Trouilhé, M.-C.; Robinea, E. Effects of Disinfection on Legionella spp., Eukarya, and Biofilms in a Hot Water System. Appl. Environ. Microbiol. 2012, 78, 6850–6858. [Google Scholar] [CrossRef]
- Lin, Y.E.; Stout, J.E.; Yu, V.L. Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods. Infect. Control. Hosp. Epidemiol. 2011, 32, 166–173. [Google Scholar] [CrossRef]
- Nisar, M.A.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. Water Stagnation and Flow Obstruction Reduces the Quality of Potable Water and Increases the Risk of Legionelloses. Front. Environ. Sci. 2020, 8, 611611. [Google Scholar] [CrossRef]
- Legionella Control Association webinar—Legionella Dangers Emerging from Lockdown. Available online: https://www.legionellacontrol.org.uk/video-media/view/?id=17 (accessed on 15 January 2024).
- Health and Safety Executive. Legionnaires’ Disease. L8. The Control of Legionella Bacteria in Water Systems Approved Code of Practice and Guidance. Available online: https://www.hse.gov.uk/pubns/books/l8.htm (accessed on 25 July 2024).
- BS7592:2022; Sampling for Legionella Bacteria in Water Systems—Code of Practice. BSI Knowledge: London, UK, 2022.
- BS EN ISO 11731:2017; Water Quality—Enumeration of Legionella. ISO: Geneva, Switzerland, 2017.
- UK Institute for Government. Timeline of UK Government Coronavirus Lockdowns and Restrictions. Available online: https://www.instituteforgovernment.org.uk/data-visualisation/timeline-coronavirus-lockdowns (accessed on 29 July 2024).
- Water Regulations Approval Scheme Ltd.—Product Approvals. Available online: https://www.wrasapprovals.co.uk (accessed on 1 August 2024).
