A Comparative Analysis of Bio-Oil Collected Using an Electrostatic Precipitator from the Pyrolysis of Douglas Fir, Eucalyptus, and Poplar Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feedstocks Characterization
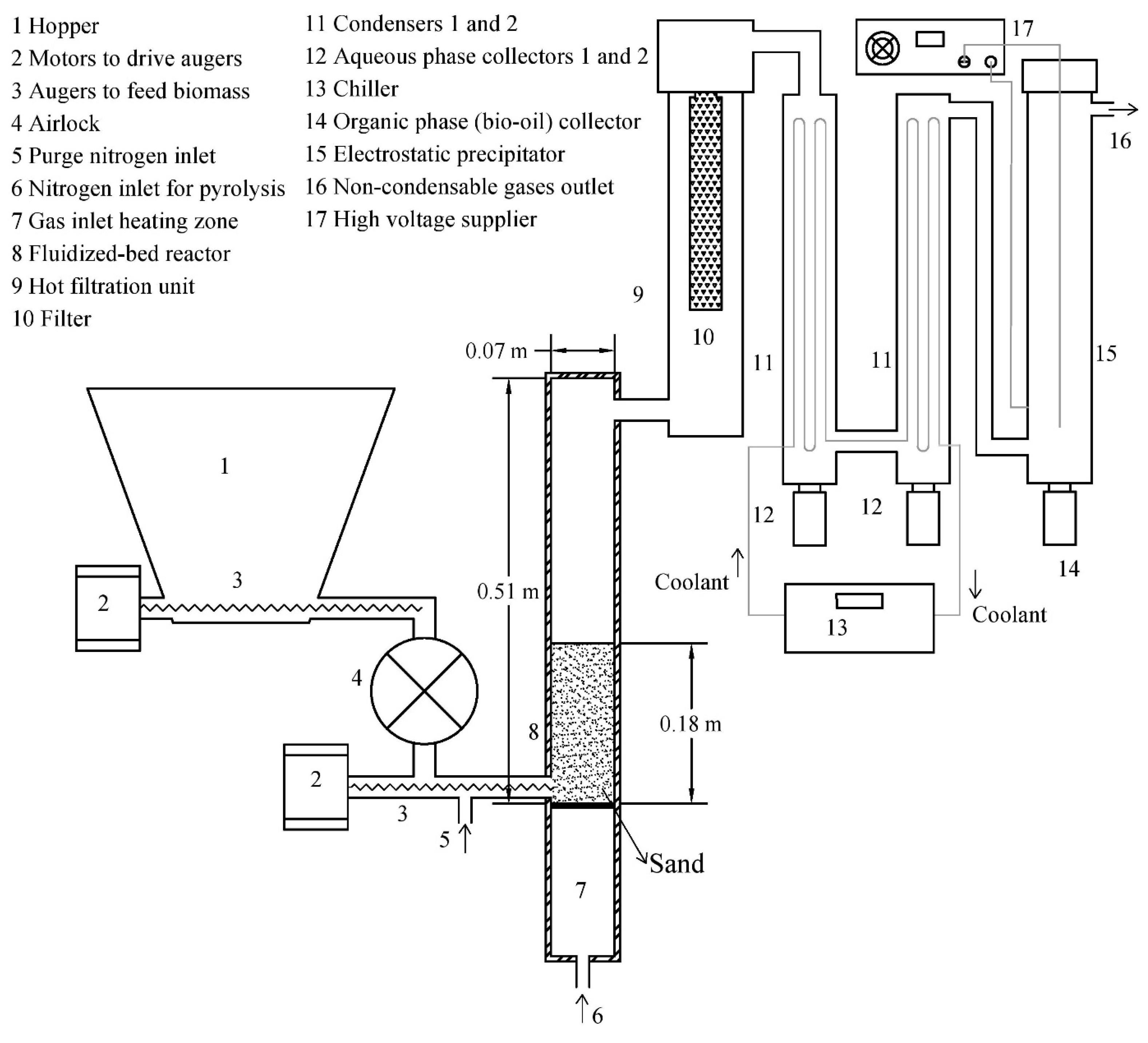
2.3. Pyrolysis Procedure
2.4. Characterization of Pyrolysis Products
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Feedstocks
3.2. Effects of Biomass on Pyrolysis
3.3. Characterization of Bio-Oils
3.3.1. Ultimate and Proximate Analyses
3.3.2. High Heating Value (HHV)
3.3.3. Total Acid Number (TAN)
3.3.4. Density and Kinematic Viscosity Analysis
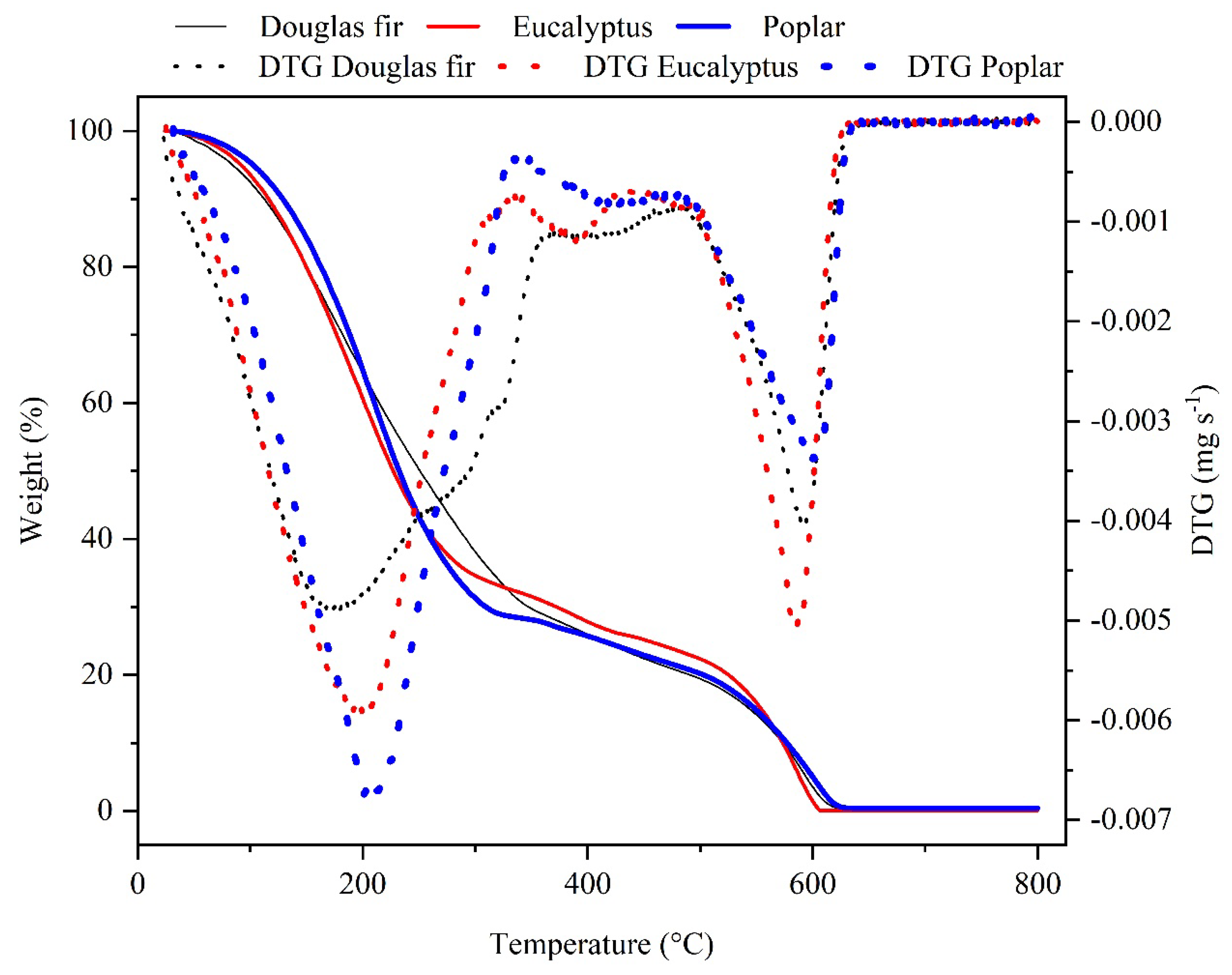
3.3.5. Thermogravimetric Analysis (TGA)
3.3.6. Fourier Infrared Transform (FTIR) Analysis
3.3.7. Gas Chromatography–Mass Spectroscopy (GC-MS) Analysis
3.3.8. Phosphorous Nuclear Magnetic Resonance (31P-NMR) Analysis
3.4. Characterization of Bio-Chars
3.4.1. Ultimate and Proximate Analyses
3.4.2. High Heating Value (HHV)
3.5. Reaction Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barreto, R.A. Fossil Fuels, Alternative Energy and Economic Growth. Econ. Model. 2018, 75, 196–220. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Bansode, A.; Barde, M.; Asafu-Adjaye, O.; Patil, V.; Hinkle, J.; Via, B.K.; Adhikari, S.; Adamczyk, A.J.; Farag, R.; Elder, T.; et al. Synthesis of Biobased Novolac Phenol–Formaldehyde Wood Adhesives from Biorefinery-Derived Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 10990–11002. [Google Scholar] [CrossRef]
- Mu, L.; Dong, Y.; Li, L.; Gu, X.; Shi, Y. Achieving High Value Utilization of Bio-Oil from Lignin Targeting for Advanced Lubrication. ES Mater. Manuf. 2021, 11, 72–80. [Google Scholar] [CrossRef]
- Pappa, C.; Feghali, E.; Vanbroekhoven, K.; Triantafyllidis, K.S. Recent Advances in Epoxy Resins and Composites Derived from Lignin and Related Bio-Oils. Curr. Opin. Green Sustain. Chem. 2022, 38, 100687. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.-C.; Dai, M.; Chen, B.; Qiao, Y.; Deng, H.; Zhang, D.; Zhang, Y.; Villas Bôas de Almeida, C.M.; Chiu, A.S.F.; et al. Shifting from Fossil-Based Economy to Bio-Based Economy: Status Quo, Challenges, and Prospects. Energy 2021, 228, 120533. [Google Scholar] [CrossRef]
