Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
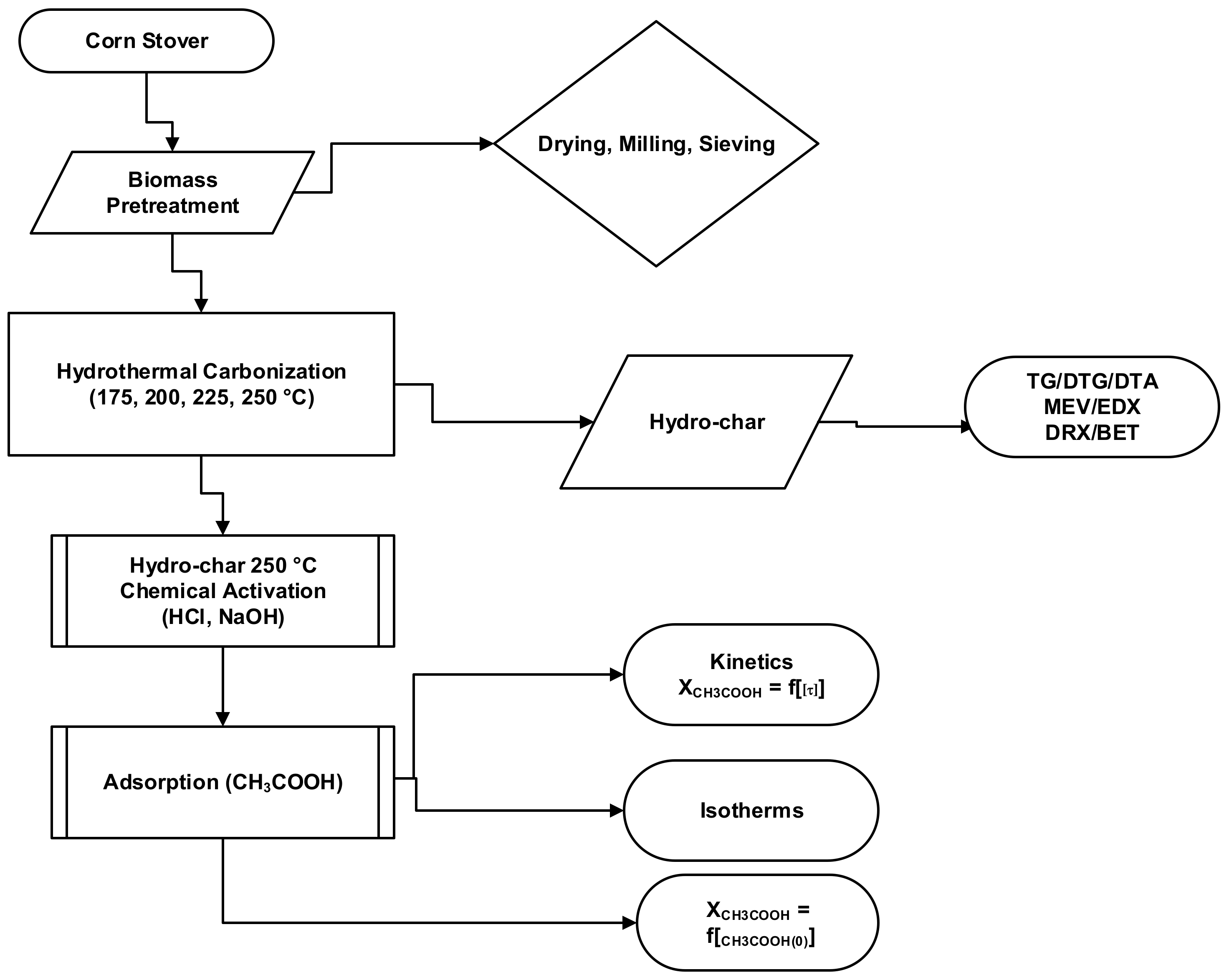
2.1. Methodology
2.2. Materials, Pre-Treatment, and Physicochemical Characterization of Corn Stover
2.3. Experimental Apparatus and Procedures
Hydrothermal Carbonization
2.4. Adsorption of CH3COOH
2.4.1. Adsorption Isotherm of CH3COOH
2.4.2. Adsorption Apparatus and Procedures
2.5. Morphological, Crystalline, and Textural Characterization of Hydro-Chars
Thermo-Gravimetric Analysis (TG/DTG/DTA)
3. Results
3.1. Morphological, Crystalline, and Textural Characterization of Hydro-Chars
3.1.1. Thermo-Gravimetric Analysis (TG/DTG/DTA)
3.1.2. SEM Analysis
3.1.3. EDX Analysis
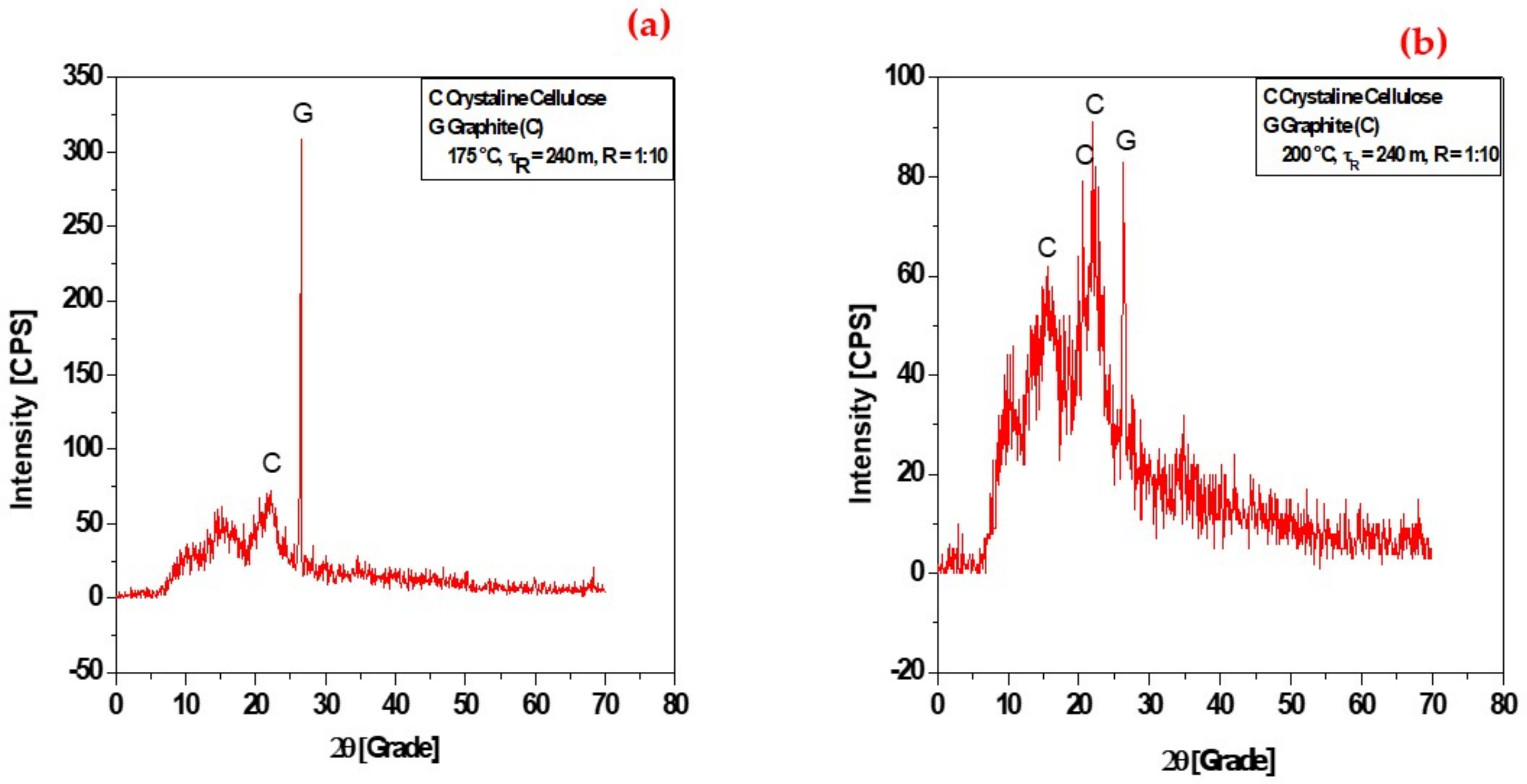
3.1.4. XRD Analysis
3.1.5. BET
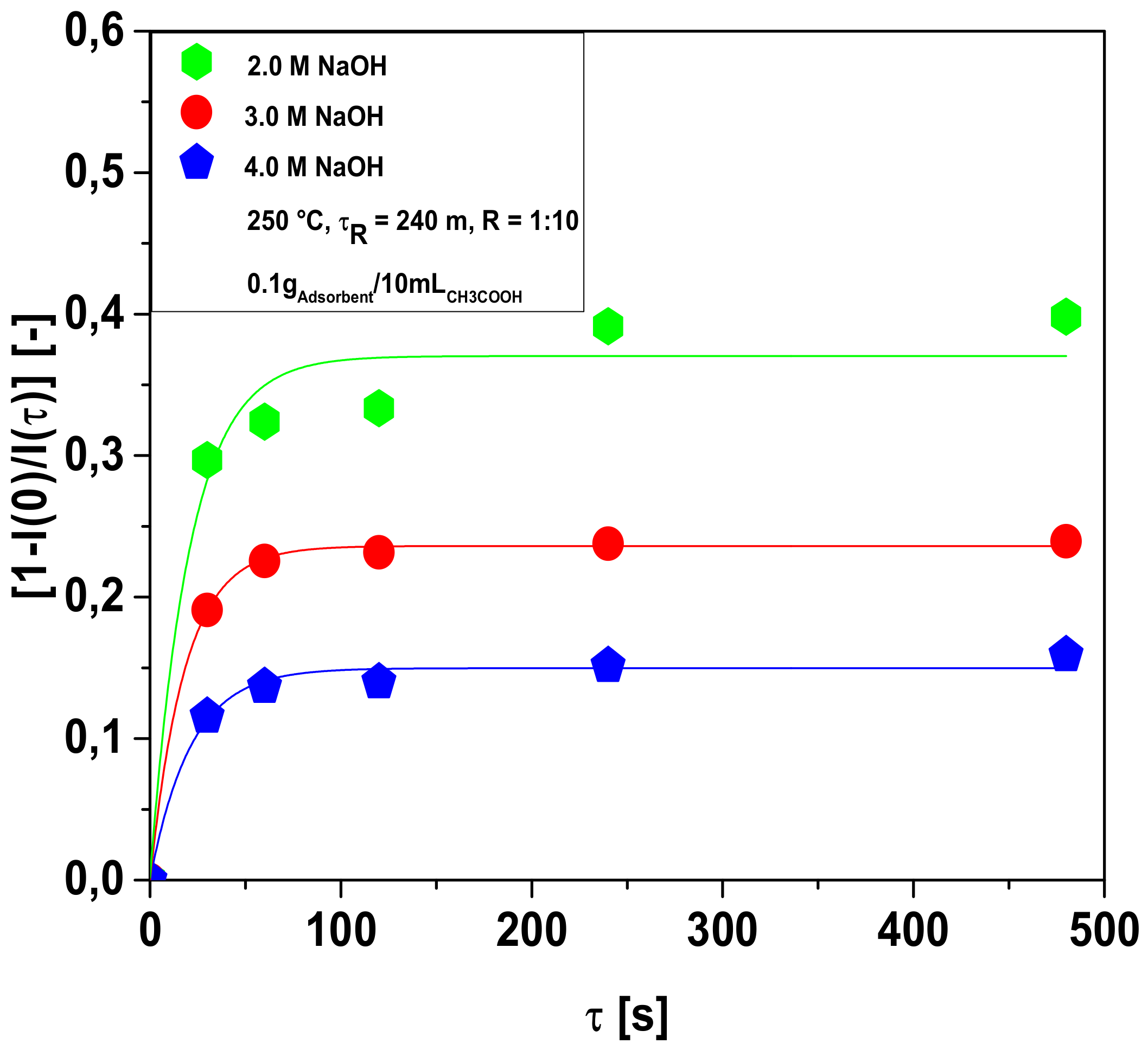
3.2. Adsorption Kinetics of Acetic Acid (CH3COOH) on Hydro-Char
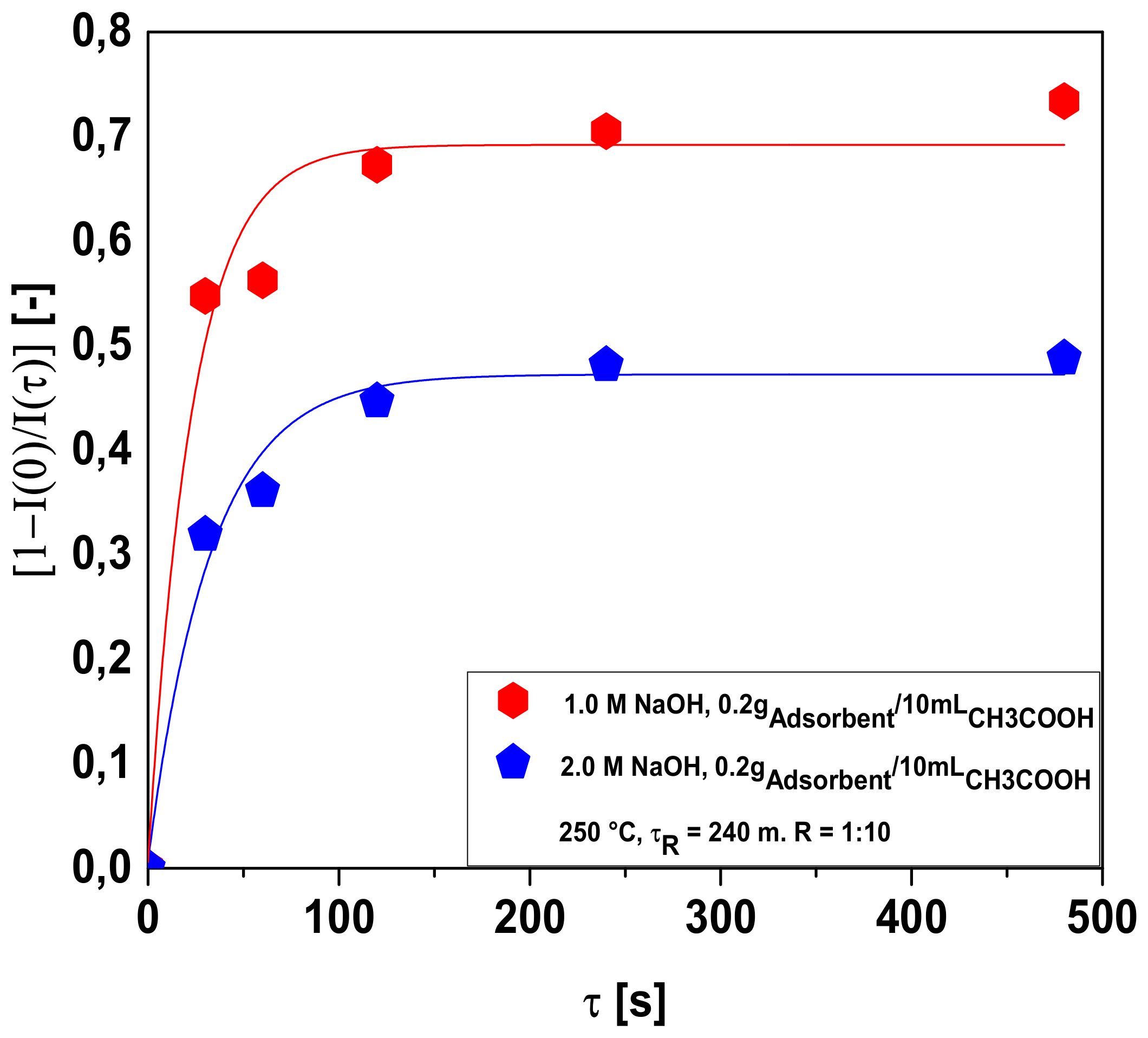
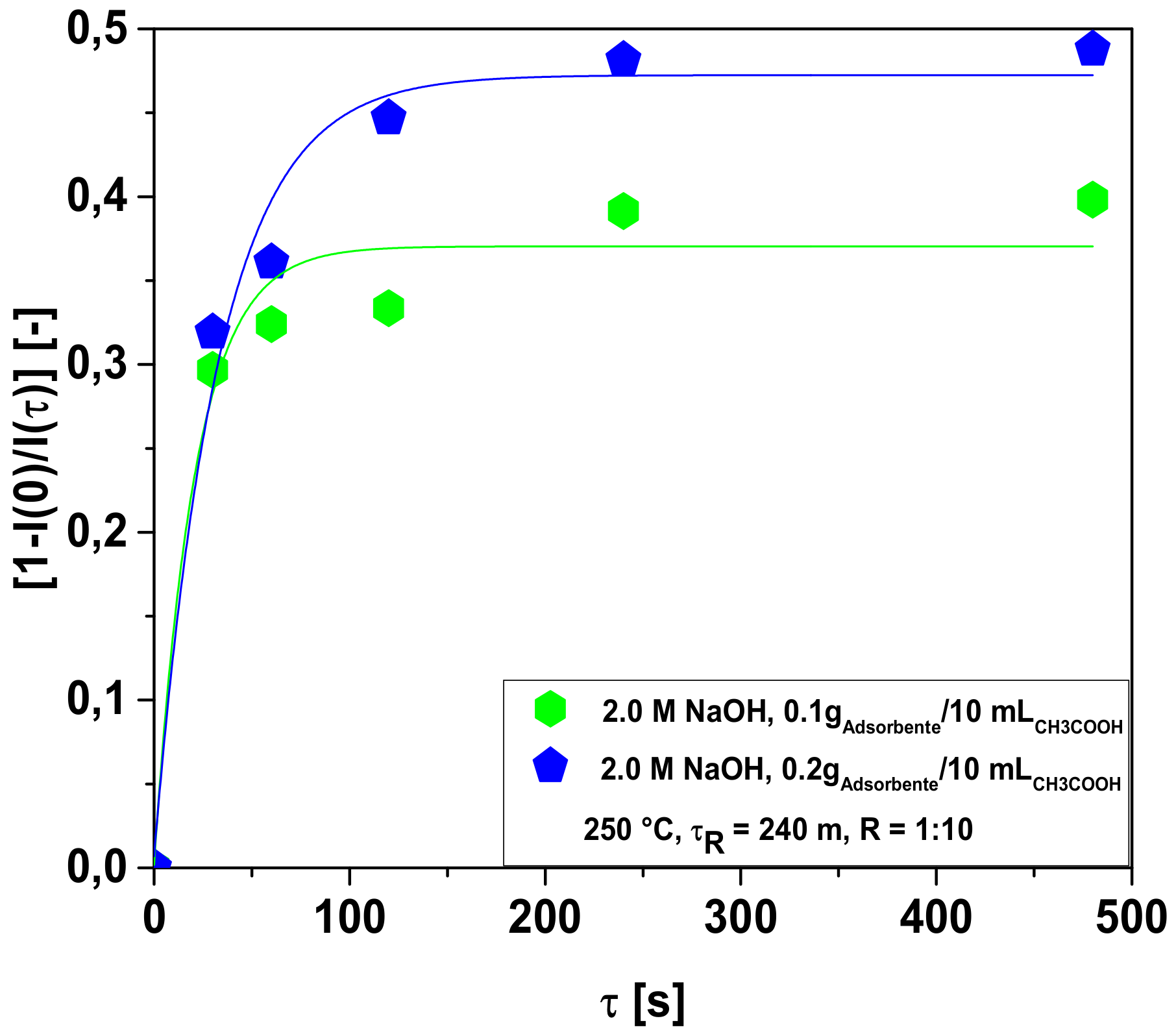
3.2.1. Bio-Adsorbent Activation with NaOH
Influence of Acetic Acid Concentration
Influence of Adsorbent-to-Solution Ratio
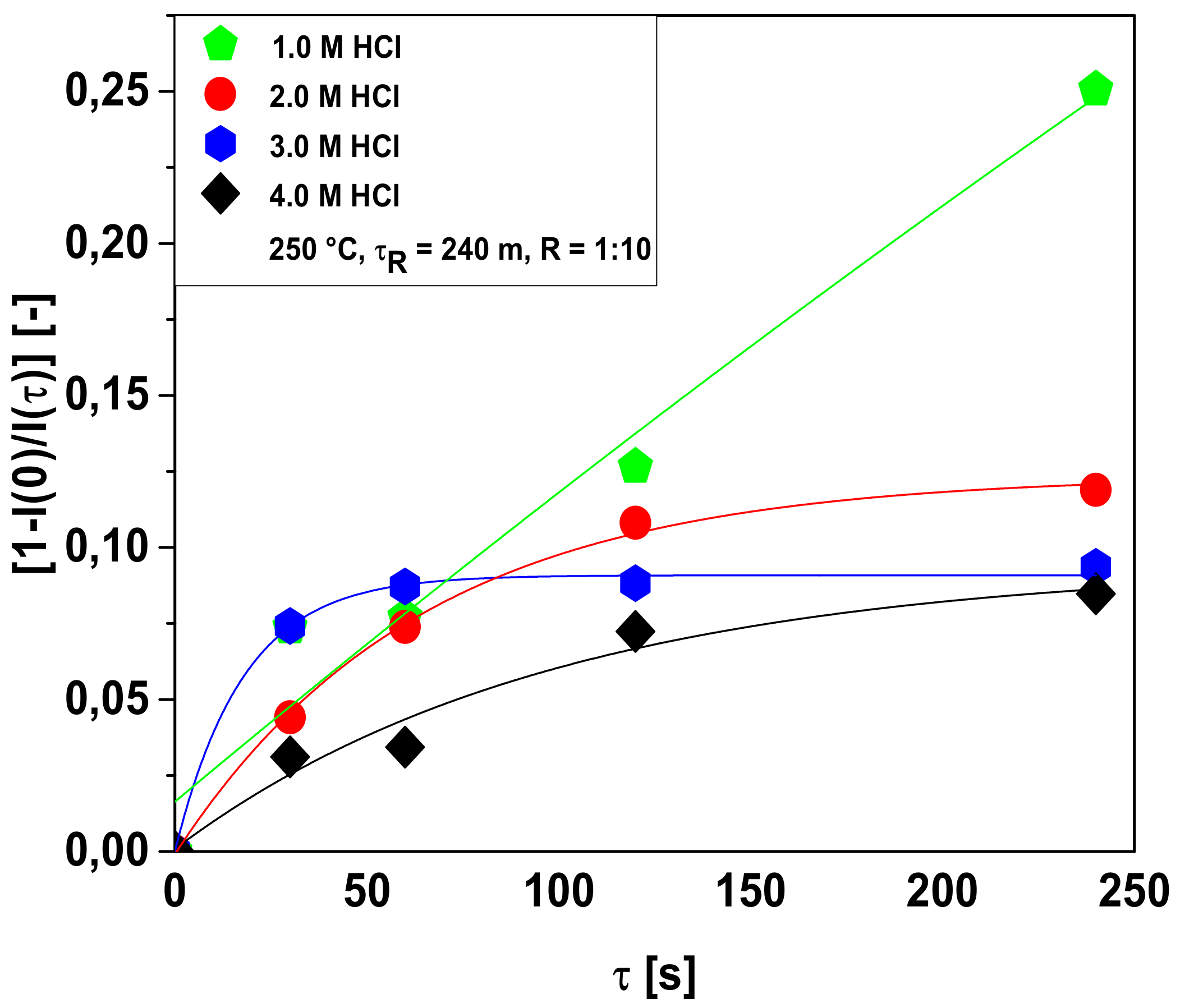
3.2.2. Bio-Adsorbent Activation with HCl
Influence of Hydrochloric Acid Concentration
3.2.3. Adsorption Equilibrium Isotherms of Acetic Acid (CH3COOH) on Hydro-Char
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTC | Hydrothermal carbonization |
| SEM | Scanning electron microscopy |
| TG/DTG/DTA | Thermogravimetric analysis |
| EDX | Energy dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| BET | Surface area and pore size distribution analysis |
| AAEMs | Alkaline and alkaline earth metals |
| HMF | Hydroxymethylfurfural |
| CH3COOH | Acetic acid |
References
- Kumar, S.; A Loganathan, V.; Gupta, R.B.; Barnett, M.O. An Assessment of U(VI) removal from groundwater using biochar produced from hydrothermal carbonization. J. Environ. Manag. 2011, 92, 2504–2512. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis Africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Machado, N.; Castro, D.; Queiroz, L.; Santos, M.; Costa, C. Production and Characterization of Energy Materials with Adsorbent Properties by Hydrothermal Processing of Corn Stover with Subcritical H2O. J. Appl. Solut. Chem. Model. 2016, 5, 117–130. [Google Scholar] [CrossRef]
