WildTrack: An IoT System for Tracking Passive-RFID Microchipped Wildlife for Ecology Research
Abstract
:1. Introduction
| Tracking Technique | Description | Information Obtained | How Information Is Obtained | Spatial Accuracy | Minimum Body Size (g) * | Lifespan of Device | Cost per Unit | Pros and Cons |
|---|---|---|---|---|---|---|---|---|
| Indirect measures (i.e., scats, tracks and hair samples) [21,22,23] | Scats: identify species using faeces. Tracks: sand or ink pads to identify species from foot tracks. Hair funnels: sticky funnels or hoops which capture hair for species identification. | Presence data | Opportunistically obtained | Exact | n/a | n/a | Variable | Useful for confirming presence of a species but not individual identification. Genetic analysis is time-consuming and expensive. Track analysis is weather-dependent. |
| Camera traps [24,25] | Camera placed in the environment that is automatically triggered typically by passive infrared motion sensor | Presence data. Time and location data. | Requires animal to trigger the camera by passing through a sensor | Exact | n/a | Days to months | USD 100–USD 800 | Useful in picking up difficult-to-catch or cryptic species. Does not require animal to carry a device. Image sorting is labour-intensive. Rarely useful in individual identification. |
| Individual identification tags [26] | Small individually numbered metal or plastic tags attached to the animal (i.e., ears, legs or wings) | Location and survival at subsequent re-captures | Requires capture and attachment of tags, followed by recapture | Exact | 1 g | Years | Minimal <USD 0.20/ tag | Cheap (can deploy large numbers). Suitable for small species. Usually lasts the animal’s lifetime. Requires re-capture. Labour intensive. |
| VHF (very high frequency) or transponder radio telemetry [3,27,28,29] | Uses the transmission of radio signals to locate a transmitter attached to the animal | Time and location data | Requires capture and attachment of tag. Data obtained when animal is near an antenna or receiver (<5 m) | 10 m | 6.6 g | From days to months dependant on battery size, transmitter power and update rate [30] | USD 100–USD 300 | Relatively inexpensive. Transmitters come in a variety of sizes. Smaller tags have shorter battery life. Labour intensive, but can be automated with towers and fixed receivers for less mobile species. Lower spatial accuracy. |
| Satellite or transponder telemetry (GPS and Argos tracking) [31,32] | Transmitter is attached to animal and sends a signal to orbiting satellites. The satellites retransmit the data back to be stored on the transmitter or sent to a receiving station. | Time and location dat.a | Requires capture and attachment of tag. Data transmitted to satellites. Requires recapture with some tags to retrieve stored data. | 250–1500 m | 120 g | From months to 1 year dependant on battery size of transmitter | USD 2000–USD 7000 | Expensive and large. Reduced labour as data is collected automatically. All Argos tags transmit but not all GPS do. Archival GPS tags store data on tag and hence require retrieval. Tags that transmit information do not need to be retrieved and data can be accessed by computer. Reduced spatial accuracy. |
| Radio frequency identification (RFID) tags [8,33] | RFID tags or microchips can be glued or implanted on the animal. PIT (passive integrated transponder) tags are not powered; instead, the chip is activated when close to an antenna. Hence, they are some of the smallest tags available (3–60 mg). | Time animal was near receiver (location if multiple receivers are used) | When animal is close to a receiver (<1 m) | Exact | 0.03 g | Years | <USD 2/ chip | Receivers can be costly USD 2,000 and minimal expense per tag (can deploy large numbers). Tags are battery-less, lightweight and can last the animals lifetime. Suitable for very small species. Needs to come close to a receiver to transmit data |
2. System Design and Implementation
3. Field Testing Methodology
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linhoff, L.J.; Soorae, P.S.; Harding, G.; Donnelly, M.A.; Germano, J.M.; Hunter, D.A.; McFadden, M.; Mendelson, J.R., III; Pessier, A.P.; Sredl, M.J.; et al. IUCN Guidelines for Amphibian Reintroductions and Other Conservation Translocations. 2021. Available online: https://policycommons.net/artifacts/1529413/iucn-guidelines-for-amphibian-reintroductions-and-other-conservation-translocations/ (accessed on 20 July 2022).
