Abstract
The purpose of the article was to conduct an in-depth literature review on the possibilities of managing combustion by-products (mainly fly ash) in the context of a closed-loop economy. First, information on the chemical composition of fly ash in Poland was collected and compared with the composition of fly ash in other European countries. The authors concentrated on describing methods for synthesizing geopolymers and zeolites using fly ash as a substrate. By-products of zeolite synthesis, which are strongly alkaline solutions, can be used as a substrate in the synthesis of geopolymers. A concept has been proposed to combine the synthesis of zeolites and geopolymers into a single process to close the material loop. The search for comprehensive technological solutions that take into account the ideas of a closed-loop economy is essential in an era of resource depletion, and this literature review encapsulates this topic area.
1. Introduction
Hard coal and lignite are raw materials characterised by a high elemental carbon content in their structure, which makes them hugely potent. Thermal processing of mine raw materials produces combustion by-products. They include bottom ash and fly ash, as well as boiler slag and residues from combustion gases desulfurization [1]. In addition to ash, obtained from the combustion of hard coal and lignite, millions of tons of combustion gases such as CO2, SO2, and NOx are emitted into the atmosphere. Other harmful substances produced by the energy sector include by-products of combustion [2,3]. It has been estimated recently that the annual output of fly ash derived from the coal and lignite sector will amount to 750 million tons [4].
Another source of fly ash is the municipal solid waste (MSW) thermal treatment industry. The composition of fly ash, despite some similarities to fly ash from coal combustion, shows a different proportion of metal oxides [5]. The properties of fly ash from MSW combustion also makes it possible to use it as a substitute raw material in the manufacturing process. Nevertheless, it should be noted here that the amount of certain chemical compounds contained in fly ash prevents its use in this way. The pretreatment of these compounds is required, which does not necessarily involve a large financial investment [6].
Unfortunately, as with the combustion of coal and lignite, the thermal treatment of MSW produces millions of tons of fly ash, which exhibits high toxicity. Among these toxic products is the emission of dioxins and furans, as well as polychlorinated biphenyls into the atmosphere. They are generated as a result of improper thermal processing of municipal waste. These chemical compounds belong to the group of halogenated aromatic hydrocarbons containing a chlorine atom in their molecule. As fly ash exhibits a diverse chemical composition and its particle size is nonuniform, it can freely enter the atmosphere and/or biosphere. Not only would improper storage truly lead to air contamination, but it would also cause water and soil contamination [7,8,9]. Fly ash is characterised by a rich and varied crystalline structure; its physicochemical properties are strictly dependent on the amount of a particular oxide whose content in the fly ash is the main component. One way to analyse and group fly ash is its division according to the amount of silicon oxide, aluminium oxide, and calcium oxide. Fly ash is categorized into: silicate ash, aluminium ash, and calcium ash [10]. Fly ash with a high content of silicon and aluminium compounds (mainly their oxides), exhibit very extensively developed pozzolanic properties, making it possible to combine with lime water, because it is possible to obtain silicate compounds and calcium aluminates [11].
Because of the rich crystalline phase of fly ash, it is possible to use it as a raw material in various chemical processes. The use of fly ash as a new material fits perfectly with the idea of a circular economy (CE). Given that the average use of fly ash worldwide is only 16%, manufacturers of construction materials (cement) and also the chemical industry producing sorbent materials are increasingly attempting to initiate combustion by-products in their plants [12]. Nowadays, consumers are increasingly paying attention to products that are recycled. In doing so, they are guided not only by concern for the health of themselves and their relatives but also by a focus on protecting the environment. Firstly, in order to successfully implement a circular economy, it is necessary to abandon classical linear production [13]. This provides companies with legal security while complying with national regulations, but it also brings greater financial benefits. The Corporate Sustainability Reporting Directive (CSRD) includes a review of companies demonstrating sustainable development practises [14].
Sustainability and the idea of a circular economy aim to reduce the quantity of natural resources used as much as possible by replacing them with waste materials. The rich chemical composition of combustion by-products, including fly ash (FA), enables their use in the synthesis of geopolymers and zeolites. Chemical activation of fly ash with alkaline solutions makes it possible to obtain new materials with improved sorption capacity and mechanical strength. Fly ash, because of its diverse crystalline composition, exhibits many chemical and physical properties, so it is possible to reuse it in the production cycle as a raw material and/or substrate. As mentioned above, the idea of a circular economy is to use industrial waste as much as possible, minimising the use of natural resources, e.g., metakaolin, from which geopolymers were originally synthesised [15,16].
Geopolymers are inorganic amorphous chemical compounds that can be synthesised either from mineral resources (mine waste, clays) or from industrial waste: fly ash, blast furnace slag, and sludge from water treatment processes. Geopolymers are made up of long chains: silicon and aluminium oxide copolymers and metal cations (Na+, K+), which play a stabilising role in the structure of the geopolymer. In the synthesis of geopolymers, the most important factor that is responsible for the crystalline structure of the geopolymer is the Si/Al ratio [17]. Another possibility for fly ash usage is the zeolite synthesis process. These are minerals found in the environment naturally, whose crystal structure is mainly based on hydrated alkali elemental aluminosilicates with a large number of micropores. Zeolites are widely used as sorbents, due to their high ability to exchange ions and sorption properties [18,19]. The geopolymerization process consists of the following steps: dissolution of silicon and aluminium compounds in a strongly alkaline solution, diffusion of the dissolved substances, polycondensation, and hardening of the gel phase. The zeolite synthesis process is very similar to the geopolymerization process; however, it consists of three steps: dissolution of silicon compounds (aluminosilicate glass), formation of the aluminosilicate gel, and crystallisation of the zeolite [20,21].
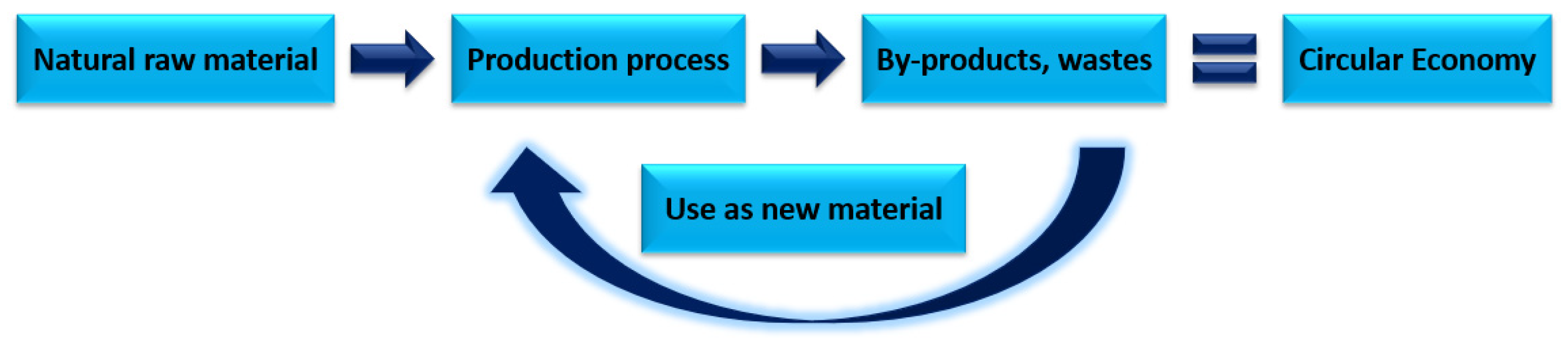
The aim of the article was to focus on the possibility of using fly ash in the context of a circular economy. The authors focused on an in-depth analysis of scientific journals on not only the characteristics of combustion by-products but also their reintroduction into the cycle. The concept of a closed-loop economy makes it possible to make the most out of waste materials not only from industries, but also from households as a new raw material which, due to its properties, can replace natural raw materials used in the chemical and construction industries Figure 1.
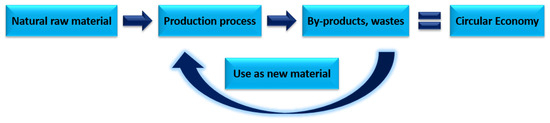
Figure 1.
Idea of circular economy (CE).
The high content of silicon and aluminium oxides allows FA to be used in the synthesis of geopolymers and zeolites as a substrate. The authors provided a broad review of the literature primarily concerning the use of fly ash in the synthesis of geopolymers and zeolites. The authors presented selected methods for the synthesis of geopolymers and zeolites. Since the idea of a circular economy is constantly being modernized, the authors propose to move away from the classic linear model of synthesizing zeolites and geopolymers using fly ash as much as possible, replacing it with a hybrid method.
2. Fly Ash—Production, Characteristics, Properties
2.1. Polish Fly Ash
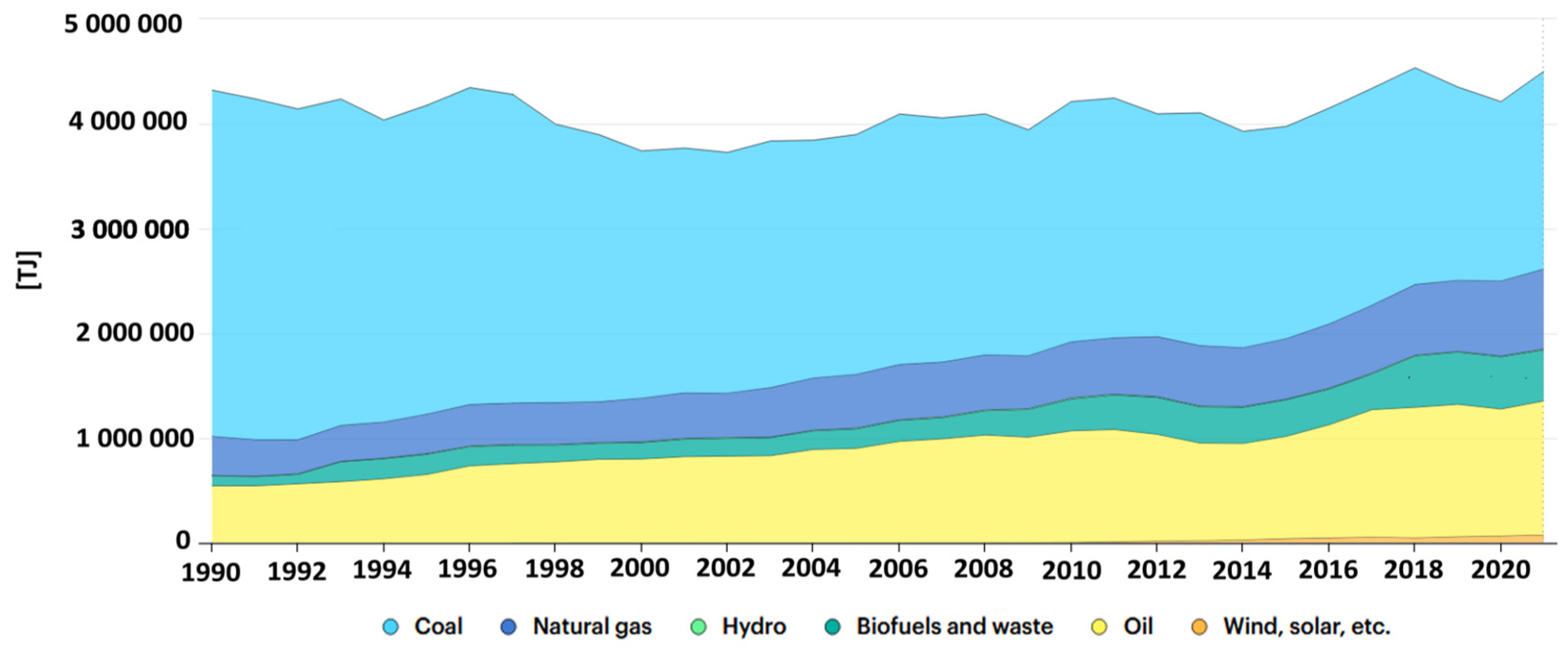
Despite many attempts to modernise the energy market in Poland, starting with renewable energy sources such as water, sun, wind, biomass, etc., the Polish energy market is still largely based on the generation of electricity and heat using hard coal and lignite (Figure 2). As a result of the combustion of fossil raw materials, the amount of inorganic matter increases. The by-products of combustion (including, to a large extent, fly ash) exhibit a diverse chemical composition that significantly affects their properties [22]. In addition to the chemical composition of the by-products of combustion (including fly ash), another characteristic of fly ash is its particle size granularity. According to the literature, fly ash granule size is mainly influenced by the combustion process temperature, the size of the feed fraction, and also the combustion technique. In the case of a fluidised bed reactor, the process temperature is between 1073 and 1323 K and the entire process is carried out at a pressure of 1–1.2 MPa. The fraction of fuel supplied is very fine (1–5 mm). Another combustion method is the use of a pulverised fuel boiler, the fuel processed consists of a very fine fraction, less than 0.1 mm, and the entire process is carried out at a temperature of 1473 to 1873 K, at a pressure of 2 to 8 MPa [23]. The method of classifying by-product is the size of their grain fraction, fly ash can be divided into three groups, based on grain size [24]:
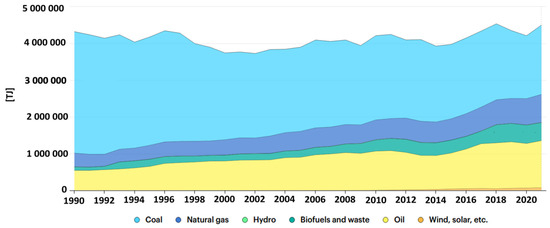
Figure 2.
Energy supply in Poland 1990–2020 (https://www.iea.org/countries/poland, accessed on 20 September 2023): Yellow—oil; green—biofuels and waste; dark blue—natural gas; blue—coal. (Source: IEA World Energy Balances 2022. All rights reserved).
- Fine-grained—where the grain size is <0.075 mm and its content is less than 25%;
- Medium-grained—where the grain size is <0.075 mm and the total content in the in the fraction is between 40 and 75%;
- Coarse-grained—the size of a single grain is <0.075 mm and the amount of particles of this size particle size does not exceed 40% of the total volume.
The Polish energy economy today relies on around 80% of its energy generation from hard coal. One of the end products of the thermal processing of coal is fly ash [25]. Other products of the combustion of energy raw materials include:
- Bottom ash—by-products of combustion, characterised by irregular grain shape and varying physical and chemical properties. They show very pozzulanic properties, high resistance to external forces. They contain a large amount of alkaline compounds in their structure and have a high hydrophilicity. They are mainly used in the mining, energy, and construction industries [26].