- Bhattarai, A.; Kemp, A.; Jahromi, H.; Kafle, S.; Adhikari, S. Thermochemical Characterization and Kinetics of Biomass, Municipal Plastic Waste, and Coal Blends and Their Potential for Energy Generation via Gasification. ACS Omega 2023, 8, 45985–46001. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.; Malolan, R.; AjaySrinivaasan, P. Hydrothermal Liquefaction and Pyrolysis of Amphiroa Fragilissima Biomass: Comparative Study on Oxygen Content and Storage Stability Parameters of Bio-Oil. Bioresour. Technol. Rep. 2020, 11, 100465. [Google Scholar] [CrossRef]
- Liaw, S.-S.; Wang, Z.; Ndegwa, P.; Frear, C.; Ha, S.; Li, C.-Z.; Garcia-Perez, M. Effect of Pyrolysis Temperature on the Yield and Properties of Bio-Oils Obtained from the Auger Pyrolysis of Douglas Fir Wood. J. Anal. Appl. Pyrolysis 2012, 93, 52–62. [Google Scholar] [CrossRef]
- Wang, G.; Fan, B.; Chen, H.; Li, Y. Understanding the Pyrolysis Behavior of Agriculture, Forest and Aquatic Biomass: Products Distribution and Characterization. J. Energy Inst. 2020, 93, 1892–1900. [Google Scholar] [CrossRef]
- Wan Sulaiman, W.R.; Lee, E.S. Pyrolysis of Eucalyptus Wood in a Fluidized-Bed Reactor. Res. Chem. Intermed. 2012, 38, 2025–2039. [Google Scholar] [CrossRef]
- Aboelela, D.; Saleh, H.; Attia, A.M.; Elhenawy, Y.; Majozi, T.; Bassyouni, M. Recent Advances in Biomass Pyrolysis Processes for Bioenergy Production: Optimization of Operating Conditions. Sustainability 2023, 15, 11238. [Google Scholar] [CrossRef]
- Bok, J.P.; Choi, Y.S.; Choi, S.K.; Jeong, Y.W. Fast Pyrolysis of Douglas Fir by Using Tilted-Slide Reactor and Characteristics of Biocrude-Oil Fractions. Renew. Energy 2014, 65, 7–13. [Google Scholar] [CrossRef]
- Heidari, A.; Stahl, R.; Younesi, H.; Rashidi, A.; Troeger, N.; Ghoreyshi, A.A. Effect of Process Conditions on Product Yield and Composition of Fast Pyrolysis of Eucalyptus Grandis in Fluidized Bed Reactor. J. Ind. Eng. Chem. 2014, 20, 2594–2602. [Google Scholar] [CrossRef]
- Oh, S.; Seok Choi, H.; Choi, I.-G.; Weon Choi, J. Evaluation of Hydrodeoxygenation Reactivity of Pyrolysis Bio-Oil with Various Ni-Based Catalysts for Improvement of Fuel Properties. RSC Adv. 2017, 7, 15116–15126. [Google Scholar] [CrossRef]
