Sulfur Loading as a Manufacturing Key Factor of Additive-Free Cathodes for Lithium-Sulfur Batteries Prepared by Composite Electroforming
Abstract
:1. Introduction
2. Materials and Methods
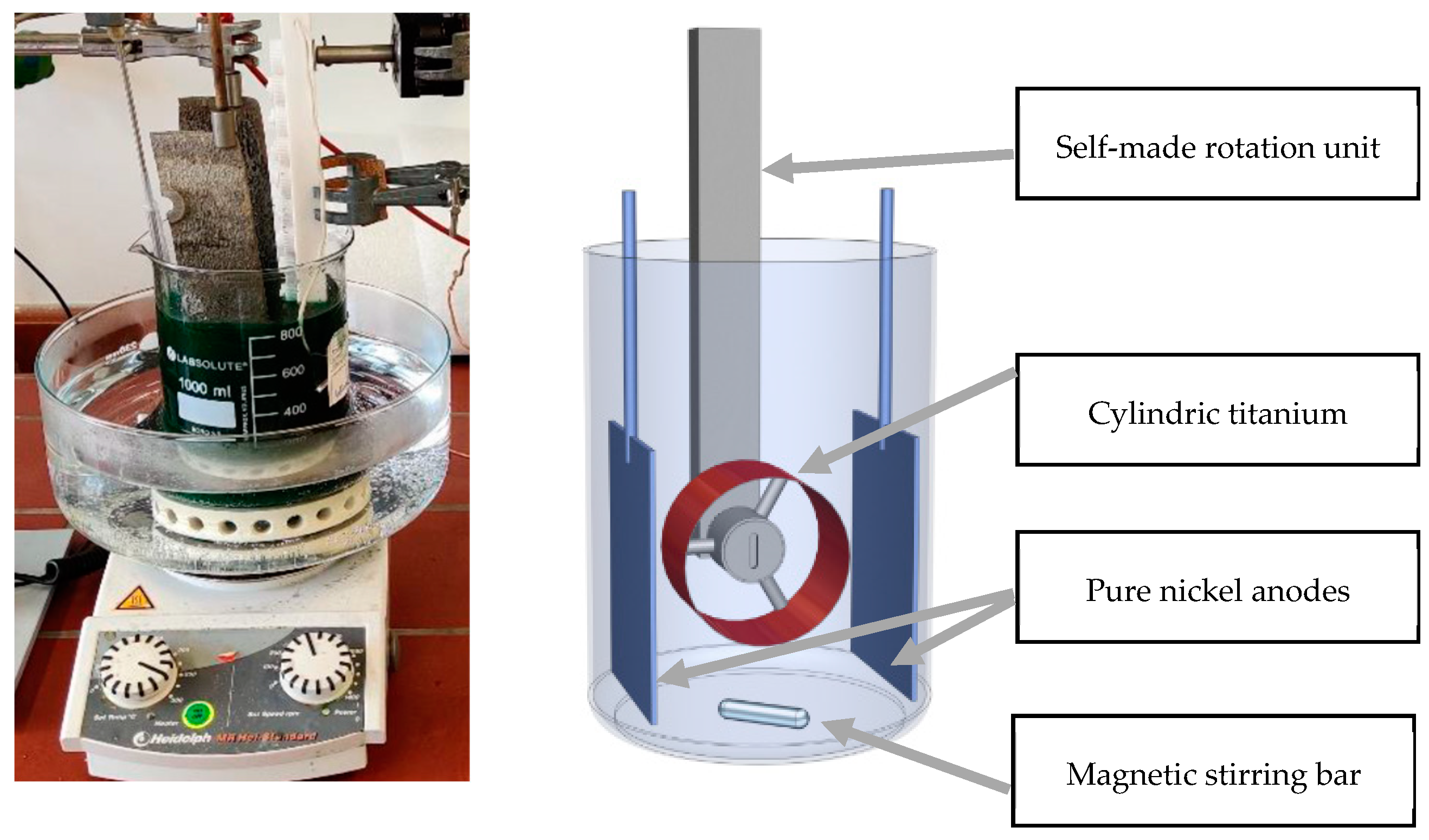
2.1. Composite Electroforming of Ni–AlSi10Mg0.4
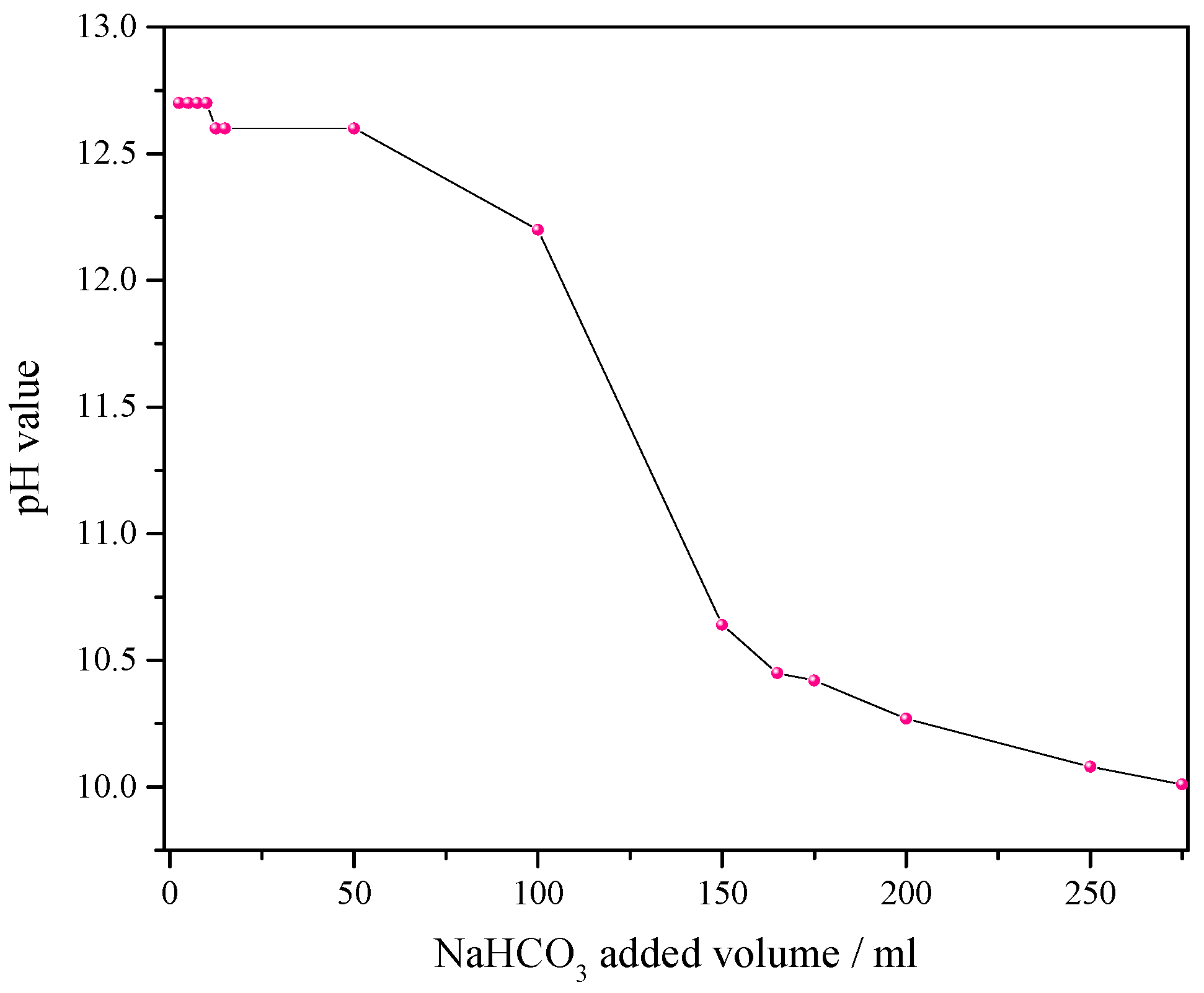
2.2. Etching of the AlSi10Mg0.4 on the AlSi10Mg0.4@Ni Composite Electroformed Foil
2.3. Sulfur Loading
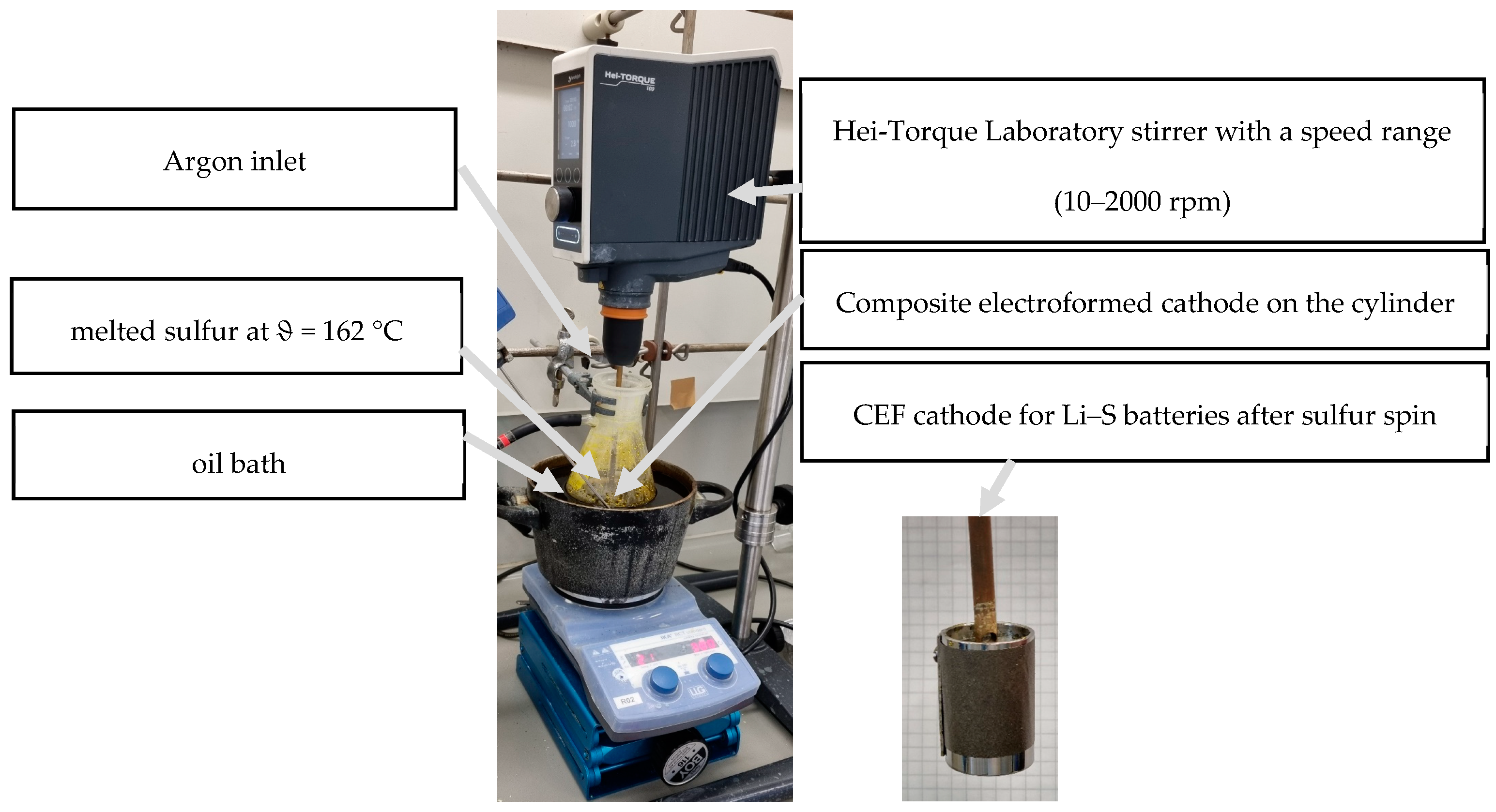
2.3.1. Sulfur Loading by Spin Coating
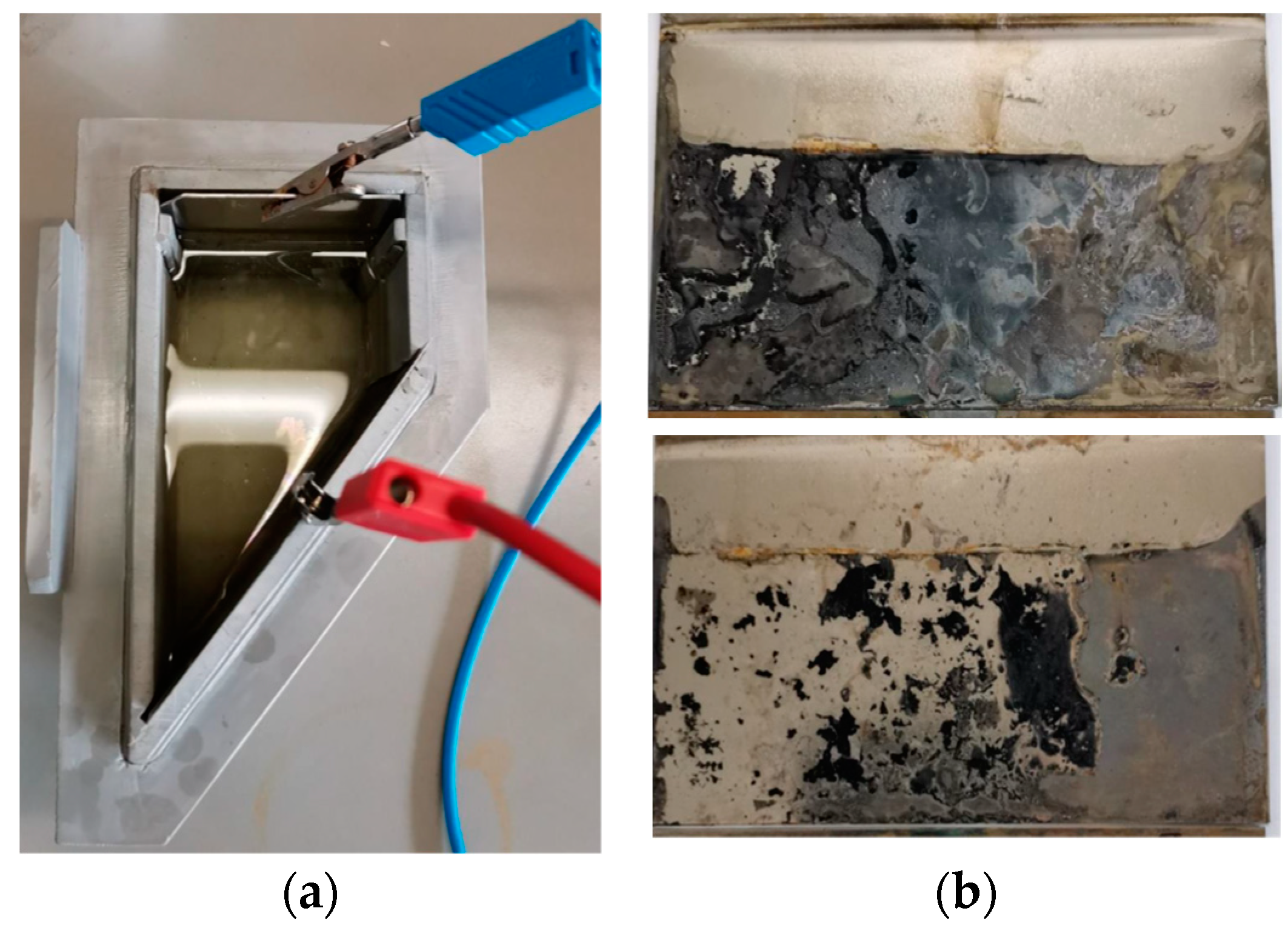
2.3.2. Sulfur Loading by Electrochemical Deposition
3. Results and Discussion
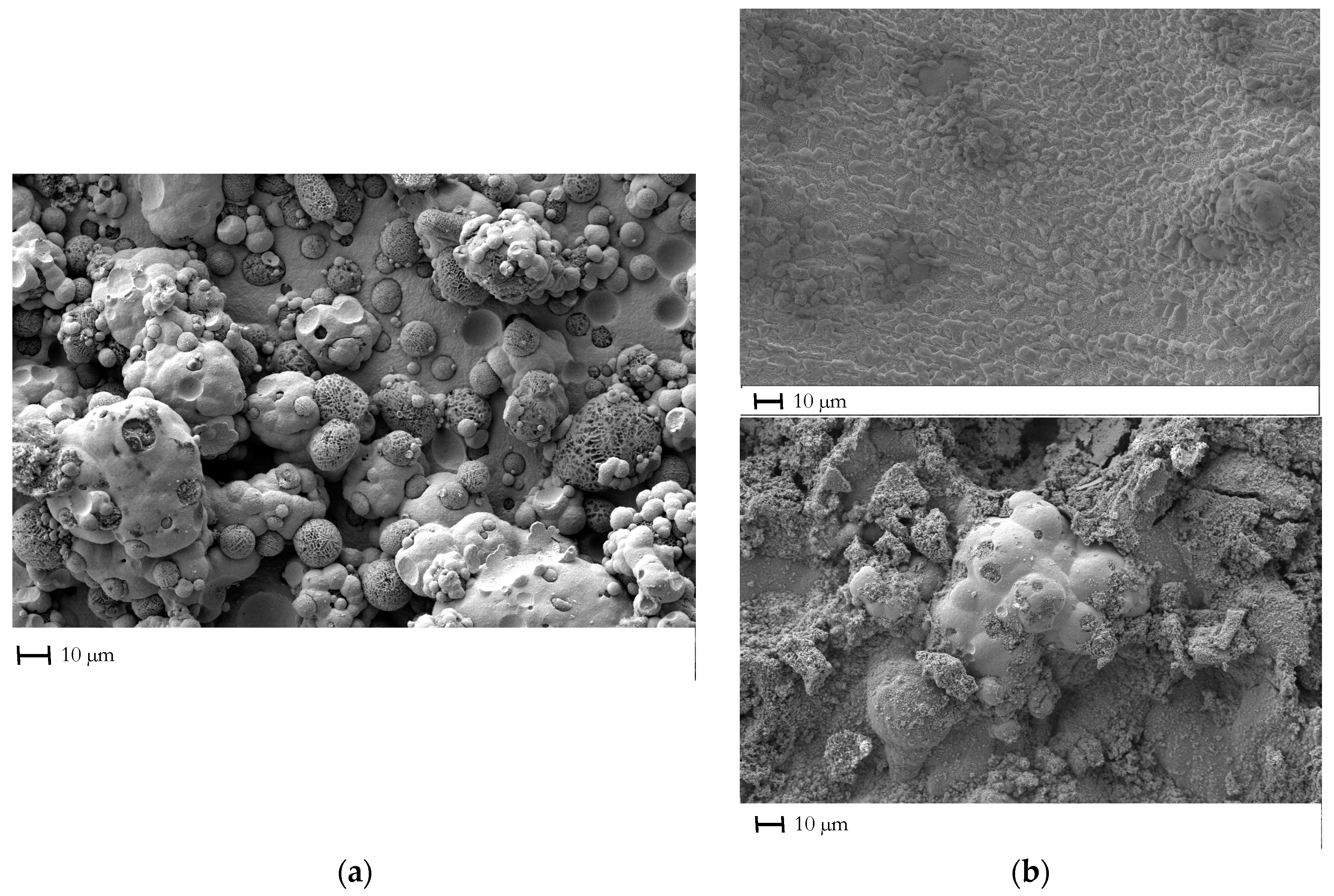
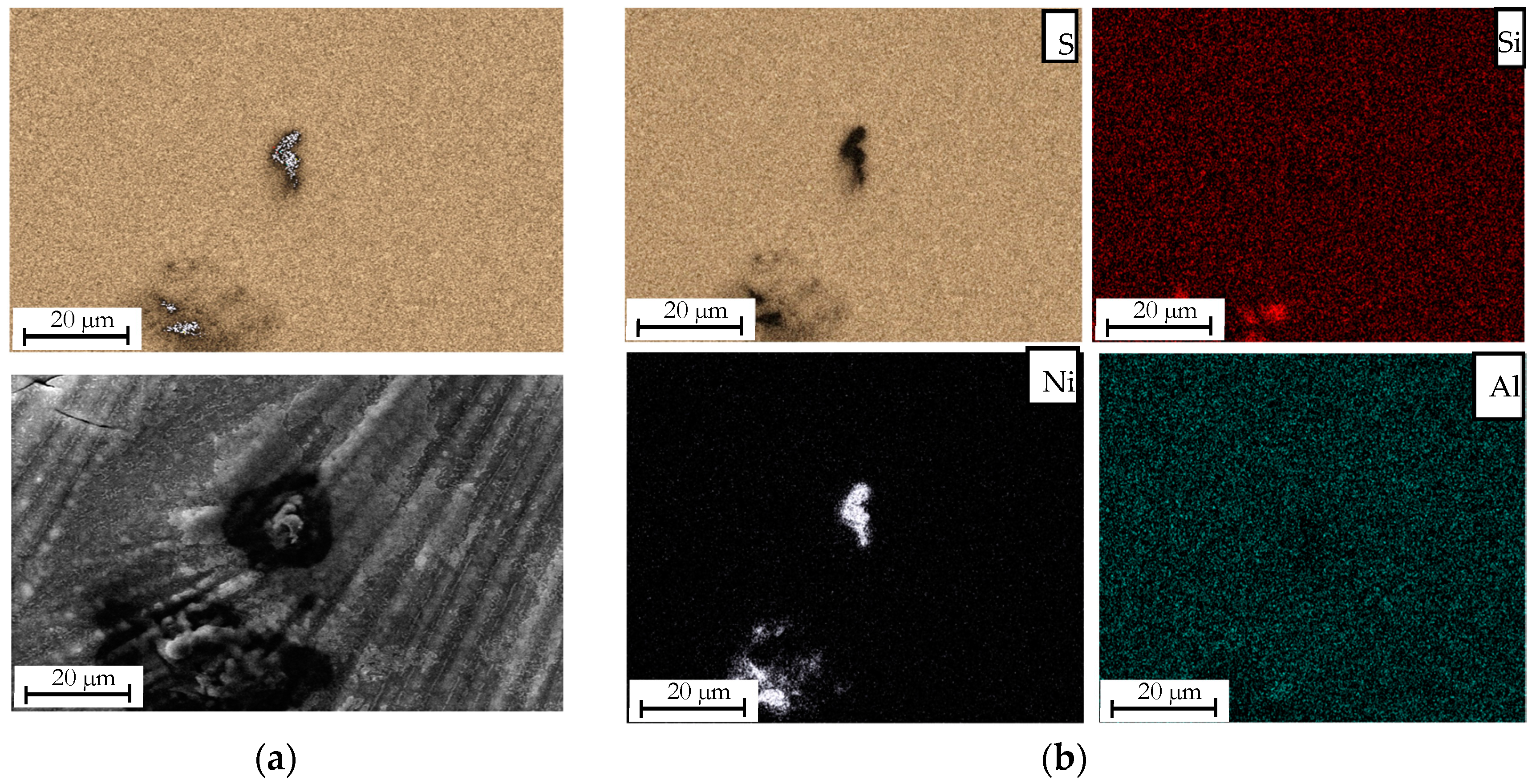
3.1. Effect of the Loading Method on the Morphological Properties of the Composite Electroformed Cathodes
