Injectability of Partially Hydrolyzed Polyacrylamide Solutions Improved by Anionic-Nonionic Surfactant in Medium and Low Permeability Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fluid Flow Tests
2.3. Hall Derivative Method
- (1)
- Based on the flow tests and data in (Section 2.2), we used the following formula to calculate the Hall integral corresponding to the ith data point:where, Ih,i is the Hall integral at the ith data point (I ≥ 2); MPa·min, Δti is the time interval between the i−1th data point and the ith data point, min; Pi−1 is the injection pressure at the i−1th data point, MPa; Pi is the injection pressure at the i-th data point, MPa; and n is the total number of data points.
- (2)
- Based on the calculation result of step (1), drawing the curve of the Hall integral versus the cumulative injection amount, the Hall curve was obtained.
- (3)
- We used the following formula to calculate the Hall derivative:where, f(Ih,i) is the Hall derivative at the i-th data point (I ≥ 2), MPa·min·mL−1; Ih,i−1 is the Hall integral at the i1th data point, MPa·min; Qi is the cumulative injection volume at the ith data point, mL; and Qi−1 is the cumulative injection volume at the i1th data point, mL.
- (4)
- Based on the calculation result of step (3), a curve of Hall derivative versus cumulative injection amount was drawn, then the Hall derivative curve was obtained.
3. Results and Discussion
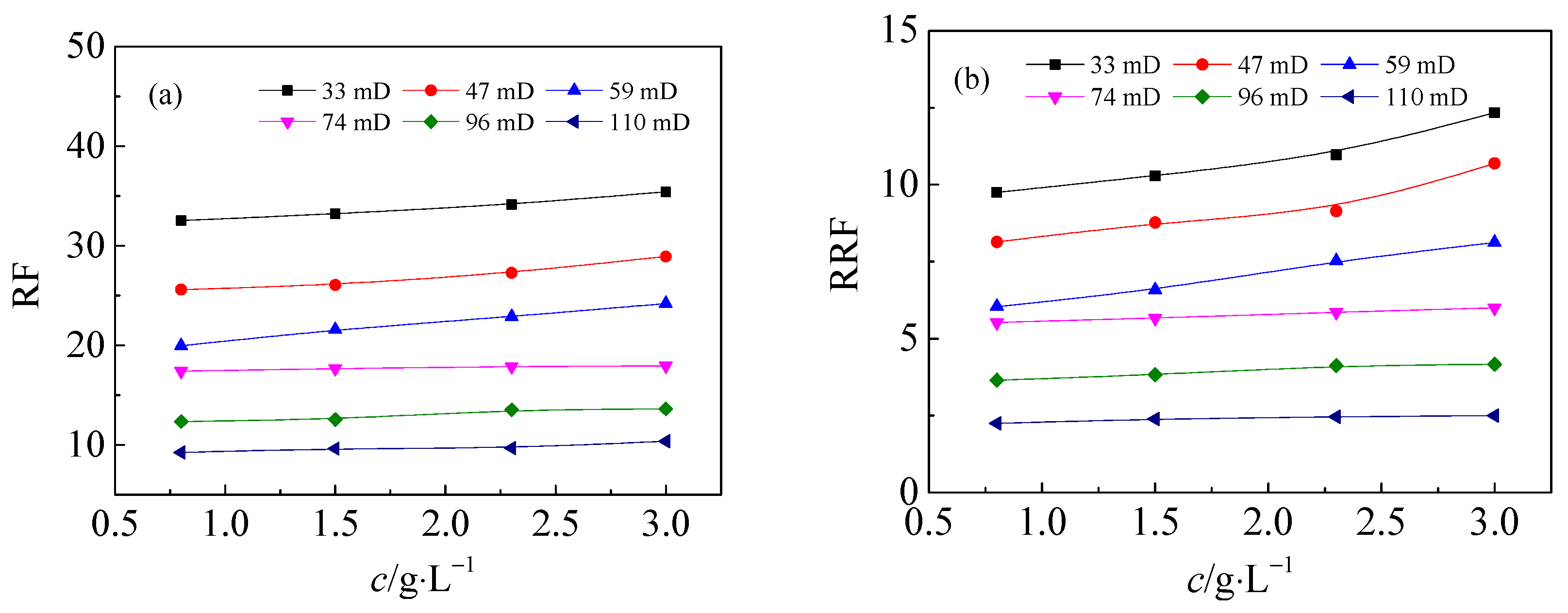
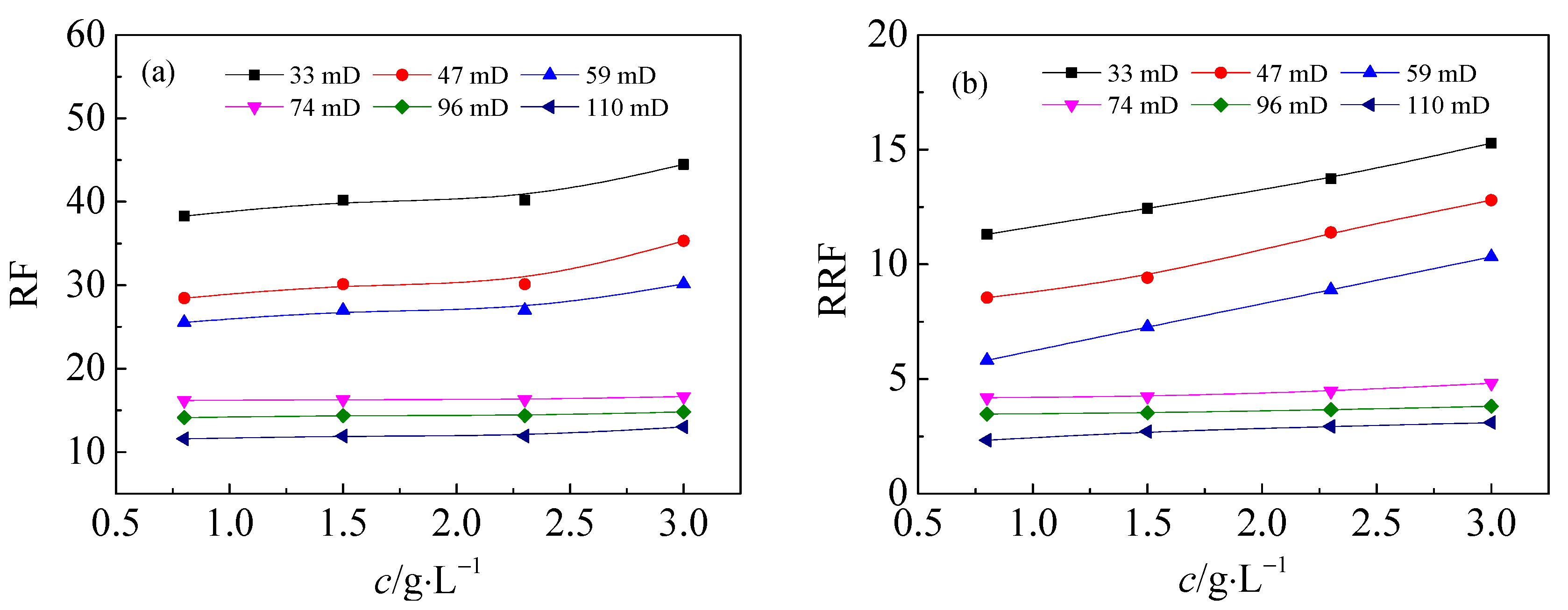
3.1. Flow Performance of HPAM Solutions in Medium and Low Permeability Cores