- Libra, J.; Ro, K.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Benhouria, A.; Asif, M.; Hameed, B.H. Methylene blue adsorption on factory-rejected tea activated carbon prepared by conjunction of hydrothermal carbonization and sodium hydroxide activation processes. J. Taiwan Inst. Chem. Eng. 2015, 52, 57–64. [Google Scholar] [CrossRef]
- Islam, A.; Tan, I.A.W.; Benhouria, A.; Asif, M.; Hameed, B.H. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem. Eng. J. 2015, 270, 187–195. [Google Scholar] [CrossRef]
- Sun, K.; Tang, J.; Gong, Y.; Zhang, H. Characterization of potassium hydroxide (KOH) modified hydrochars from different feedstocks for enhanced removal of heavy metals from water. Environ. Sci. Pollut. Res. 2015, 22, 16640–16651. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X. Production and optimization of bamboo hydrochars for adsorption of Congo red and 2-naphthol. Bioresour. Technol. 2016, 207, 379–386. [Google Scholar] [CrossRef]
- Han, L.; Ro, K.S.; Sun, K.; Sun, H.; Wang, Z.; Libra, J.A.; Xing, B. New evidence for high sorption capacity of hydrochar for hydrophobic organic pollutants. Environ. Sci. Technol. 2016, 50, 13274–13282. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Ro, K.; Sun, K.; Libra, J.; Xing, B. Removal of antimony (III) and cadmium (II) from aqueous solution using animal manure-derived hydrochars and pyrochars. Bioresour. Technol. 2017, 234, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Pellera, F.-M.; Giannis, A.; Kalderis, D.; Anastasiadou, K.; Stegmann, R.; Wang, J.-Y.; Gidarakos, E. Adsorption of Cu(II) ions from aqueous solutions on biochars prepared from agricultural by-products. J. Environ. Manag. 2012, 96, 35–42. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Xi, J.; Yao, D.; Zhou, Z.; Tian, Y.; Lu, X. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017, 232, 204–210. [Google Scholar] [CrossRef]
- Kirschhöfer, F.; Sahin, O.; Becker, G.C.; Meffert, F.; Nusser, M.; Anderer, G.; Kusche, S.; Klaeusli, T.; Kruse, A.; Brenner-Weiss, G. Wastewater treatment-adsorption of organic micropollutants on activated HTC-carbon derived from sewage sludge. Water Sci. Technol. 2015, 73, 607–616. [Google Scholar] [CrossRef]
- Koottatep, T.; Fakkaew, K.; Tajai, N.; Polprasert, C. Isotherm models and kinetics of copper adsorption by using hydrochar produced from hydrothermal carbonization of faecal sludge. J. Water, Sanit. Hyg. Dev. 2017, 7, 102–110. [Google Scholar] [CrossRef]
