A Process Intensification Approach for CO2 Absorption Using Amino Acid Solutions and a Guanidine Compound
Abstract
:1. Introduction
2. Materials and Methods
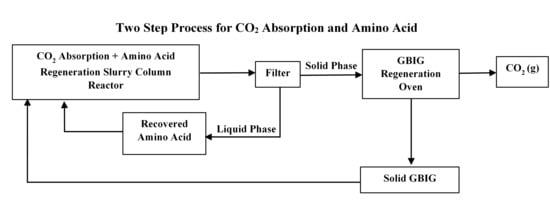
2.1. Model Organization
2.2. Reaction Scheme
2.3. Reactive Mass Transfer
2.4. Slurry Bubble Column Model
2.4.1. Mass Balances
2.4.2. Parameter Estimation
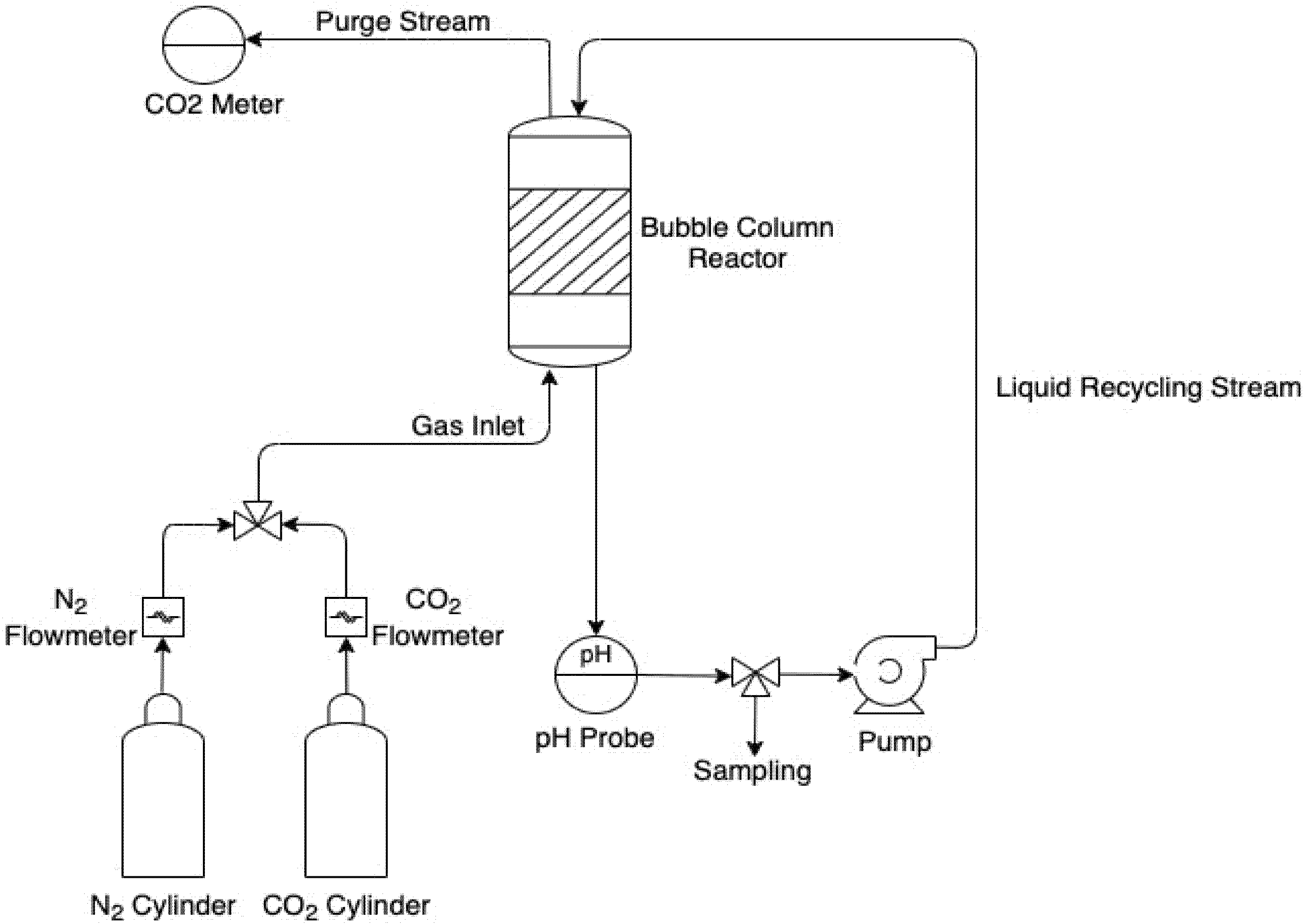
2.5. Experimental Part
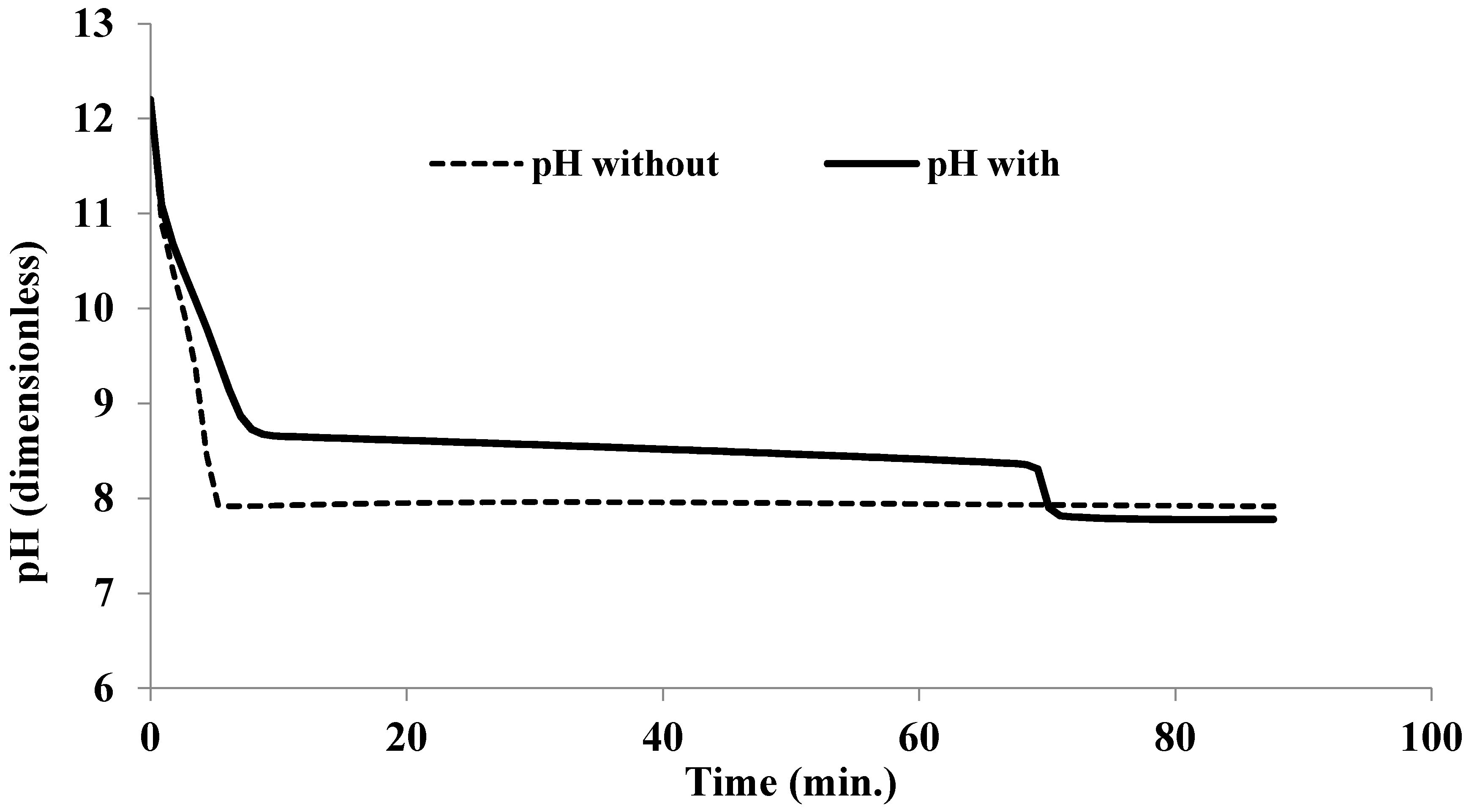
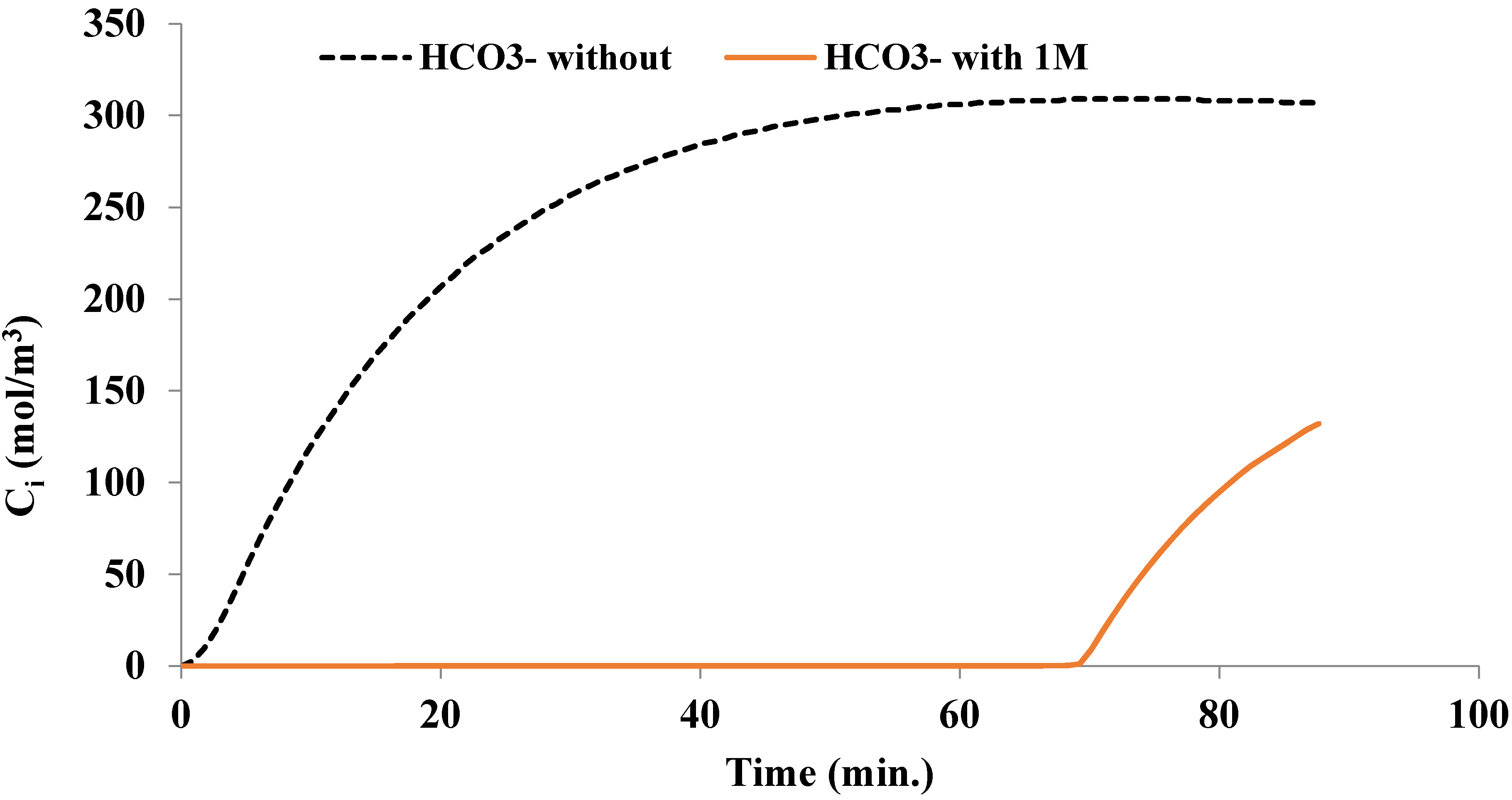
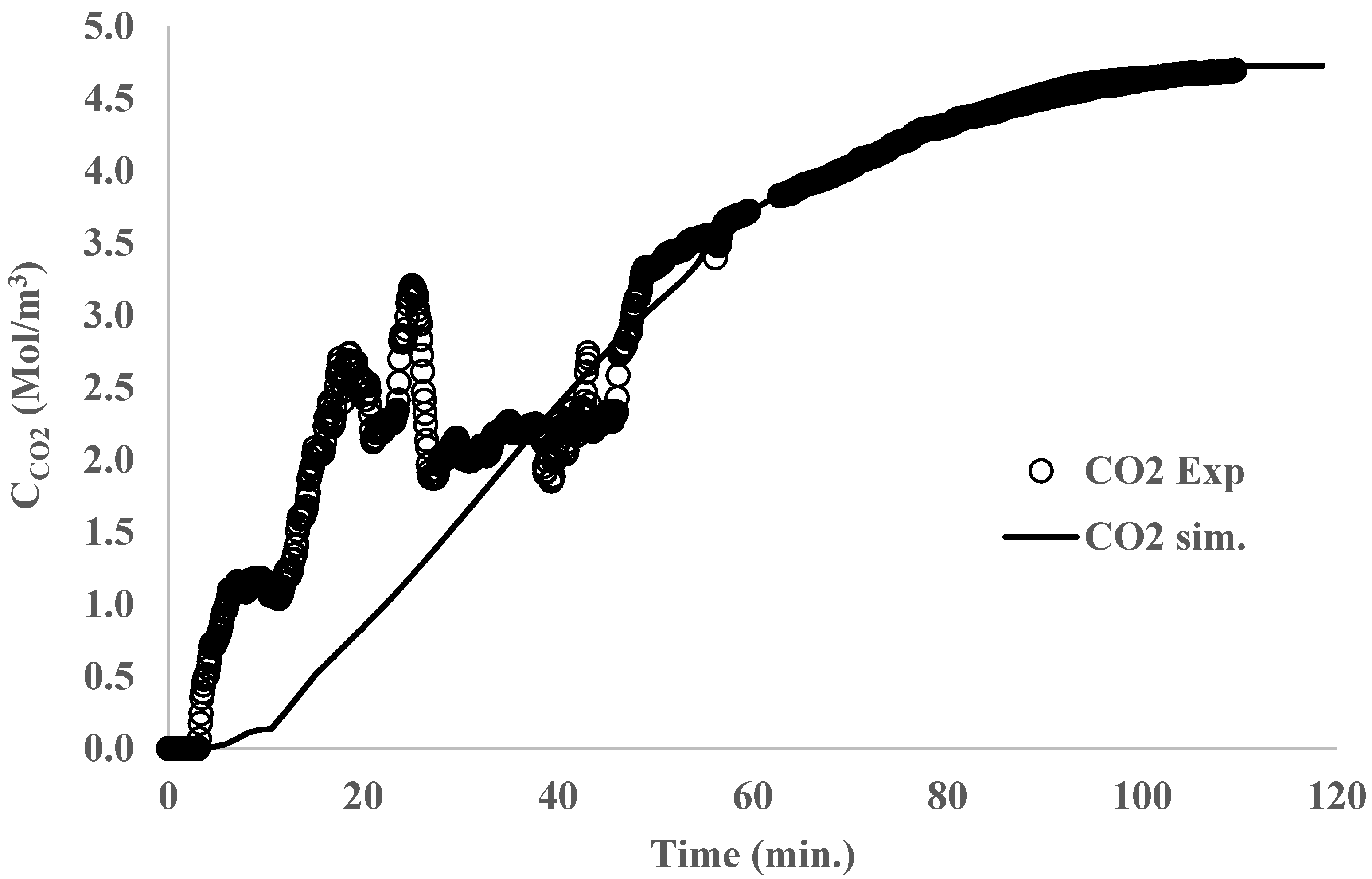
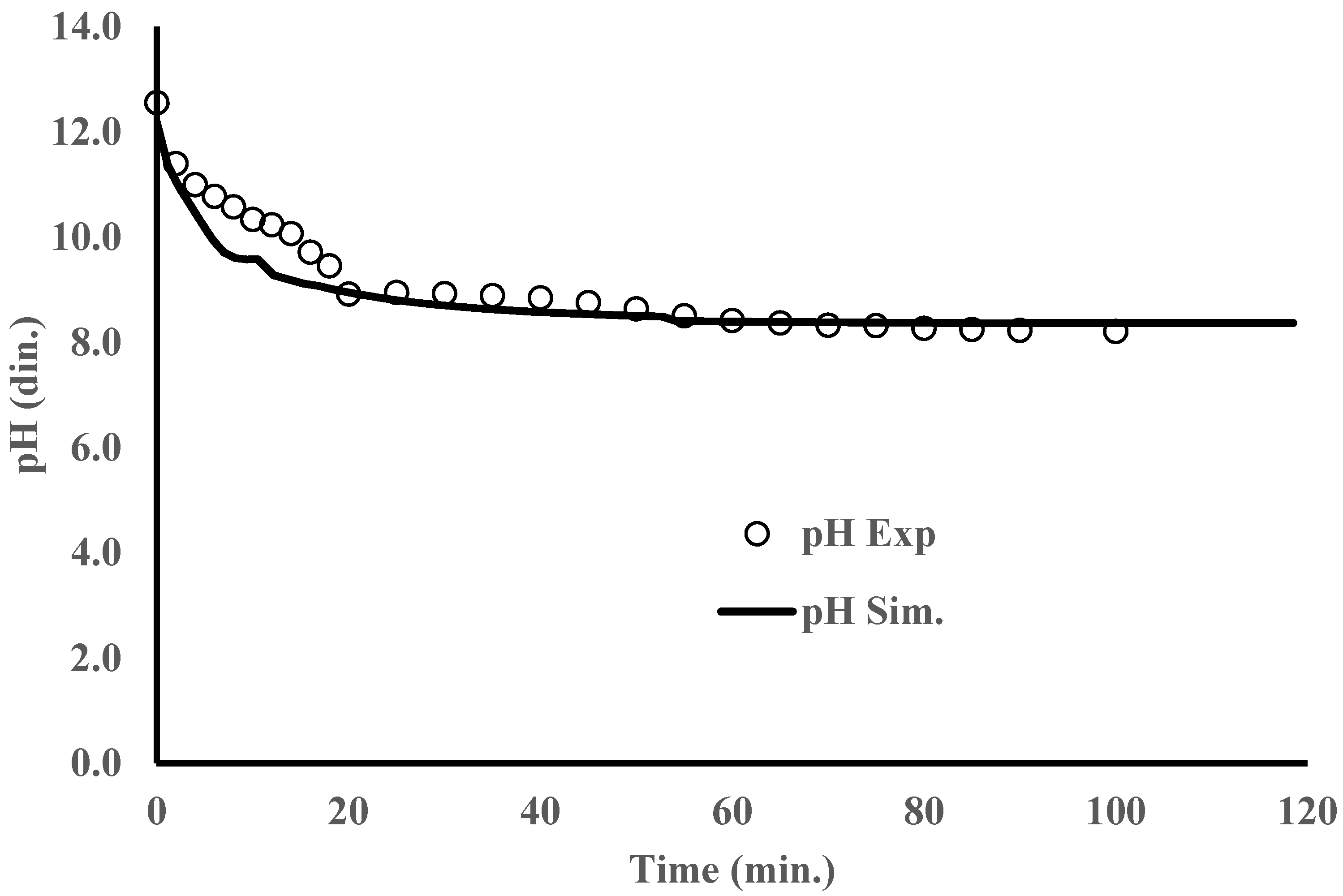
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix
| Compound N° | Gas | Liquid |
|---|---|---|
| 1 | N2 (g) | SAR- |
| 2 | O2 (g) | SAR−(CO2−) |