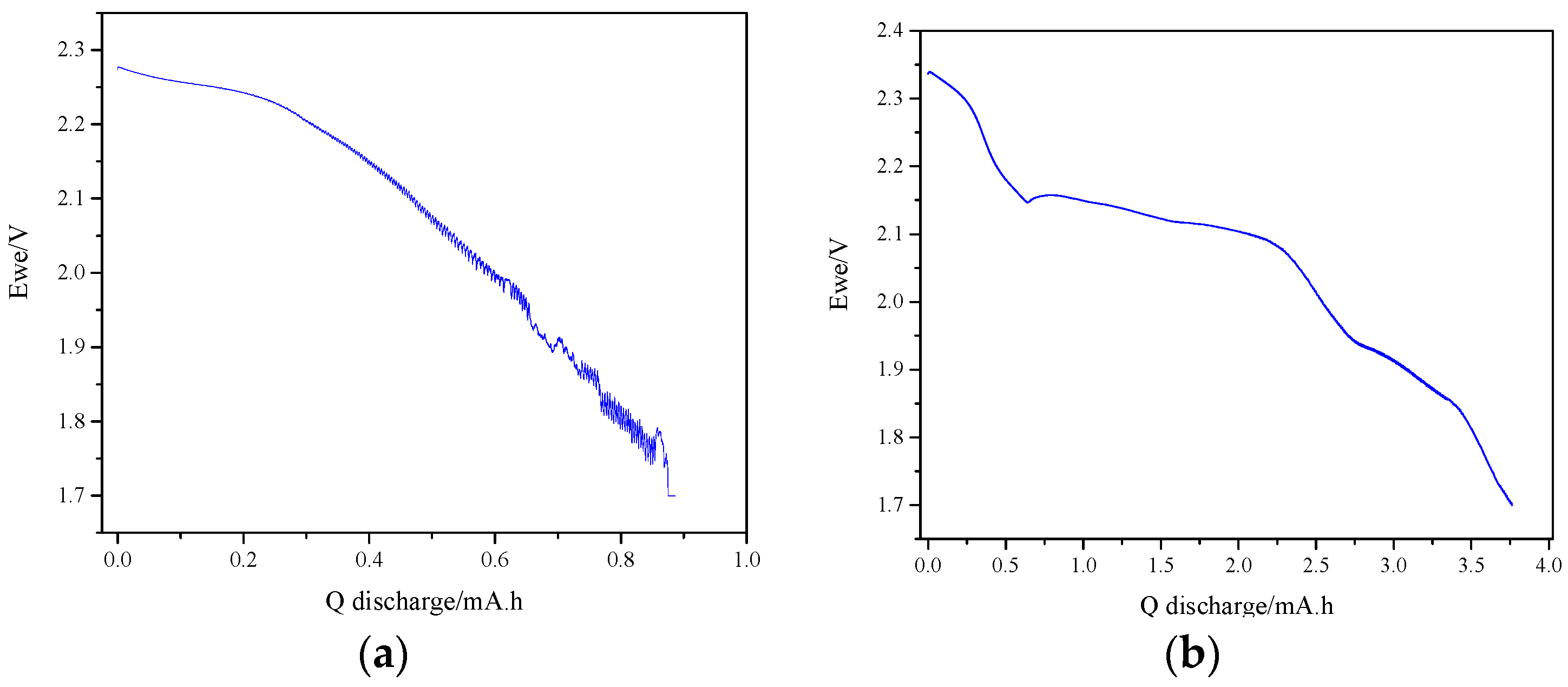
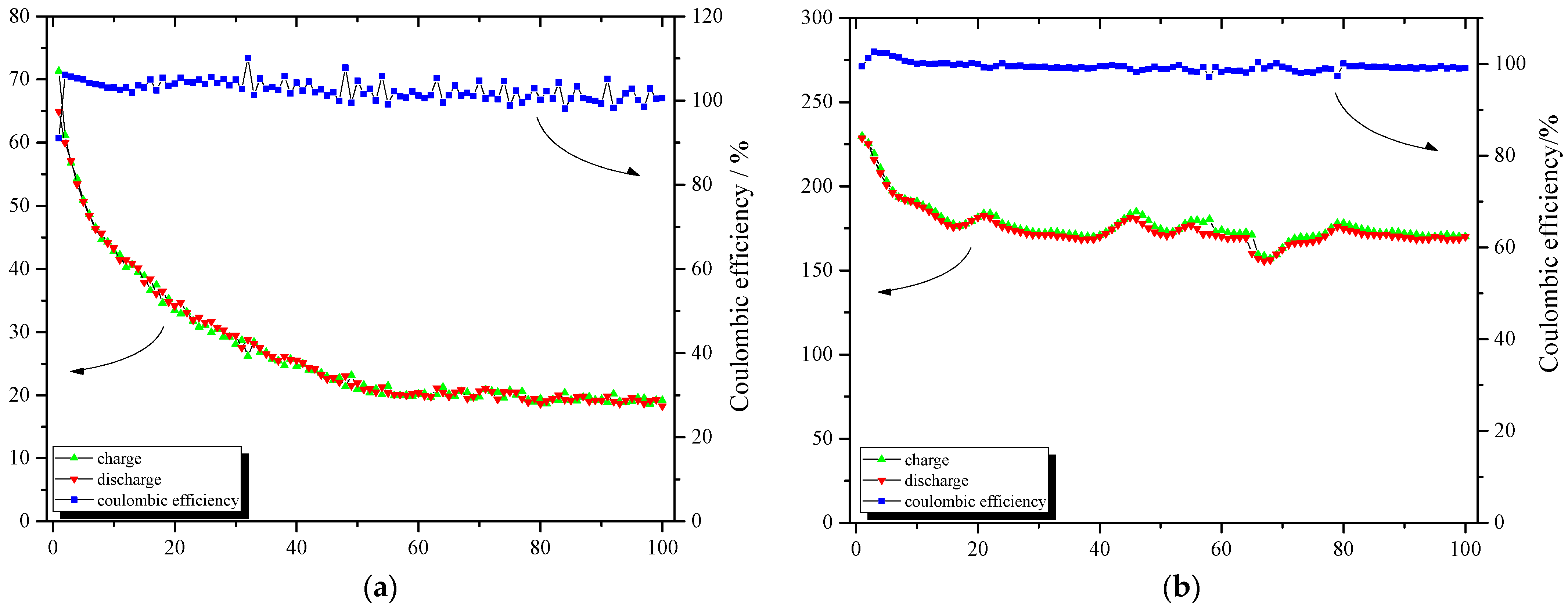
3.2. Effect of the Loading Method on the Electrochemical Performance of the Composite Electroformed Cathodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedorková, A.; Oriňáková, R.; Čech, O.; Sedlaříková, M. New Composite Cathode Materials for Li/S Batteries: A Review. Int. J. Electrochem. Sci. 2013, 8, 10308–10319. [Google Scholar]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Liu, Z.; Zheng, B. Interaction Mechanisms between Lithium Polysulfides/Sulfide and Small Organic Molecules. ACS Omega 2021, 6, 4995–5000. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shaw, L. Recent advances in lithium–sulfur batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Waluś, S.; Offer, G.; Hunt, I.; Patel, Y.; Stockley, T.; Williams, J.; Purkayastha, R. Volumetric expansion of Lithium-Sulfur cell during operation—Fundamental insight into applicable characteristics. Energy Storage Mater. 2018, 10, 233–245. [Google Scholar] [CrossRef]
- Hencz, L.; Chen, H.; Ling, H.; Wang, Y.; Lai, C.; Zhao, H.; Zhang, S. Housing Sulfur in Polymer Composite Frameworks for Li–S Batteries. Nano-Micro Lett. 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörgel, T.; Meinhard, S.; Sörgel, Ş. Film Composite Material. EP 3114721, 16 January 2019. [Google Scholar]
- Erhardt, C.; Sörgel, Ş.; Meinhard, S.; Sörgel, T. Proof of concept for a novel, binder-free and conducting carbon-free sulfur battery cathode: Composite electroformation of copper foil with incorporated polythiophene wrapped sulfur particles. J. Power Sources 2015, 296, 70–77. [Google Scholar] [CrossRef]
- Sörgel, T.; Meyer, J. Chemische und elektrochemische Dispersionsbeschichtung. WOMag 2013, 9, 24–33. [Google Scholar]
- Kang, N.; Lin, Y.; Yang, L.; Lu, D.; Xiao, J.; Qi, Y.; Cai, M. Cathode porosity is a missing key parameter to optimize lithium-sulfur battery energy density. Nat. Commun. 2019, 10, 4597–4607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Choi, S.; Kim, J.; Lee, J. Cryogenic mechanical behavior of 5000- and 6000-series aluminum alloys: Issues on application to offshore plants. Cryogenics 2015, 68, 44–58. [Google Scholar] [CrossRef]