- Sun, K.; Gao, B.; Ro, K.; Novak, J.M.; Wang, Z.; Herbert, S.; Xing, B. Assessment of herbicide sorption by biochars and organic matter associated with soil and sediment. Environ. Pollut. 2012, 163, 167–173. [Google Scholar] [CrossRef]
- Eibisch, N.; Schroll, R.; Fuß, R.; Mikutta, R.; Helfrich, M.; Flessa, H. Pyrochars and hydrochars differently alter the sorption of the herbicide isoproturon in an agricultural soil. Chemosphere 2015, 119, 155–162. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Zhou, C.; Luo, G.; Zhang, S.; Chen, J. A novel porous carbon derived from hydrothermal carbon for efficient adsorption of tetracycline. Carbon 2014, 77, 627–636. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hy-drochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Sun, K.; Ro, K.; Guo, M.; Novak, J.; Mashayekhi, H.; Xing, B. Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour. Technol. 2011, 102, 5757–5763. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Chen, J.; Zimmerman, A.R. Hydrochars derived from plant biomass under various conditions: Characteri-zation and potential applications and impacts. Chem. Eng. J. 2015, 267, 253–259. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Zimmerman, A.R.; Ro, K.S.; Chen, J. Physically (CO2) activated hydrochars from hickory and peanut hull: Preparation, characterization, and sorption of methylene blue, lead, copper, and cadmium. RSC Adv. 2016, 6, 24906–24911. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb(II) onto modified hydrochar: Modeling and mechanism analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, M.; Khanday, W.A.; Asif, M.; Hameed, B. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Petrović, J.; Stojanović, M.; Milojković, J.V.; Petrović, M.; Šoštarić, T.; Laušević, M.D.; Mihajlović, M.L. Alkali modified hydrochar of grape pomace as a perspective adsorbent of Pb 2+ from aqueous solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Lv, B.W.; Xu, H.; Guo, J.Z.; Bai, L.Q.; Li, B. Efficient adsorption of methylene blue on carboxylate-rich hydrochar prepared by one-step hydrothermal carbonization of bamboo and acrylic acid with ammonium persulphate. J. Hazard. Mater. 