- Cavallaro, A.; Rhoads, W.J.; Sylvestre, E.; Marti, T.; Walser, J.-C.; Hammes, F. Legionella relative abundance in shower hose biofilms is associated with specific microbiome members. FEMS Microbes 2023, 4, xtad016. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Summary | References |
|---|---|---|
| L. anisa as a colonizer of water distribution systems | After L. pneumophila, L. anisa is the species most frequently detected in the environment, including from both hot water systems and cooling-tower systems | [14,21,22,23,24,25,26,27,28,29] |
| Case reports of colonization of cooling towers, hotel decorative fountain, and restaurant water feature | [5,6,30,31] | |
| Colonization in cooling towers associated with protozoan Acanthamoeba | [5,32] | |
| 9/20 (45%) hospital water systems in 14 US states colonized with L. anisa—sample positivity rate of 57/674 (8.5%) | [33] | |
| 29/108 (27%) electronic tap-water samples are L. anisa-positive compared to 25/108 (23%) L. pneumophila-positive (USA) | [34] | |
| Japanese studies >40% samples from public buildings contaminated with L. pneumophila and L. anisa; L. anisa persisted in hospital water for at least 6 years, causing sporadic contamination of shower heads | [25,35] | |
| Frequency of environmental detection of L. anisa | 13.8% of environmental water sample isolates, but only 0.8% of clinical isolates | [21] |
| 10/8066 (<0.1%) confirmed clinical cases of legionellosis in New Zealand, Australia, Europe, USA, and Japan attributable to L. anisa | [9] | |
| In 508 community-acquired pneumonia cases, 0.2% cases were attributable to L. anisa, and 91.5% to L. pneumophila | [17] | |
| Non-pneumonic conditions attributable to L. anisa infection | Chronic endocarditis, pleural infection, osteomyelitis, and mycotic aortic aneurysm | [11,36,37,38] |