- Berger-Tal, O.; Blumstein, D.; Swaisgood, R.R. Conservation translocations: A review of common difficulties and promising directions. First Edition. Int. Union Conserv. Nat. 2020, 23, 121–131. [Google Scholar]
- Kays, R.; Crofoot, M.C.; Jetz, W.; Wikelski, M. Terrestrial animal tracking as an eye on life and planet. Science 2015, 348, 1222–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikes, R.S.; Care, A. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Carver, S.; Browne, E.; Thai, B.S. WomBot: An exploratory robot for monitoring wombat burrows. SN Appl. Sci. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Lunadei, L. The role of RFID in agriculture: Applications, limitations and challenges. Comput. Electron. Agric. 2011, 79, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Brown-Brandl, T.M.; Adrion, F.; Maselyne, J.; Kapun, A.; Hessel, E.; Saeys, W.; Van Nuffel, A.; Gallmann, E. A review of passive radio frequency identification systems for animal monitoring in livestock facilities. Appl. Eng. Agric. 2019, 35, 579–591. [Google Scholar] [CrossRef]
- Rafiq, K.; Appleby, R.G.; Edgar, J.P.; Radford, C.; Smith, B.P.; Jordan, N.R.; Dexter, C.E.; Jones, D.N.; Blacker, A.R.; Cochrane, M. WildWID: An open-source active RFID system for wildlife research. Methods Ecol. Evol. 2021, 12, 1580–1587. [Google Scholar] [CrossRef]
- Scott, N.L.; Hansen, B.; LaDue, C.A.; Lam, C.; Lai, A.; Chan, L. Using an active Radio Frequency Identification Real-Time Location System to remotely monitor animal movement in zoos. Anim. Biotelemetry 2016, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Barlow, S.E.; O’Neill, M.A.; Pavlik, B.M. A prototype RFID tag for detecting bumblebee visitations within fragmented landscapes. J. Biol. Eng. 2019, 13, 1–6. [Google Scholar] [CrossRef]
- Bonter, D.N.; Bridge, E.S. Applications of radio frequency identification (RFID) in ornithological research: A review. J. Field Ornithol. 2011, 82, 1–10. [Google Scholar] [CrossRef]
- van Harten, E.; Reardon, T.; Lumsden, L.F.; Meyers, N.; Prowse, T.A.; Weyland, J.; Lawrence, R. High detectability with low impact: Optimizing large PIT tracking systems for cave-dwelling bats. Ecol. Evol. 2019, 9, 10916–10928. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Lindholm, A.K.; Lopes, P.C.; Dobay, A.; Steinert, S.; Buschmann, F.J.U. A system for automatic recording of social behavior in a free-living wild house mouse population. Anim. Biotelemetry 2015, 3, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Walter, T.; Couzin, I.D. TRex, a fast multi-animal tracking system with markerless identification, and 2D estimation of posture and visual fields. eLife 2021, 10, e64000. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Vayssade, J.A.; Troupe, W.; Arquet, R. Outdoor animal tracking combining neural network and time-lapse cameras. Comput. Electron. Agric. 2020, 168, 105150. [Google Scholar] [CrossRef]
- Ross, R.; Parsons, L.; Thai, B.S.; Hall, R.; Kaushik, M. An IOT smart rodent bait station system utilizing computer vision. Sensors 2020, 20, 4670. [Google Scholar] [CrossRef] [PubMed]
- Duda, N.; Nowak, T.; Hartmann, M.; Schadhauser, M.; Cassens, B.; Wägemann, P.; Nabeel, M.; Ripperger, S.; Herbst, S.; Meyer-Wegener, K.; et al. BATS: Adaptive ultra low power sensor network for animal tracking. Sensors 2018, 18, 3343. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Li, H.; Tian, C.; Myjak, M.J.; Xiao, J.; Bellgraph, B.J.; Cartmell, S.S.; Deng, Z.D. A Frequency-Programmable Miniaturized Radio Frequency Transmitter for Animal Tracking. Sensors 2021, 21, 6683. [Google Scholar] [CrossRef]