2022, 421, 126741. [Google Scholar]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Marx, S.; Venter, R.J.; Louw, A.; Dewah, C.T. Upgrading of the aqueous product stream from hydrothermal liquefaction: Simultaneous removal of minerals and phenolic components using waste-derived hydrochar. Biomass-Bioenergy 2021, 151, 106170. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.M. Carboxylate-rich carbonaceous materials via one-step hy-drothermal carbonization of glucose in the presence of acrylic acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Ghadikolaei, N.F.; Kowsari, E.; Balou, S.; Moradi, A.; Taromi, F.A. Preparation of porous biomass-derived hydrothermal carbon modified with terminal amino hyperbranched polymer for prominent Cr(VI) removal from water. Bioresour. Technol. 2019, 288, 121545. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Han, B.; Zhang, E.; Cheng, G.; Zhang, L.; Wang, D.; Wang, X. Hydrothermal carbon superstructures enriched with carboxyl groups for highly efficient uranium removal. Chem. Eng. J. 2018, 338, 734–744. [Google Scholar] [CrossRef]
- Liu, J.-L.; Qian, W.-C.; Guo, J.-Z.; Shen, Y.; Li, B. Selective removal of anionic and cationic dyes by magnetic Fe3O4-loaded amine-modified hydrochar. Bioresour. Technol. 2021, 320, 124374. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environ. Pollut. 2021, 268, 115910. [Google Scholar] [CrossRef]
- Li, B.; Guo, J.; Lv, K.; Fan, J. Adsorption of methylene blue and Cd(II) onto maleylated modified hydrochar from water. Environ. Pollut. 2019, 254, 113014. [Google Scholar] [CrossRef]
- Li, B.; Lv, J.-Q.; Guo, J.-Z.; Fu, S.-Y.; Guo, M.; Yang, P. The polyaminocarboxylated modified hydrochar for efficient capturing methylene blue and Cu(II) from water. Bioresour. Technol. 2019, 275, 360–367. [Google Scholar] [CrossRef]
- Li, H.Z.; Zhang, Y.N.; Guo, J.Z.; Lv, J.Q.; Huan, W.W.; Li, B. Preparation of hydrochar with high adsorption perfor-mance for methylene blue by cohydrothermal carbonization of polyvinyl chloride and bamboo. Bioresour. Technol. 2021, 337, 125442. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-pot synthesis and characterization of engineered hy-drochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere 2020, 254, 126866. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I.; Hassan, E.B. Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Tran, H.N.; Chao, H.-P.; Lin, C.