A 2D Modelling Approach for Predicting the Response of a Two-Chamber Microbial Fuel Cell to Substrate Concentration and Electrolyte Conductivity Changes
Abstract
:1. Introduction
2. Materials and Methods
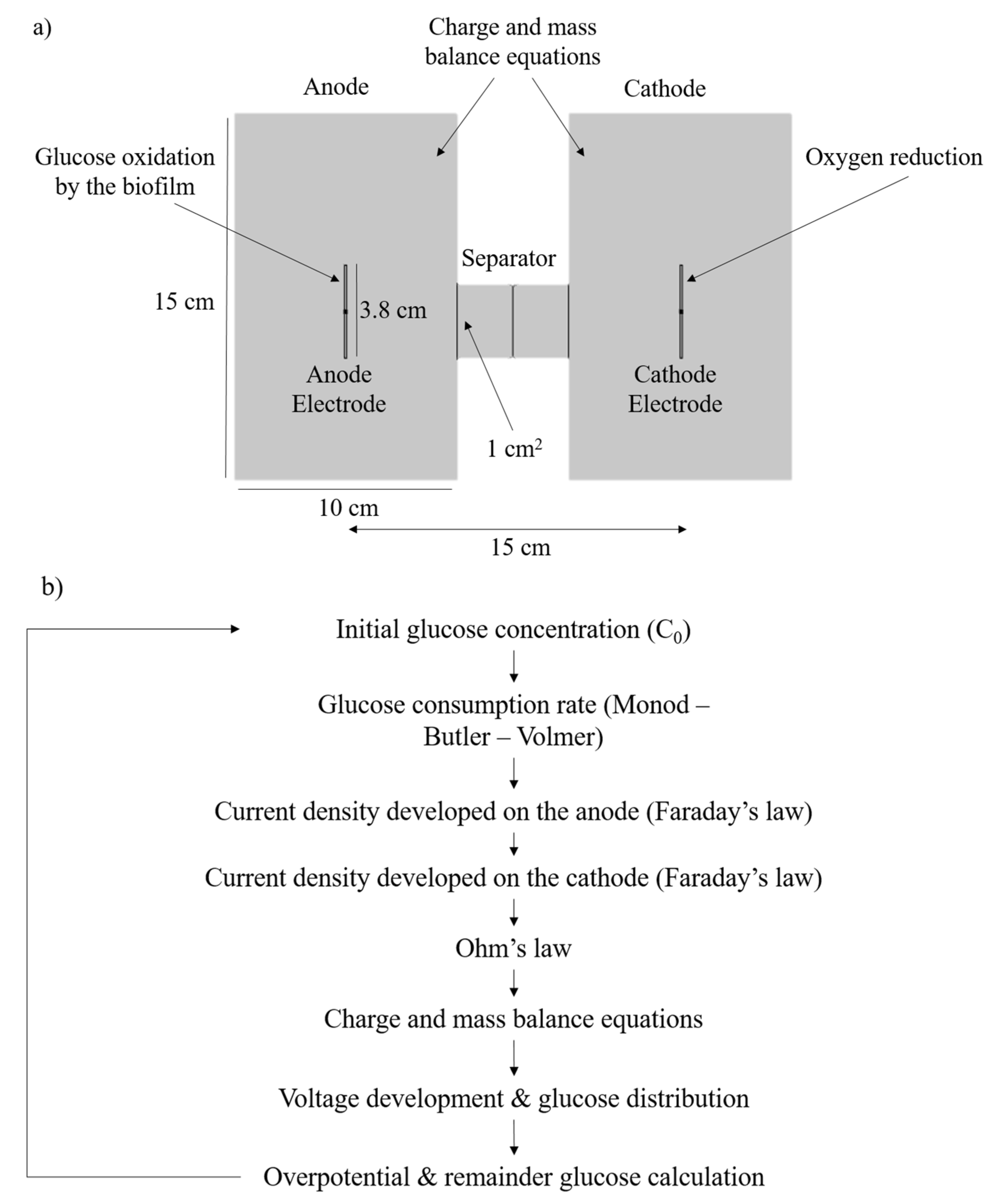
2.1. Experimental Setup
2.2. Model Description
2.3. Mass and Charge Transfer Equations
3. Results
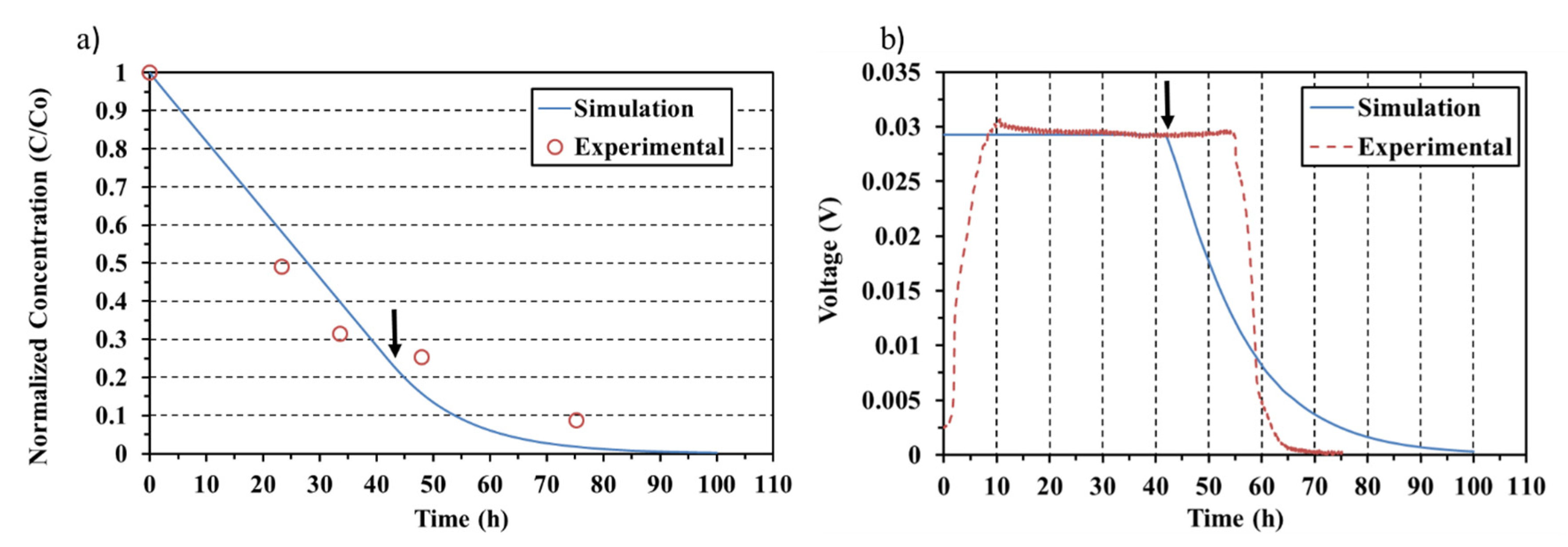
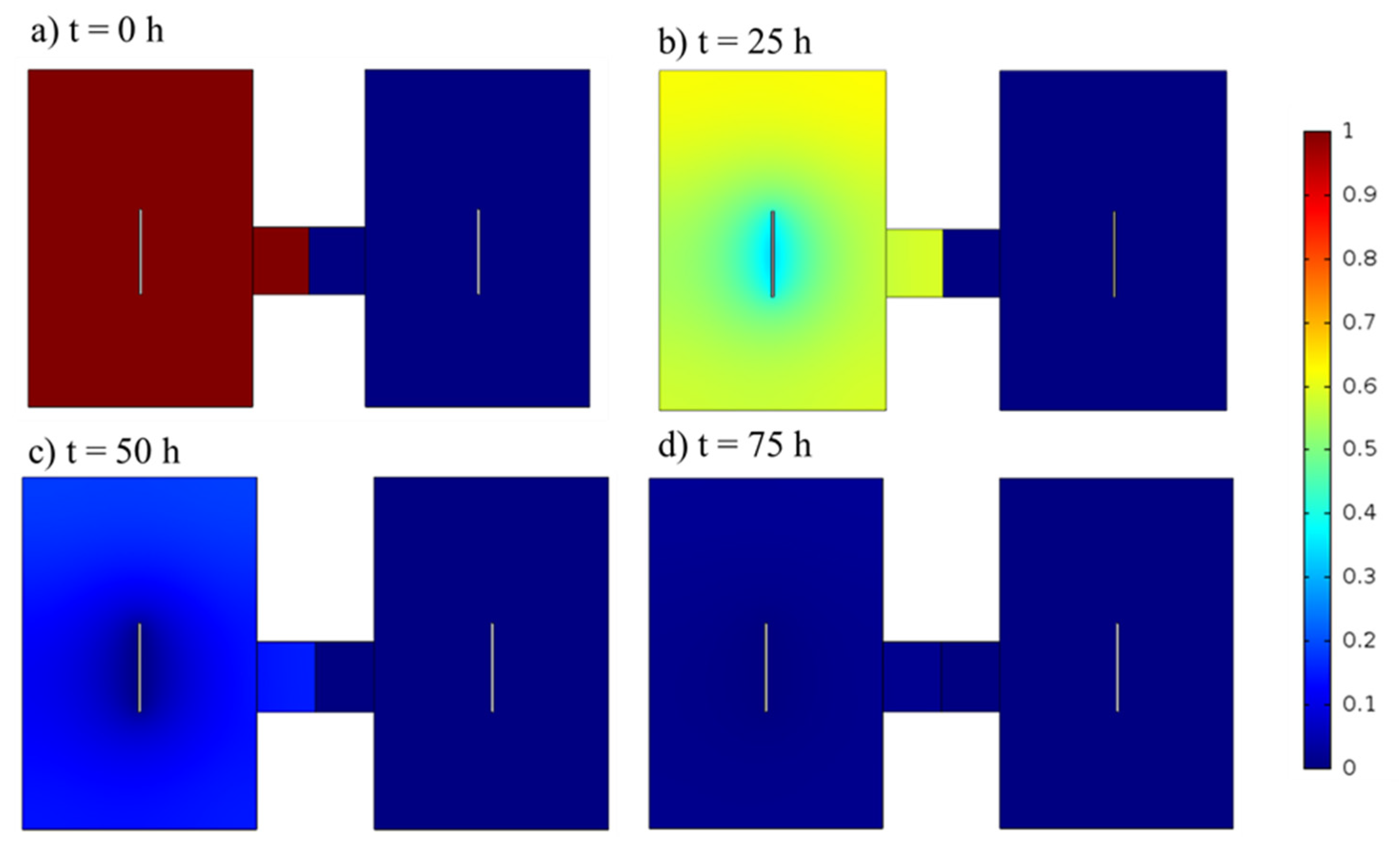
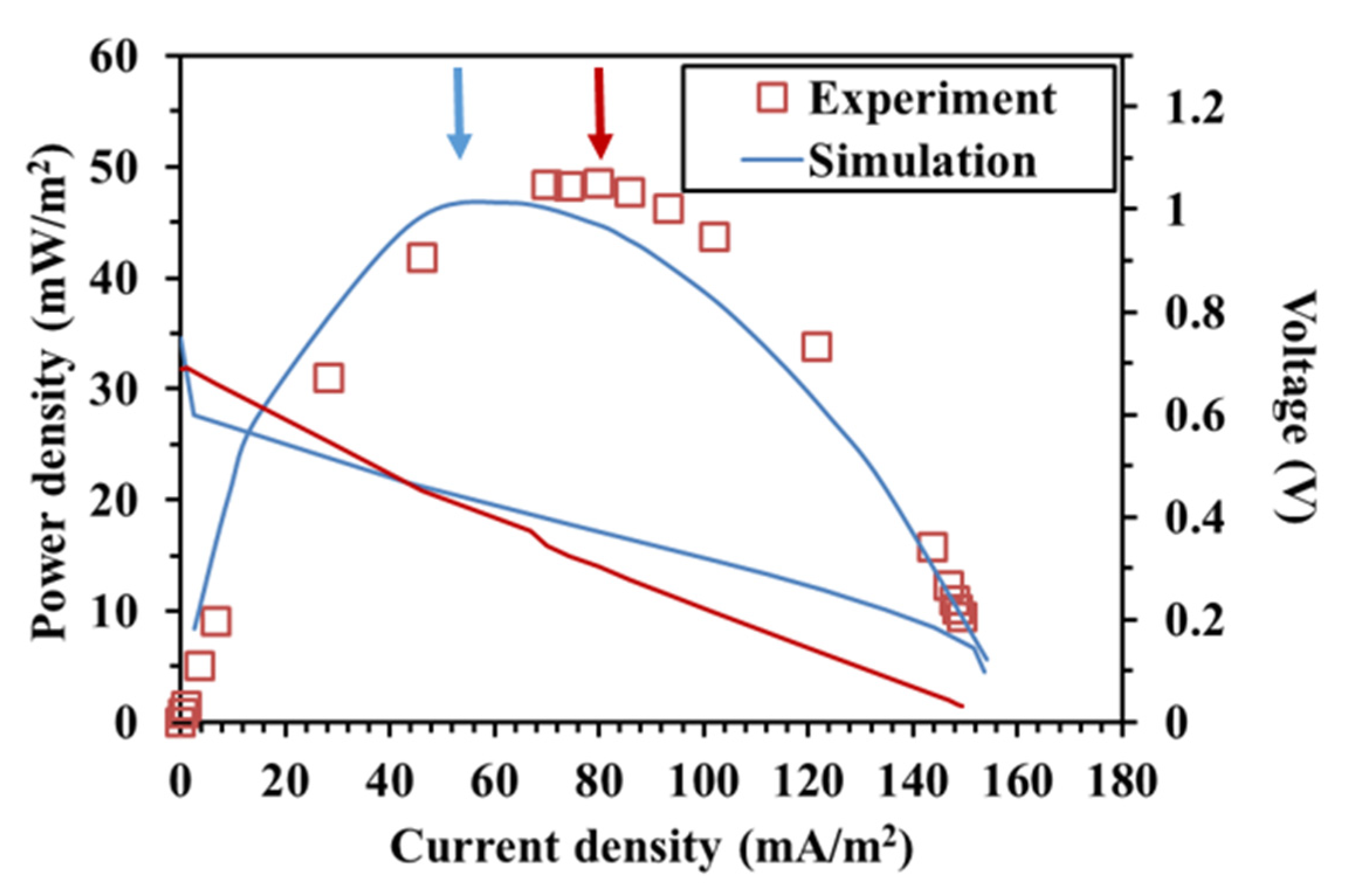
3.1. Model Results and Validation
| Symbol | Description | Unit | Value | Source |
|---|---|---|---|---|
| Maximum specific growth rate | mol m−2 h−1 | 6 × 10−3 | Calculated by Aquasim model [28] | |
| Half–velocity rate constant for glucose | mol m−3 | 3 × 10−4 | ||
| Anode transfer coefficient | – | 0.05 | ||
| Forward rate constant of cathode reaction | m12 mol−4 h−1 | 9.19 × 10−5 | ||
| Half–velocity rate constant for oxygen | mol m−3 | 4 × 10−3 | ||
| Cathode transfer coefficient | – | 0.7 | ||
| Anode Capacitance | F m−2 | 13,721 | ||
| Cathode Capacitance | F m−2 | 500 | ||