| 3 | CO2 (g) | SAR−(CO2H) |
| 4 | H2O (g) | CO2 (aq.) |
| 5 | - | H2O |
| 6 | - | HCO3− |
| 7 | - | OH− |
| 8 | - | H+ |
| 9 | - | CO32− |
| 10 | - | SARH (aq.) |
| 11 | - | GBIGH22+(aq.) |
| 12 | - | GBIGH2(HCO3)2(H2O)2 |
| Eqn. No/Reaction | Forward Rate (kfi) | Equilibrium Constant | Reverse Rate (kri) |
|---|---|---|---|
| A1 (1 + 2) | k1f = 9.5 × 1011 (mM−1s−1) exp(−7348/Tl) | K1 = 11.78 × exp(721.7/Tl) × 10(909.1/Tl) | k1r = k1f/K1 |
| A2 (4) | k2f = 3.59 × 105 (mM−1s−1) exp(−7400/Tl) | K2 = k2f/k2r | k2r = 4931.6 (mM−1 s−1) × exp(−8004/Tl) (s−1) |
| A3 (5) | k3f = 4.52 × 107 (mM−1 s−1) × 10(−909.1/Tl) | K3 = 4.52E10 (M−1) × 10(−909.1/Tl) (mM−1) | k3r = (k3f)/K3 |