-C. Effect of nitric acid oxidation on the surface of hydrochars to sorb methylene blue: An adsorption mechanism comparison. Adsorpt. Sci. Technol. 2019, 37, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Parra-Marfíl, A.; Ocampo-Pérez, R.; Collins-Martínez, V.H.; Flores-Vélez, L.M.; Gonzalez-Garcia, R.; Medellín-Castillo, N.A.; Labrada-Delgado, G.J. Synthesis and characterization of hydrochar from industrial Capsicum annuum seeds and its application for the adsorptive removal of methylene blue from water. Environ. Res. 2020, 184, 109334. [Google Scholar] [CrossRef]
- Qian, W.-C.; Luo, X.-P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef]
- Ronix, A.; Pezoti, O.; de Souza, L.S.; Souza, I.P.A.F.; Bedin, K.C.; Souza, P.S.C.; Silva, T.L.; Melo, S.A.R.; Cazetta, A.L.; Almeida, V.C. Hydrothermal carbonization of coffee husk: Optimization of experimental parameters and adsorption of methylene blue dye. J. Environ. Chem. Eng. 2017, 5, 4841–4849. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kineticmodeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Insight into adsorption mechanism of cationic dye onto agricultural residues-derived hydrochars: Negligible role of π-π interaction. Korean J. Chem. Eng. 2017, 34, 1708–1720. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Guo, J.-Z.; Huan, W.-W.; Liu, L. Sorption of methyl orange from aqueous solution by protonated amine modified hydrochar. Bioresour. Technol. 2018, 268, 454–459. [Google Scholar] [CrossRef]
- Spataru, A.; Jain, R.; Chung, J.W.; Gerner, G.; Krebs, R.; Lens, P.N.L. Enhanced adsorption of orthophosphate and copper onto hydrochar derived from sewage sludge by KOH activation. RSC Adv. 2016, 6, 101827–101834. [Google Scholar] [CrossRef]
- Flora, J.F.; Lu, X.; Li, L.; Flora, J.R.; Berge, N.D. The effects of alkalinity and acidity of process water and hydrochar washing on the adsorption of atrazine on hydrothermally producedhydrochar. Chemosphere 2013, 93, 1989–1996. [Google Scholar] [CrossRef]
- Machado, N.; de Castro, D.; Santos, M.; Araújo, M.; Lüder, U.; Herklotz, L.; Werner, M.; Mumme, J.; Hoffmann, T. Process analysis of hydrothermal carbonization of corn Stover with subcritical H2O. J. Supercrit. Fluids 2018, 136, 110–122. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Zielinska, B.; Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass-Convers. Biorefinery 2012, 3, 113–126. [Google Scholar] [CrossRef]