- Pienihäkkinen, E.; Leijenhorst, E.J.; Wolters, W.; Lindfors, C.; Lahtinen, J.; Ohra-aho, T.; Oasmaa, A. Valorization of Eucalyptus, Giant Reed Arundo, Fiber Sorghum, and Sugarcane Bagasse via Fast Pyrolysis and Subsequent Bio-Oil Gasification. Energy Fuels 2022, 36, 12021–12030. [Google Scholar] [CrossRef]
- Kim, K.H.; Eom, I.Y.; Lee, S.M.; Choi, D.; Yeo, H.; Choi, I.-G.; Choi, J.W. Investigation of Physicochemical Properties of Biooils Produced from Yellow Poplar Wood (Liriodendron Tulipifera) at Various Temperatures and Residence Times. J. Anal. Appl. Pyrolysis 2011, 92, 2–9. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.-K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Hu, S.; Wan, C.; Li, Y. Production and Characterization of Biopolyols and Polyurethane Foams from Crude Glycerol Based Liquefaction of Soybean Straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef]
- Uprety, B.K.; Reddy, J.V.; Dalli, S.S.; Rakshit, S.K. Utilization of Microbial Oil Obtained from Crude Glycerol for the Production of Polyol and Its Subsequent Conversion to Polyurethane Foams. Bioresour. Technol. 2017, 235, 309–315. [Google Scholar] [CrossRef]
- Xu, J.; Brodu, N.; Mignot, M.; Youssef, B.; Taouk, B. Synthesis and Characterization of Phenolic Resins Based on Pyrolysis Bio-Oil Separated by Fractional Condensation and Water Extraction. Biomass Bioenergy 2022, 159, 106393. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Boateng, A.A.; Brown, A.; Timko, M.T. Flash Distillation of Bio-Oils for Simultaneous Production of Hydrocarbons and Green Coke. Ind. Eng. Chem. Res. 2019, 58, 1794–1802. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Park, Y.-K.; Ryu, C.; Suh, D.J.; Suh, Y.-W.; Yim, J.-H.; Kim, S.-S. Bio-Oil Production from Fast Pyrolysis of Waste Furniture Sawdust in a Fluidized Bed. Bioresour. Technol. 2010, 101, S91–S96. [Google Scholar] [CrossRef]
- Alaejos, J.; Tapias, R.; López, F.; Romero, D.; Ruiz, F.; Fernández, M. Biomass Production and Quality of Twelve Fast-Growing Tree Taxa in Short Rotation under Mediterranean Climate. Forests 2023, 14, 1156. [Google Scholar] [CrossRef]