- Slag—the main chemical compounds forming the crystalline structure are silicon oxide and aluminium oxide. Slag can be divided into two varieties. The first includes unburned slag, its characteristic feature is its dark grey colour, and the carbon in it is only partially burnt, while the grains are glassy. The second variety of slag includes burnt slag, which has a brick-red colour. Coal firing results in a large amount of sinter; the grain fraction is relatively small. In addition to the enamel content, the substances included in the chemical composition of the slag are mullite crystals, fused quartz, anorthite, melilite, burnt clay rock and clayey ironstone, magnetite, and gypsum [27,28].
- Flue gases, the resulting by-products of combustion in the gaseous state. They include primarily CO2 and also SO2, NOx [29].
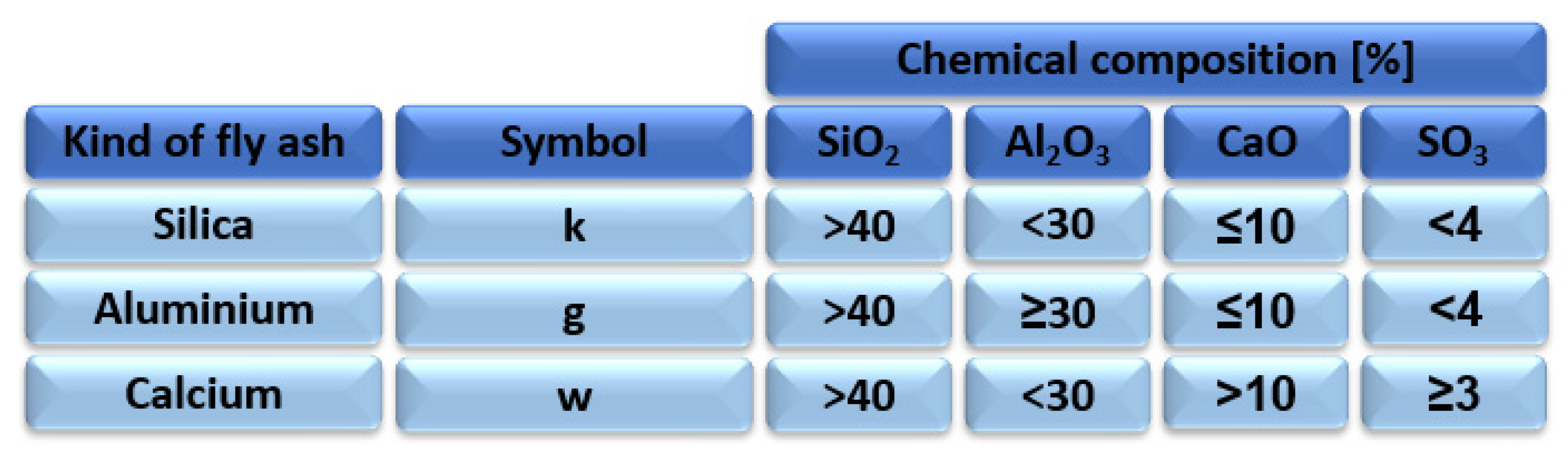
Due to the large production of fly ash in the energy sector, the idea of reusing fly ash in industry is constantly being improved. Since the fact that the development of fly ash as a raw material is restricted by the restrictions and regulations issued by the state authorities, fly ash is dumped in large amounts in heaps or landfills. Improper management of fly ash can lead to contamination of the atmosphere, earth, water, and air [30]. With regard to the restrictions in place, Poland has divided ash into three types Figure 3.
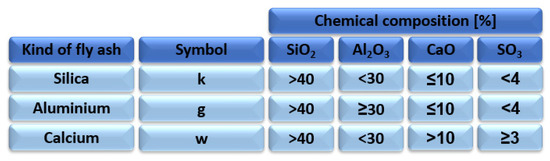
Figure 3.
Division of fly ash into categories depending on its chemical composition according to standard BN-79/6732.09 [31].
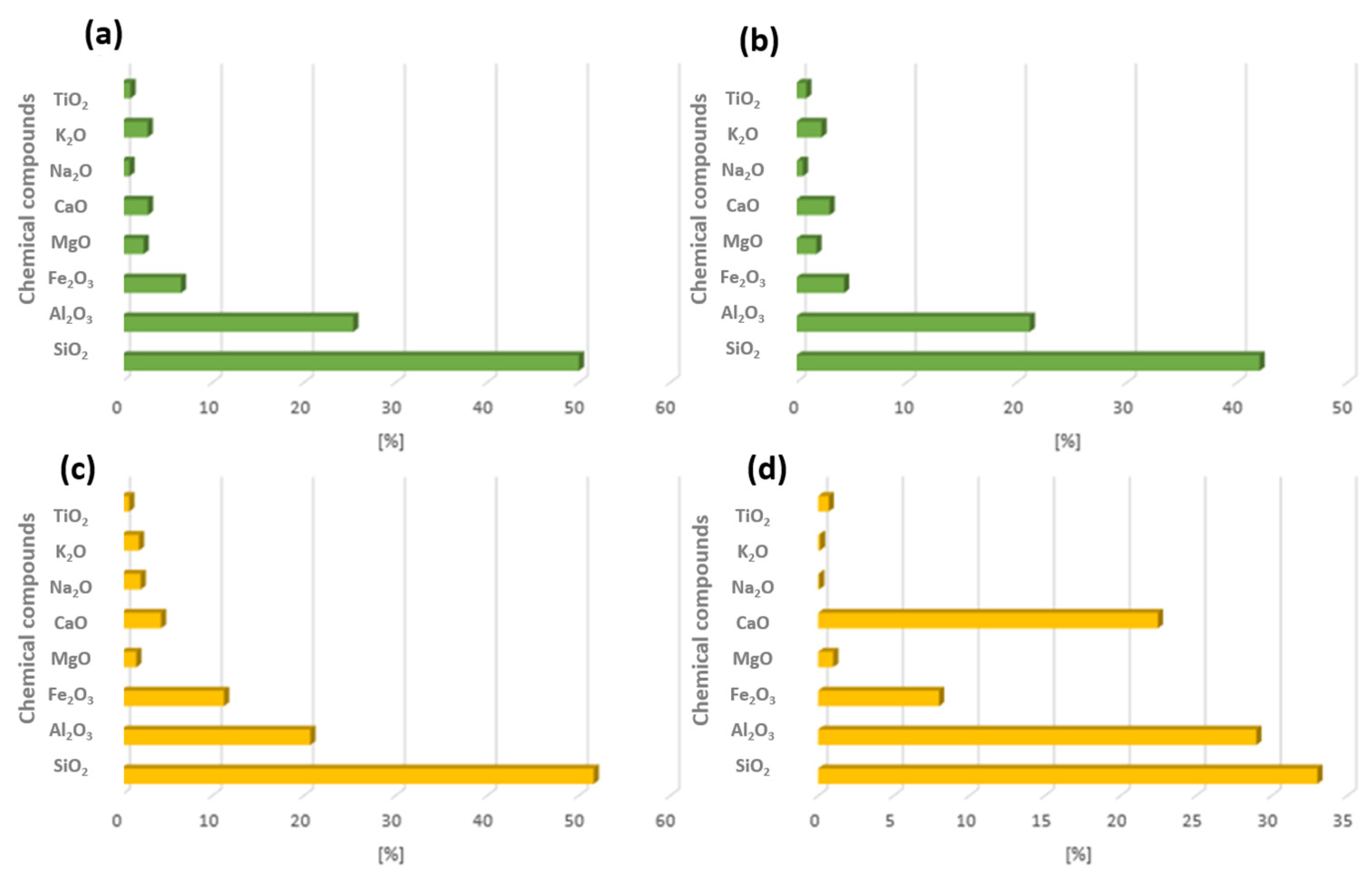
In the Polish energy sector, electricity and heat are obtained mainly from two raw materials: hard coal and lignite. Each of these raw materials has a different chemical structure, which significantly affects energy efficiency and also the composition of combustion by-products. The classification of fly ash can also be carried out by taking into account the raw material that has been burnt. Fly ash that is the product of hard coal combustion belongs to class F, whereas fly ash obtained from lignite coal belongs to group C (ASTM C-618) [32]. In the case of similar raw materials, the chemical composition of fly ash also varies [33]. The main components of fly ash from both hard coal and lignite include silicon oxide, aluminium oxide, iron oxide, and calcium oxide. Figure 4 shows the chemical composition of fly ash from several Polish power stations [22].
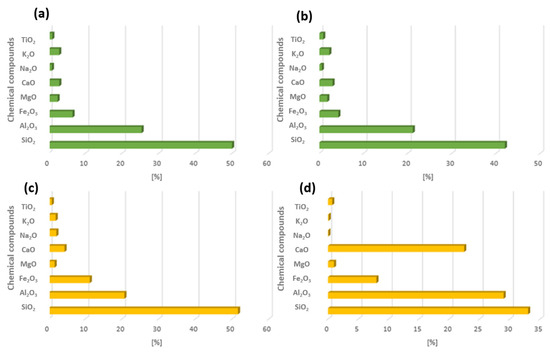
Figure 4.
Example of chemical composition from combustion of energy resources hard coal and lignite from Polish energy plants: (a) Chemical composition of FA from coal (1), (b) chemical composition of FA from coal (2), (c) chemical composition of FA from lignite (1), (d) chemical composition of FA from lignite (2) [22].
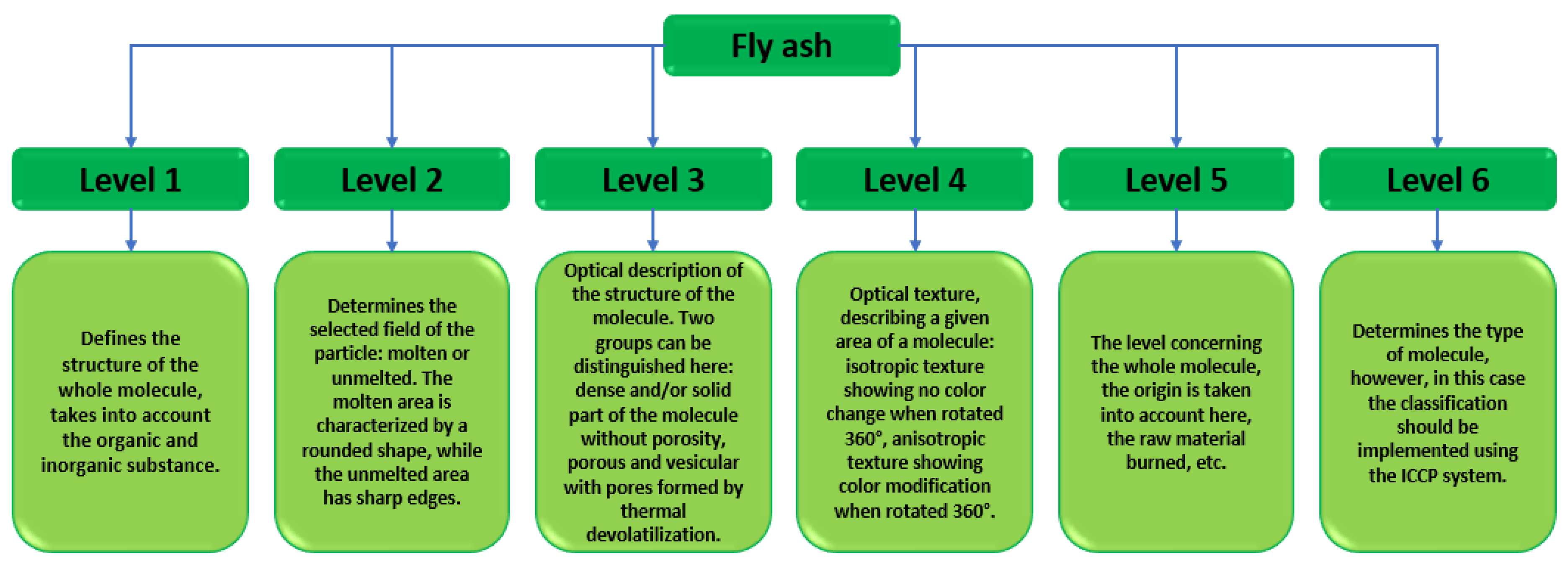
As a member of the European Union, Poland is subject to strict restrictions on the extraction and development of energy resources. In 2017, an announcement on critical raw material resources was introduced. The purpose of this document was a broad analysis of the amount of energy raw materials extracted. The main coal mining region in Poland is the Silesia Region and it is the most extensive mining area. The list of critical raw materials primarily aims to initiate the idea of a circular economy, which can be understood as the replacement of as many natural resources as possible, using industrial waste, including fly ash [34,35]. The classifications of fly ash are being upgraded at a rapid pace. The number of scientific publications devoted to fly ash is increasing each year. The authors have presented a study on fly ash classification taking into account multiple parameters ‘Identification and Petrographic Classification of Fly Ash Components Working Group, Commission III—ICCP’. In their research paper, the authors detailed the classification of fly ash into six levels: the first three define a given field in the fly ash particle, while the next three present the identification of the entire molecule Figure 5 [36].
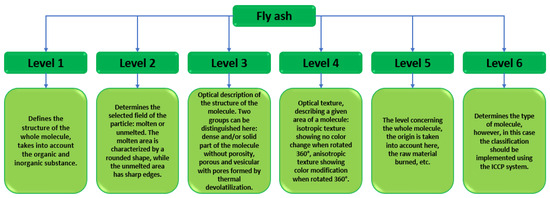
Figure 5.
Classification of fly ash according to the ICCP system [37].
Table 1 shows a partial qualitative and quantitative analysis of fly ash produced in Poland. According to previous comments, the main chemical compound that builds the crystalline structure is silicon oxide, and its average content in each fly ash is about 50%. The second chemical compound to be considered is aluminium oxide, its content ranges from 20 to 30%. These two chemical compounds make it possible to use the by-products of combustion that are fly ash for use in the synthesis of geopolymers and zeolites. Other important chemical compounds include calcium oxide. Its content in fly ash samples from hard coal does not exceed 10%; making it almost twice as low. Fly ash with a high calcium content shows very high hydraulic strength, so it can be used in construction as a substitute for cement, thereby reducing the cost of its production. Due to the high content of calcium compounds, construction materials show high mechanical strength and chemical resistance.

Table 1.
Chemical composition of fly ash from Polish power plants [31]. Adapted with permission from Ref. [31]. Copyright 2020, Elsevier.