- Lech-Grega, M.; Szymañski, W.; Płonka, B.; Boczkal, S.; Gawlik, M.; Bigaj, M.; Korczak, P. The Structure and Properties of Wrought Aluminium Alloys Series 6xxx with Vanadium for Automotive Industry. In Light Metals 2013; Springer: Cham, Switzerland, 2013; pp. 527–532. [Google Scholar]
- Steier, V.; Ashiuchi, E.; Reißig, L.; Araújo, J. Effect of a deep cryogenic treatment on wear and microstructure of a 6101-aluminum alloy. Adv. Mater. Sci. Eng. 2016, 10, 1155–1167. [Google Scholar]
- Fujii, H.; Oka, T. Development of Lightweight Forged Piston Material by Optimizing Size of Needle-type Intermetallic Compound. SAE Int. J. Mater. 2009, 1, 450–458. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.; Corona, A.; Archer, L. Porous Hollow Carbon@Sulfur Composites for High-Power Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.; Zhang, Y. Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Yamin, H.; Peled, E. Electrochemistry of a nonaqueous lithium/sulfur cell. J. Power Sources 1983, 9, 281–287. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.; He, G.; Parkin, I. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Lu, S.; Chen, Y.; Wu, X.; Wang, Z.; Lingyuan, L.; Qin, W.; Jiang, L. Binder-free cathodes based on sulfur–carbon nanofibers composites for lithium–sulfur batteries. RSC Adv. 2014, 4, 18052. [Google Scholar] [CrossRef]
- Ding, K.; Liu, Q.; Bu, Y.; Meng, K.; Wang, W.; Yuan, D.; Wang, Y. High surface area porous polymer frameworks: Potential host material for lithium-sulfur batteries. J. Alloys Compd. 2016, 657, 626–630. [Google Scholar] [CrossRef]