- Habedank, A.; Urmersbach, B.; Kahnau, P.; Lewejohann, L. O mouse, where art thou? The Mouse Position Surveillance System (MoPSS)—An RFID-based tracking system. Behav. Res. Methods 2022, 54, 676–689. [Google Scholar] [CrossRef]
- Sinha, R.S.; Wei, Y.; Hwang, S.H. A survey on LPWA technology: LoRa and NB-IoT. ICT Express 2017, 3, 14–21. [Google Scholar] [CrossRef]
- Klare, U.; Kamler, J.F.; Macdonald, D.W. A comparison and critique of different scat-analysis methods for determining carnivore diet. Mammal Rev. 2011, 41, 294–312. [Google Scholar] [CrossRef]
- Gužvica, G.; Bošnjak, I.; Bielen, A.; Babić, D.; Radanović-Gužvica, B.; Šver, L. Comparative analysis of three different methods for monitoring the use of green bridges by wildlife. PLoS ONE 2014, 9, e106194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, D.; Harris, B.; Claridge, A.; Barry, S. Efficacy of hair-sampling techniques for the detection of medium-sized terrestrial mammals. I. A comparison between hair-funnels, hair-tubes and indirect signs. Wildl. Res. 2002, 29, 379–387. [Google Scholar] [CrossRef]
- Fonteyn, D.; Vermeulen, C.; Deflandre, N.; Cornelis, D.; Lhoest, S.; Houngbégnon, F.G.; Doucet, J.L.; Fayolle, A. Wildlife trail or systematic? Camera trap placement has little effect on estimates of mammal diversity in a tropical forest in Gabon. Remote. Sens. Ecol. Conserv. 2021, 7, 321–336. [Google Scholar] [CrossRef]
- Rovero, F.; Zimmermann, F.; Berzi, D.; Meek, P. “Which camera trap type and how many do I need?” A review of camera features and study designs for a range of wildlife research applications. Hystrix 2013, 24, 148–156. [Google Scholar]
- Rivalan, P.; Godfrey, M.H.; PRÉVOT-JULLIARD, A.C.; Girondot, M. Maximum likelihood estimates of tag loss in leatherback sea turtles. J. Wildl. Manag. 2005, 69, 540–548. [Google Scholar] [CrossRef]
- Kays, R.; Tilak, S.; Crofoot, M.; Fountain, T.; Obando, D.; Ortega, A.; Kuemmeth, F.; Mandel, J.; Swenson, G.; Lambert, T.; et al. Tracking animal location and activity with an automated radio telemetry system in a tropical rainforest. Comput. J. 2011, 54, 1931–1948. [Google Scholar] [CrossRef]
- Ojo, M.O.; Viola, I.; Baratta, M.; Giordano, S. Practical experiences of a smart livestock location monitoring system leveraging gnss, lorawan and cloud services. Sensors 2021, 22, 273. [Google Scholar] [CrossRef]
- Liu, X.; Yang, T.; Yan, B. Internet of things for wildlife monitoring. In Proceedings of the 2015 IEEE/CIC International Conference on Communications in China-Workshops (CIC/ICCC), Shenzhen, China, 2–4 November 2015; pp. 62–66. [Google Scholar]
- Dyo, V.; Ellwood, S.A.; Macdonald, D.W.; Markham, A.; Mascolo, C.; Pásztor, B.; Trigoni, N.; Wohlers, R. Wildlife and environmental monitoring using RFID and WSN technology. In Proceedings of the 7th ACM Conference on Embedded Networked Sensor Systems, Berkley, CA, USA, 4–6 November 2009; pp. 371–372. [Google Scholar]
- Coyne, M.; Godley, B. Satellite Tracking and Analysis Tool (STAT): An integrated system for archiving, analyzing and mapping animal tracking data. Mar. Ecol. Prog. Ser. 2005, 301, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.; Holland, J.D.; Minot, E.O. Wildlife tracking technology options and cost considerations. Wildl. Res. 2011, 38, 653–663. [Google Scholar] [CrossRef]
- Floyd, R.E. RFID in animal-tracking applications. IEEE Potentials 2015, 34, 32–33. [Google Scholar] [CrossRef]
- Neff, E.P. An RFID-based tracking system gives mice their say. Lab Anim. 2021, 50, 282. [Google Scholar] [CrossRef]
- Lie, R. LoRa. 2018. Available online: https://lora.readthedocs.io/en/latest/ (accessed on 20 May 2022).