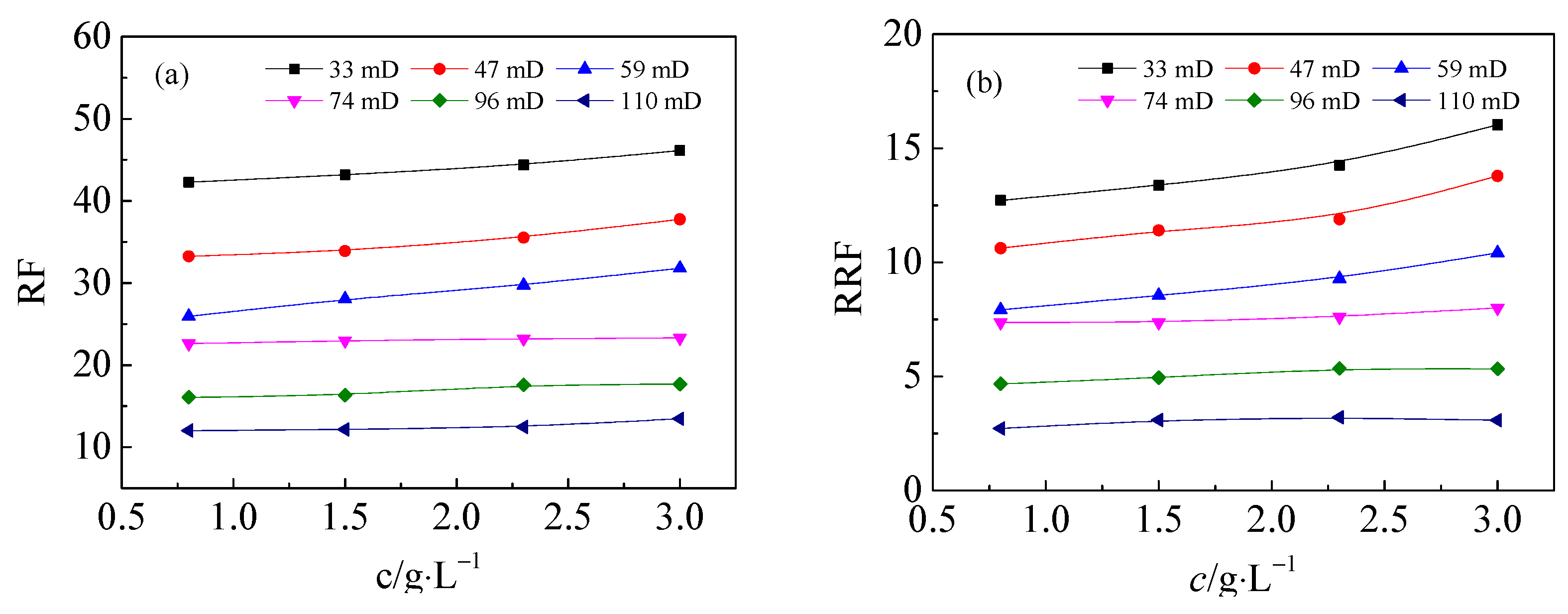
3.2. Effect of ANS on the Flow Performance of HPAM Solutions in Medium and Low Permeability Cores
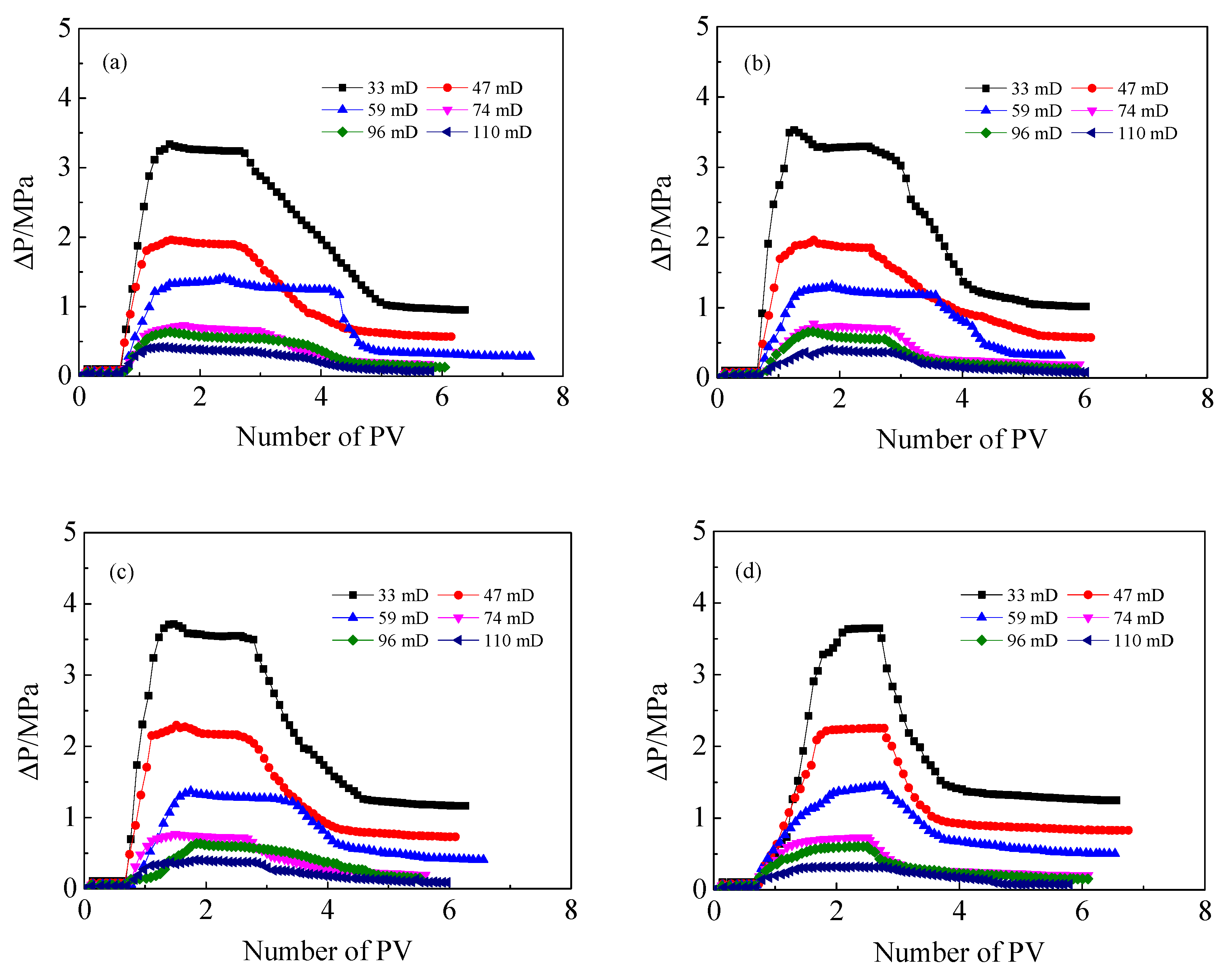
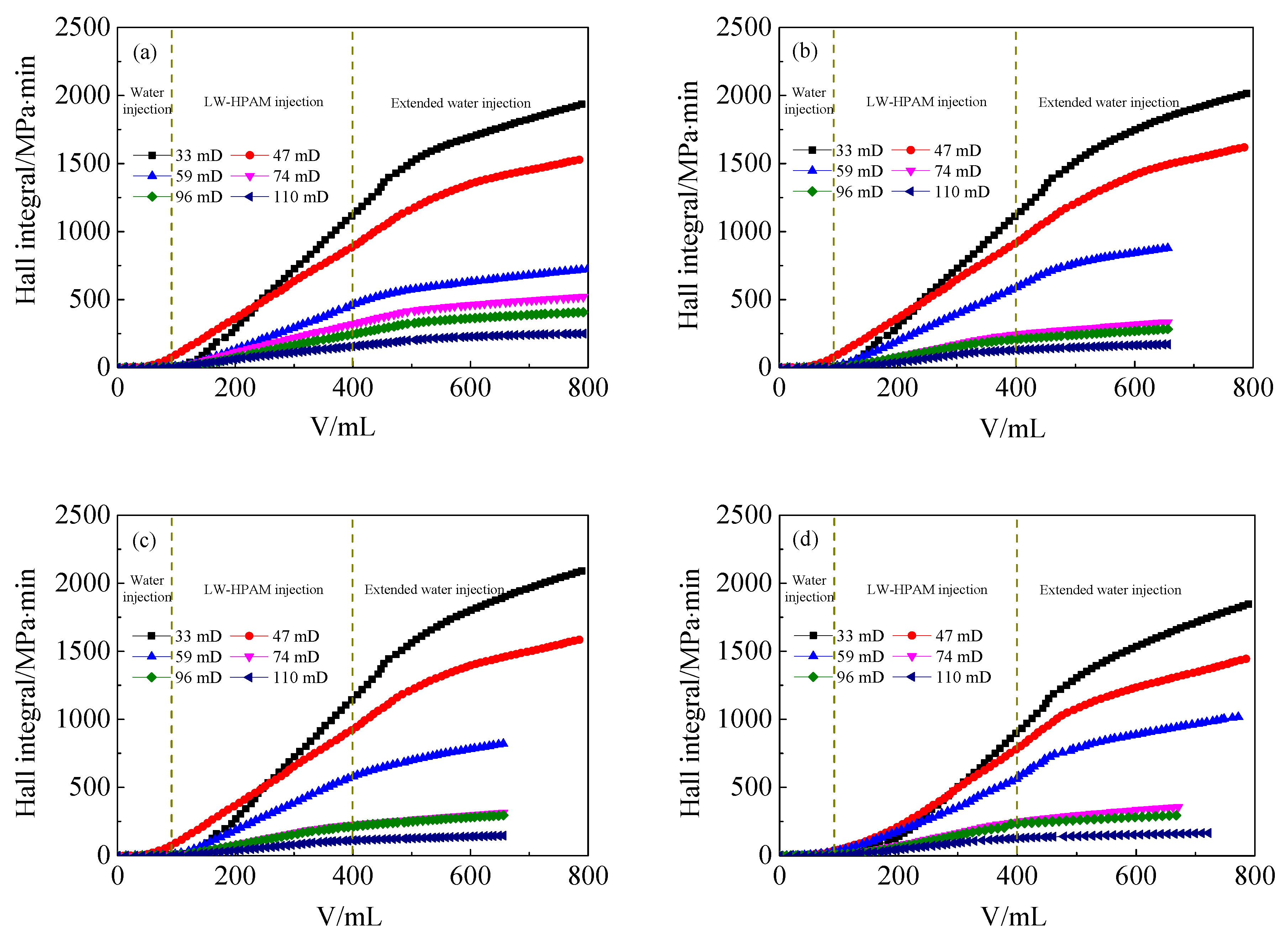
3.3. Injection Performance of HPAM Solutions in Medium and Low Permeability Cores
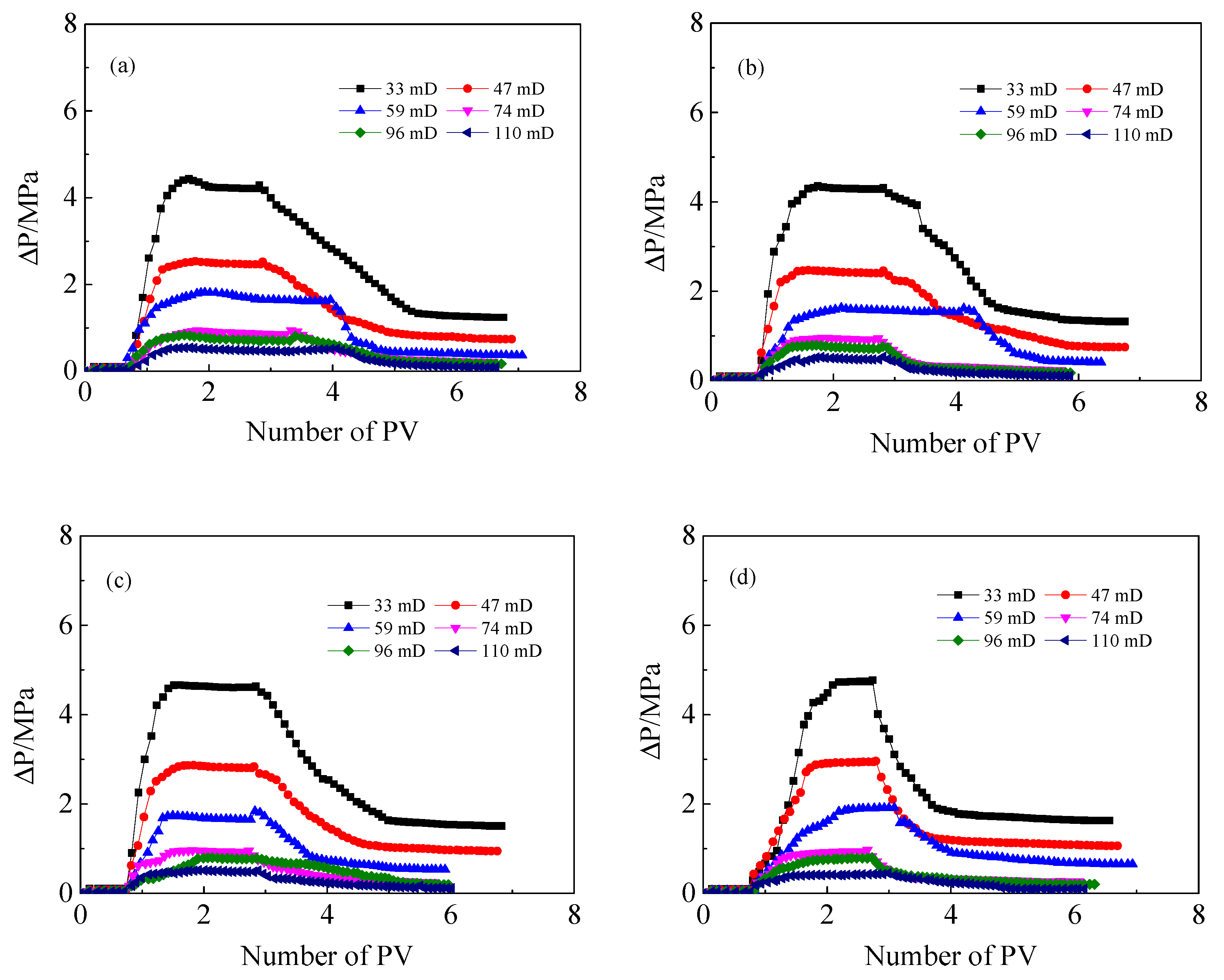
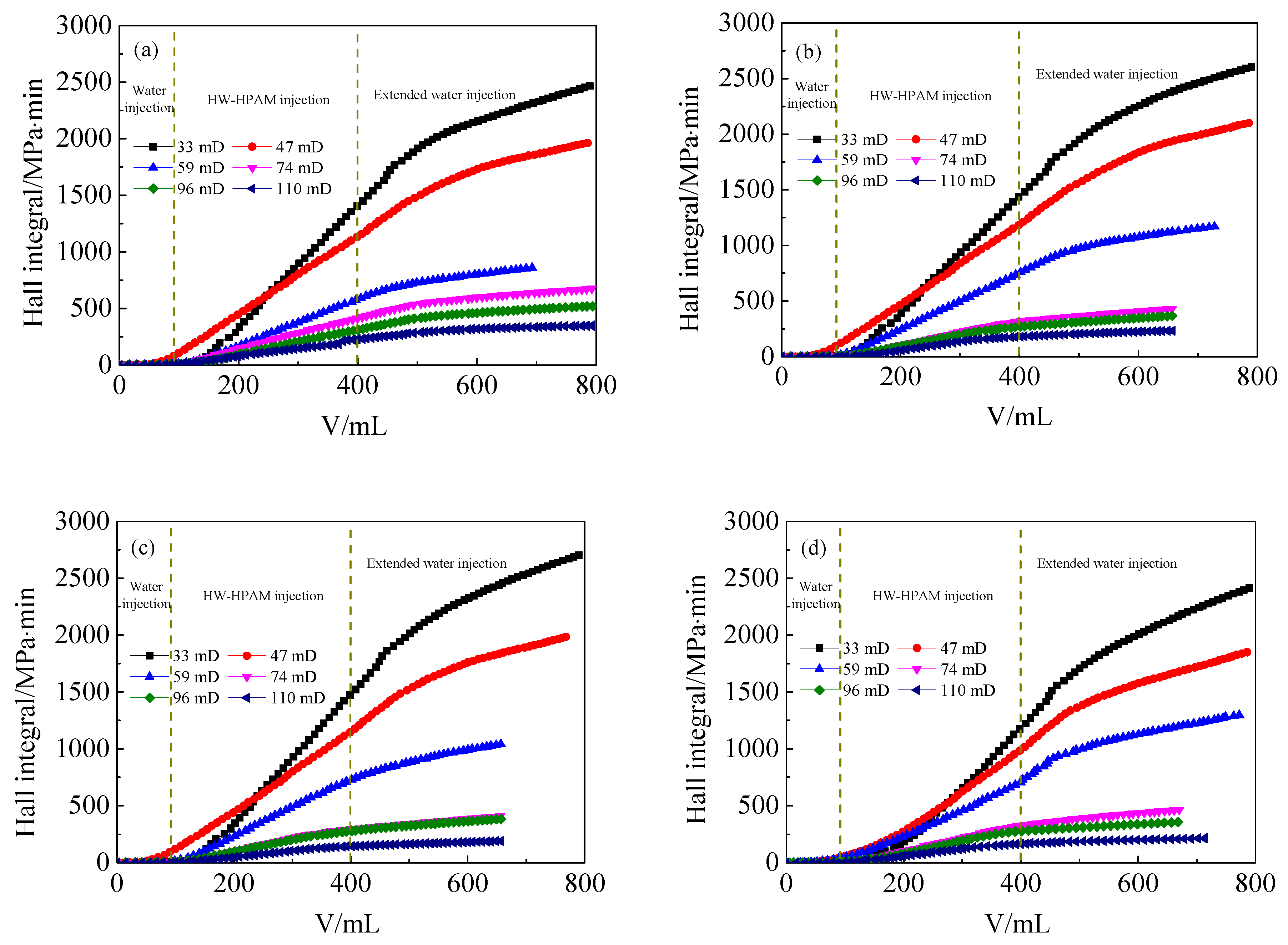
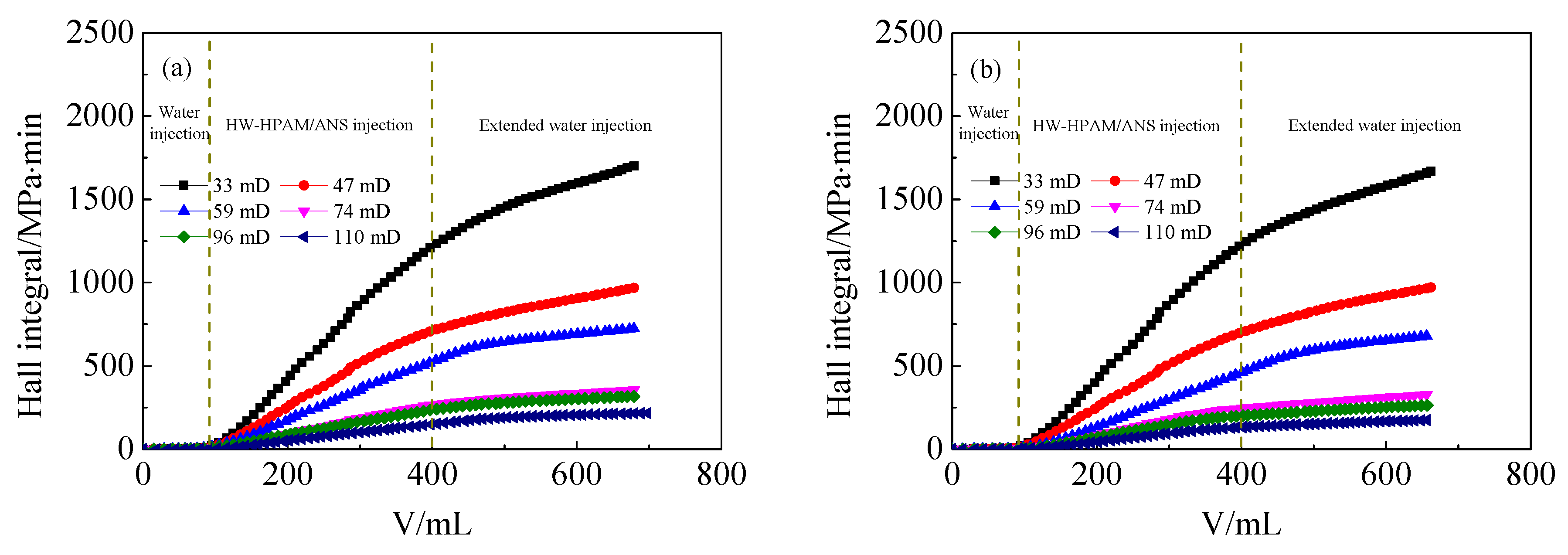
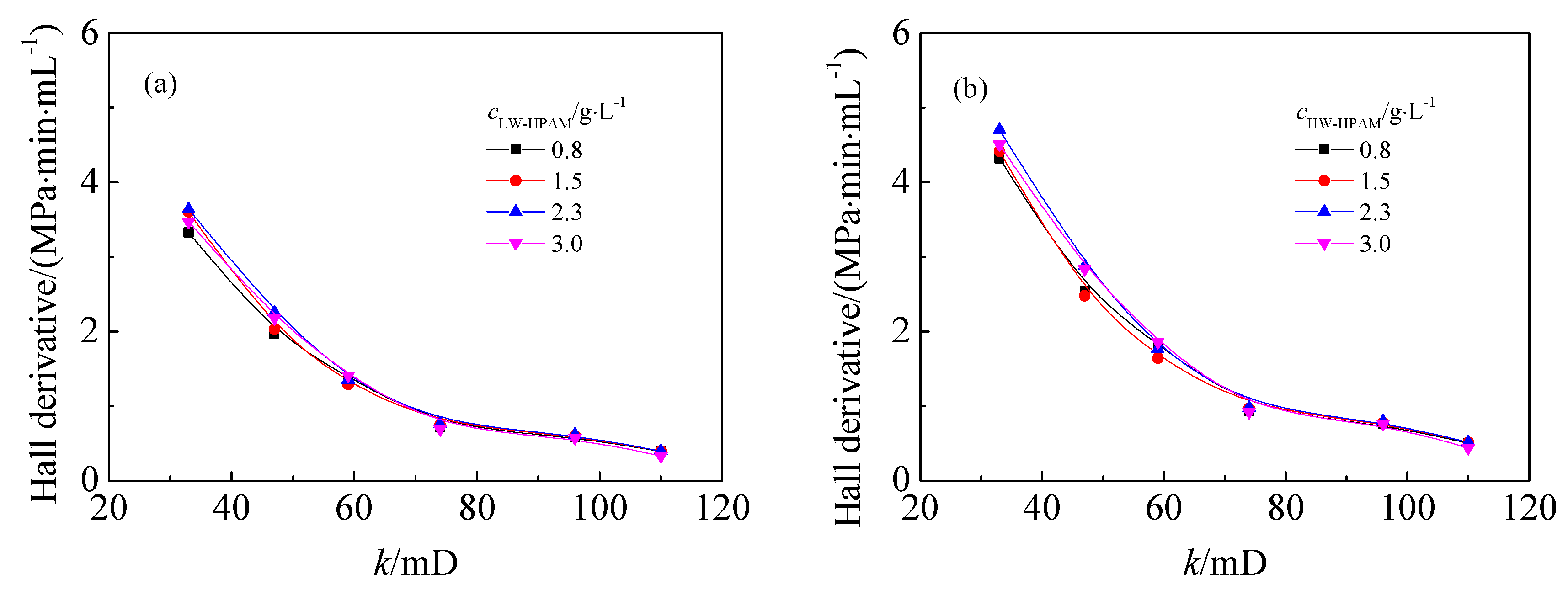
3.4. Effect of ANS on the Injection Performance of HPAM Solutions in Medium and Low Permeability Cores