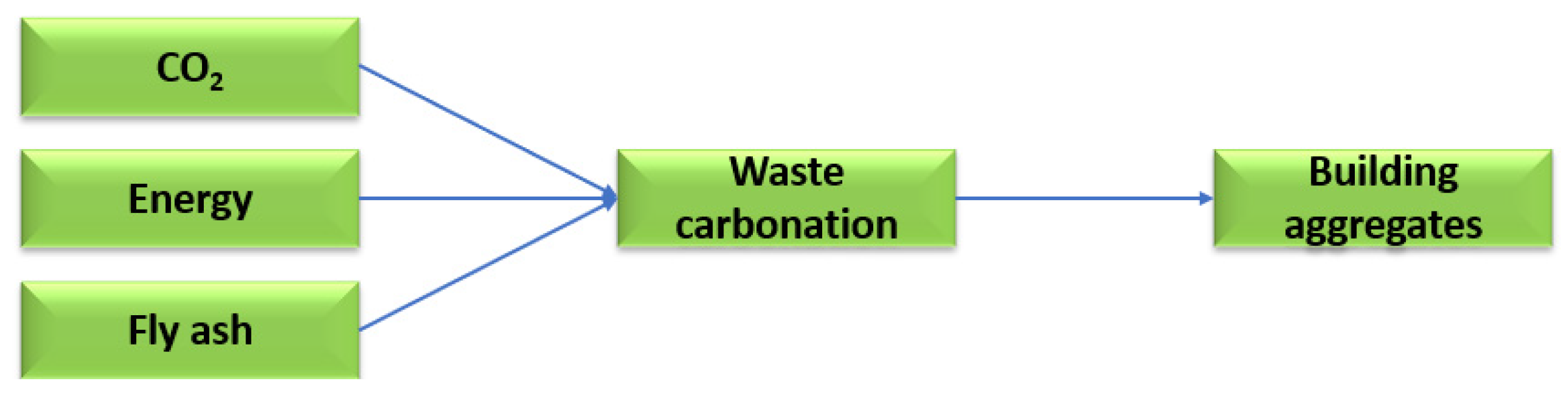
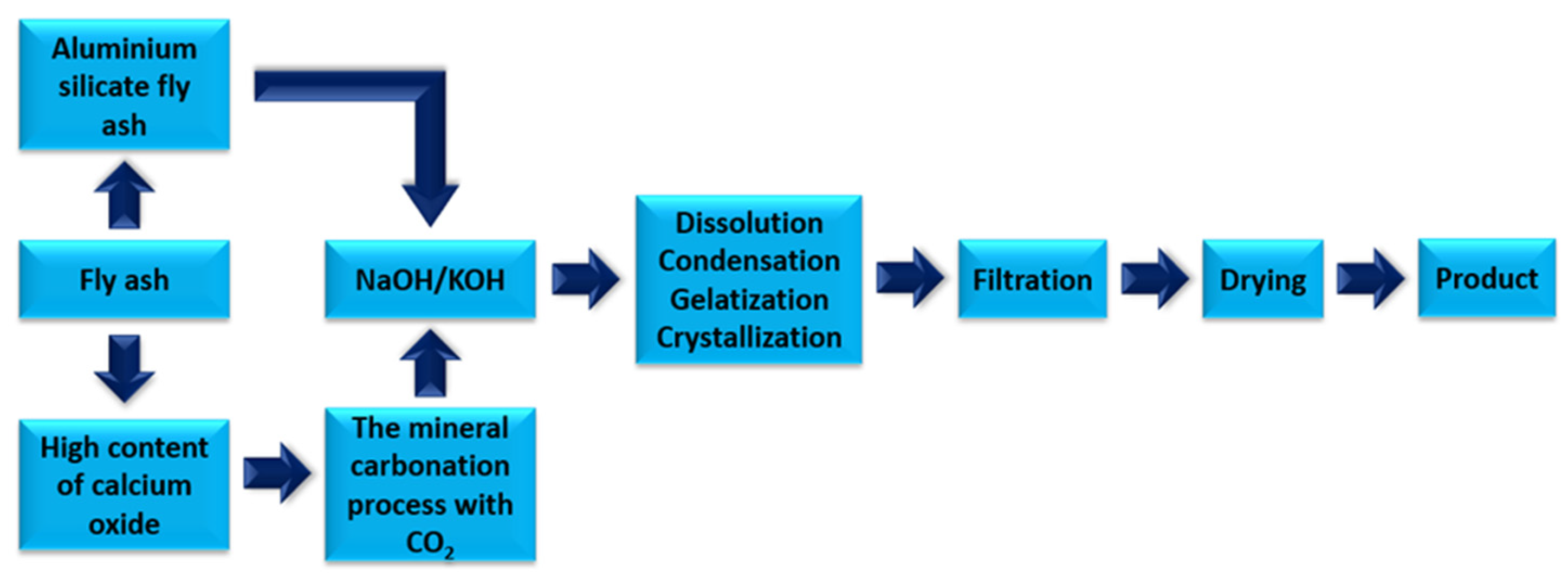
Technological solutions are being searched for to reduce the amount of calcium oxide in fly ash in favour of calcium carbonate, which is restricted both in Poland and in European Union countries. One such solution is the mineral carbonation process using carbon dioxide. The mineral carbonation process makes it possible to manage high-calcium fly ash as a raw material for the production of building materials. Carbon dioxide can be used in the mineral carbonation process to reduce calcium oxide in favour of low-energy carbonate molecules. Figure 6 shows a scheme for the saturation of waste from thermal treatment of energy raw materials with carbon dioxide [38].
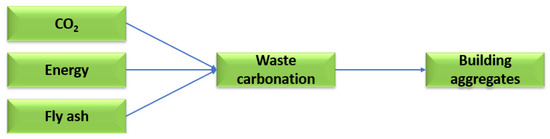
Figure 6.
The process of saturating high-calcium fly ash with carbon dioxide.
2.2. Energy Sector in Europe—Fly Ash
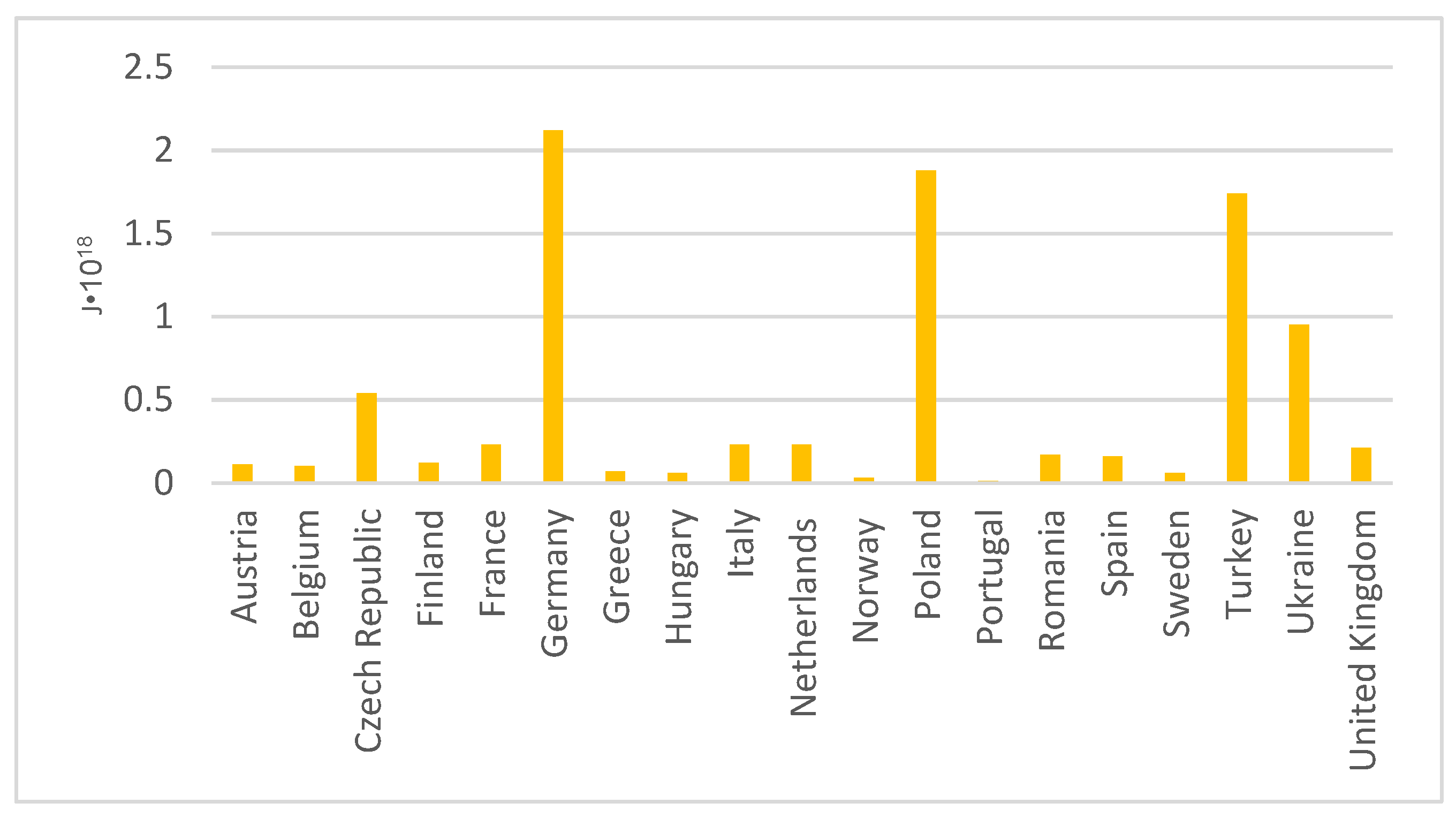
Greenhouse gas emissions and the production of combustion by-products in Europe have prompted attempts to modernise the energy market. As the energy sector is widely developed, there are many options for obtaining electricity in European Union countries. These include primary energy, natural gas, renewable energy sources, nuclear energy, and oil. Although there are so many different options available to countries in Europe, the power sector still uses coal. Figure 7 shows the amount of coal-fired energy generation in Europe [39].
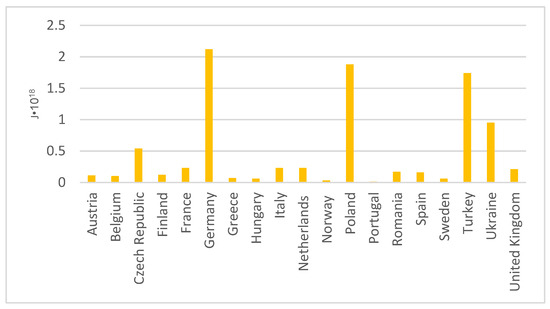
Figure 7.
Primary energy derived from hard coal in Europe.
The European Union’s goal is to achieve climate neutrality by 2050. The plans related to the European Green Deal include the development of modern environmental technologies as a priority in their activities. The Green Deal will not only improve the quality of the environment but will also bring many other benefits. Examples include creating more jobs, increasing company revenues, and being independent from other businesses [40]. The use of fly ash as a raw material for the production of building materials, e.g., cement, will make it possible to reduce the use of natural resources and thus reduce the costs of the entire process. Another positive aspect of using fly ash for the production of building materials is that it achieves zero carbon dioxide emissions. Fly ash shows very good pozzulanic properties. During the synthesis process, it reacts with the activating agent to form so-called C-S-H gels. In addition to their pozzolanic properties, fly ash also exhibits high hydraulic activity, especially fly ash from the HCFA group (high calcium fly ash). This fly ash is mainly used in the production of hydraulic binders. Plants using fly ash for cement production must comply with the applicable standards (PN-EN 197-1:2002/A3 2007 and PN-EN 450-1+A1:2009). In addition to the use of fly ash for cement production, it has found extensive use in: road building, mining, and land stabilisation and reclamation. The use of fly ash as an additive in the concrete production process reduces the amount of water required to produce a mix with a certain workability. The granulation of fly ash has a positive effect on the workability of the concrete, as its particles are so small that they easily lodge between the cement particles. Another positive aspect of using fly ash as a cement substitute is that it increases the mechanical strength of the concrete and its chemical resistance, to weather conditions like acid rains. The use of FA in the production of concrete reduces the rate and amount of energy released during the hydration process, thus avoiding cracks in the concrete [41,42,43].
Table 2 shows the content of selected metal oxides that form the crystalline structure of fly ash from European countries. It can be seen that the content of each oxide varies greatly from country to country. This is mainly due to the type of raw material used, the combustion temperature, and the technology. Fly ash must meet appropriate standards in order to be used in industry, the first of which is its silicon and aluminium oxide content. The silica content is usually between 40 and 60%, but in the case of fly ash from Germany and Greece, it varies greatly, from 20 to even 80%. The second oxide that plays a significant role in both the synthesis of geopolymers and zeolites is alumina. It is mainly in the range of 10 to 30%. The compound that prevents fly ash from being used as a substrate is calcium oxide. According to accepted European restrictions, its presence in fly ash must not exceed certain values (according to the norm PN-EN 197-1). As some fly ash has a high calcium oxide content, e.g., in Poland, Turkey, and Bulgaria, initiatives are being taken to reduce this compound in favour of calcium carbonate, which is not harmful to the environment. Mineral carbonation is an example of this process.

Table 2.
Chemical composition of fly ash from Europe % wt. [44]. Adapted with permission from Ref. [44]. Copyright 2022, Elsevier.
