Photocatalytic Evolution of Hydrogen Peroxide: A Minireview
Abstract
:1. Introduction
2. Redox Reactions during Photocatalytic Synthesis of H2O2–Early Considerations
3. Functional Photocatalytic Systems for H2O2 Evolution
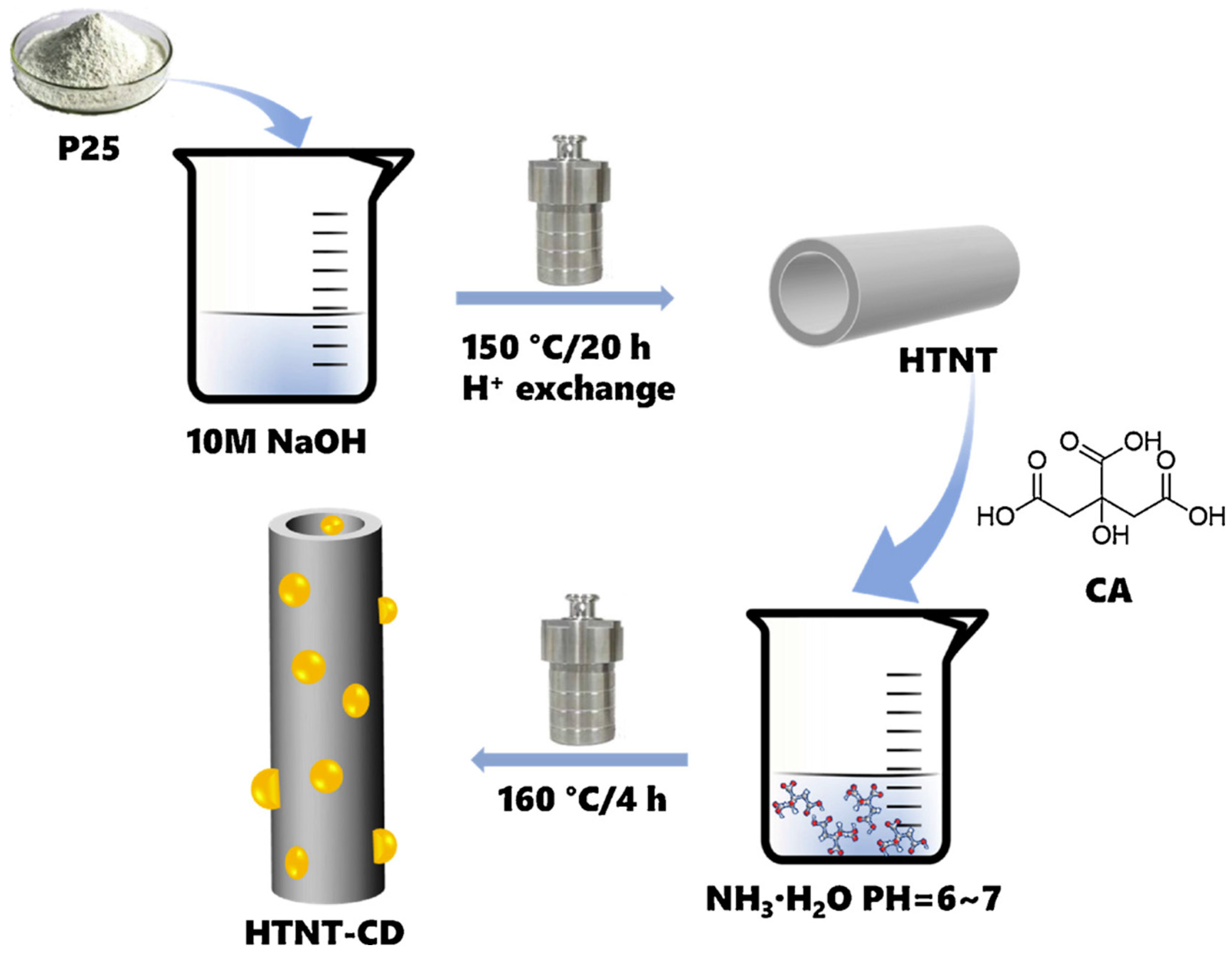
3.1. Titanium Dioxide (TiO2)
3.2. Metal Nanoparticle-Decorated Titania
3.3. Other Oxides
3.4. Metal Sulfides
3.5. Metal-Organic Frameworks (MOFs)
3.6. Carbon-Based Semiconductors
3.7. Graphitic Carbon Nitride (g-C3N4) Systems
4. State-of-the-Art and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AQ | Anthraquinone |
| Tris | tris(hydroxymethyl) aminomethane |
| TNT | TiO2 nanotubes |
| CA | Citric acid |
| CD | Carbon dots |
| NP | Nanoparticles |
| rGO | Reduced graphene oxide |
| MOF | Metal-organic framework |
| OPA | Organophosphonic acid |
| BP | Biphenyl |
| DPA | Diphenylacetylene |
| DPDA | Diphenyldiacetylene |
| CHF | Covalent heptazine framework |
| COF | Covalent organic framework |
| NHE | Normal hydrogen electrode |
| BN | Boron nitride |
| CNT | Carbon nanotubes |
| ORR | Oxygen reduction reaction |
| WOR | Water oxidation reaction |
References
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [PubMed]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B: Environ. 2017, 210, 235–254. [Google Scholar]
- Hou, H.; Zeng, X.; Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef]
- Prat, C.; Vicente, M.; Esplugas, S. Treatment of bleaching waters in the paper industry by hydrogen peroxide and ultraviolet radiation. Water Res. 1988, 22, 663–668. [Google Scholar] [CrossRef]
- Ksibi, M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Engin. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Nishimi, T.; Kamachi, T.; Kato, K.; Kato, T.; Yoshizawa, K. Mechanistic study on the production of hydrogen peroxide in the anthraquinone process. Eur. J. Org. Chem. 2011, 2011, 4113–4120. [Google Scholar] [CrossRef]
- Liu, T.; Pan, Z.; Kato, K.; Wu, B.; Yamakata, A.; Katayama, K.; Chen, B.; Chu, C.; Domen, K. Overall photosynthesis of H2O2 by an inorganic semiconductor. Nat. Commun. 2022, 13, 1034. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconduction photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar]
- Harbour, J.R.; Tromp, J.; Hair, M.L. Photogeneration of hydrogen peroxide in aqueous TiO2 dispersions. Can. J. Chem. 1985, 63, 204–208. [Google Scholar] [CrossRef]
- Cai, R.; Kubota, Y.; Fujishima, A. Effect of copper ions on the formation of hydrogen peroxide from photocatalytic titanium dioxide particles. J. Catal. 2003, 219, 214–218. [Google Scholar] [CrossRef]
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 photocatalysis of oxygenated and deoxygenated aqueous systems: A probe for photocatalytically produced hydroxyl radicals. J. Phys. Chem. C 2014, 118, 10083–10087. [Google Scholar] [CrossRef]
- Muraki, H.; Saji, T.; Fujihira, M.; Aouagui, S. Photocatalytic oxidation of water to hydrogen peroxide by irradiation of aqueous suspensions of TiO2. J. Electroanal. Chem. 1984, 169, 319–323. [Google Scholar] [CrossRef]
- Cormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of H2O2 and organic peroxides in aqueous suspensions of TiO2, ZnO and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Goto, H.; Hanada, Y.; Ohno, T.; Matsumura, M. Quantitative analysis of superoxide ion and hydrogen peroxide produced from molecular oxygen in photoirradiated TiO2 particles. J. Catal. 2004, 225, 223–229. [Google Scholar] [CrossRef]
- Mrowetz, M.; Selli, E. Photocatalytic degradation of formic and benzoic acids and hydrogen peroxide evolution in TiO2 and ZnO water suspensions. J. Photochem. Photobiol. A Chem. 2006, 180, 15–22. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Tsukamoto, D.; Shiro, A.; Sugano, Y.; Hirai, T. Selective hydrogen peroxide formation by titanium dioxide photocatalysis with benzylic alcohols and molecular oxygen in water. ACS Catal. 2013, 3, 2222–2227. [Google Scholar] [CrossRef]
- Hirakawa, T.; Nosaka, Y. Selective production of superoxide ions and hydrogen peroxide over nitrogen- and sulfur-doped TiO2 photocatalysts with visible light in aqueous suspension systems. J. Phys. Chem. C 2008, 112, 15818–15823. [Google Scholar] [CrossRef]
- Ma, R.; Wang, L.; Liu, Z.; Xing, M.; Zhu, L.; Meng, X.; Xiao, F.-S. Solid acids accelerate the photocatalytic hydrogen peroxide synthesis over a hybrid catalyst of titania nanotube with carbon dot. Appl. Catal. B Environ. 2019, 244, 594–603. [Google Scholar]
- Zheng, L.; Zhang, J.; Hu, Y.H.; Long, M. Enhanced photocatalytic production of H2O2 by Nafion coatings on S,N-codoped graphene-quantum-dots-modified TiO2. J. Phys. Chem. C 2019, 123, 13693–13701. [Google Scholar] [CrossRef]
- Lee, T.; Bui, H.T.; Yoo, J.; Ra, M.; Han, S.H.; Kim, W.; Kwon, W. Formation of TiO2@carbon core/shell nanocomposites from a single molecular layer of aromatic compounds for photocatalytic hydrogen peroxide generation. ACS Appl. Mater. Interf. 2019, 11, 41196–41203. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Zhao, S.; Gong, Y.; Lin, S.; Zhao, X. Highly-efficient photocatalytic hydrogen peroxide production over polyoxometalates covalently immobilized onto titanium dioxide. Appl. Catal. A 2020, 591, 117271. [Google Scholar] [CrossRef]
- Maurino, V.; Minero, C.; Mariella, G.; Pelizzetti, E. Sustained production of H2O2 on irradiated TiO2-fluoride systems. Chem. Commun. 2005, 2627–2629. [Google Scholar] [CrossRef]
- Teranishi, M.; Naya, S.; Tada, H. In situ liquid phase synthesis of hydrogen peroxide from molecular oxygen using gold nanoparticle-loaded titanium(IV) dioxide photocatalyst. J. Amer. Chem. Soc. 2010, 132, 7850–7851. [Google Scholar] [CrossRef]
- Teranishi, M.; Naya, S.; Tada, H. Temperature- and pH-dependence of hydrogen peroxide formation from molecular oxygen by gold nanoparticle-loaded titanium(IV) oxide photocatalyst. J. Phys. Chem. C 2016, 120, 1083–1088. [Google Scholar] [CrossRef]
- Tsukamoto, D.; Shiro, A.; Shiraishi, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Photocatalytic H2O2 production from ethanol/O2 system using TiO2 loaded with Au−Ag bimetallic alloy nanoparticles. ACS Catal. 2012, 2, 599–603. [Google Scholar] [CrossRef]