- Silva, C.D.M.S.D.; de Castro, D.A.R.; Santos, M.C.; Almeida, H.D.S.; Schultze, M.; Lüder, U.; Hoffmann, T.; Machado, N.T. Process analysis of main organic compounds dissolved in aqueous phase by hydrothermal processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of process temperature, biomass-to-water ratio, and production scales. Energies 2021, 14, 5608. [Google Scholar] [CrossRef]
- Mota, S.; Mancio, A.D.A.; Lhamas, D.; de Abreu, D.; da Silva, M.; dos Santos, W.; de Castro, D.; de Oliveira, R.; Araújo, M.; Borges, L.E.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Almeida, H.D.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; de Castro, D.; Pereira, M.; Pereira, L.; Mâncio, A.D.A.; Santos, M.; Souza, J.D.S.; et al. Production of biofuels by thermal catalytic cracking of scum from grease traps in pilot scale. J. Anal. Appl. Pyrolysis 2016, 118, 20–33. [Google Scholar] [CrossRef]
- Serrão, A.C.M.; Silva, C.M.S.; Assunção, F.P.D.C.; Ribeiro, H.J.D.S.; Santos, M.C.; Almeida, H.D.S.; Junior, S.D.; Borges, L.E.P.; de Castro, D.A.R.; Machado, N.T. Análise do processo de pirólise de sementes de açaí (euterpe oleracea, mart): Influência da temperatura no rendimento dos produtos de reação e nas propriedades físico-químicas do bio-óleo/process analysis of pyrolise of açaí (euterpe oleracea, mart) seeds: Influence of temperature on the yield of reaction products and physico-chemical properties of bio-oil. Braz. J. Dev. 2021, 7, 18200–18220. [Google Scholar] [CrossRef]
- Sittisun, P.; Tippayawong, N.; Wattanasiriwech, D. Thermal Degradation characteristics and kinetics of oxy combustion of corn residues. Adv. Mater. Sci. Eng. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Li, B.; Cai, J.-X.; Liu, C.; McAdam, K.; Zhang, K. Thermal & chemical analyses of hydrothermally derived carbon materials from corn starch. Fuel Process. Technol. 2016, 153, 43–49. [Google Scholar]
- Kumar, S.; Kothari, U.; Kong, L.; Lee, Y.; Gupta, R.B. Hydrothermal pretreatment of switchgrass and corn stover for production of ethanol and carbon microspheres. Biomass-Bioenergy 2011, 35, 956–968. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–98. [Google Scholar] [CrossRef]