3. The Process of the Synthesis of Geopolymers Using Different Fly Ashes
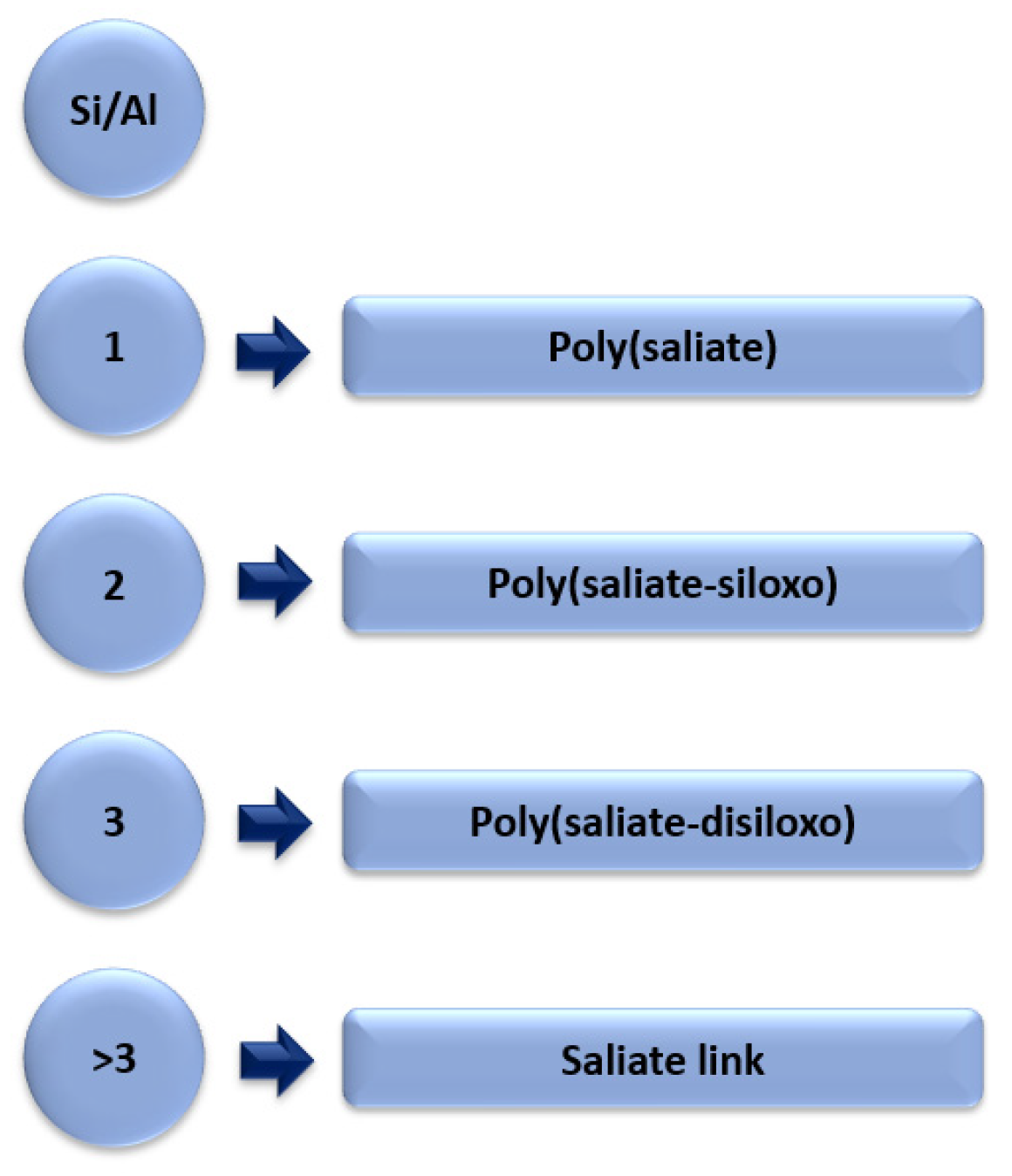
Geopolymers are inorganic aluminosilicate compounds. As previously mentioned, geopolymers were originally obtained from metakaolin. The synthetics geopolymers are obtained by chemicals activating fly ash together with an activating agent mainly NaOH [17]. Geopolymers are made up of long chains: silicon and aluminium oxide copolymers and metal cations (Na+, K+), which play a stabilising role in the structure of the geopolymer. In the synthesis of geopolymers, the most important factor that is responsible for the crystalline structure of the geopolymer is the Si/Al ratio. Figure 8 shows the Si/Al ratio and the different chains of aluminosilicate oligopolymers.
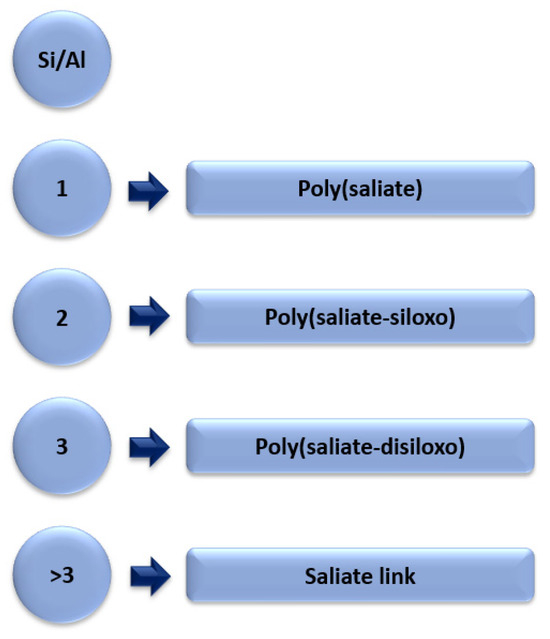
Figure 8.
Different Si/Al ratios in aluminosilicate oligopolymers, forming a geopolymer.
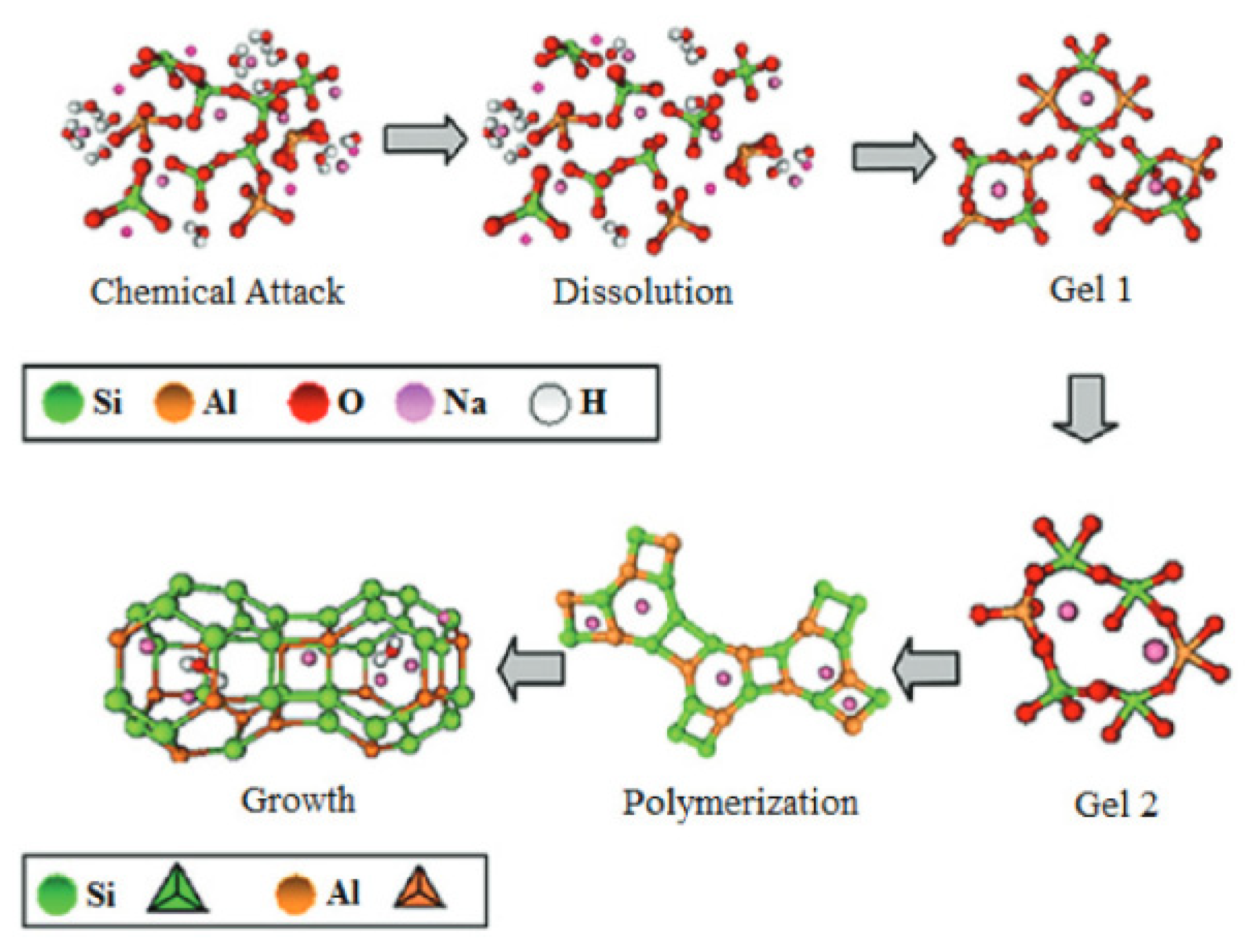
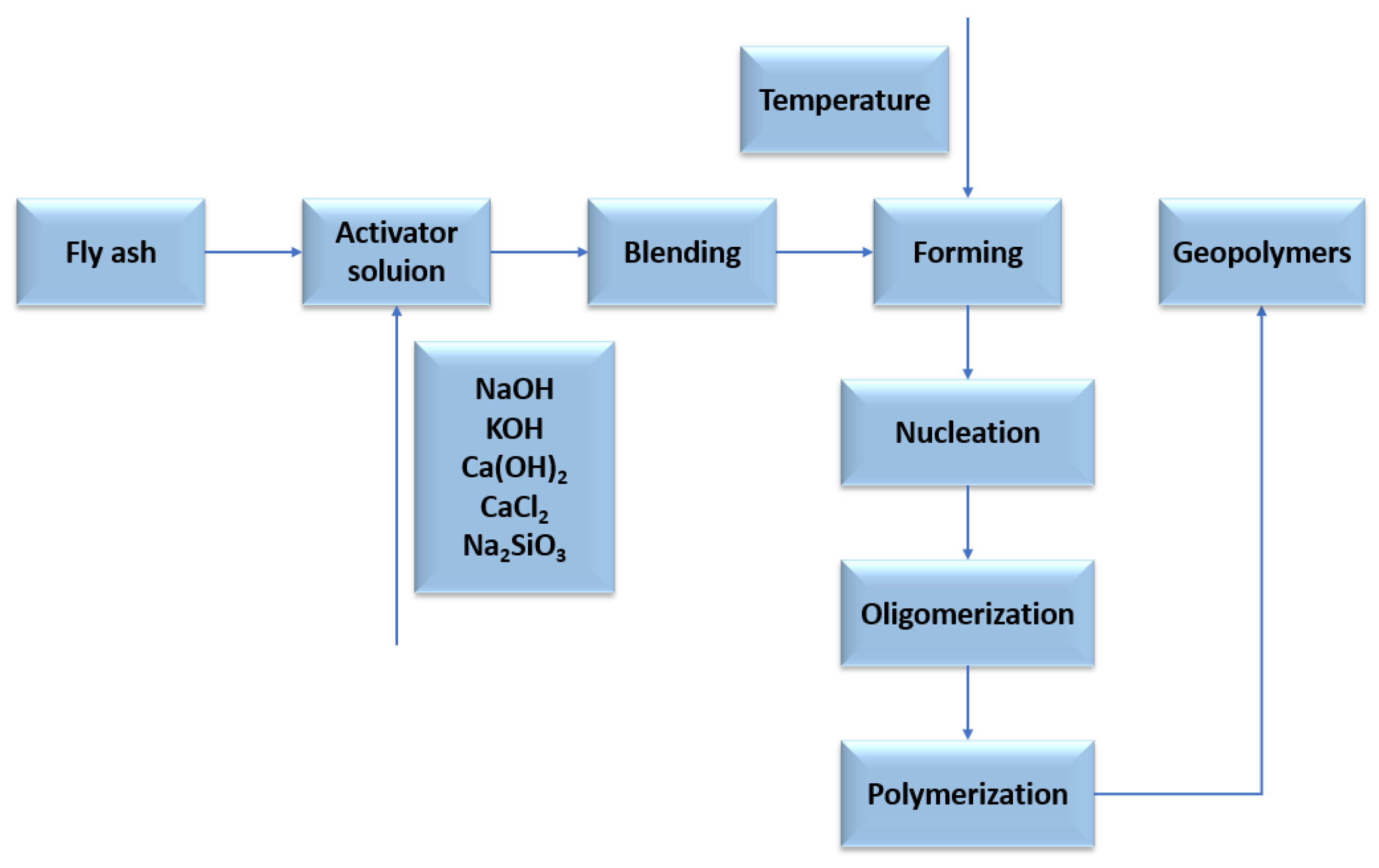
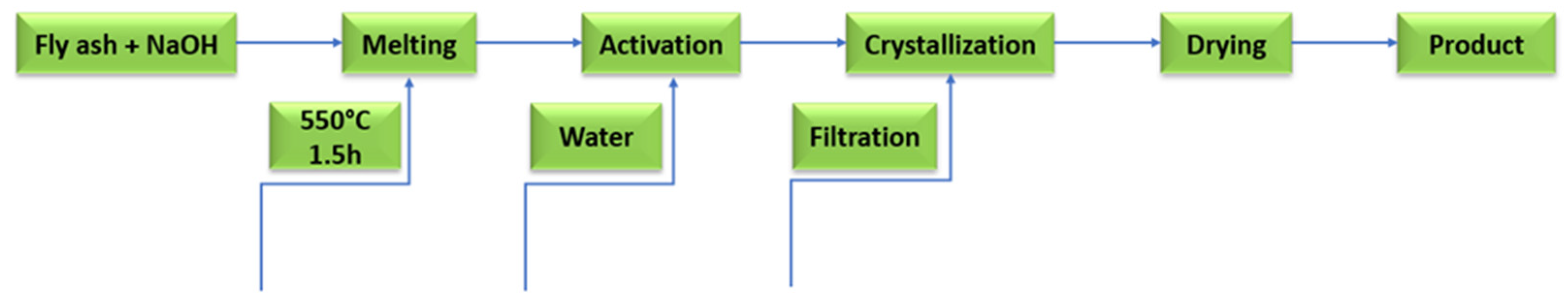
The geopolymerization process using FA or metakaolin involves complex and simultaneous steps in the formation of a three-dimensional amorphous geopolymer network. The dissolution of silicon and aluminium compounds depends primarily on: process kinetics, physicochemical, and thermodynamic parameters. The next step is reorganisation: crystalline networks of different sizes are formed, thus providing the physical properties of the geopolymer. The metal cations (silicon and aluminium) in the structure of the geopolymer gels are coordinated by oxygen bridges, resulting in the formation of a negatively charged ion, which is balanced by the metal cations (sodium and/or potassium) from the activator. An aqueous environment with high pH enables the geopolymerization process, in which reactive aluminosilicate compounds are dissolved and in the next stage (polycondensation) tetrahedra [SiO4]4− and [AlO4]5− combine with other ions [45,46]. Depending on the composition of the raw material used in the geopolymerization process, the end product can consist of different phases: amorphous, gel, etc. The chemical activation of fly ash containing large amounts of aluminosilicates proceeds quite differently from the hydration of classic Portland cement. The binding phase of the cement is calcium silicate hydrate (C-(A)-S-H) gel, while the chemical activation of fly ash results in sodium aluminosilicate hydrate as the main phase: N-A-S-H. The geopolymerization process is based on the hydrolysis–condensation of compounds rich in silicon and aluminum. A crystalline network is then formed, in whose pores water molecules are trapped. Aluminosilicate gels are obtained by the sol–gel process, spherical polymer units that are densely packed. The speed of the process depends primarily on the amount of water, or more precisely, its expulsion into larger pores, as a result of which the network is reorganized [47,48,49,50]. The process of geopolymer network formation is shown in Figure 9 and Figure 10. The crystal network of geopolymers is made up of an irregular aluminosilicate network. The sialate network is built between the [SiO4]4− and [AlO4]5− tetrahedra linked by oxygen atoms [51]. The geopolymerization process is based on the preparation of alumosilicate samples (e.g., metakaolin, clays, fly ash) with a well-defined Si/Al ratio and the preparation of aqueous solutions of activating agents (usually highly concentrated sodium hydroxide) [52,53,54]. The prepared paste is cast into special moulds used to shape the geopolymer and then heated for a period of 24 h at a temperature of approximately 25–70 °C [55].