- Brückner, J.; Thieme, S.; Grossmann, H.; Dörfler, S.; Althues, H.; Kaskel, S. Lithium–sulfur batteries: Influence of C-rate, amount of electrolyte and sulfur loading on cycle performance. J. Power Sources 2014, 268, 82–87. [Google Scholar] [CrossRef]
- Sörgel, Ş.; Kesten, O.; Wengel, A.; Sörgel, T. Nickel/sulfur composite electroplated nickel foams for the use as 3D cathode in lithium/sulfur batteries—A proof of concept. Energy Storage Mater. 2018, 10, 223–232. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Ni concentration from Ni(NH2SO3)2 [g/L] | 110 |
| NiCl2·6H2O concentration [g/L] | 3.3 |
| B(OH)3 [g/L] | 30 |
| AlSi10Mg0.4 concentration [g/L] | 2 |
| pH | 4 |
| Electrolyte temperature [°C] | 50 |
| Substrate rotation speed [rpm] | 1 |
| 30 mm stirring bar rotation speed [rpm] | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mofid, W.; Sörgel, T. Sulfur Loading as a Manufacturing Key Factor of Additive-Free Cathodes for Lithium-Sulfur Batteries Prepared by Composite Electroforming. Energies 2023, 16, 1134. https://doi.org/10.3390/en16031134
El Mofid W, Sörgel T. Sulfur Loading as a Manufacturing Key Factor of Additive-Free Cathodes for Lithium-Sulfur Batteries Prepared by Composite Electroforming. Energies. 2023; 16(3):1134. https://doi.org/10.3390/en16031134
Chicago/Turabian StyleEl Mofid, Wassima, and Timo Sörgel. 2023. "Sulfur Loading as a Manufacturing Key Factor of Additive-Free Cathodes for Lithium-Sulfur Batteries Prepared by Composite Electroforming" Energies 16, no. 3: 1134. https://doi.org/10.3390/en16031134