- Kaynan, N.; Berke, B.A.; Hazut, O.; Yerushalmi, R. Sustainable photocatalytic production of hydrogen peroxide from water and molecular oxygen. J. Mater. Chem. A 2014, 2, 13822–13826. [Google Scholar] [CrossRef]
- Kim, K.; Park, J.; Kim, H.; Jung, G.Y.; Kim, M.G. Solid-phase photocatalysts: Physical vapor deposition of Au nanoislands on porous TiO2 films for millimolar H2O2 production within a few minutes. ACS Catal. 2019, 9, 9206–9211. [Google Scholar] [CrossRef]
- Zuo, G.; Li, B.; Guo, Z.; Wang, L.; Yang, F.; Hou, W.; Zhang, S.; Zong, P.; Liu, S.; Meng, X.; et al. Efficient Photocatalytic Hydrogen Peroxide production over TiO2 Passivated by SnO2. Catalysts 2019, 9, 623. [Google Scholar] [CrossRef]
- Awa, K.; Naya, S.; Fujishima, M.; Tada, H. A Three-Component Plasmonic Photocatalyst Consisting of Gold Nanoparticle and TiO2−SnO2 Nanohybrid with Heteroepitaxial Junction: Hydrogen Peroxide Synthesis. J. Phys. Chem. C 2020, 124, 7797–7802. [Google Scholar] [CrossRef]
- Hoffman, A.J.; Carraway, E.R.; Hoffmann, M.R. Photocatalytic Production of H202 and Organic Peroxides on Quantum-Sized Semiconductor Colloids. Environ. Sel. Technol. 1994, 28, 776–785. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; Qiu, W.; Gao, W. Hydrogen peroxide generation and photocatalytic degradation of estrone by microstructural controlled ZnO nanorod arrays. Appl. Surf. Sci. 2012, 263, 389–396. [Google Scholar] [CrossRef]
- Hirakawa, H.; Shiota, S.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Au Nanoparticles Supported on BiVO4: Effective Inorganic Photocatalysts for H2O2 Production from Water and O2 under Visible Light. ACS Catal. 2016, 8, 4976–4982. [Google Scholar] [CrossRef]
- Teranishi, M.; Kunimoto, T.; Naya, S.; Kobayashi, H.; Tada, H. Visible-Light-Driven Hydrogen Peroxide Synthesis by a Hybrid Photocatalyst Consisting of Bismuth Vanadate and Bis(hexafluoroacetylacetonato)copper(II) Complex. J. Phys. Chem. C 2020, 124, 3715–3721. [Google Scholar] [CrossRef]
- Dhabarde, N.; Carrillo-Ceja, O.; Tian, S.; Xiong, G.; Raja, K.; Subramanian, V.R. Bismuth Vanadate Encapsulated with Reduced Graphene Oxide: A Nanocomposite for Optimized Photocatalytic Hydrogen Peroxide Generation. J. Phys. Chem. C 2021, 125, 23669–23679. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Vertova, A.; Minguzzi, A.; Rondinini, S. Photoelectrochemical and photocatalytic systems based on titanates for hydrogen peroxide formation. J. Electroanal. Chem. 2018, 808, 395–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.J. Formation of hollow MoO3/SnS2 heterostructured nanotubes for efficient light-driven hydrogen peroxide production. J. Mater. Chem. A 2018, 6, 20304–20312. [Google Scholar] [CrossRef]
- Thakur, S.; Kshetri, T.; Kim, N.H.; Lee, J.H. Sunlight-driven sustainable production of hydrogen peroxide using a CdS–graphene hybrid photocatalyst. J. Catal. 2017, 345, 78–86. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, X. Photocatalytic production of hydrogen peroxide over Z-scheme Mn3O4/ Co9S8 with p-n heterostructure. Appl. Catal. B Environ. 2021, 298, 120516. [Google Scholar] [CrossRef]