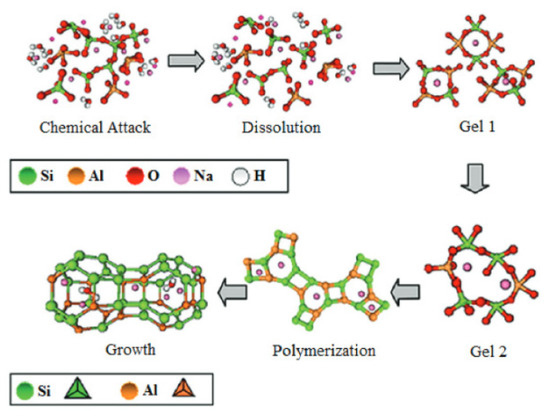
Figure 9.
Mechanism of the geopolymerization process [56].
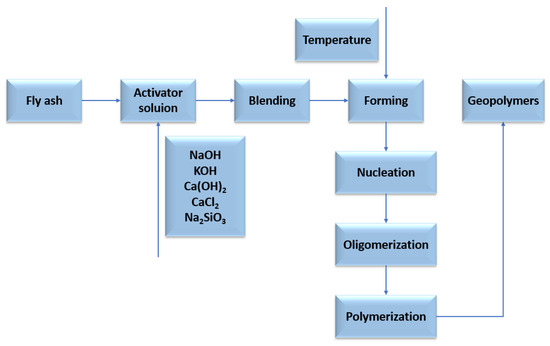
Figure 10.
Idea of obtaining geopolymers from fly ash with different activators.
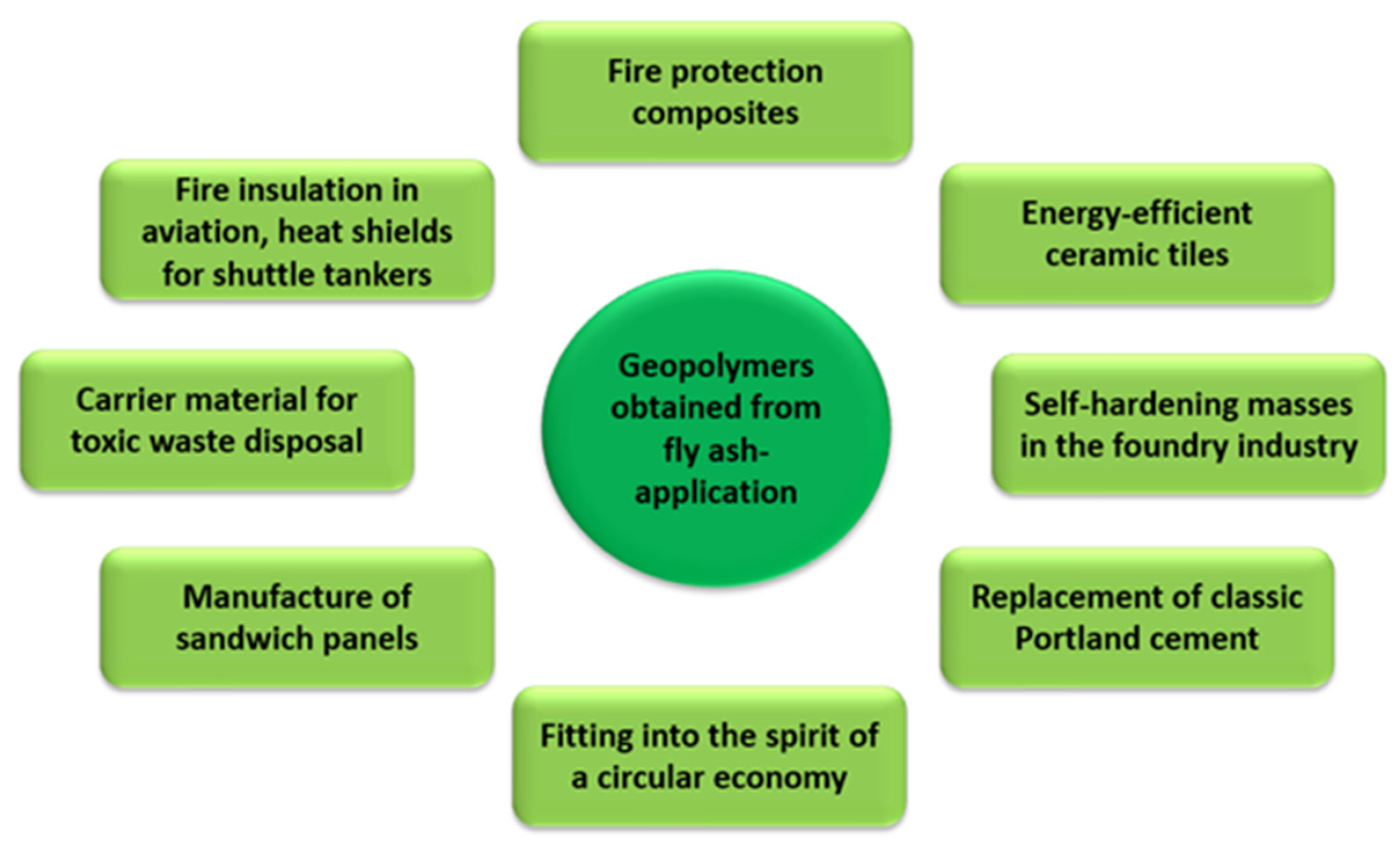
The use of fly ash in geopolymerization has many positive aspects for environmental engineering. Firstly, by using it as a substrate in the geopolymer synthesis process, the amount of landfilled fly ash from various industrial sectors is reduced. Geopolymers show properties far superior to those of classic Portland cement. Depending on the course of the geopolymerization process (the concentration of the activating agent, fly ash composition, and stabilising additives must be taken into account here), the compressive strength of geopolymers ranges from 25 to 100 MPa, while the flexural strength ranges from 5 to 25 MPa [57,58,59,60,61,62]. They are also highly resistant to acids, chlorine, and sulphur compounds. They are also resistant to weather conditions (high frost resistance) and high heat resistance of up to 800–900 °C. Figure 11 shows the possibilities of using geopolymers in industry.
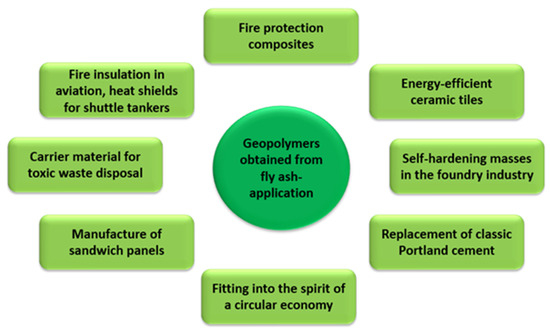
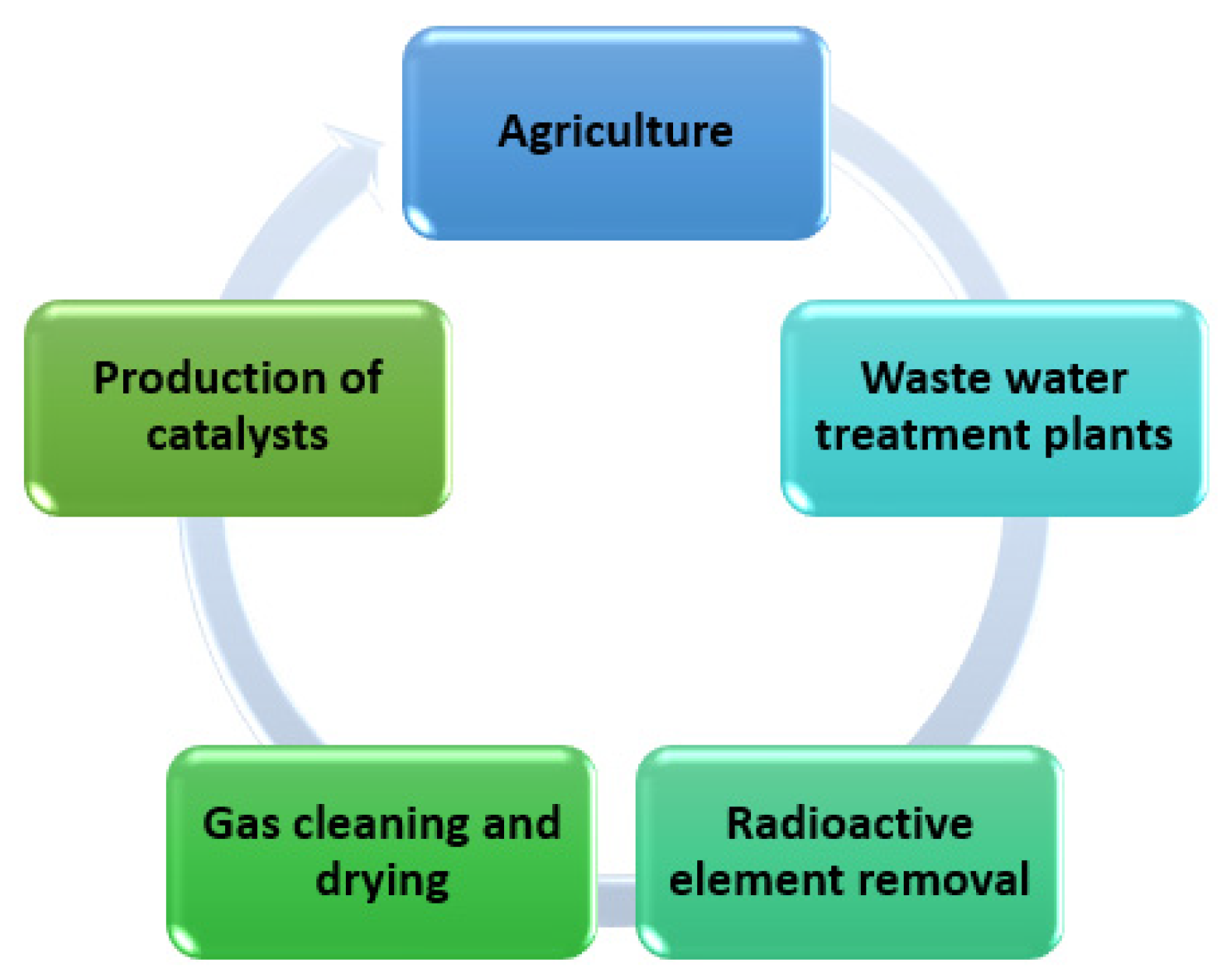
Figure 11.
Use of geopolymers in industry.
Table 3 shows the parameters of the geopolymerization process of the selected authors, along with the subjects of the tests that were carried out on the geopolymers obtained. Rattanasak et al. [63] confirmed the thesis that during the geopolymerization process the use of admixtures is as conducive as possible to the formation of geopolymer chains. The addition of calcium chloride reduces the setting time both at the beginning and at the end of the process. The addition of Na2SO4 increases the bonding time due to the formation of ettringite. The addition of CaSO4 did not affect the setting time of the paste. The compressive strength for the control paste was 25.8 MPa, while the addition of 1 wt.% admixture resulted in higher compressive strength, interestingly the addition of 2 wt.% admixture has almost no effect on compressive strength. Somna et al. [64] carried out the synthesis of geopolymers using fly ash and sodium hydroxide at different concentrations. The compressive strength of the obtained geopolymers was very similar to classic Portland cement. As the geopolymers aged, their compressive strength increased. The highest growth compressive strength for the obtained geopolymers was observed when NaOH concentrations ranging from 4.5 to 9.5 mol/dm3 were applied. The concentration of the NaOH solution affects the strength of the geopolymer. The highest compressive strength was observed for the geopolymer sample where NaOH with a concentration of 14 mol/dm3 was applied, after a time of 60 days it was 25.5 MPa. There are also publications where not only fly ash is subjected to mechanical activation to obtain geopolymer, but also bottom ash after pretreatment (grinding). The mass ratio of fly ash and bottom ash to NaOH and Na2SiO3 was 15:4:6. The obtained products were subjected to compression testing. Geopolymers obtained from fly ash showed almost twice the compressive strength of geopolymers obtained from bottom ash. The most resistant material was a sample of geopolymer obtained from fly ash and sodium hydroxide at a concentration of 10 mol/dm3. The compressive strength was 35 MPa [65].

Table 3.
Research on the synthesis of geopolymers from fly ash and the determination of their properties.
Different experiments have been carried out on geopolymers, one of which was to test the resistance of geopolymers to sulfate. Bhutta et al. [66] proposed the effect of the exposure to a 5% Na2SO4 solution on two samples (OPC—ordinary Portland cement and BFAGC—blended fuel ash geopolymer concrete). The test samples were immersed in a solution containing SO42− anions for a period of 18 months. After the presumed running time, the samples were examined. A large mass loss was observed in the OPC geopolymer sample—it was 20%. The sodium sulfate solution did not affect the strength of BFAGC geopolymer, with the passage of time its strength increased, the sample was resistant up to 35 MPa. The OPC sample behaved in the opposite way, with the passage of time its compressive strength decreased from 22 to 10 MPa. A similar study was conducted by Long et al. and Lakhssassi [67,68].
As mentioned above, ash with a high calcium content can be used in the mineral carbonation process. The concept of introducing such a technology will fill another gap in the material loop of obtaining geopolymers. However, this process will significantly modify the composition of the fly ash; therefore, research studies are being conducted to determine the impact of this process on the subsequent synthesis of geopolymer materials. Two samples were subjected to the carbon dioxide sorption process: Portland cement and a geopolymer obtained by activation with sodium hydroxide. The process of mineral carbonation on the test samples proceeded in different ways. The compressive strength of the PCC (Portland cement concrete) sample increased sharply in the first few days, but stabilized after 7 days and was about 63 MPa. In the case of the geopolymer sample obtained from fly ash, the compressive strength decreased in the first seven days and then began to increase from 33 to 44 MPa. The strength of the samples before the mineral carbonation process was 49 MPa for PCC and 46 MPa for FGC (fly ash geopolymeric concrete) [69]. Other authors have attempted to carry out mineral carbonization on geopolymers obtained from fly ash and ground granulated blast furnace slag with strict ratios. The kinetics of the mineral carbonation process depends on how the samples are prepared. A sample prepared from fly ash and GGBFS (ground granulated blast furnace slag), 70:30 without Na2SiO3 admixture, showed similar CO2 sorption process performance to Portland cement. The applied methods of instrumental analysis detected a change in the composition of the samples tested; the presence of calcium carbonates formed was confirmed [70].