- Coleman, M.D.; Shaw, T.M.; Kimsey, M.J.; Moore, J.A. Nutrition of Douglas-Fir in the Inland Northwest. Soil Sci. Soc. Am. J. 2014, 78, S11–S22. [Google Scholar] [CrossRef]
- Fonseca, F.G.; Funke, A.; Niebel, A.; Soares Dias, A.P.; Dahmen, N. Moisture Content as a Design and Operational Parameter for Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2019, 139, 73–86. [Google Scholar] [CrossRef]
- Uzun, B.B.; Pütün, A.E.; Pütün, E. Composition of Products Obtained via Fast Pyrolysis of Olive-Oil Residue: Effect of Pyrolysis Temperature. J. Anal. Appl. Pyrolysis 2007, 79, 147–153. [Google Scholar] [CrossRef]
- Mariyam, S.; Alherbawi, M.; Pradhan, S.; Al-Ansari, T.; McKay, G. Biochar Yield Prediction Using Response Surface Methodology: Effect of Fixed Carbon and Pyrolysis Operating Conditions. Biomass Convers. Biorefinery 2023, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Khanal, S.K. Bioenergy: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-56831-6. [Google Scholar]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Li, M.; Ragauskas, A.J. Elucidating Structural Characteristics of Biomass Using Solution-State 2 D NMR with a Mixture of Deuterated Dimethylsulfoxide and Hexamethylphosphoramide. ChemSusChem 2016, 9, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Sakhakarmy, M.; Kafle, S.; Adhikari, S. Upcycling of Pine and Sodium Silicate Composites through Pyrolysis: Effects of Pyrolysis Temperature and Sodium Silicate Content. Energy Convers. Manag. X 2024, 23, 100615. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Ragauskas, A.J.; Tuskan, G.A. Poplar as a Feedstock for Biofuels: A Review of Compositional Characteristics. Biofuels Bioprod. Biorefining 2010, 4, 209–226. [Google Scholar] [CrossRef]
- Wu, S.-R.; Chang, C.-C.; Chang, Y.-H.; Wan, H.-P. Comparison of Oil-Tea Shell and Douglas-Fir Sawdust for the Production of Bio-Oils and Chars in a Fluidized-Bed Fast Pyrolysis System. Fuel 2016, 175, 57–63. [Google Scholar] [CrossRef]
- Santos, J.; Ouadi, M.; Jahangiri, H.; Hornung, A. Valorisation of Lignocellulosic Biomass Investigating Different Pyrolysis Temperatures. J. Energy Inst. 2020, 93, 1960–1969. [Google Scholar] [CrossRef]