- Tian, Z.; Han, C.; Zhao, Y.; Dai, W.; Lian, X.; Wang, Y.; Zheng, Y.; Shi, Y.; Pan, X.; Huang, Z.; et al. Efficient photocatalytic hydrogen peroxide generation coupled with selective benzylamine oxidation over defective ZrS3 nanobelts. Nat. Commun. 2021, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Y.; Nie, H.; Wei, K.; Cao, J.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Interface photo-charge kinetics regulation by carbon dots for efficient hydrogen peroxide production. J. Mater. Chem. A 2021, 9, 515. [Google Scholar] [CrossRef]
- Chen, X.; Kondo, Y.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. Metal–organic framework-based nanomaterials for photocatalytic hydrogen peroxide production. Phys. Chem. Chem. Phys. 2020, 22, 14404. [Google Scholar] [CrossRef] [PubMed]
- Isaka, Y.; Kondo, Y.; Kawase, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Photocatalytic production of hydrogen peroxide through selective two-electron reduction of dioxygen utilizing amine-functionalized MIL-125 deposited with nickel oxide nanoparticles. Chem. Commun. 2018, 54, 9270. [Google Scholar] [CrossRef]
- Liu, C.; Bao, T.; Yuan, L.; Zhang, C.; Wang, J.; Wan, J.; Yu, C. Semiconducting MOF@ZnS Heterostructures for Photocatalytic Hydrogen Peroxide Production: Heterojunction Coverage Matters. Adv. Funct. Mater. 2022, 32, 2111404. [Google Scholar] [CrossRef]
- Isaka, Y.; Kawase, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Two-Phase System Utilizing Hydrophobic Metal-Organic Frameworks for Photocatalytic Synthesis of Hydrogen Peroxide. Angew. Chem. Int. Ed. 2019, 58, 5402–5406. [Google Scholar] [CrossRef]
- Kawase, Y.; Isaka, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Ti cluster-alkylated hydrophobic MOFs for photocatalytic production of hydrogen peroxide in two-phase systems. Chem. Commun. 2019, 55, 6743. [Google Scholar] [CrossRef]
- Chen, X.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. Introduction of a secondary ligand into titanium-based metal–organic frameworks for visible-light-driven photocatalytic hydrogen peroxide production from dioxygen reduction. J. Mater. Chem. A 2021, 9, 2815–2821. [Google Scholar] [CrossRef]
- Chen, X.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. Hydrophobic Titanium Doped Zirconium-based Metal Organic Framework for Photocatalytic Hydrogen Peroxide Production in Two-phase System. J. Mater. Chem. A 2020, 8, 1904–1910. [Google Scholar] [CrossRef]
- Wecławski, M.K.; Jakesova, M.; Charyton, M.; Demitri, N.; Koszarna, B.; Oppelt, K.; Sariciftci, S.; Gryko, D.T.; Głowacki, E.D. Biscoumarin-containing acenes as stable organic semiconductors for photocatalytic oxygen reduction to hydrogen peroxide. J. Mater. Chem. A 2017, 5, 20780–20788. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, M.; Sun, Y.; Zhou, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Carbon-Supported Oxygen Vacancy-Rich Co3O4 for Robust Photocatalytic H2O2 Production via Coupled Water Oxidation and Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2019, 2, 8737–8746. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Takii, T.; Hagi, T.; Mori, S.; Kofuji, Y.; Kitagawa, Y.; Tanaka, S.; Ichikawa, S.; Hirai, T. Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to hydrogen peroxide energy conversion. Nat. Mater. 2019, 18, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Hagi, T.; Matsumoto, M.; Tanaka, S.; Ichikawa, S.; Hirai, T. Solar-to-hydrogen peroxide energy conversion on resorcinol–formaldehyde resin photocatalysts prepared by acid-catalysed polycondensation. Commun. Chem. 2020, 3, 169. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Matsumoto, M.; Ichikawa, S.; Tanaka, S.; Hirai, T. Polythiophene-Doped Resorcinol−Formaldehyde Resin Photocatalysts for Solar-to-Hydrogen Peroxide Energy Conversion. J. Amer. Chem. Soc. 2021, 143, 12590–12599. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, Y.; Cao, J.; Sun, Y.; Liao, F.; Liu, Y.; Huang, H.; Shao, M.; Kang, Z. A function-switchable metal-free photocatalyst for the efficient and selective production of hydrogen and hydrogen peroxide. J. Mater. Chem. A 2020, 8, 11773–11780. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, J.; Wang, X.; Liu, Y.; Zhao, Y.; Wang, H.; Liu, Y.; Huang, H.; Liao, F.; Shao, M.; et al. A metal-free photocatalyst for highly efficient hydrogen peroxide photoproduction in real seawater. Nat. Commun. 2021, 12, 483. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Wan, Y.; Zhang, Y.; Qi, Z.; Wu, X.; Xu, H. Acetylene and Diacetylene Functionalized Covalent Triazine Frameworks as Metal-Free Photocatalysts for Hydrogen Peroxide Production: A New Two-Electron Water Oxidation Pathway. Adv. Mater. 2020, 32, 1904433. [Google Scholar] [CrossRef]
- Cheng, H.; Lv, H.; Cheng, J.; Wang, L.; Wu, X.; Xu, H. Rational Design of Covalent Heptazine Frameworks with Spatially Separated Redox Centers for High-Efficiency Photocatalytic Hydrogen Peroxide Production. Adv. Mater. 2022, 34, 2107480. [Google Scholar] [CrossRef]
- Kou, M.; Wang, Y.; Xu, Y.; Ye, L.; Huang, Y.; Jia, B.; Li, H.; Ren, J.; Deng, Y.; Chen, J.; et al. Molecularly Engineered Covalent Organic Frameworks for Hydrogen Peroxide Photosynthesis. Angew. Chem. Int. Ed. 2022, 16, e202200413. [Google Scholar]
- Liu, L.; Gao, M.Y.; Yang, H.; Wang, X.; Li, X.; Cooper, A.I. Linear Conjugated Polymers for Solar-Driven Hydrogen Peroxide Production: The Importance of Catalyst Stability. J. Amer. Chem. Soc. 2021, 143, 19287–19293. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Li, Y.; Zhao, H.; Wang, J.; Huang, H.; Liu, Y.; Kang, Z. Converting water impurity in organic solvent into hydrogen and hydrogen peroxide by organic semiconductor photocatalyst. Appl. Catal. B Environ. 2022, 305, 121047. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Sampaio, M.J.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Recent Strategies for Hydrogen Peroxide Production by Metal-Free Carbon Nitride Photocatalysts. Catalysts 2019, 9, 990. [Google Scholar] [CrossRef]