4. Zeolites as Part of a Circular Economy
Fly ash, due to its diverse crystalline composition, exhibits many different chemical and physical properties. As a result, it is possible to reuse it in the production cycle as a raw material and/or substrate. As mentioned earlier, the idea of a circular economy is to use industrial waste as much as possible, thus minimizing the use of natural resources [15]. One of the options for using fly ash obtained from the thermal treatment of fossil fuels or unconventional fuels is the zeolite synthesis process. Zeolites are minerals found in the environment naturally, whose crystal structure is based mainly on hydrated alkali elemental aluminosilicates with a large number of micropores. Fly ash is most often used to synthesize zeolites [71]. Among the most well-known methods for the synthesising of zeolites using fly ash as the raw material is the hydrothermal method under atmospheric pressure, as shown in Figure 12. It is a multi-step physical and chemical process. The whole process takes place in a high pH environment in the liquid phase. Most often, sodium or potassium hydroxide is used. The physical parameter that affects the performance of the process is mainly temperature. The zeolitization process is carried out at 80–200 °C [72,73]. The next most widely used method for the synthesis of zeolites from fly ash waste materials is the fusion method (Figure 13). Fly ash with a high silicon oxide content is activated together with solid sodium hydroxide at a temperature around 600 °C [74,75]. Zeolites are largely used as sorbents, due to their high ability to ion exchange and sorption properties [18,19,76,77].
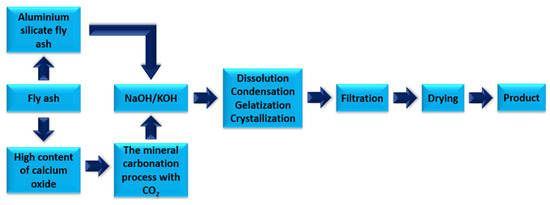
Figure 12.
The hydrothermal process of synthesis zeolites.
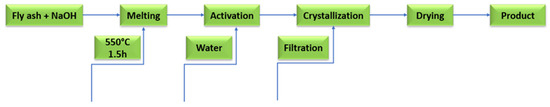
Figure 13.
The fusion method for synthesis of zeolites.
Table 4 shows the synthesis processes of zeolites obtained by two methods. The hydrothermal method uses solutions of aqueous sodium hydroxide and potassium hydroxide of varying concentrations. Depending on the chemical composition of the fly ash and the process parameters (temperature and crystallisation time), different types of zeolites can be obtained, e.g., Na-A, Na-P1 or K-H or Na-X. In the fusion method, it is also possible to obtain similar products, but this method is primarily more time-consuming and complicated. Pretreatment of the fly ash, desorption of the water so that it does not react with the solid sodium hydroxide, and time spent obtaining the melt in an elevated-temperature environment are all necessary. By initiating a circular economy as one way of disposing of combustion by-products, including fly ash, it is possible to reintroduce them into the industrial cycle and also to use the resulting product as a new material (Figure 14).

Table 4.
Fly ash, method of synthesis parameters and product reference.
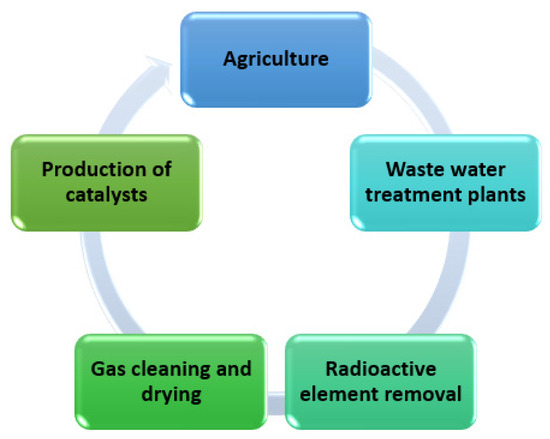
Figure 14.
Examples of the use of synthetic zeolites obtained from fly ash in industry.
Physicochemical Properties of Synthetic Zeolites
The scientific literature on the synthesis of zeolites from fly ash is extensive. As synthetic zeolites are characterised by different physicochemical properties, the topics related to them are diverse. Synthetically obtained zeolites are very often used in industry as sorbents. Zeolites obtained from fly ash can be used as a substitute for natural zeolites or those obtained from pure chemical reagents. Because the processing of raw materials for energy such as hard coal and especially lignite produces large amounts of flue gases that contain large amounts of SO2, Czuma et al. [87] attempted to perform SO2 sorption on zeolites obtained from fly ash using a fusion method. The SO2 sorption process was carried out at 25 °C on zeolites X and A. The F700-0.8-6 sample showed a higher SO2 sorption capacity, and it was also observed in the reverse process (desorption) that only in the first stage the sorption capacity decreases, while in the other stages it remains the same. The decrease in sorption capacity was due to the fact that SO2 was bound to fly ash which did not completely react in the zeolite synthesis process. For sample F550-0.8-6, the sorption capacity was lower, but the sample behaved similarly in sorption and desorption measurements as the F700-0.8-6 sample. The higher sorption capacity of SO2 is due to the fact that the test sample contained more zeolite X and A.
Pedrolo et al. [88] carried out production of synthetic zeolites using a hydrothermal method using potassium hydroxide as the activating agent. The concentration range was 3–5 mol/dm3 and they investigated the sorption capacity of SO2 zeolites at ambient temperature and determined the specific surface area of SBET zeolites and their CEC cation exchange capacity. The sorption process was carried out on synthetic zeolites and on commercial zeolite Y. The system temperature was 25 °C. Nitrogen was dosed for 2 h to remove moisture from the samples. Subsequently, SO2 sorption studies were initiated, SO2 sorption tests were carried out as follows: a mixture of SO2 with N2 was dosed at 100 cm3 min−1. The zeolites obtained by the hydrothermal method showed a much higher SO2 sorption capacity than the commercial zeolite Y. The authors also showed that the specific surface area BET (SBET) and cation exchange capacity increased with increasing KOH concentration and synthesis time. In comparison, the SBET for the fly ash raw material was 3.4 m2 g−1, while the CEC was equal to 0 meq. NH4+ g−1. For the synthetic zeolite obtained by mechanical activation of fly ash with KOH at a concentration of 5 mol/dm3, the SBET was 102.4 m2 g−1 while the CEC was 1.9 meq. NH4+ g−1.
In addition to the use of synthetic zeolites for flue gas cleaning, they are also used as air purifiers for gases such as CO and NOx. Siddharth et al. [89] obtained zeolite from fly ash by a fusion method at 600 °C where the NaOH/Fa ratio was 1:12. The synthesis yielded two types of zeolites: Na-P1 and A. The efficiency of the sorption process was determined by X-ray photoelectron spectroscopy (XPS). Quantitative analysis showed that the zeolite obtained from the fly ash was capable of adsorbing gases such as NO, NO2, N2O, as well as CO and CO2.
Research on hydrogen storage in materials with very high porosity began about 50 years ago. It has been proven that hydrogen can be stored on, for example, activated carbons under cryogenic conditions. Czarna-Juszkiewicz et al. [90] decided to conduct a literature review aimed at finding an equivalent activated carbon on which hydrogen could be stored. Due to continuous modernization and expanding knowledge of the circular economy, the focus was on zeolites obtained from fly ash. Since the process of synthesising zeolites does not require a large financial investment, economical synthetic zeolites can serve as a material for hydrogen storage. Under low-pressure cryogenic conditions, the average sorption capacity of H2 on zeolites is 2 wt.%. A higher sorption capacity of H2 (7.3 wt.%) is shown by zeolite-templated carbons (ZTC) [90].
Yang et al. [91] conducted a proof-of-concept experiment to synthesise zeolite from fly ash by the fusion method using sodium hydroxide and various admixtures (aluminium compounds) and then subjected the resulting product to the As (V) sorption effect. Kunecki et al. [92] have attempted a hybrid zeolite method along with modification with silver and iron ions. The X and A zeolites obtained from fly ash class C and F were activated with AgNO3 and Fe(NO3)3∙9H2O. The final product was used to capture mercury from the gas stream. The results of crystallographic analysis presented that the zeolites that were obtained differ in chemical composition and structure. The crystal structure of a zeolite significantly affects its physicochemical properties, such as SBET and CEC. Zeolites that were activated with iron ions did not show the ability to sorb mercury; one possible reason for this is that it was the form of iron that was used to activate the zeolite. Zeolites activated with silver ions have shown the ability to sorb mercury, especially zeolite X [92].
5. Synergy of Zeolite and Geopolymer Synthesis Using Fly Ash
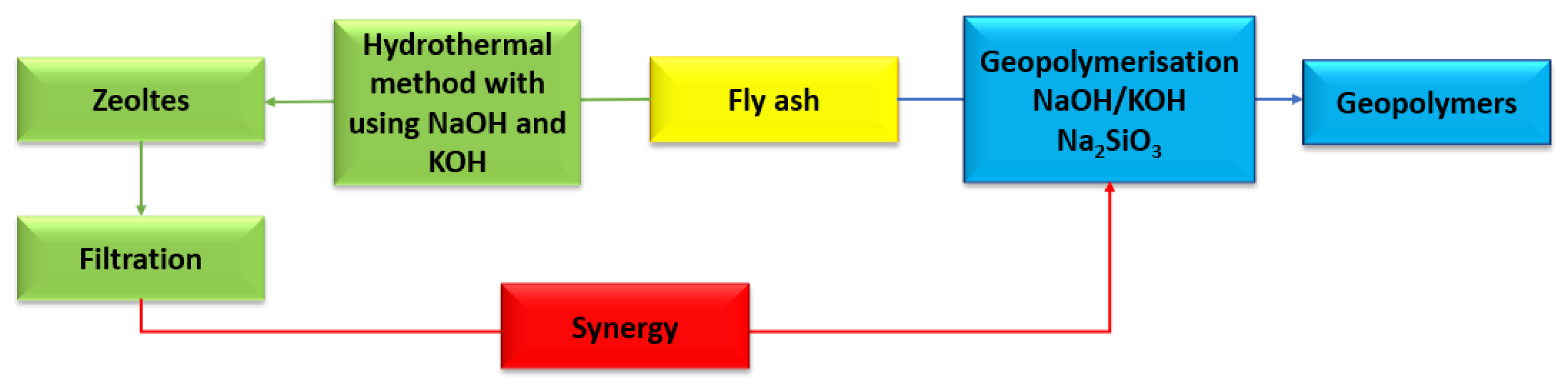
The circular economy is a broad scientific branch that enables the greatest possible reduction in the use of natural resources. By using fly ash (class F) for the synthesis of zeolites and geopolymers, landfilling and environmental pollution can be greatly reduced. However, the question that needs to be asked here is: What about the residues and by-products that result from the zeolite and geopolymer synthesis processes. As with most processes, the research and experimentation process generates unwanted substances, which also need to be managed. The solution is not to store them, but to use them. The hydrothermal method for the synthesis of zeolites from fly ash uses aqueous solutions of sodium hydroxide and potassium hydroxide at a range of concentrations. In addition to the obtained zeolite, a large amount of highly alkaline liquid is produced [93,94,95]. The idea of a circular economy is about using all waste as much as possible. Therefore, the filtrate obtained in the process of hydrothermal synthesis of zeolites can be used as a substitute for sodium hydroxide in the synthesis of geopolymers (Figure 15) [96]. Another example of synergy (hybrid method) is the preparation of a zeolite–geopolymer composite. During the geopolymerization process (chemical activation with NaOH and/or KOH), in addition to the amorphous product (gel), geopolymers can contain unreacted material and form other porous compounds such as zeolites. Zeolite–geopolymer composites can complement each other to give a final product with improved physical and chemical properties. The gel phase of the geopolymer can increase the mechanical properties of the zeolite and, at the same time, the resulting zeolite can increase the specific surface area of the geopolymer, making it have greater sorption properties [97]. The idea of a synergy process (hybrid method) makes it possible to obtain a new porous material. Liu et. al., 2023, in their research paper, present a zeolite–geopolymer composite as a material with highly developed sorption properties. The composite consists of a geopolymer and has a strong substrate, while the zeolite portion serves as an adsorbent. In the synthesis of the zeolite–geopolymer composite, clinoptilolite was used. The obtained composite was subjected to adsorption of heavy metals (ions: Cr3+, Pb2+, Ni2+, Cu2+ and Cd2+). The efficiency of heavy metal ion removal ranged from 87 to as much as 99% [98]. Another literature item also confirms the thesis that zeolite–geopolymer composites have high mechanical strength, a well-developed specific surface area, and also high ion exchange capacity, e.g., Pb2+. Yang et. al., 2022, in their scientific publication, presented the possibility of obtaining such a composite using fly ash and metakaolin as sorbents. In their study, they proved that the synthetic composite contained microporous, mesoporous, and microporous pores and its specific surface area was larger than that from natural zeolite (heulandite). The compressive mechanical strength of the resulting composite was 6.4 MPa, with a removal efficiency of almost 450% for Pb2+ ions in solution [99]. Zeolite-geopolymer composites can not only be used as adsorbents for metal ions but can also be carved out for gas adsorption. Papa et. al., 2023, in their scientific work, presented the possibility of obtaining a composite using metakaolin and commercial zeolite Na4A. Geopolymer slurries were prepared with metakaolin and a concentrated solution of sodium potassium hydroxide or potassium silicate. The Si/Al ratio was 1.2 or 2.0. Geopolymer matrices act as binders that enable the shaping the zeolite and defining different chemical compositions, thus increasing the mechanical properties of the resulting product. The CO2 adsorption process was carried out on a pressure apparatus using the volumetric method. A higher amount of adsorbed CO2 was noticed on the composite using sodium hydroxide as an activator. At a pressure of 1 bar, the yield of adsorbed CO2 was 2.6 mmol g−1. The sorption capacity for the composite obtained using potassium hydroxide at the same pressure value was only 0.003 mmol g−1. This may be due to the fact that the zeolite Na4A was cancelled out by potassium ions during ion exchange [100].
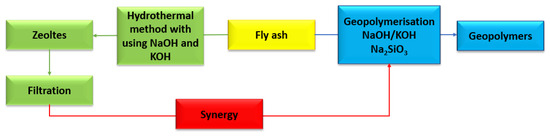
Figure 15.
Synergy of synthesis zeolites and geopolymers from fly ash.