| Clinical testing for L. anisa by urinary antigen tests (UATs) | UAT-sensitive and -specific but target L. pneumophila Sg1, possibly missing up to 40% of cases, and limited for matching environmental samples | [2,9,33,39,40,41] |
| Clinical testing for L. anisa by polymerase chain reaction (PCR) | >80% LD cases in Denmark diagnosed by PCR—likely to increase detection in non-Sg1 cases; thus, non-L. pneumophila species found to cause at least 4.5% of all LD cases | [22,42] |
| Spanish study of 210 urine samples from suspected pneumonia, and only 4 (1.9%) tested positive for Sg1 by UAT, while by PCR, these 4 tested positive for Sg1 plus 10 more positive for unidentifiable Legionella species and 1 positive for L. anisa | [8] | |
| In a Colombian study of HIV-associated pneumonia patients, 17 had Legionella co-infection—all culture negative, but 6/17 PCR positive for L. anisa and 3/17 for L. pneumophila | [43] |
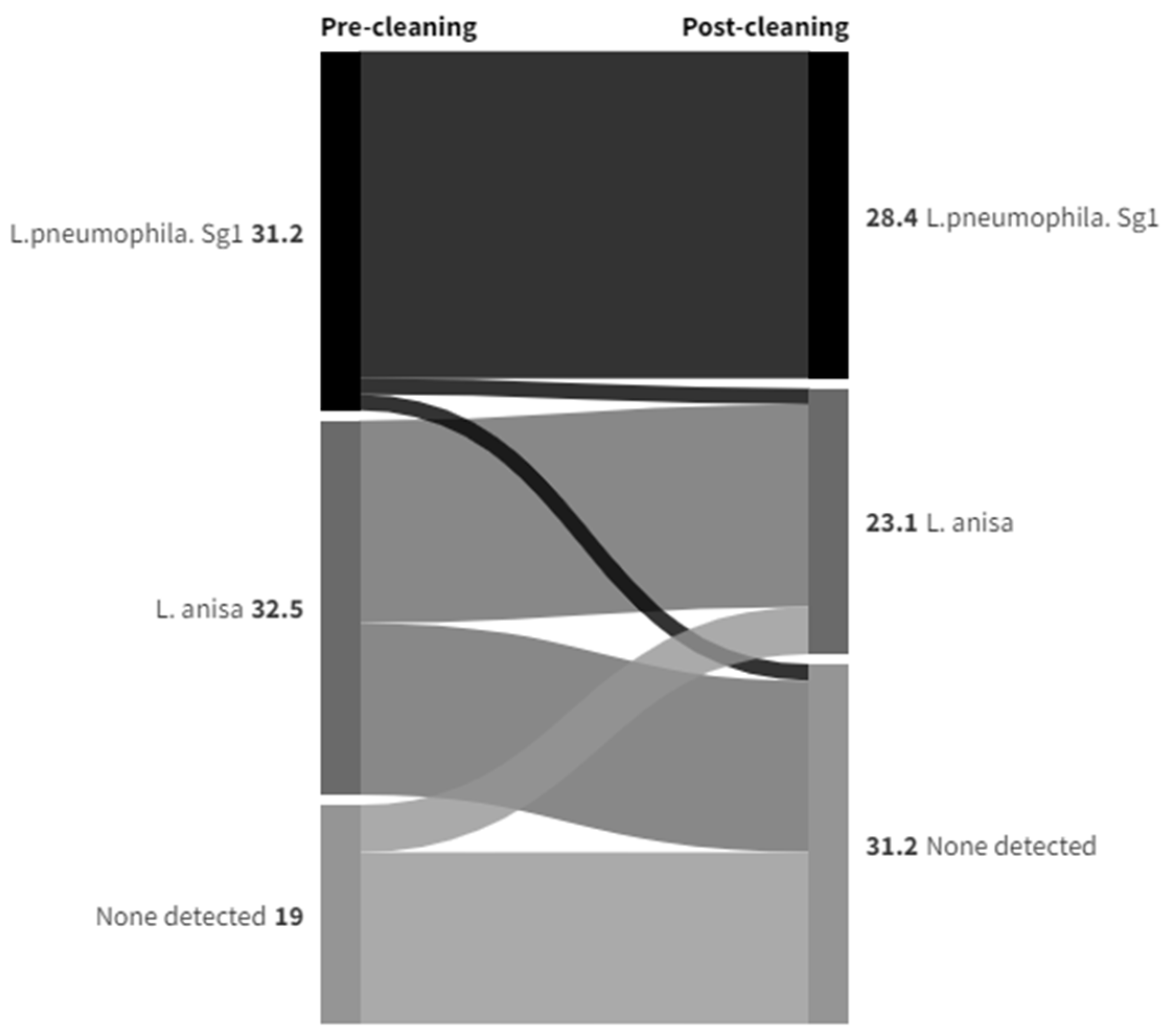
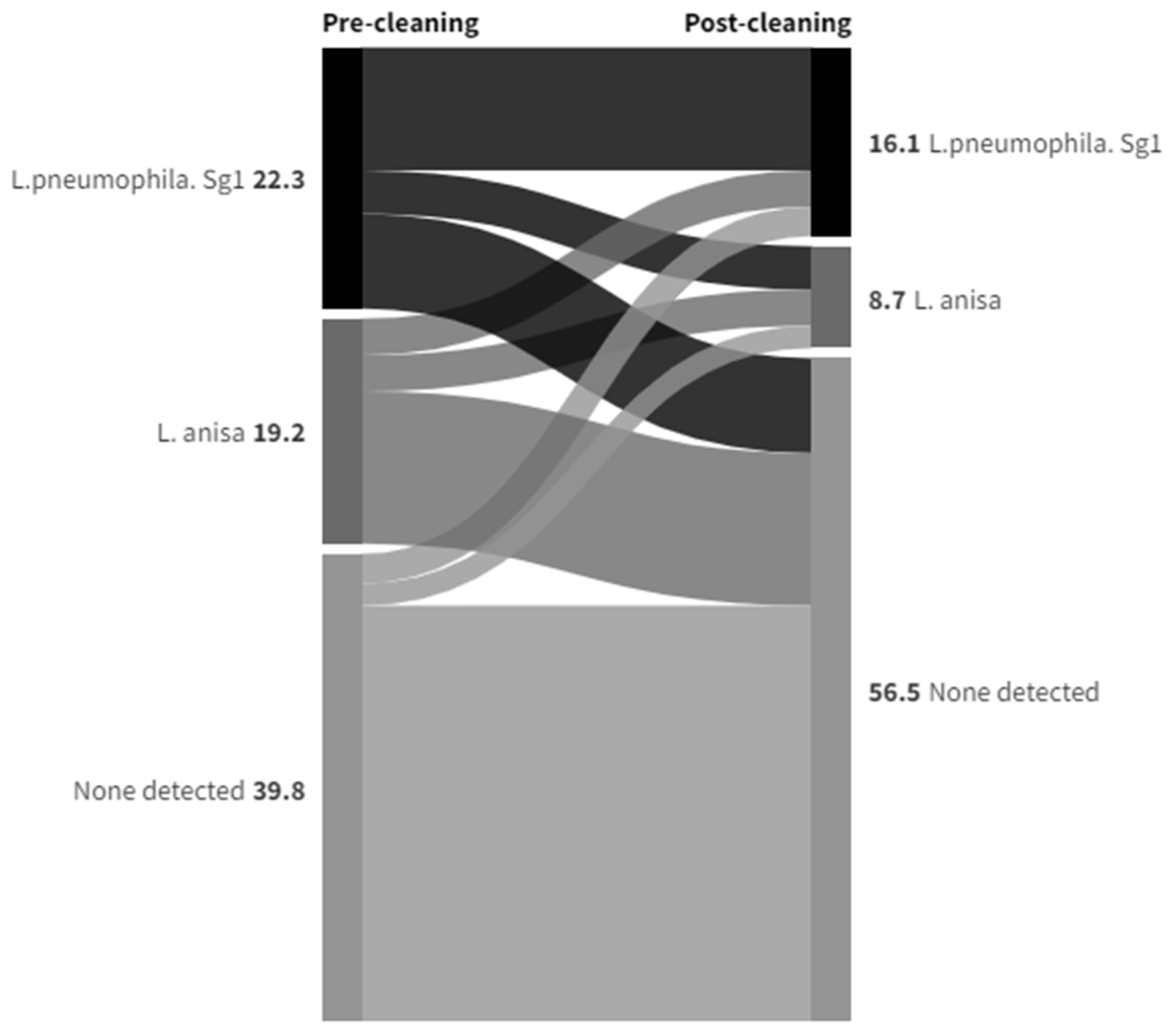
| Post-Cleaning Sample | Grand Total | |||||
|---|---|---|---|---|---|---|
| Anisa | Pneumophila | Other | ND | |||
| Pre-Cleaning Sample | Anisa | 18 | 6 | 3 | 34 | 61 |
| Pneumophila | 8 | 54 | 4 | 15 | 81 | |
| Other | 6 | 6 | 4 | 9 | 25 | |
| None Detected | 6 | 5 | 1 | 103 | 116 | |
| Grand Total | 38 | 71 | 12 | 162 | 283 | |
| Date | Pre-Cleaning Sample Colonies | Post-Cleaning Sample Colonies | ||||||
|---|---|---|---|---|---|---|---|---|
| Anisa | Pneumophila | Other | Total | Anisa | Pneumophila | Other | Total | |
| Jan 2020 | 40 | 1520 | 0 | 1560 | ||||
| Feb 2020 | 12,920 | 80 | 1268 | 14,268 | ||||
| Mar 2020 | 5820 | 360 | 2400 | 8580 | ||||
| May 2020 | 2920 | 2120 | 1460 | 6500 | ||||
| Jun 2020 | 4762 | 7630 | 0 | 12,392 | 3081 | 1540 | 0 | 4621 |
| Jul 2020 | 3180 | 460 | 6400 | 10,040 | 3340 | 0 | 180 | 3520 |