- Haider, Z.; Cho, H.; Moon, G.; Kim, H. Minireview: Selective production of hydrogen peroxide as a clean oxidant over structurally tailored carbon nitride photocatalysts. Catal. Today 2019, 335, 55–64. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.; Xu, Y. Amorphous and Crystalline 2D Polymeric Carbon Nitride Nanosheets for Photocatalytic Hydrogen/Oxygen Evolution and Hydrogen Peroxide Production. Chem. Asian J. 2020, 15, 2329–2340. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Sugano, Y.; Tsukamoto, D.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Highly Selective Production of Hydrogen Peroxide on Graphitic Carbon Nitride (g-C3N4) Photocatalyst Activated by Visible Light. ACS Catal. 2014, 4, 774–780. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Kofuji, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Sunlight-Driven Hydrogen Peroxide Production from Water and Molecular Oxygen by Metal-Free Photocatalysts. Angew. Chem. Int. Ed. 2014, 126, 13672–13677. [Google Scholar] [CrossRef]
- Kofuji, Y.; Ohkita, S.; Shiraishi, Y.; Sakamoto, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Graphitic Carbon Nitride Doped with Biphenyl Diimide: Efficient Photocatalyst for Hydrogen Peroxide Production from Water and Molecular Oxygen by Sunlight. ACS Catal. 2016, 6, 7021–7029. [Google Scholar] [CrossRef]
- Kofuji, Y.; Isobe, Y.; Shiraishi, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Hydrogen Peroxide Production on Carbon Nitride–Boron Nitride– Reduced Graphene Oxide Hybrid Photocatalyst under Visible Light. ChemCatChem 2018, 10, 2070–2077. [Google Scholar] [CrossRef]
- Cao, J.; Wang, H.; Zhao, Y.; Liu, Y.; Wu, Q.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Phosphorus-doped porous carbon nitride for efficient sole production of hydrogen peroxide via photocatalytic water splitting with a two-channel pathway. J. Mater. Chem. A 2020, 8, 3701–3707. [Google Scholar] [CrossRef]
- Feng, C.; Tang, L.; Deng, Y.; Wang, J.; Luo, J.; Liu, Y.; Ouyang, X.; Yang, H.; Yu, J.; Wang, J. Synthesis of Leaf-Vein-Like g-C3N4 with Tunable Band Structures and Charge Transfer Properties for Selective Photocatalytic H2O2 Evolution. Adv. Funct. Mater. 2020, 30, 2001922. [Google Scholar] [CrossRef]
- Ye, Y.X.; Pan, J.; Xie, F.; Gong, L.; Huang, S.; Ke, Z.; Zhu, F.; Xu, J.; Ouyang, G. Highly efficient photosynthesis of hydrogen peroxide in ambient conditions. Proc. Nat. Acad. Sci. USA 2021, 118, e2103964118. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhang, Q.; Yang, H.; Kato, K.; Yang, W.; Lu, Y.R.; Liu, S.; Wang, C.; Yamakata, A.; Su, C.; et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 2021, 4, 374–384. [Google Scholar] [CrossRef]
- Moon, G.; Fujitsuka, M.; Kim, S.; Majima, T.; Wang, X.; Choi, W. Eco-Friendly Photochemical Production of H2O2 through O2 Reduction over Carbon Nitride Frameworks Incorporated with Multiple Heteroelements. ACS Catal. 2017, 7, 2886–2895. [Google Scholar] [CrossRef]
- Fattahimoghaddam, H.; Mahvelati-Shamsabadi, T.; Jeong, C.S.; Lee, B.K. Coral-like potassium and phosphorous doped graphitic carbon nitride structures with enhanced charge and mass transfer dynamics toward photocatalytic hydrogen peroxide production and microbial disinfection. J. Coll. Interf. Sci. 2022, 617, 326–340. [Google Scholar] [CrossRef]