| Glucose inhibition constant | mol m−3 | 37 × 10−3 | ||
| F | Faraday’s constant | Coulombs mol−1 | 96,485 | [29] |
| R | Gas constant | J mol−1 K−1 | 8.31 | |
| Dissolved oxygen concentration in the cathode chamber | mol m−3 | 0.3125 | [30] | |
| Glucose diffusion coefficient | m2 s−1 | 0.5 × 10−9 | ||
| Electrolyte conductivity | S m−1 | 1.2 | Experimental values | |
| Electrode conductivity | S m−1 | 10 | ||
| Open Circuit Voltage | V | 0.75 | ||
| Electrode surface | cm2 | 19 | ||
| Separator surface | cm2 | 1 | ||
| Electrode distance | cm | 17 |
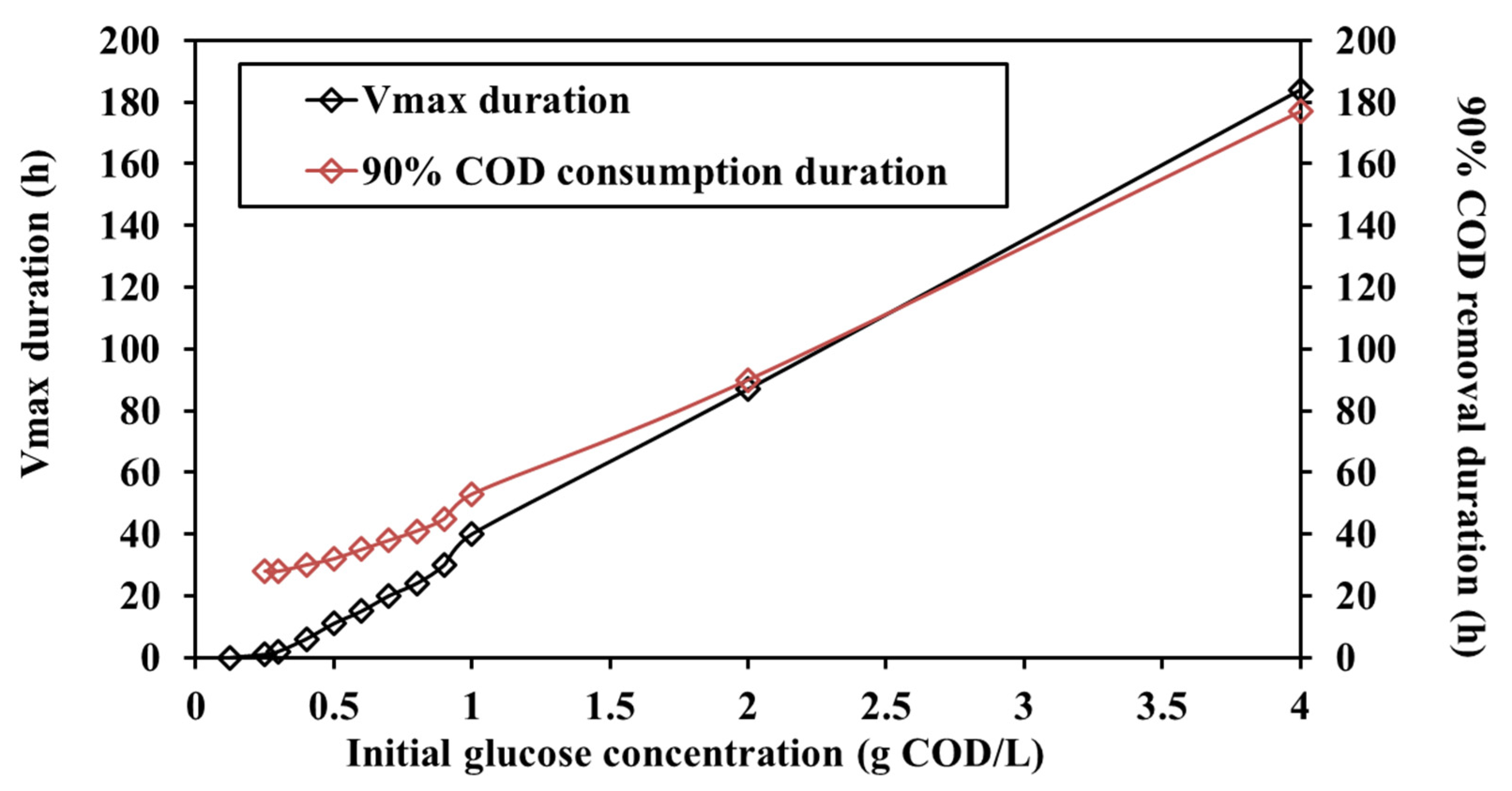
3.2. Different Initial Substrate Concentrations
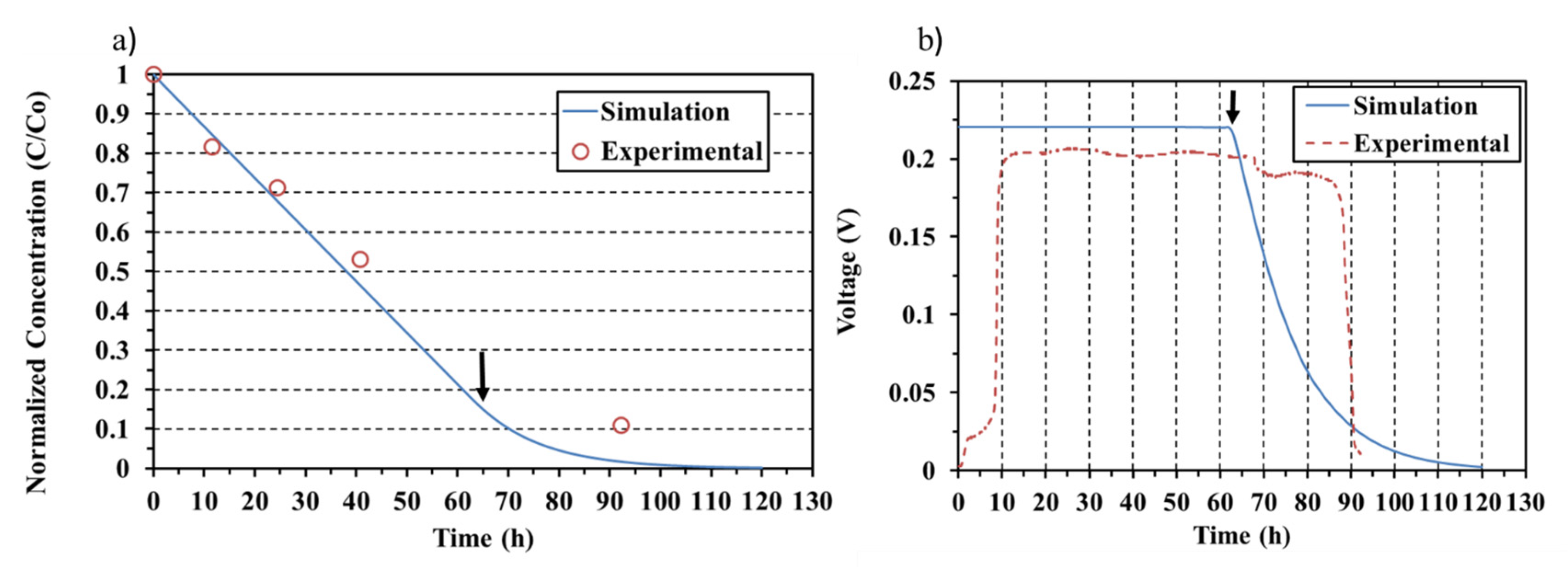
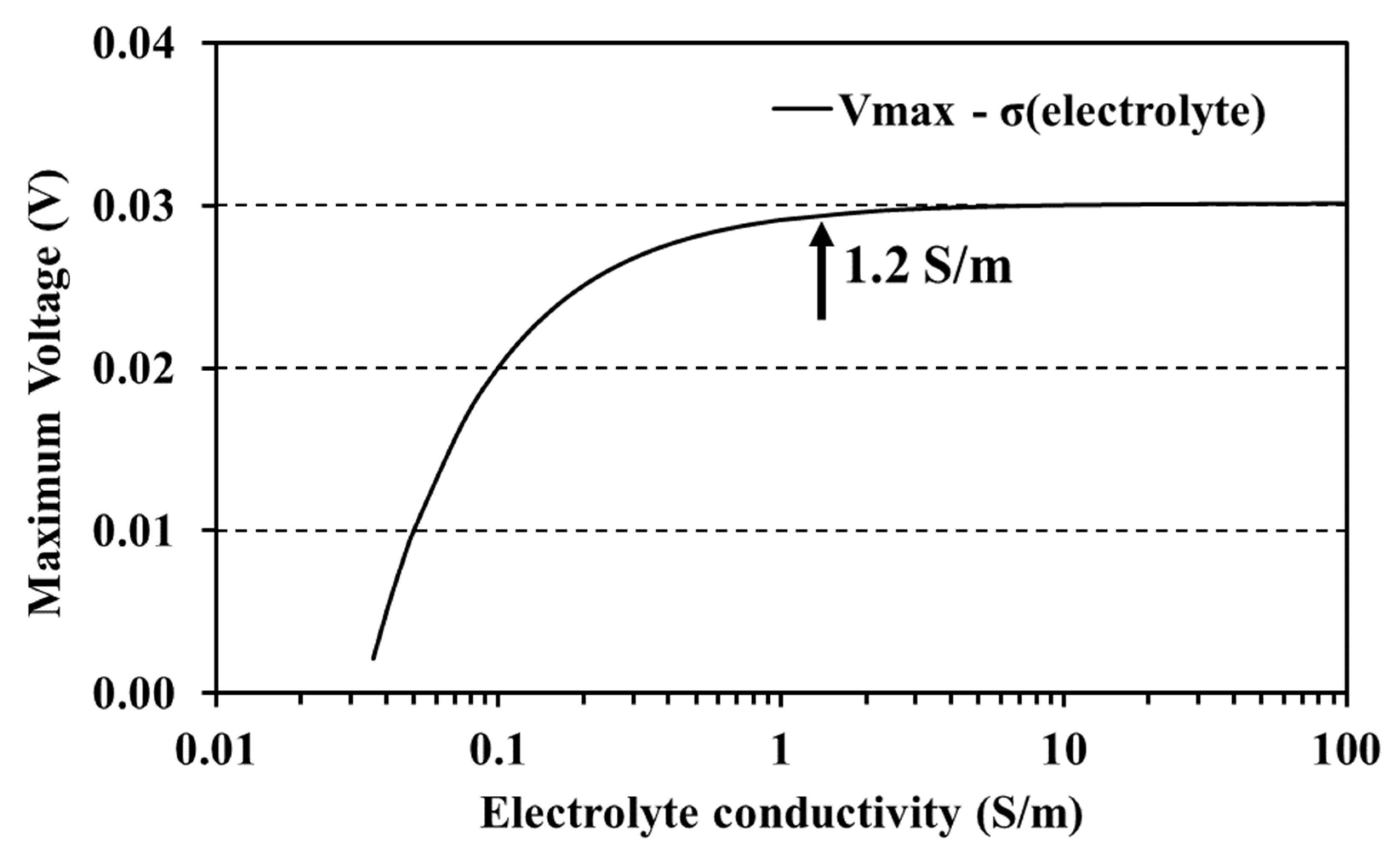
3.3. Different Initial Electrolyte Conductivities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Benneton, X.D.; Strik, D.P.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Afsharian, Y.P.; Rahimnejad, M. Functional dynamics of microbial communities in bioelectrochemical systems: The importance of eco-electrogenic treatment of complex substrates. Curr. Opin. Electrochem. 