| Nov 2020 | 11,080 | 25,080 | 4200 | 40,360 | 1260 | 10,820 | 1400 | 13,480 |
| Dec 2020 | 2520 | 8280 | 0 | 10,800 | 120 | 3180 | 0 | 3300 |
| Feb 2021 | 2821 | 26,496 | 0 | 29,317 | 408 | 19,635 | 4400 | 24,443 |
| Mar 2021 | 11,673 | 50,127 | 2120 | 63,920 | 8937 | 37,380 | 0 | 46,317 |
| Apr 2021 | 28,875 | 39,480 | 17,260 | 85,615 | 940 | 11,540 | 3860 | 16,340 |
| May 2021 | 3400 | 111,440 | 0 | 114,840 | 0 | 20,880 | 200 | 21,080 |
| Jun 2021 | 6680 | 53,540 | 9700 | 69,920 | 11,460 | 15,240 | 3160 | 29,860 |
| Total | 74,991 | 322,533 | 39,680 | 437,204 | 51,246 | 124,295 | 18,328 | 193,869 |
| Species Detected | Count | (Proportion) |
|---|---|---|
| Legionella pneumophila | 17,617 | (29.3%) |
| Legionella anisa | 32,517 | (54.1%) |
| Legionella species | 4162 | (6.9%) |
| Other | 1992 | (3.3%) |
| Blank (no species identified) | 3787 | (6.3%) |
| Total | 60,075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crook, B.; Young, C.; Rideout, C.; Smith, D. The Contribution of Legionella anisa to Legionella Contamination of Water in the Built Environment. Int. J. Environ. Res. Public Health 2024, 21, 1101. https://doi.org/10.3390/ijerph21081101
Crook B, Young C, Rideout C, Smith D. The Contribution of Legionella anisa to Legionella Contamination of Water in the Built Environment. International Journal of Environmental Research and Public Health. 2024; 21(8):1101. https://doi.org/10.3390/ijerph21081101
Chicago/Turabian StyleCrook, Brian, Charlotte Young, Ceri Rideout, and Duncan Smith. 2024. "The Contribution of Legionella anisa to Legionella Contamination of Water in the Built Environment" International Journal of Environmental Research and Public Health 21, no. 8: 1101. https://doi.org/10.3390/ijerph21081101