- Varga, E.; Szengyel, Z.; Réczey, K. Chemical pretreatments of corn stover for enhancing enzymatic digestibility. Appl. Biochem. Biotechnol. 2002, 98, 73–87. [Google Scholar] [CrossRef]
- Mohammed, I.; Na, R.; Kushima, K.; Shimizu, N. Investigating the effect of processing parameters on the products of hydrothermal carbonization of corn stover. Sustainability 2020, 12, 5100. [Google Scholar] [CrossRef]
- Xing, X.; Fan, F.; Shi, S.; Li, Y.; Zhang, X.; Yang, J. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of corn straw. BioResources 2016, 11, 9190–9204. [Google Scholar] [CrossRef] [Green Version]
- Hoskinson, R.L.; Karlen, D.L.; Birrell, S.J.; Radtke, C.W.; Wilhelm, W.W. Engineering, nutrient removal, and feedstock conversion evaluations of four corn stover harvest scenarios. Biomass Bioenergy 2007, 31, 126–136. [Google Scholar] [CrossRef]
- Kang, K.; Sonil, N.; Guotao, S.; Ling, Q.; Yongqing, G.; Tianle, Z.; Mingqiang, Z.; Runcang, S. Microwaveassisted hydrothermal carbonizatijon of corn stalk for solid biofuel production: Optimization of process parameters and characterization of hydrochar. Energy 2019, 186, 115–125. [Google Scholar] [CrossRef]
- Becker, R.; Dorgerloh, U.; Helmis, M.; Mumme, J.; Diakité, M.; Nehls, I. Hydrothermally carbonized plant materials: Patterns of volatile organic compounds detected by gas chromatography. Bioresour. Technol. 2013, 130, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Wirth, B.; Lueder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, Y.; Zhang, J. Effect of solution pH on the carbon microsphere synthesized by hydrothermal carbonization. Procedia Environ. Sci. 2011, 11, 1322–1327. [Google Scholar] [CrossRef] [Green Version]








| Hydro-Chars | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 175 °C | 200 °C | 225 °C | 250 °C | |||||||||
| Chemical Elements | Mass (wt.%) | Atomic Mass (wt.%) | SD | Mass (wt.%) | Atomic Mass (wt.%) | SD | Mass (wt.%) | Atomic Mass (wt.%) | SD | Mass (wt.%) | Atomic Mass (wt.%) | SD |
| C | 60.69 | 67.56 | 0.503 | 71.98 | 77.43 | 0.818 | 73.89 | 79.17 | 0.654 | 76.04 | 81.05 | 0.631 |
| O | 38.57 | 32.24 | 0.504 | 27.83 | 22.47 | 0.819 | 25.73 | 20.70 | 0.656 | 23.59 | 18.87 | 0.632 |
| Si | 0.136 | 0.065 | 0.020 | 0.194 | 0.089 | 0.043 | - | - | - | - | - | - |
| Ca | 0.181 | 0.061 | 0.018 | - | - | - | 0.374 | 0.120 | 0.032 | - | - | - |