2022, 31, 100816. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.A.; Henrich, A.W. Engineering microbial electrocatalysis for chemical and fuel production. Curr. Opin. Biotechnol. 2014, 29, 93–98. [Google Scholar] [CrossRef]
- Madiraju, K.S.; Lyew, D.; Kok, R.; Raghavan, V. Carbon neutral electricity production by Synechocystis sp. PCC6803 in a microbial fuel cell. Bioresour. Technol. 2012, 110, 214–218. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Seelam, J.S.; Vanbroekhoven, K.; Pant, D. An enriched electroactive homoacetogenic biocathode for the microbial electrosynthesis of acetate through carbon dioxide reduction. Faraday Discuss. 2015, 183, 445–462. [Google Scholar] [CrossRef]
- Imoro, A.Z.; Mensah, M.; Buamah, R. Developments in the microbial desalination cell technology: A review. Water-Energy Nexus 2021, 4, 76–87. [Google Scholar] [CrossRef]
- Minteer, S.D.; Liaw, B.Y.; Cooney, M.J. Enzyme-based biofuel cells. Curr. Opin. Biotechnol. 2007, 18, 228–234. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef] [Green Version]
- Kundu, A.; Sahu, J.N.; Redzwan, G.; Hashim, M.A. An overview of cathode material and catalysts suitable for generating hydrogen in microbial electrolysis cell. Int. J. Hydrogen Energy 2013, 38, 1745–1757. [Google Scholar] [CrossRef]
- Mohan, S.V.; Mohanakrishna, G.; Velvizhi, G.; Babu, V.L.; Sarma, P. Bio-catalyzed electrochemical treatment of real field dairy wastewater with simultaneous power generation. Biochem. Eng. J. 2010, 51, 32–39. [Google Scholar] [CrossRef]
- Rabaey, K.; Angenent, L.; Schröder, U.; Keller, J. Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application, 1st ed.; IWA Publishing: London, UK, 2010; pp. 401–430. ISBN 9781843392330. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, D.A.; Carmona-Martínez, A.A.; Chendake, A.D.; Pandit, S.; Pant, D. Modeling and optimization strategies towards performance enhancement of microbial fuel cells. Bioresour. Technol. 2021, 320, 124256. [Google Scholar] [CrossRef] [PubMed]
- Recio-Garrido, D.; Perrier, M.; Tartakovsky, B. Modeling, optimization and control of bioelectrochemical systems. Chem. Eng. J. 2016, 289, 180–190. [Google Scholar] [CrossRef]