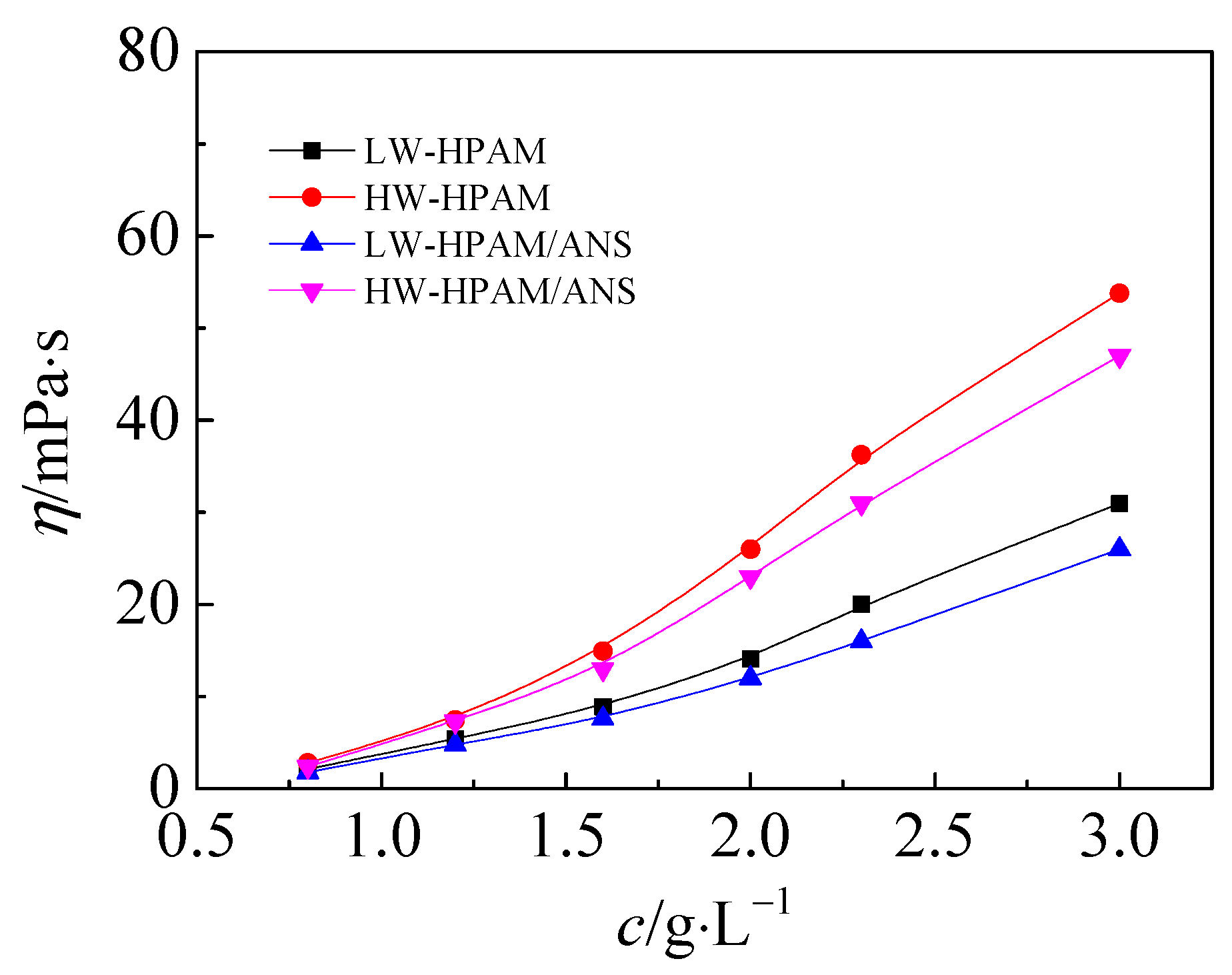
3.5. Mechanism of Improving HPAM Injectability by Anionic-Nonionic Surfactant
4. Conclusions
- (1)
- During the flow process, the HPAM concentration has little effect on the pressure gradient. As the core permeability decreases, the stable pressure gradient of HPAM increases. The capacity of HPAM with higher molecular weight in permeability reduction is stronger with higher residual resistance factor.
- (2)
- In medium and low permeability reservoirs, higher molecular weight increases the viscosity of HPAM solution, but also reduces its injectability. Therefore, the sweep coefficient and injectability need to be comprehensively considered when selecting HPAM type for enhanced oil recovery.
- (3)
- In the presence of ANS, the residual resistance factor and the injection critical point of HPAM decrease, either for LW-HPAM or HW-HPAM. This confirms that ANS can reduce HPAM injection pressure and enhance the injectability of HPAM in medium and low permeability reservoirs.