| A4 (6) | k4f = 1.88 × 1012 × exp[−7698./Tl] (mM−1s−1) | K4 = k4f/k4r | k4r = 2.491 × 1016 × exp[−1.367E4/Tl] (s−1) |
| A5 (7) | k5f = 4.32 × 10−7 (s−1) | K5 =10(3404.7/Tl ) + 0.032786 × Tl + 14.8435) (mM) | K5r = (k5f)/K6 |
| A6 (-) | k6f = 3.6 × 10−10 (s−1 mM) | K6 = 1.58 × 10−3 (mM2) × exp(−6832/Tl) | k6r = (k6f)/K6 |
| A7 (-) | k7f = 0.1 (mM1s−1) | K7= 4.474 × 10−3 (mM−1) × exp(5325./T) | k7r = (k7f)/K7 |
| A8 (-) | k8f = 0.1 (mM1s−1) | K8= 2.83 × 10−5 (mM−1) × exp(6832./T) | k8r = (k8f)/K8 |
| A9 (8) | k9f = 2 × 10−5 Tl (mM−2 s−1) | K9= 2.26 × 10−5 (mM−1) | k9r = (k9f)/K9 |
| A10 (9) | k10f = 8.95 × 10−3 (mM1s−1) | K10 = 0.54 (mM−3) | k10r = (k10f)/K10 |
| Notation | Parameter | Value/Range | Units |
|---|---|---|---|