- Zhang, X.; Halme, A. Modelling of a Microbial Fuel Cell Process Xia-Chang Zhang and Aarne Halme Automation Technology Laboratory; Helsinki University of Technology: Espoo, Finland, 1995; Volume 17, pp. 809–814. [Google Scholar]
- Zeng, Y.; Choo, Y.F.; Kim, B.-H.; Wu, P. Modelling and simulation of two-chamber microbial fuel cell. J. Power Sources 2010, 195, 79–89. [Google Scholar] [CrossRef]
- Belleville, P.; Merlin, G.; Ramousse, J.; Deseure, J. Two-dimensional modelling of syntrophic glucose conversion in bioanodes for coulombic efficiency optimization. Bioresour. Technol. Rep. 2019, 6, 15–25. [Google Scholar] [CrossRef]
- Serra, P.; Espírito-Santo, A.; Magrinho, M. A steady-state electrical model of a microbial fuel cell through multiple-cycle polarization curves. Renew. Sustain. Energy Rev. 2020, 117, 109439. [Google Scholar] [CrossRef]
- Day, J.R.; Heidrich, E.S.; Wood, T.S. A scalable model of fluid flow, substrate removal and current production in microbial fuel cells. Chemosphere 2021, 291, 132686. [Google Scholar] [CrossRef]