- Singh, Y. Comparison of okumura, hata and cost-231 models on the basis of path loss and signal strength. Int. J. Comput. Appl. 2012, 59. [Google Scholar] [CrossRef]
- Ojo, M.O.; Adami, D.; Giordano, S. Experimental evaluation of a LoRa wildlife monitoring network in a forest vegetation area. Future Internet 2021, 13, 115. [Google Scholar] [CrossRef]




| Enclosure | No. of Detections | No. of Base Stations that Made Detections (No. Installed) | Date of Detections | Comments |
|---|---|---|---|---|
| 1 | 31 | 5 (9) | 12 January–7 March 2021 | 2 mice released 10 January, 1 on 11 January, 1 on 16 January |
| 2 | 32 | 2 (9) | 11 January–14 March 2021 | 1 mouse released 10 January, 1 on 11 January, 2 on 12 January |
| 3 | 31 | 5 (8) | 17 January–19 March 2021 | 2 mice released 16 January, 2 on 17 January |
| 4 | 8 | 2 (10) | 13 January–6 March 2021 | 1 mouse released 10 January, 3 on 18 January. One mouse escaped and was detected in enclosure 8 |
| 5 | 53 | 6 (10) | 10 January–22 March 2021 | 2 mice released 10 January, 2 on 18 January. One mouse escaped and was detected in enclosure 8, then returned to enclosure 5 |
| 7 | 34 | 5 (8) | 13 January 21–22 March 2021 | 2 mice released 10 January, 2 on 16 January |
| Position: Edge of Antenna | ||
|---|---|---|
| Angle to Antenna Plane | Mean Distance (mm) | Standard Deviation |
| 0 (‖) Tangent | 38 | 9.7 |
| 0 (‖) 90 Tangent | 105.8 | 3.8 |
| 45 (∠) Tangent | 89.2 | 2.9 |
| 45 (∠) 90 Tangent | 66 | 6.5 |
| 90 (⊥) | 117.6 | 2.5 |
| Position: 40 mm from Antenna Edge | ||
| Angle to Antenna Plane | Mean Distance (mm) | Standard Deviation |
| 0 (‖) min(Secant) | 46 | 6.5 |
| 0 (‖) max(Secant) | 85 | 5 |
| 45 (∠) min(Secant) | 59 | 7.4 |
| 45 (∠) max(Secant) | 129 | 2.2 |
| 90 (⊥) | 144 | 4.2 |
| Position: Antenna Center | ||
| Angle to Antenna Plane | Mean Distance (mm) | Standard Deviation |
| 0 (‖) | 38 | 14.4 |
| 45 (∠) | 125 | 3.5 |
| 90 (⊥) | 153 | 2.7 |
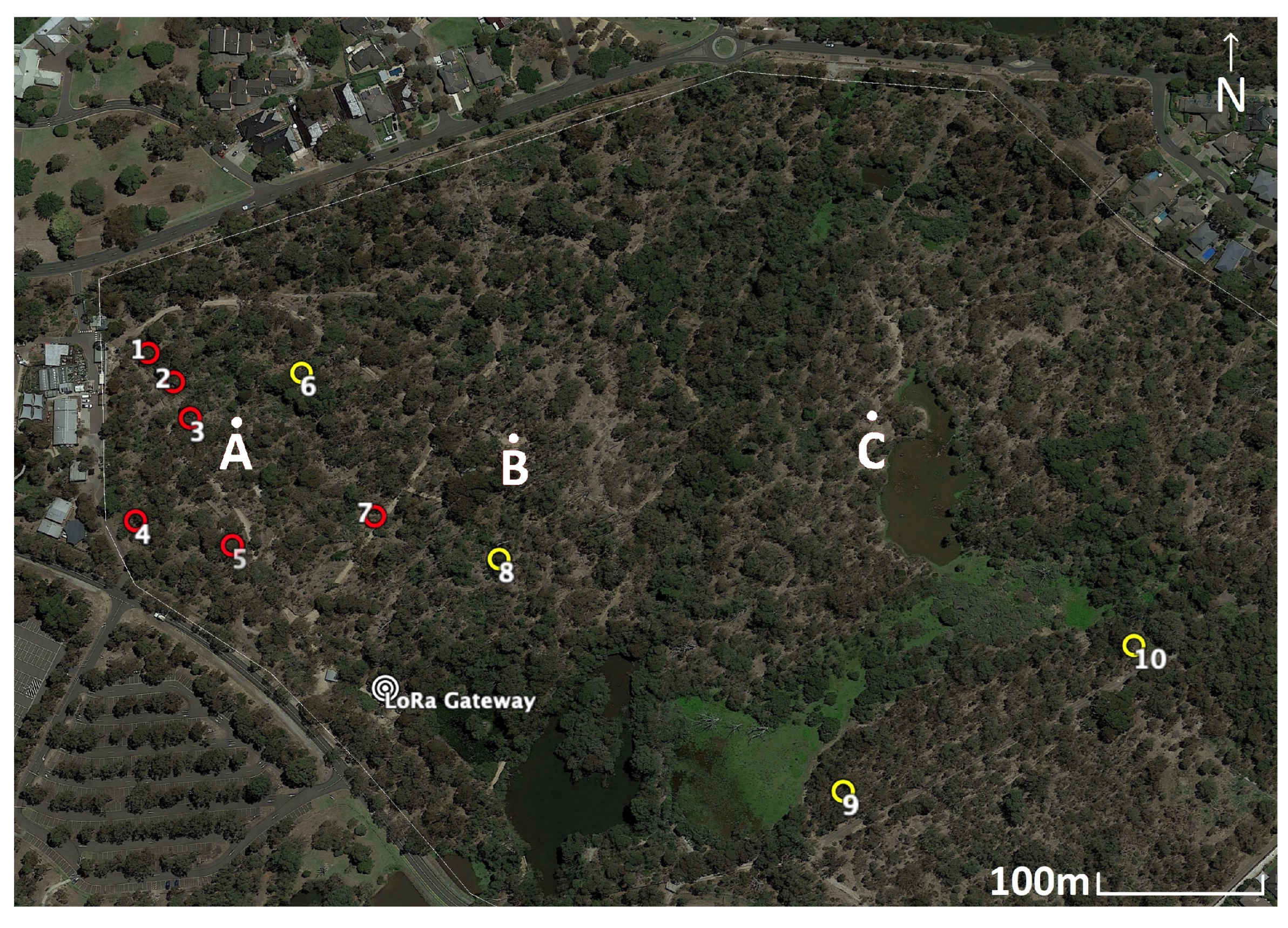