6. Conclusions
The potential of using fly ash as a substrate for the production of porous materials, in particular geopolymers and zeolites, has been extensively discussed in the scientific literature. The process of synthesising geopolymers and zeolites using fly ash fits into the idea of a closed-loop economy, which aims to maximise the use of waste materials. The authors of this study noted the importance of a hybrid process for the synthesis of geopolymers and zeolites, using the filtrate from the zeolitisation process as a substrate in the geopolymer synthesis process (Figure 16). Since chemical activation of fly ash with concentrated solutions of sodium and/or potassium hydroxide is very often used for hydrothermal synthesis of zeolites, highly alkaline aqueous by-products are formed during the process in addition to the final product. Due to the high content of hydroxide ions, it is possible to use the zeolite process waste (filtrate) as an activating substance in the synthesis of geopolymers. The hybrid process model for the synthesis of geopolymers and zeolites enables a continuous closed loop where no by-product is unused. The hybrid model of the chemical activation of fly ash, in addition to the use of by-products in the new cycle, also enables the formation of zeolite–geopolymer composites. The proposition to develop a method for the hybrid synthesis of zeolites from fly ash and then geopolymers fits into the idea of a closed-loop economy. First of all, it makes it possible to use the by-products of combustion as a new raw material and thus make use of the waste obtained at the synthesis stage. The resulting materials exhibit specific physicochemical properties, thus enabling them to be used in industry. Research on geopolymers and synthetic zeolites derived from fly ash shows that the resulting porous materials can find applications in a variety of industries, from environmental engineering to the building materials industry. Activation of fly ash with sodium or potassium hydroxide solutions provides the possibility of trapping heavy metal oxides in the matrix of geopolymers, so they do not have a negative impact on the environment.
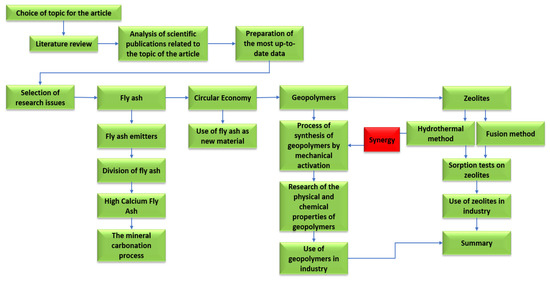
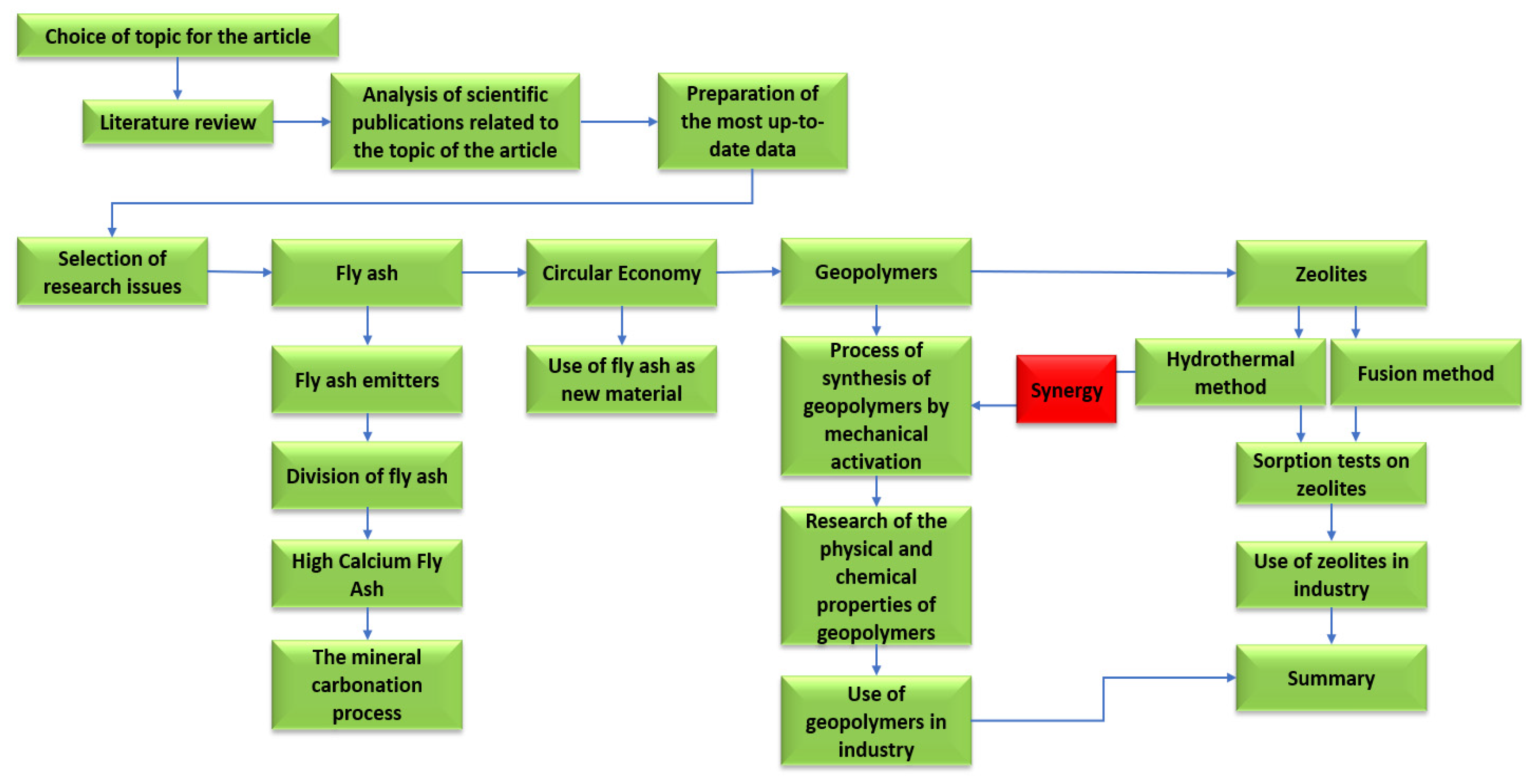
Figure 16.
Detailed plan for the preparation of the review article.
Author Contributions
Conceptualization, P.B. and K.Z.; methodology, J.S. (Jakub Szczurowski); software, J.S. (Jakub Sobala); validation, K.Z. and J.S. (Jakub Szczurowski); formal analysis, K.Z. and J.S. (Jakub Szczurowski); investigation, J.S. (Jakub Sobala); resources, J.S. (Jakub Sobala); data curation, P.B.; writing—original draft preparation, J.S. (Jakub Sobala); writing—review and editing, K.Z. and J.S. (Jakub Szczurowski); visualization, J.S. (Jakub Sobala) and J.S. (Jakub Szczurowski); supervision, K.Z.; project administration, K.Z.; funding acquisition, K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This project was carried out within OPUS 2022. This research was fully funded by the National Science Centre, Poland, grant number OPUS-22 UMO-2021/43/B/ST8/01636.
Data Availability Statement
Data will be available after contacting the authors of the publication.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Ju, T.; Meng, Y.; Han, S.; Lin, L.; Jiang, J. On the State of the Art of Crystalline Structure Reconstruction of Coal Fly Ash: A Focus on Zeolites. Chemosphere 2021, 283, 131010. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, H.; Zhang, J. Understanding the Effect of High-Volume Fly Ash on Micro-Structure and Mechanical Properties of Cemented Coal Gangue Paste Backfill. Constr. Build. Mater. 2023, 378, 131202. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, S.; Finkelman, R.B.; Graham, I.T.; French, D.; Hower, J.C.; Li, X. Leaching Characteristics of Alkaline Coal Combustion By-Products: A Case Study from a Coal-Fired Power Plant, Hebei Province, China. Fuel 2019, 255, 115710. [Google Scholar] [CrossRef]
- Zimar, Z.; Robert, D.; Zhou, A.; Giustozzi, F.; Setunge, S.; Kodikara, J. Application of Coal Fly Ash in Pavement Subgrade Stabilisation: A Review. J. Environ. Manag. 2022, 312, 114926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, M.; Lv, Y.; Ting, Z.J.; Zhao, S.; Liu, Z.; Zhang, X.; Yang, Y.; You, Y.; Yuan, W. Utilization of Municipal Solid Waste Incineration Fly Ash as Construction Materials Based on Geopolymerization. Resour. Conserv. Recycl. Adv. 2023, 19, 200162. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Poon, C.S.; Jiang, W.H.; Ma, Z.H.; Chen, X.; Lu, J.X.; Dong, H.X. Physicochemical and Pozzolanic Properties of Municipal Solid Waste Incineration Fly Ash with Different Pretreatments. Waste Manag. 2023, 160, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, X. Detoxification, Solidification and Recycling of Municipal Solid Waste Incineration Fly Ash: A Review. Chem. Eng. J. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the Management of MSW Incineration Ashes from Gas Cleaning: New Perspectives on Recovery of Secondary Raw Materials and Circular Economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Liu, B. Degradation Technologies and Mechanisms of Dioxins in Municipal Solid Waste Incineration Fly Ash: A Review. J. Clean Prod. 2020, 250, 119507. [Google Scholar] [CrossRef]
- Wang, C.Q.; Zeng, Z.Y.; Wang, A.M.; Gao, S.H.; Huang, J.S. Basic Properties, Characteristic Heavy Metals Leaching and Migration of Coal Incineration Fly Ash-Based Mortar. Structures 2023, 54, 1179–1195. [Google Scholar] [CrossRef]
- Ustabaş, İ.; Kaya, A. Comparing the Pozzolanic Activity Properties of Obsidian to Those of Fly Ash and Blast Furnace Slag. Constr. Build. Mater. 2018, 164, 297–307. [Google Scholar] [CrossRef]
- Borowski, G.; Ozga, M. Comparison of the Processing Conditions and the Properties of Granules Made from Fly Ash of Lignite and Coal. Waste Manag. 2020, 104, 192–197. [Google Scholar] [CrossRef]
- Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M. Mapping of Research Lines on Circular Economy Practices in Agriculture: From Waste to Energy. Renew. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- Opferkuch, K.; Caeiro, S.; Salomone, R.; Ramos, T.B. Circular Economy Disclosure in Corporate Sustainability Reports: The Case of European Companies in Sustainability Rankings. Sustain. Prod. Consum. 2022, 32, 436–456. [Google Scholar] [CrossRef]
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. Fly Ash for Sustainable Construction: A Review of Fly Ash Concrete and Its Beneficial Use Case Studies. Clean. Mater. 2022, 6, 100143. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Calero-Rodríguez, A.; Bonet-Martínez, E.; Pérez-Villarejo, L.; Sánchez-Soto, P.J. Geopolymers Made from Metakaolin Sources, Partially Replaced by Spanish Clays and Biomass Bottom Ash. J. Build. Eng. 2021, 40, 102761. [Google Scholar] [CrossRef]
- Stefańska, A.; Łach, M.; Mikuła, J. Geopolimery Jako Przykład Możliwości Zagospodarowania Odpadów. In Nowoczesne technologie XXI w. – przegląd, trendy i badania. Tom 1; Wydawnictwo Naukowe TYGIEL sp. z o.o: Lublin, Poland, 2019. [Google Scholar]
- Belviso, C. State-of-the-Art Applications of Fly Ash from Coal and Biomass: A Focus on Zeolite Synthesis Processes and Issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible Applications of Coal Fly Ash in Wastewater Treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Fedyna, M.; Marzec, M.; Panek, R.; Szerement, J.; Marcińska-Mazur, L.; Jarosz, R.; Bajda, T.; Franus, W.; Mierzwa-Hersztek, M. The Influence of Zeolite X Ion-Exchangeable Forms and Impregnation with Copper Nitrate on the Adsorption of Phosphate Ions from Aqueous Solutions. J. Water Process Eng. 2022, 50, 103299. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Strzałkowska, E. Rare Earth Elements and Other Critical Elements in the Magnetic Fraction of Fly Ash from Several Polish Power Plants. Int. J. Coal Geol. 2022, 258, 104015. [Google Scholar] [CrossRef]
- Chmielniak, T.; Stelmach, S. Współczesne technologie zgazowania węgla. Probl. Ekol. 2009, 13, 69–76. [Google Scholar]
- Łuczak-Wilamowska, B. Możliwość Zastosowania Popiołów-odpadów Przemysłu energetycznego-do uszczelniania i rekultywacji składowisk odpadów. Biuletyn Państwowego Instytutu Geologicznego 2011, 446, 477–482. [Google Scholar]
- Kudełko, M. Modeling of Polish Energy Sector—Tool Specification and Results. Energy 2021, 215, 119149. [Google Scholar] [CrossRef]
- Tamanna, K.; Raman, S.N.; Jamil, M.; Hamid, R. Coal Bottom Ash as Supplementary Material for Sustainable Construction: A Comprehensive Review. Constr. Build. Mater. 2023, 389, 131679. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Harat, A.; Jaguś, A.; Porszke, A. Environmental Aspects of the Leaching of Mineral components from Power Plant’s Fly Ashes and Slags. Pol. J. Mater. Environ. Eng. 2022, 4, 21–29. [Google Scholar] [CrossRef]
- Piotr Smarzewski, D.B.-H. Mechanical and Microstructural Properties of High Performance Concrete with Burnt Coal Cinder. Available online: https://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-89ece729-bee0-4391-a0d5-def18c3572e8 (accessed on 20 October 2023).
- Mu, L.; Wang, S.; Lu, J.; Liu, G.; Zhao, L.; Lan, Y. Effect of Flue Gas Condensing Waste Heat Recovery and Its Pressure Drop on Energy Saving and Carbon Reduction for Refinery Heating Furnace. Energy 2023, 279, 128081. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.; Song, W. Mechanical Model and Strength Development Evolution of High Content Fly Ash–Cement Grouting Material. Constr. Build. Mater. 2023, 398, 132492. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Białecka, B.; Nowak, J.; Klupa, A. Assessment of the Potential of Polish Fly Ashes as a Source of Rare Earth Elements. Ore Geol. Rev. 2020, 124, 103638. [Google Scholar] [CrossRef]
- Shon, C.-S.; Kim, Y.-S. Evaluation of West Texas Natural Zeolite as an Alternative of ASTM Class F Fly Ash. Constr. Build. Mater. 2013, 47, 389–396. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal Fly Ash as a Resource for Rare Earth Elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [PubMed]
- Claudia, B.; Darina, B.; Andrea, B.G.; Constantin, C.; Jo, D.; Patricia, D.; Yildirim, K.; Latunussa, C.E.L.; Lucia, M.; Simone, M.; et al. Methodology for Establishing the EU List of Critical Raw Materials: Guidelines; EU Publications: Luxembourg, 2017; ISBN 9789279680519. [Google Scholar]
- Jarosinski, A.; Kulczycka, J. Possibilities of Obtaining Certain Critical Raw Materials in Poland in the Contex of Circular Economy Implementation. Inz. Miner. 2018, 19, 315–324. [Google Scholar]
- Pgdn, P.; Suárez-Ruiz, I.; Valentim, B. Atlas of Fly Ash Occurrences Identification and Petrographic Classification of Fly Ash Components Working Group. In Commission III-ICCP International Committee for Coal and Organic Petrology-ICCP Go to “Main Menu of Contents”; Porto University: Portugal, 2015; ISBN 978-84-608-1416-0. [Google Scholar]
- Suárez-Ruiz, I.; Valentim, B.; Borrego, A.G.; Bouzinos, A.; Flores, D.; Kalaitzidis, S.; Malinconico, M.L.; Marques, M.; Misz-Kennan, M.; Predeanu, G.; et al. Development of a Petrographic Classification of Fly-Ash Components from Coal Combustion and Co-Combustion. (An ICCP Classification System, Fly-Ash Working Group—Commission III.). Int. J. Coal Geol. 2017, 183, 188–203. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use Creating Value from Emissions; IEA: Paris, France, 2019. [Google Scholar]
- The Statistical Review of World Energy Analysesdata on World Energy Markets from the Prior Year. The Review Has Been Providing Timely, Comprehensive and Objective Data to the Energy Community since 1952. In Bp Statistical Review of World Energy; Energy Institute (EI): London, UK, 2022.