- Matsena, M.T.; Chirwa, E.M. Hexavalent chromium-reducing microbial fuel cell modeling using integrated Monod kinetics and Butler-Volmer equation. Fuel 2022, 312, 122834. [Google Scholar] [CrossRef]
- Sindhuja, M.; Kumar, N.S.; Sudha, V.; Harinipriya, S. Equivalent circuit modeling of microbial fuel cells using impedance spectroscopy. J. Energy Storage 2016, 7, 136–146. [Google Scholar] [CrossRef]
- Oliveira, V.; Simões, M.; Melo, L.; Pinto, A. A 1D mathematical model for a microbial fuel cell. Energy 2013, 61, 463–471. [Google Scholar] [CrossRef]
- Skiadas, I.V.; Lyberatos, G. The periodic anaerobic baffled reactor. Water Sci. Technol. 1998, 38, 401–408. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewateri, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; ISBN 978-0875532356. [Google Scholar]
- Al-Baghdadi, M.A.S. Modelling of proton exchange membrane fuel cell performance based on semi-empirical equations. Renew. Energy 2005, 30, 1587–1599. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Logan, B.E.; Murano, C.; Scott, K.; Gray, N.; Head, I. Electricity generation from cysteine in a microbial fuel cell. Water Res. 2005, 39, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Tremouli, A. Development of an Innovative Single Chamber Microbial Fuel Cell for Wastewater Treatment. Ph.D. Thesis, University of Patras, School of Chemical Engineering, Patras, Greece, 2013. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics: A Read-Reference Book of Chemical and Physical Data, 82; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.; Siegrist, H.A.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
| Component | Concentration (mg/L) |
|---|---|
| Solution A | |
| CaCl2·2H2O | 22,500 |
| NH4Cl | 35,900 |
| MgCl2·2H2O | 16,200 |
| KCl | 117,000 |
| MnCl2·4H2O | 1800 |
| CoCl2·6H2O | 2700 |
| H3BO3 | 513 |
| CuCl2·2H2O | 243 |
| Na2MoO4·2H2O | 230 |
| ZnCl2 | 189 |
| NiCl2·6H2O | 200 |
| H2WO4 | 10 |
| Solution B | |
| FeSO4 | 700 |
| Solution C | |