| Gas phase flow rate | 1–5 | L min−1 | |
| Liquid phase flow rate | 0.1–1 | L min−1 | |
| Column height | 0.9 | m | |
| Column diameter | 0.08 | m | |
| Gas bubble Sauter diameter | 1 × 10−3–5 × 10−3 | m | |
| Solid volume fraction | 0.04–0.1 | (-) | |
| Initial CO2 volume fraction in gas-phase | 0.05–0.14 | (-) | |
| Liquid feed tank volume | 0.5 × 10−3–4 ×10−3 | m3 | |
| Initial amino acid concentration | 0.1–1 | M | |
| Initial GBIG concentration | 0.1–1 | M | |
| Total pressure | 1.1 × 10−5 | Pa | |
| Temperature | 293 | K | |
| pH | Potential of hydrogen | 10.2–12.5 | (-) |
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global Warming of 1.5 °C; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Blunden, J.; Arndt, D.S. State of the climate in 2018. Bull. Am. Meteorol. Soc. 2019, 100, Si–S306. [Google Scholar] [CrossRef] [Green Version]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Greer, T. Modeling and Simulation of Post Combustion CO2 Capturing. Master’s Thesis, Telemark University College, Faculty of Technology, Porsgrunn, Norway, 2008. [Google Scholar]
- Bosch, H.; Versteeg, G.F.; van Swaaij, W.P.M. Gas-Liquid Mass Transfer with Parallel Reversible Reactions-III. Absorption of CO2 into Solutions of Blends of Amines. Chem. Eng. Sci. 1989, 44, 2745–2750. [Google Scholar] [CrossRef] [Green Version]
- Hagewiesche, D.P.; Ashour, S.S.; Al-Ghawas, H.A.; Sandall, O.C. Absorption of Carbon Dioxide into Aqueous Blends of Monoethanolamine and Methyldiethanolamine. Chem. Eng. Sci. 1995, 50, 1071–1079. [Google Scholar] [CrossRef]
- Sakwattanapong, R.; Aroonwilas, A.; Veawab, A. Reaction Rate of CO2 in Aqueous MEA-AMP Solution: Experiment and Modeling. Energ. Procedia 2009, 1, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Gabitto, J.; Tsouris, C. Carbon Dioxide Absorption Modeling for Off-Gas Treatment in the Nuclear Fuel Cycle. Int. J. Chem. Eng. 2018, 2018, 3158147. [Google Scholar] [CrossRef]
- Versteeg, G.F.; Holst, J.V.; Politiek, P.P.; Niederer, J.P. CO2 Capture from Flue Gas using Amino Acid Salt Solutions [Online], June 2006. Available online: http://www.co2-cato.nl/doc.php?lid=317 (accessed on 4 September 2018).