- (4)
- For interaction of ANS and HPAM, on the one side, ANS decreases the viscosity of the HPAM aqueous solution while, on the other side, the adsorption capacity of HPAM on the core pore wall is weakened due to the preferential adsorption of ANS on the rock surface. Consequently, the adsorption and retention of the HPAM in the in medium and low permeability reservoirs are reduced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of polymer-flooding technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Qiu, Z.; Gong, H.; Fan, H.; Zhu, T.; Zhang, H.; Dong, M. Hydrophobic effect further improves the rheological behaviors and oil recovery of polyacrylamide/nanosilica hybrids at high salinity. Chem. Eng. Sci. 2021, 232, 116369. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Zhao, S. Which one is more important in chemical flooding for enhanced court heavy oil recovery, lowering interfacial tension or reducing water mobility? Energy Fuels 2010, 24, 1829–1836. [Google Scholar] [CrossRef]
- Liang, X.; Shi, L.; Cheng, L.; Wang, X.; Ye, Z. Optimization of polymer mobility control for enhanced heavy oil recovery: Based on response surface method. J. Pet. Sci. Eng. 2021, 206, 109065. [Google Scholar] [CrossRef]
- Zhou, W.; Xin, C.; Chen, S.; Yu, Q.; Wang, K. Polymer-enhanced foam flooding for improving heavy oil recovery in thin reservoirs. Energy Fuels 2020, 34, 4116–4128. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Yu, L.; Gong, H.; Li, Y. The displacement efficiency and rheology of welan gum for enhanced heavy oil recovery. Polym. Adv. Technol. 2015, 25, 1122–1129. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Gong, H.; Ding, B.; Dong, M.; Li, Y. A microbial exopolysaccharide produced by Sphingomonas species for enhanced heavy oil recovery at high temperature and high salinity. Energy Fuels 2017, 31, 3960–3969. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, Z.; Gong, H.; Zhu, C.; Dong, M. Synergy of microbial polysaccharides and branched-preformed particle gel on thickening and enhanced oil recovery. Chem. Eng. Sci. 2019, 208, 115138. [Google Scholar] [CrossRef]
- Seright, R.S. The effects of mechanical degradation and viscoelastic behavior on injectivity of polyacrylamide solutions. SPE J. 1983, 23, 475–485. [Google Scholar] [CrossRef]
- Zhang, G.; Seright, R.S. Effect of concentration on hpam retention in porous media. SPE J. 2014, 19, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Hirasaki, G.J.; Pope, G.A. Analysis of factors influencing mobility and adsorption in the flow of polymer solution through porous media. SPE J. 1974, 14, 337–346. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Bedrikovetsky, P.; Lotfollahi, M.; Lake, L.W. Simultaneous sorption and mechanical entrapment during polymer flow through porous media. Water Resour. Res. 2016, 52, 2279–2298. [Google Scholar] [CrossRef]
- Shang, X.; Bai, Y.; Lv, K.; Dong, C. Experimental study on viscosity and flow characteristics of a clay-intercalated polymer. J. Mol. Liq. 2021, 322, 114931. [Google Scholar] [CrossRef]
- Wang, Z.; Le, X.; Feng, Y.; Zhang, C. The role of matching relationship between polymer injection parameters and reservoirs in enhanced oil recovery. J. Pet. Sci. Eng. 2013, 111, 139–143. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Pang, M.; Fang, T.; Zhao, S.; Wang, Z.; Zhou, Y. In research on plugging mechanism and optimisation of plug removal measure of polymer flooding response well in Bohai oilfield. In Proceedings of the International Petroleum Technology Conference, Virtual, 23 March–1 April 2021. [Google Scholar]
- Liu, H.; Yang, G.; Fangchao, L. In an overview of polymer broken down for increasing injectability in polymer flooding. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 22–24 October 2013. [Google Scholar]
- Jolma, I.W.; Strand, D.; Stavland, A.; Fjelde, I.; Hatzignatiou, D. When size matters—Polymer injectivity in chalk matrix. In Proceedings of the 19th European Symposium on Improved Oil Recovery, Stavanger, Norway, 24–27 April 2017; pp. 1–11. [Google Scholar]
- Jung, J.C.; Zhang, K.; Chon, B.H.; Choi, H.J. Rheology and polymer flooding characteristics of partially hydrolyzed polyacrylamide for enhanced heavy oil recovery. J. Appl. Polym. Sci. 2013, 127, 4833–4839. [Google Scholar] [CrossRef]
- Dupas, A.; Hénaut, I.; Rousseau, D.; Poulain, P.; Tabary, R.; Argillier, J.-F.; Aubry, T. In impact of polymer mechanical degradation on shear and extensional viscosities: Towards better injectivity forecasts in polymer flooding operations. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 8–10 April 2013. [Google Scholar]
- Ghosh, P.; Mohanty, K.K. Laboratory treatment of HPAM polymers for injection in low permeability carbonate reservoirs. J. Pet. Sci. Eng. 2020, 185, 106574. [Google Scholar] [CrossRef]
- Al-Shakry, B.; Skauge, T.; Shaker Shiran, B.; Skauge, A. Impact of mechanical degradation on polymer injectivity in porous media. Polymers 2018, 10, 742. [Google Scholar] [CrossRef]
- Al Hashmi, A.R.; Al Maamari, R.S.; Al Shabibi, I.S.; Mansoor, A.M.; Zaitoun, A.; Al Sharji, H.H. Rheology and mechanical degradation of high-molecular-weight partially hydrolyzed polyacrylamide during flow through capillaries. J. Pet. Sci. Eng. 2013, 105, 100–106. [Google Scholar] [CrossRef]
- Sorbie, K.S.; Roberts, L.J. In a model for calculating polymer injectivity including the effects of shear degradation. In Proceedings of the SPE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 16–18 April 1984. [Google Scholar]
- Sorbie, K.S. Polymer retention in porous media. In Polymer-Improved Oil Recovery; Sorbie, K.S., Ed.; Springer: Dordrecht, The Netherlands, 1991; pp. 126–164. [Google Scholar]
- Garrepally, S.; Jouenne, S.; Leuqeux, F.; Olmsted, P.D. In polymer flooding—Towards a better control of polymer mechanical degradation at the near wellbore. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020. [Google Scholar]