| (NH4)2PO4 | 7210 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamperidis, T.; Tremouli, A.; Peppas, A.; Lyberatos, G. A 2D Modelling Approach for Predicting the Response of a Two-Chamber Microbial Fuel Cell to Substrate Concentration and Electrolyte Conductivity Changes. Energies 2022, 15, 1412. https://doi.org/10.3390/en15041412
Kamperidis T, Tremouli A, Peppas A, Lyberatos G. A 2D Modelling Approach for Predicting the Response of a Two-Chamber Microbial Fuel Cell to Substrate Concentration and Electrolyte Conductivity Changes. Energies. 2022; 15(4):1412. https://doi.org/10.3390/en15041412
Chicago/Turabian StyleKamperidis, Theofilos, Asimina Tremouli, Antonis Peppas, and Gerasimos Lyberatos. 2022. "A 2D Modelling Approach for Predicting the Response of a Two-Chamber Microbial Fuel Cell to Substrate Concentration and Electrolyte Conductivity Changes" Energies 15, no. 4: 1412. https://doi.org/10.3390/en15041412
APA StyleKamperidis, T., Tremouli, A., Peppas, A., & Lyberatos, G. (2022). A 2D Modelling Approach for Predicting the Response of a Two-Chamber Microbial Fuel Cell to Substrate Concentration and Electrolyte Conductivity Changes. Energies, 15(4), 1412. https://doi.org/10.3390/en15041412