- van Holst, J.; Versteeg, G.F.; Brilman, D.W.F.; Hogendoorn, J.A. Kinetic Study of CO2 with Various Amino Acid Salts in Aqueous Solution. Chem. Eng. Sci. 2009, 64, 59–68. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Konduru, P.; Vaidyanathan, M.; Kenig, E.Y. Kinetics of Carbon Dioxide Removal by Aqueous Alkaline Amino Acid salts. Ind. Eng. Chem. Res. 2010, 49, 11067–11072. [Google Scholar] [CrossRef]
- Portugal, A.F.; Derks, P.W.J.; Versteeg, G.F.; Magalhães, F.D.; Mendes, A. Characterization of Potassium Glycinate for Carbon Dioxide Absorption Purposes. Chem. Eng. Sci. 2007, 62, 6534–6547. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.N.; Bian, Y.; Zhao, Y. Kinetics of CO2 Absorption into Aqueous Basic Amino Acid Salt: Potassium Salt of Lysine Solution. Environ. Sci. Technol. 2016, 50, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Benamor, A.; JaberAl-Marri, M.; Khraisheh, M.; Nasser, M.S.; Tontiwachwuthikul, P. Reaction Kinetics of Carbon Dioxide in Aqueous Blends of N-methyl-diethanolamine and Glycine using the Stopped Flow Technique. J. Nat. Gas Sci. Eng. 2016, 33, 186–195. [Google Scholar] [CrossRef]
- Mahmud, N.; Benamor, A.; Nasser, M.S.; Al-Marri, M.J.; Qiblawey, H.; Tontiwachwuthikul, P. Reaction Kinetics of Carbon Dioxide with Aqueous Solutions of L-Arginine, Glycine & Sarcosine using the Stopped Flow Technique. Int. J. Greenh. Gas Control 2017, 63, 47–58. [Google Scholar]
- Brethomé, F.M.; Williams, N.J.; Seipp, C.A.; Kidder, M.K.; Custelcean, R. Direct Air Capture of CO2 via Aqueous-Phase Absorption and Crystalline-Phase Release using Concentrated Solar Power. Nat. Energy 2018, 3, 553–559. [Google Scholar] [CrossRef]
- Kasturi, A.S.; Ladshaw, A.; Yiacoumi, S.; Gabitto, J.; Garrabrant, K.; Custelcean, R.; Tsouris, C. CO2 Absorption from Simulated Flue Gas in a Bubble Column. Sep. Sci. Technol. 2019, 54, 2034–2046. [Google Scholar] [CrossRef]
- Jockenhöevel, T.; Schneider, R.; Rode, H. Development of an Economic Post-Combustion Carbon Capture Process. Energy Procedia 2009, 1, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Gabitto, J.; Custelcean, R.; Tsouris, C. Simulation of Carbon Dioxide Absorption by Amino Acids in Two-Phase Batch and Bubble Column Reactors. Sep. Sci. Technol. 2019, 54, 2013–2025. [Google Scholar] [CrossRef]
- Seipp, C.A.; Williams, N.J.; Kidder, M.K.; Custelcean, R. CO2 Capture from Ambient Air by Crystallization with a Guanidine Sorbent. Angew. Chem. Int. Ed. Engl. 2017, 56, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.J.; Seipp, C.A.; Brethomé, F.M.; Ma, Y.-Z.; Ivanov, A.S.; Bryantsev, V.S.; Kidder, M.K.; Martin, H.J.; Holguin, E.; Garrabrant, K.A.; et al. CO2 Capture via Crystalline Hydrogen-Bonded Bicarbonate Dimers. Chem 2019, 5, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Kasturi, A.; Gabitto, J.; Tsouris, C.; Custelcean, R. Carbon Dioxide Capture with Aqueous Amino Acids: Mechanistic Study of Amino Acid Regeneration by Guanidine Crystallization and Process Intensification. Sep. Purif. Technol. 2021, 271, 118839. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Park, L.K.; Yiacoumi, S.; Tsouris, C. Modular Chemical Process Intensification: A Review. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Brilman, D.W.F.; Mengers, H.; Nijmeijer, K.; Wessling, M. Kinetics of CO2 Absorption in Aqueous Sarcosine Salt Solutions: Influence of Concentration, Temperature, and CO2 Loading. Ind. Eng. Chem. Res. 2010, 49, 9693–9702. [Google Scholar] [CrossRef]