- Tian, J.; Wang, D.; Li, S.; Pei, Y.; Qiao, M.; Li, Z.; Zhang, J.; Zong, B. KOH-Assisted Band Engineering of Polymeric Carbon Nitride for Visible Light Photocatalytic Oxygen Reduction to Hydrogen Peroxide. ACS Sustain. Chem. Eng. 2020, 8, 594–603. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Cao, Y.; Du, R.; Bao, Z.; Zhang, S.; Shao, F.; Ji, W.; Yang, J.; Zhuang, G.; et al. Trace water triggers high-efficiency photocatalytic hydrogen peroxide production. J. Energy Chem. 2022, 64, 47–54. [Google Scholar] [CrossRef]
- Tian, J.; Wu, T.; Wang, D.; Pei, Y.; Qiao, M.; Zong, B. One-pot synthesis of potassium and phosphorus-doped carbon nitride catalyst derived from urea for highly efficient visible light-driven hydrogen peroxide production. Catal. Today 2019, 330, 171–178. [Google Scholar] [CrossRef]
- Liu, B.; Bie, C.; Zhang, Y.; Wang, L.; Li, Y.; Yu, J. Hierarchically Porous ZnO/g-C3N4 S-Scheme Heterojunction Photocatalyst for Efficient H2O2 Production. Langmuir 2021, 37, 14114–14124. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Chen, S.; Qua, X. Enhanced Photocatalytic H2O2 Production over Carbon Nitride by Doping and Defect Engineering. ACS Catal. 2020, 10, 14380–14389. [Google Scholar] [CrossRef]
- Wang, X.; Han, Z.; Yu, L.; Liu, C.; Liu, Y.; Wu, G. Synthesis of Full-Spectrum-Response Cu2(OH)PO4/g-C3N4 Photocatalyst with Outstanding Photocatalytic H2O2 Production Performance via a “Two Channel Route”. ACS Sustain. Chem. Eng. 2018, 6, 14542–14553. [Google Scholar] [CrossRef]
- Zhao, X.; You, Y.; Huang, S.; Wu, Y.; Ma, Y.; Zhang, G.; Zhang, Z. Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light. Appl. Catal. B Environ. 2020, 278, 119251. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Liu, Y.; Chen, H.; Lei, J.; Zhang, J. Visible-light-driven photocatalytic H2O2 production on g-C3N4 loaded with CoP as a noble metal free cocatalyst. Eur. J. Inorg. Chem. 2017, 40, 4797–4802. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, M.; Zhang, Z.; Yao, W.; Tan, H.; Zhu, Y. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ. Sci. 2018, 11, 2581–2589. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.; Yang, Z.; Li, S.; Chu, C.; Chen, B. Simultaneously Tuning Band Structure and Oxygen Reduction Pathway toward High-Efficient Photocatalytic Hydrogen Peroxide Production Using Cyano-Rich Graphitic Carbon Nitride. Adv. Funct. Mater. 2021, 31, 2105731. [Google Scholar] [CrossRef]
- Kim, H.; Choi, Y.; Hu, S.; Choi, W.; Kim, J.H. Photocatalytic hydrogen peroxide production by anthraquinone-augmented polymeric carbon nitride. Appl. Catal. B Environ. 2018, 229, 121–129. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, T.; Li, X.; Xu, T.; Yang, B.; Zhao, X. Carbon nanotubes covalent combined with graphitic carbon nitride for photocatalytic hydrogen peroxide production under visible light. Appl. Catal. B Environ. 2018, 224, 725–732. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, C.; Zhang, M.; Zhu, C.; Li, H.; Wang, H.; Song, Y.; Huang, H.; Liu, Y.; Kang, Z. Photocatalytic H2O2 and H2 Generation from Living Chlorella vulgaris and Carbon Micro Particle Comodified g-C3N4. Adv. Energy Mater. 