| Zn | - | - | - | - | - | - | - | - | - | 0.366 | 0.072 | 0.065 |
| Cu | 0.210 | 0.044 | 0.042 | - | - | - | - | - | - | - | - | - |
| Parameters | CCH3COOH (mg/mL) | ||
|---|---|---|---|
| 2 | 3 | 4 | |
| Pseudo-first-order | |||
| (-) | 0.3694 | 0.2361 | 0.1497 |
| κ (s−1) | 0.0479 | 0.0545 | 0.0468 |
| r2 | 0.969 | 0.999 | 0.988 |
| Parameters | CCH3COOH (mg/mL) | |
|---|---|---|
| 1 | 2 | |
| Pseudo-first-order | ||
| (-) | 0.4689 | 0.3694 |
| κ (s−1) | 0.0305 | 0.0479 |
| r2 | 0.982 | 0.969 |
| Parameters | CCH3COOH (mg/mL) | |
|---|---|---|
| 2 | 2 | |
| Pseudo-first-order | 0.1 gAdsorbent/10 mLCH3COOH | 0.2 gAdsorbent/10 mLCH3COOH |
| (-) | 0.4689 | 0.6889 |
| κ (s−1) | 0.0305 | 0.0430 |
| r2 | 0.982 | 0.973 |
| Parameters | CCH3COOH (mg/mL) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Pseudo-first-order | ||||
| (-) | - | 0.1241 | 0.0908 | 0.0941 |
| κ (s−1) | - | 0.0156 | 0.0563 | 0.0101 |
| r2 | 0.969 | 0.998 | 0.978 | 0.967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.E.G.; da Costa Assunção, F.P.; Teribele, T.; Pereira, L.M.; de Castro, D.A.R.; Santo, M.C.; da Costa, C.E.F.; Shultze, M.; Hofmann, T.; Machado, N.T. Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions. Energies 2021, 14, 8154. https://doi.org/10.3390/en14238154
Costa MEG, da Costa Assunção FP, Teribele T, Pereira LM, de Castro DAR, Santo MC, da Costa CEF, Shultze M, Hofmann T, Machado NT. Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions. Energies. 2021; 14(23):8154. https://doi.org/10.3390/en14238154
Chicago/Turabian StyleCosta, Maria Elizabeth Gemaque, Fernanda Paula da Costa Assunção, Tiago Teribele, Lia Martins Pereira, Douglas Alberto Rocha de Castro, Marcelo Costa Santo, Carlos Emerson Ferreira da Costa, Maja Shultze, Thomas Hofmann, and Nélio Teixeira Machado. 2021. "Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions" Energies 14, no. 23: 8154. https://doi.org/10.3390/en14238154
APA StyleCosta, M. E. G., da Costa Assunção, F. P., Teribele, T., Pereira, L. M., de Castro, D. A. R., Santo, M. C., da Costa, C. E. F., Shultze, M., Hofmann, T., & Machado, N. T. (2021). Characterization of Bio-Adsorbents Produced by Hydrothermal Carbonization of Corn Stover: Application on the Adsorption of Acetic Acid from Aqueous Solutions. Energies, 14(23), 8154. https://doi.org/10.3390/en14238154