- Commision to the European Parlament; The European Council; The Council; The European Economic and Social Committee; Committe of the Regions. The European Green Deal; Office of the European Union: Brussels, Belgium, 2020. [Google Scholar]
- Sanjuán, M.Á.; Argiz, C. Fineness of Coal Fly Ash for Use in Cement and Concrete. Fuels 2021, 2, 471–486. [Google Scholar] [CrossRef]
- Lu, L.; Liu, C.; Qu, S.; Zhang, M. Experimental Study on the Mechanical and Hydraulic Behaviour of Fibre-Reinforced Cemented Soil with Fly Ash. Constr. Build. Mater. 2022, 321, 126374. [Google Scholar] [CrossRef]
- Gazdič, D.; Kulísek, K.; Fridrichová, M.; Dvořák, K. Fired Hydraulic Binder Based on Fluidized Combustion Fly Ash. Procedia Eng. 2017, 172, 319–324. [Google Scholar] [CrossRef]
- Gao, K.; Iliuta, M.C. Trends and Advances in the Development of Coal Fly Ash-Based Materials for Application in Hydrogen-Rich Gas Production: A Review. J. Energy Chem. 2022, 73, 485–512. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; van Deventer, J.S.J. Molecular Mechanisms Responsible for the Structural Changes Occurring during Geopolymerization: Multiscale Simulation. AIChE J. 2012, 58, 2241–2253. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly Ash-Based Geopolymer: Clean Production, Properties and Applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A Review on Alkaline Activation: New Analytical Perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef]
- Ettahiri, Y.; Bouargane, B.; Fritah, K.; Akhsassi, B.; Pérez-Villarejo, L.; Aziz, A.; Bouna, L.; Benlhachemi, A.; Novais, R.M. A State-of-the-Art Review of Recent Advances in Porous Geopolymer: Applications in Adsorption of Inorganic and Organic Contaminants in Water. Constr. Build. Mater. 2023, 395, 132269. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Davaabal, B.; Bayarzul, U.; Ankhtuya, A.; Jadambaa, T.; Mackenzie, K.J.D. Utilization of Radioactive High-Calcium Mongolian Flyash for the Preparation of Alkali-Activated Geopolymers for Safe Use as Construction Materials. Ceram. Int. 2014, 40, 16475–16483. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Zhernovskaya, I.V.; Danakin, D.N.; Teslya, A.Y.; Kozhukhova, M.I. Effect of Structure and Stereochemistry on Metakaolin Reactivity When Geopolymerization. In XIII General Meeting of the Russian Mineralogical Society and the Fedorov Session; Marin, Y., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 484–491. [Google Scholar]
- Kozhukhova, N.; Strokova, V.; Zhernovsky, I.; Sobolev, K. Geopolymerization and Structure Formation in Alkali Activated Aluminosilicates with Different Crystallinity Degree. In Proceedings of the 14th International Congress for Applied Mineralogy (ICAM2019), Belgorod, Russia, 23–27 September 2019. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, J.; Jiang, Y.; Zang, J.; Wu, T.; Ma, F.; Qian, B.; Wang, L.; Hu, Y.; Ma, B. The Performance of Micropore-Foamed Geopolymers Produced from Industrial Wastes. Constr. Build. Mater. 2021, 304, 124636. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology. In Cement Based Materials; InTech: Gandhinagar, India, 2018. [Google Scholar]
- Mikuła, J.S. Rozwiązania Proekologiczne w Zakresie Produkcji: Praca Zbiorowa. T. 1, Nowoczesne Materiały Kompozytowe Przyjazne Środowisku; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2014; ISBN 9788372427809. [Google Scholar]
- Raza, M.H.; Zhong, R.Y. A Sustainable Roadmap for Additive Manufacturing Using Geopolymers in Construction Industry. Resour. Conserv. Recycl. 2022, 186, 106592. [Google Scholar] [CrossRef]
- Ge, X.; Hu, X.; Shi, C. The Effect of Different Types of Class F Fly Ashes on the Mechanical Properties of Geopolymers Cured at Ambient Environment. Cem. Concr. Compos. 2022, 130, 104528. [Google Scholar] [CrossRef]
- Bezerra, B.P.; Morelli, M.R.; Luz, A.P. Effect of Reactive Silica Sources on the Properties of Na-Metakaolin-Based Geopolymer Binder. Constr. Build. Mater. 2023, 364, 129989. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Zhang, Y.; Ma, J.; Xiao, R.; Guo, F.; Bai, Y.; Huang, B. Influence of Size Effect on the Properties of Slag and Waste Glass-Based Geopolymer Paste. J. Clean. Prod. 2023, 383, 135428. [Google Scholar] [CrossRef]
- Tang, J.; Ji, X.; Liu, X.; Zhou, W.; Chang, X.; Zhang, S. Mechanical and Microstructural Properties of Phosphate-Based Geopolymers with Varying Si/Al Molar Ratios Based on the Sol-Gel Method. Mater. Lett. 2022, 308, 131178. [Google Scholar] [CrossRef]
- Rattanasak, U.; Pankhet, K.; Chindaprasirt, P. Effect of Chemical Admixtures on Properties of High-Calcium Fly Ash Geopolymer. Int. J. Miner. Metall. Mater. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-Activated Ground Fly Ash Geopolymer Cured at Ambient Temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative Study on the Characteristics of Fly Ash and Bottom Ash Geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Hussin, W.M.; Azreen, M.; Tahir, M.M. Sulphate Resistance of Geopolymer Concrete Prepared from Blended Waste Fuel Ash. J. Mater. Civ. Eng. 2014, 26, 04014080. [Google Scholar] [CrossRef]
- Long, T.; Wang, Q.; Guan, Z.; Chen, Y.; Shi, X. Deterioration and Microstructural Evolution of the Fly Ash Geopolymer Concrete against MgSO4 Solution. Adv. Mater. Sci. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- Lakhssassi, M.Z.; Alehyen, S.; Alouani, M.E.; Taibi, M. The Effect of Aggressive Environments on the Properties of a Low Calcium Fly Ash Based Geopolymer and the Ordinary Portland Cement Pastes. Mater. Today Proc. 2019, 13, 1169–1177. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, X.S.; Wang, Q.Y.; Tang, L. The Influence of Carbonization on the Performances of Fly Ash Geopolymeric Concrete. Appl. Mech. Mater. 2015, 744–746, 1519–1526. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a Blended Slag-Fly Ash Geopolymer Concrete in Field Conditions after 8 Years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Motak, M.; Gálvez, M.E.; Da Costa, P. Ni/Zeolite X Derived from Fly Ash as Catalysts for CO2 Methanation. Fuel 2020, 267, 117139. [Google Scholar] [CrossRef]
- Murayama, N.; Takahashi, T.; Shuku, K.; Lee, H.H.; Shibata, J. Effect of Reaction Temperature on Hydrothermal Syntheses of Potassium Type Zeolites from Coal Fly Ash. Int. J. Miner. Process. 2008, 87, 129–133. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Beran, E.; Czímerová, A. Properties and Potential Applications of Zeolitic Materials Produced from Fly Ash Using Simple Method of Synthesis. Powder Technol. 2006, 166, 47–54. [Google Scholar] [CrossRef]
- Kirdeciler, S.K.; Akata, B. One Pot Fusion Route for the Synthesis of Zeolite 4A Using Kaolin. Adv. Powder Technol. 2020, 31, 4336–4343. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Xu, D.; Han, L.; Niu, D.; Tian, B.; Zhang, J.; Zhang, L.; Wu, W. Removal of Ammonium from Aqueous Solutions Using Zeolite Synthesized from Fly Ash by a Fusion Method. Desalination 2011, 271, 111–121. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of Zeolites from Coal Fly Ash: An Overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Park, M.; Choi, L.; Lim, W.T.; Kim, M.C.; Choi, J.; Ho Heo, N. Molten-Salt Method for the Synthesis of Zeolitic Materials II. Charact. Zeolitic Mater. 2000, 37, 91–98. [Google Scholar]
- Hollman, G.G.; Steenbruggen, G.; Janssen-Jurkovičová, M. A Two-Step Process for the Synthesis of Zeolites from Coal Fly Ash. Fuel 1999, 78, 1225–1230. [Google Scholar]
- Mimura, H.; Yokota, K.; Akiba, K.; Onodera, Y. Alkali Hydrothermal Synthesis of Zeolites from Coal Fly Ash and Their Uptake Properties of Cesium Ion. J. Nucl. Sci. Technol. 2001, 38, 766–772. [Google Scholar] [CrossRef][Green Version]
- Steenbruggen, G.; Hollman, G.G. The Synthesis of Zeolites from Fly Ash and the Properties of the Zeolite Products. J. Geochem. Explor. 1998, 62, 305–309. [Google Scholar] [CrossRef]
- Querol, X.; Uman Äa, J.C.; Plana, F.; Alastuey, A.; Lopez-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Garcia-Rojo, E. Synthesis of Zeolites from fly Ash at Pilot Plant Scale. Examples of Potential Applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The Conversion Technology of Fly Ash into Zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, F.S.; Zhu, J.; Liu, Z. Effective Utilization of Waste Ash from MSW and Coal Co-Combustion Power Plant-Zeolite Synthesis. J. Hazard Mater. 2008, 153, 382–388. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Q.; Wu, H. Synthesis of Zeolite-like Material by Hydrothermal and Fusion Methods Using Municipal Solid Waste Fly Ash. Procedia Environ. Sci. 2016, 31, 662–667. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujii, A. Effect of Stirring on the Dissolution of Coal Fly Ash and Synthesis of Pure-Form Na-A and -X Zeolites by Two-Step Process. Adv. Powder Technol. 2009, 20, 473–479. [Google Scholar] [CrossRef]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.F.; et al. Synthesis of High Quality Zeolites from Coal Fly Ash: Mobility of Hazardous Elements and Environmental Applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Baran, P. Analysis of the Influence of Fusion Synthesis Parameters on the SO2 Sorption Properties of Zeolites Produced out of Fly Ash. In Proceedings of the E3S Web of Conferences, Kraków, Poland, 17–19 May 2016. [Google Scholar]
- Pedrolo, D.R.S.; De Menezes Quines, L.K.; De Souza, G.; Marcilio, N.R. Synthesis of Zeolites from Brazilian Coal Ash and Its Application in SO2 Adsorption. J. Environ. Chem. Eng. 2017, 5, 4788–4794. [Google Scholar] [CrossRef]
- Siddharth; Maiti, S.; Raj, H.; Bisht, R.S.; Minocha, A.K.; Panigrahi, S.K.; Alexander, S.; Sameer; Singh, M. X-Ray Photoelectron Spectroscopy Study on Adsorption Property of Harmful Air Pollutants on Zeolite Prepared from Fly Ash. Mater. Res. Express 2018, 5, 085507. [Google Scholar] [CrossRef]
- Czarna-Juszkiewicz, D.; Cader, J.; Wdowin, M. From Coal Ashes to Solid Sorbents for Hydrogen Storage. J. Clean. Prod. 2020, 270, 122355. [Google Scholar] [CrossRef]
- Yang, T.; Han, C.; Liu, H.; Yang, L.; Liu, D.; Tang, J.; Luo, Y. Synthesis of Na-X Zeolite from Low Aluminum Coal Fly Ash: Characterization and High Efficient As (V) Removal. Adv. Powder Technol. 2019, 30, 199–206. [Google Scholar] [CrossRef]
- Kunecki, P.; Wdowin, M.; Hanc, E. Fly Ash-Derived Zeolites and Their Sorption Abilities in Relation to Elemental Mercury in a Simulated Gas Stream. J. Clean. Prod. 2023, 391, 136181. [Google Scholar] [CrossRef]
- Czarna, D.; Baran, P.; Kunecki, P.; Panek, R.; Żmuda, R.; Wdowin, M. Synthetic Zeolites as Potential Sorbents of Mercury from Wastewater Occurring during Wet FGD Processes of Flue Gas. J. Clean. Prod. 2016, 172, 2636–2645. [Google Scholar] [CrossRef]
- Czuma, N.; Casanova, I.; Baran, P.; Szczurowski, J.; Zarębska, K. CO2 Sorption and Regeneration Properties of Fly Ash Zeolites Synthesized with the Use of Differentiated Methods. Sci. Rep. 2020, 10, 1825. [Google Scholar] [CrossRef] [PubMed]
- Zarębska, K.; Szczurowski, J.; Gazda-Grzywacz, M.; Wróbel, W.; Bator, J.; Baran, P. Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor. Energies 2023, 16, 5188. [Google Scholar] [CrossRef]
- Baran, P.; Nazarko, M.; Włosińska, E.; Kanciruk, A.; Zarębska, K. Synthesis of Geopolymers Derived from Fly Ash with an Addition of Perlite. J. Clean. Prod. 2021, 293, 126112. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-Zeolite Composites: A Review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Qiu, X.; Meng, Y.; Wang, H.; Zhou, S.; Qiao, Q.; Yan, C. Clinoptilolite Based Zeolite-Geopolymer Hybrid Foams: Potential Application as Low-Cost Sorbents for Heavy Metals. J. Environ. Manag. 2023, 330, 117167. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Gao, M.; Bai, H.; Nagasaka, T. Synthesis of Zeolite-Geopolymer Composites with High Zeolite Content for Pb(II) Removal by a Simple Two-Step Method Using Fly Ash and Metakaolin. J. Clean. Prod. 2022, 378, 134528. [Google Scholar] [CrossRef]
- Papa, E.; Minelli, M.; Marchioni, M.C.; Landi, E.; Miccio, F.; Natali Murri, A.; Benito, P.; Vaccari, A.; Medri, V. Metakaolin-Based Geopolymer—Zeolite NaA Composites as CO2 Adsorbents. Appl. Clay Sci. 2023, 237, 106900. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).