- Seright, R.S. In how much polymer should be injected during a polymer flood? In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–16 April 2016. [Google Scholar]
- Zhu, S.; Zhang, S.; Xue, X.; Zhang, J.; Xu, J.; Liu, Z. Influencing factors for effective establishment of residual resistance factor of polymer solution in porous media. J. Polym. Res. 2022, 29, 210. [Google Scholar] [CrossRef]
- Wang, X.; Ge, L.; Liu, D.; Zhu, Q.; Zheng, B. Experimental study on influencing factors of resistance coefficient and residual resistance coefficient in oilfield Z. World J. Eng. Technol. 2019, 7, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Hall, H.N. How to analyze waterflood injection Well Performance. World Oil 1963, 157, 128–130. [Google Scholar]
- Buell, R.S.; Kazeml, H.; Poettmann, F.H. Analyzing Injectivity off Polymer Solutions with the Hall Plot. SPE Reserv. Eng. 1990, 5, 41–46. [Google Scholar] [CrossRef]
- Pasikki, R.; Pasaribu, H. In application of hydraulic stimulation to improve well injectivity. In Proceedings of the 14th Indonesia International Geothermal Convention & Exhebition, Jakarta, Indonesia, 2–3 June 2014. [Google Scholar]
- Ratnaningsih, D.R.; Danny, I.L. Waterflooding surveillance: Real time injector performance analysis using Hall plot method & derivative. IOP Conf. Ser. Earth Environ. Sci. 2018, 212, 012074. [Google Scholar]
- Leslie, T.; Xiao, H.; Dong, M. Tailor-modified starch/cyclodextrin-based polymers for use in tertiary oil recovery. J. Pet. Sci. Eng. 2005, 46, 225–232. [Google Scholar] [CrossRef]
- Wever, D.A.; Picchioni, F.; Broekhuis, A.A. Comblike polyacrylamides as flooding agent in enhanced oil recovery. Ind. Eng. Chem. Res. 2013, 52, 16352–16363. [Google Scholar] [CrossRef]
- Wei, B. β-Cyclodextrin associated polymeric systems: Rheology, flow behavior in porous media and enhanced heavy oil recovery performance. Carbohydr. Polym. 2015, 134, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Zaitoun, A.; Kohler, N. In Two-phase flow through porous media: Effect of an adsorbed polymer layer. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 2–5 October 1988. [Google Scholar]
- Dário, A.F.; Hortêncio, L.M.; Sierakowski, M.R.; Neto JC, Q.; Petri, D.F. The effect of calcium salts on the viscosity and adsorption behavior of xanthan. Carbohydr. Polym. 2011, 84, 669–676. [Google Scholar] [CrossRef]
- Petri, D.F.; de Queiroz Neto, J.C. Identification of lift-off mechanism failure for salt drill-in drilling fluid containing polymer filter cake through adsorption/desorption studies. J. Pet. Sci. Eng. 2010, 70, 89–98. [Google Scholar] [CrossRef]
- Tam, K.C.; Wyn-Jones, E. Insights on polymer surfactant complex structures during the binding of surfactants to polymers as measured by equilibrium and structural techniques. Chem. Soc. Rev. 2006, 35, 693–709. [Google Scholar] [CrossRef]
- Chen, S.; Han, M.; AlSofi, A.M. Synergistic Effects between Different Types of Surfactants and an Associating Polymer on Surfactant–Polymer Flooding under High-Temperature and High-Salinity Conditions. Energy Fuels 2021, 35, 14484–14498. [Google Scholar] [CrossRef]
- Jian-Xin, L.; Yong-Jun, G.; Jun, H.; Jian, Z.; Xing, L.; Xin-Ming, Z.; Xin-Sheng, X.; Ping-Ya, L. Displacement Characters of Combination Flooding Systems consisting of Gemini-Nonionic Mixed Surfactant and Hydrophobically Associating Polyacrylamide for Bohai OffshoreOilfield. Energy Fuels 2012, 26, 2858–2864. [Google Scholar] [CrossRef]
- Ma, T.; Feng, H.; Wu, H.; Li, Z.; Jiang, J.; Xu, D.; Meng, Z.; Kang, W. Property evaluation of synthesized anionic-nonionic gemini surfactants for chemical enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123800. [Google Scholar] [CrossRef]
- Glasbergen, G.; Wever, D.; Keijzer, E.; Farajzadeh, R. In Injectivity Loss in Polymer Floods: Causes, Preventions and Mitigations. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 11–14 October 2015. [Google Scholar]
- Kondyurin, A.; Naseri, P.; Fisher, K.; McKenzie, D.R.; Bilek, M.M. Mechanisms for surface energy changes observed in plasma immersion ion implanted polyethylene: The roles of free radicals and oxygen-containing groups. Polym. Degrad. Stab. 2009, 94, 638–646. [Google Scholar] [CrossRef]
- Yu, H.; Xu, H.Y.; Fan, J.; Zhu, Y.; Wang, F.; Wu, H. Transport of shale gas in microporous/nanoporous media: Molecular to pore-scale simulations. Energy Fuels 2021, 35, 911–943. [Google Scholar] [CrossRef]









| Sample No. | Length, cm | Section Size, cm | k, ×10−3 μm2 | φ, % |
|---|---|---|---|---|
| # 1 | 29.87 | 4.49 × 4.35 | 33 | 17.3 |
| # 2 | 29.80 | 4.35 × 4.49 | 47 | 17.5 |
| # 3 | 29.83 | 4.53 × 4.42 | 59 | 17.9 |
| # 4 | 30.08 | 4.53 × 4.47 | 74 | 18.1 |
| # 5 | 30.12 | 4.48 × 4.51 | 96 | 19.1 |
| # 6 | 29.31 | 4.52 × 4.43 | 110 | 19.9 |
| Compositions (mg·L−1) | Total Salinity (mg·L−1) | |||||
|---|---|---|---|---|---|---|
| Na+, K+ | Ca2+ | Mg2+ | SO42– | Cl− | HCO3– | |
| 6.786 | 756 | 27 | 4 | 11,347 | 927 | 19,847 |
| System | RF | RFF | r (μm) | e (μm) |
|---|---|---|---|---|
| LW-HPAM | 35 | 12.4 | 1.24 | 0.58 |
| HW-HPAM | 46 | 16.1 | 1.24 | 0.62 |
| LW-HPAM/ANS | 34 | 11.2 | 1.24 | 0.56 |
| HW-HPAM/ANS | 44 | 14.7 | 1.24 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wei, J.; Chen, Y.; Jia, S.; Wang, Y.; Qiao, X.; Xu, L. Injectability of Partially Hydrolyzed Polyacrylamide Solutions Improved by Anionic-Nonionic Surfactant in Medium and Low Permeability Reservoirs. Energies 2022, 15, 6866. https://doi.org/10.3390/en15196866
Wang L, Wei J, Chen Y, Jia S, Wang Y, Qiao X, Xu L. Injectability of Partially Hydrolyzed Polyacrylamide Solutions Improved by Anionic-Nonionic Surfactant in Medium and Low Permeability Reservoirs. Energies. 2022; 15(19):6866. https://doi.org/10.3390/en15196866
Chicago/Turabian StyleWang, Long, Jianguang Wei, Yinghe Chen, Shihua Jia, Yiling Wang, Xudong Qiao, and Long Xu. 2022. "Injectability of Partially Hydrolyzed Polyacrylamide Solutions Improved by Anionic-Nonionic Surfactant in Medium and Low Permeability Reservoirs" Energies 15, no. 19: 6866. https://doi.org/10.3390/en15196866