- Xiang, Q.; Fang, M.; Yu, H.; Maeder, M. Kinetics of the Reversible Reaction of CO2 (aq) and HCO3- with Sarcosine Salt in Aqueous Solution. J. Phys. Chem. A 2012, 116, 10276–10284. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.F.; van Swaaij, W.P.M. On the Kinetics between CO2 and Alkanolamines both in Aqueous and Non-Aqueous Solutions-I. Primary and Secondary Amines. Chem. Eng. Sci. 1988, 43, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Morsi, B.I.; Basha, O.M. Mass Transfer in Multiphase Systems. In Mass Transfer-Advancement in Process Modelling; Solecki, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Perry, R.H.; Green, D.W. Chemical Engineers Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Greer, T.; Bedelbayev, A.; Igreja, J.M.; Pereira Gomes, J.F.; Lie, B. A Dynamic Model for the De-Absorption of Carbon Dioxide from Monoethanolamine Solution. Proc. SIMS 2008, 2008, 7–8. [Google Scholar]
- Tavlarides, L.L.; Lin, R.; Nan, Y.; Yiacoumi, S.; Tsouris, C.; Ladshaw, A.; Sharma, K.; Gabitto, J.; DePaoli, D. Sorption Modeling and Verification for Off-Gas Treatment; NEUP: Atlanta, GA, USA, 2015. Available online: https://neup.inl.gov/ (accessed on 4 July 2021). [CrossRef]
- Fukuma, M.; Muroyama, K.; Yasunishi, A. Properties of Bubble Swarms in a Slurry Bubble Column. J. Chem. Eng. Jpn. 1987, 20, 20–33. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, R.; Behkish, A.; Sehabiague, L.; Heintz, Y.; Oukaci, R.; Morsi, B.I. An Algorithm for Predicting the Hydrodynamic and Mass Transfer Parameters in Bubble Column and Slurry in Bubble Column Reactors. Fuel Proc. Technol. 2008, 29, 322–343. [Google Scholar] [CrossRef]
- Sehabiague, L.; Lemoine, R.; Behkish, A.; Heintz, Y.; Sanoja, M.; Oukaci, R.; Morsi, B.I. Modeling and Optimization of a Large-Scale Bubble Column Reactor for Producing 10,000 bbl/day of Fischer-Tropsch Liquid Hydrocarbons. J. Chin. Inst. Chem. Eng. 2008, 38, 169–179. [Google Scholar] [CrossRef]
- Götz, M.; Lefevre, J.; Mörs, F.; Ortloff, F.; Reimert, R.; Bajohr, S. Novel Gas Holdup Correlation for Slurry Bubble Column Reactors Operated in the Homogeneous Regime. Chem. Eng. J. 2017, 308, 1209–1224. [Google Scholar] [CrossRef]
- de Swart, J.W.A.; an Vliet, R.E.; Krishna, R. Size, Structure and Dynamics of ‘Large’ Bubbles in a Two-Dimensional Slurry Bubble Column. Chem. Eng. Sci. 1996, 51, 4619. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasturi, A.; Gabitto, J.F.; Custelcean, R.; Tsouris, C. A Process Intensification Approach for CO2 Absorption Using Amino Acid Solutions and a Guanidine Compound. Energies 2021, 14, 5821. https://doi.org/10.3390/en14185821
Kasturi A, Gabitto JF, Custelcean R, Tsouris C. A Process Intensification Approach for CO2 Absorption Using Amino Acid Solutions and a Guanidine Compound. Energies. 2021; 14(18):5821. https://doi.org/10.3390/en14185821
Chicago/Turabian StyleKasturi, Abishek, Jorge F. Gabitto, Radu Custelcean, and Costas Tsouris. 2021. "A Process Intensification Approach for CO2 Absorption Using Amino Acid Solutions and a Guanidine Compound" Energies 14, no. 18: 5821. https://doi.org/10.3390/en14185821