2018, 8, 1802525. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wu, J.; Han, Y.; Huang, H.; Liu, Y.; Kang, Z. Photo-charge regulation of metal-free photocatalyst by carbon dots for efficient and stable hydrogen peroxide production. J. Mater. Chem. A 2021, 9, 25453–25462. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Labudova, M.; Danko, M.; Micusik, M.; Kovacova, M.; Spitalsky, Z.; Pavlovic, V.; Medic, M.; Todorovic Markovic, B.M. Highly efficient antioxidant F- and Cl-doped carbon quantum dots for bioimaging. ACS Sustain. Chem. Eng. 2020, 8, 16327–16338. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Sampaio, M.J.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Metal-free carbon nitride photocatalysis with in situ hydrogen peroxide generation for the degradation of aromatic compounds. Appl. Catal. B Environ. 2019, 252, 128–137. [Google Scholar] [CrossRef]
- Xie, H.; Zheng, Y.; Guo, X.; Liu, Y.; Zhang, Z.; Zhao, J.; Zhang, W.; Wang, Y.; Huang, Y. Rapid Microwave Synthesis of Mesoporous Oxygen-Doped g-C3N4 with Carbon Vacancies for Efficient Photocatalytic H2O2 Production. ACS Sustain. Chem. Eng. 2021, 9, 6788–6798. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, N.; Wang, F.; Lei, J.; Zhou, L.; Liu, Y.; Zhang, J. Carbon Vacancy Mediated Incorporation of Ti3C2 Quantum Dots in a 3D Inverse Opal g-C3N4 Schottky Junction Catalyst for Photocatalytic H2O2 Production. ACS Sustain. Chem. Eng. 2021, 9, 481–488. [Google Scholar] [CrossRef]






| Main Semiconductor | Co-Catalyst/Dopant | Band Gap (eV) | H2O2 Yield (μmol/g/h) | Reference (Year) |
|---|---|---|---|---|
| Cyano-C3N4 | Na+ | 2.53 | 16,000 | [84] (2021) |
| NH2-UiO-66(Zr)@OPA MOF | Ti4+ | nd | 13,580 | [49] (2020) |
| C3N4 | K+/Na+ | 2.63 | 10,200 | [79] (2020) |
| C3N4 | K+ | 2.75 | 10,000 | [76] (2022) |
| C3N4 | ZnO | 2.83 | 4000 | [78] (2021) |
| Anthracene/acetylene-based semiconductor | - | 2.89 | 3923 | [61] (2022) |
| Carbon support | Co3O4 | 1.84–1.97 | 3785 | [51] (2019) |
| TiO2 nanotubes | Carbon dots | 2.98 | 3420 | [20] (2019) |
| C3N4 | - | 2.68 | 3103 | [90] (2019) |
| C3N4 | WO3 | 2.60 | 2920 | [83] (2018) |
| NiTiO3 | - | 3.00 | 2500 | [37] (2018) |
| 3-[(4-ethynylphenyl) ethynyl]pyridine polymer | - | 2.34 | 2267 | [60] (2021) |
| C3N4 | N,S-doped carbon dots plus carbazole derivative | 2.58 | 2203 | [88] (2021) |
| C3N4 | (COOH)2 | 2.42 | 2008 | [91] (2021) |
| C3N4 | P | 2.58 | 1968 | [69] (2020) |
| C3N4 | Ti3C2 | 2.60 | 1847 | [92] (2021) |
| 4-methoxybenzaldehyde/procyanidin network | Carbon dots | 1.94 | 1776 | [56] (2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamoschos, N.; Tasis, D. Photocatalytic Evolution of Hydrogen Peroxide: A Minireview. Energies 2022, 15, 6202. https://doi.org/10.3390/en15176202
Karamoschos N, Tasis D. Photocatalytic Evolution of Hydrogen Peroxide: A Minireview. Energies. 2022; 15(17):6202. https://doi.org/10.3390/en15176202
Chicago/Turabian StyleKaramoschos, Nikolaos, and Dimitrios Tasis. 2022. "Photocatalytic Evolution of Hydrogen Peroxide: A Minireview" Energies 15, no. 17: 6202. https://doi.org/10.3390/en15176202