| Test Site A | ||||||
|---|---|---|---|---|---|---|
| Height: 0 m | Height: 1 m | Height: 1.5 m | ||||
| Node 1 | Node 2 | Node 1 | Node 2 | Node 1 | Node 2 | |
| RSSI | −104 [3.6] | −103 [3.2] | −100 [2.4] | −101 [3.3] | −103 [3.5] | −99 [3.6] |
| SNR | −3.9 [3.6] | −5 [2.7] | 5.6 [1.3] | 1.6 [1.8] | −1.6 [3.4] | 4.1 [4.0] |
| Test Site B | ||||||
| Height: 0 m | Height: 1 m | Height: 1.5 m | ||||
| Node 1 | Node 2 | Node 1 | Node 2 | Node 1 | Node 2 | |
| RSSI | −96.8 [1.5] | −96.4 [3.6] | −86.2 [4.6] | −89 [1.2] | −91.2 [3.9] | −90.2 [2.9] |
| SNR | 6.85 [1.9] | 7 [2.0] | 10.95 [0.9] | 10.8 [2.2] | 9.9 [1.2] | 10.1 [1.3] |
| Test Site C | ||||||
| Height: 0 m | Height: 1 m | Height: 1.5 m | ||||
| Node 1 | Node 2 | Node 1 | Node 2 | Node 1 | Node 2 | |
| RSSI | −106 [1.2] | −107 [3.5] | −105.8 [3.0] | −103.8 [2.78] | −106.4 [1.3] | −104 [2.4] |
| SNR | −5.65 [3.1] | −7.5 [2.4] | −4.6 [3.7] | −5.3 [4.3] | −4.7 [3.3] | −5.25 [5.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, R.; Anderson, B.; Bienvenu, B.; Scicluna, E.L.; Robert, K.A. WildTrack: An IoT System for Tracking Passive-RFID Microchipped Wildlife for Ecology Research. Automation 2022, 3, 426-438. https://doi.org/10.3390/automation3030022
Ross R, Anderson B, Bienvenu B, Scicluna EL, Robert KA. WildTrack: An IoT System for Tracking Passive-RFID Microchipped Wildlife for Ecology Research. Automation. 2022; 3(3):426-438. https://doi.org/10.3390/automation3030022
Chicago/Turabian StyleRoss, Robert, Ben Anderson, Brian Bienvenu, Emily L. Scicluna, and Kylie A. Robert. 2022. "WildTrack: An IoT System for Tracking Passive-RFID Microchipped Wildlife for Ecology Research" Automation 3, no. 3: 426-438. https://doi.org/10.3390/automation3030022
APA StyleRoss, R., Anderson, B., Bienvenu, B., Scicluna, E. L., & Robert, K. A. (2022). WildTrack: An IoT System for Tracking Passive-RFID Microchipped Wildlife for Ecology Research. Automation, 3(3), 426-438. https://doi.org/10.3390/automation3030022