- Han-u-domlarpyos, V.; Kuchonthara, P.; Reubroycharoen, P.; Hinchiranan, N. Quality Improvement of Oil Palm Shell-Derived Pyrolysis Oil via Catalytic Deoxygenation over NiMoS/γ-Al2O3. Fuel 2015, 143, 512–518. [Google Scholar] [CrossRef]
- Nolte, M.W.; Liberatore, M.W. Viscosity of Biomass Pyrolysis Oils from Various Feedstocks. Energy Fuels 2010, 24, 6601–6608. [Google Scholar] [CrossRef]
- Grønli, M.G.; Várhegyi, G.; Di Blasi, C. Thermogravimetric Analysis and Devolatilization Kinetics of Wood. Ind. Eng. Chem. Res. 2002, 41, 4201–4208. [Google Scholar] [CrossRef]
- Han, S.; Zhang, X.; Wang, R.; Wang, K.; Jiang, J.; Xu, J. Electrocatalytic Conversion of G-Type and S-Type Phenolic Compounds from Different Tree Species in a Heteropolyacid Fluidized System. Chem. Eng. J. 2023, 452, 139299. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Chen, M.; Li, H.; Li, L.; Zhou, Z.; Li, C.; Yang, Z.; Wang, J. Lignin Catalytic Depolymerization for Phenolic Monomers: Boosting the Selective Cleavage of β-O-4 Bonds of Lignin by Mo–O and Al(IV)–O–BO2 Interfacial Sites in B–Mo/Sepiolite. ACS Sustain. Chem. Eng. 2023, 11, 16722–16738. [Google Scholar] [CrossRef]
- Biswas, B.; Sakhakarmy, M.; Rahman, T.; Jahromi, H.; Adhikari, S.; Krishna, B.B.; Bhaskar, T.; Baltrusaitis, J.; Eisa, M.; Kouzehkanan, S.M.T.; et al. Selective Production of Phenolic Monomer via Catalytic Depolymerization of Lignin over Cobalt-Nickel-Zirconium Dioxide Catalyst. Bioresour. Technol. 2024, 398, 130517. [Google Scholar] [CrossRef]
- David, K.; Kosa, M.; Williams, A.; Mayor, R.; Realff, M.; Muzzy, J.; Ragauskas, A. 31P-NMR Analysis of Bio-Oils Obtained from the Pyrolysis of Biomass. Biofuels 2010, 1, 839–845. [Google Scholar] [CrossRef]
- Ben, H.; Wu, F.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A.J. A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks. Polymers 2019, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Ragauskas, A.J. Heteronuclear Single-Quantum Correlation–Nuclear Magnetic Resonance (HSQC–NMR) Fingerprint Analysis of Pyrolysis Oils. Energy Fuels 2011, 25, 5791–5801. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. NMR Characterization of Pyrolysis Oils from Kraft Lignin. Energy Fuels 2011, 25, 2322–2332. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis Polygeneration of Poplar Wood: Effect of Heating Rate and Pyrolysis Temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Yang, X.; Dong, C.; Zhang, Z.; Zhang, X.; Zhu, X. Influence of Pyrolysis Temperature and Time on the Cellulose Fast Pyrolysis Products: Analytical Py-GC/MS Study. J. Anal. Appl. Pyrolysis 2011, 92, 430–438. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef] [PubMed]
- Soest, P.J.V.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Celikbag, Y.; Via, B.K.; Adhikari, S.; Buschle-Diller, G.; Auad, M.L. The effect of ethanol on hydroxyl and carbonyl groups in biopolyol produced by hydrothermal liquefaction of loblolly pine: 31P-NMR and 19F-NMR analysis. Bioresour. Technol. 2016, 214, 37–44. [Google Scholar] [CrossRef]
| Analysis Type | Feedstock | ||
|---|---|---|---|
| Douglas Fir | Eucalyptus | Poplar | |
| Proximate (%) | |||
| Moisture content (wb) # | 8.78 ± 0.79 a | 7.38 ± 0.03 b | 8.37 ± 0.31 ab |
| Volatile content (db) | 90.35 ± 0.19 a | 86.86 ± 0.82 b | 88.41 ± 0.38 ab |
| Ash content (db) | 0.10 ± 0.06 a | 1.15 ± 0.19 b | 0.60 ± 0.17 c |
| Fixed carbon * | 9.55 | 12.07 | 10.99 |
| Ultimate (%, db) | |||
| C | 52.61 ± 0.13 a | 51.00 ± 0.14 b | 51.53 ± 0.18 c |
| H | 7.10 ± 0.34 a | 7.17 ± 0.15 a | 7.33 ± 0.05 a |
| N | 0.25 ± 0.04 | 0.25 ± 0.02 | 0.24 ± 0.01 |
| S | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 |
| O * | 40.03 ± 0.20 | 41.57 ± 0.22 | 40.89± 0.23 |
| Components (%, db, ash free) | |||
| Cellulose | 47.06 | 51.70 | 53.33 |
| Hemicellulose | 10.75 | 12.39 | 13.34 |
| Lignin | 27.43 | 23.80 | 18.45 |
| Biomass Type | Yield (%, Dry Basis) | ||||
|---|---|---|---|---|---|
| Condensed Liquid | Bio-Char | Gas and Loss | |||
| Organic Phase (Bio-Oil) | Aqueous Phase | Total | |||
| Douglas Fir | 16.78 | 21.15 | 37.93 | 23.58 | 38.49 |
| Eucalyptus | 17.75 | 21.87 | 39.62 | 21.13 | 39.25 |
| Poplar | 18.52 | 21.53 | 40.05 | 19.02 | 40.93 |
| Analysis Type | Douglas Fir | Eucalyptus | Poplar | |||
|---|---|---|---|---|---|---|
| Bio-Oil | Bio-Char + | Bio-Oil | Bio-Char + | Bio-Oil | Bio-Char + | |
| Proximate (%) | ||||||
| Aqueous/moisture content (wb) * | 8.36 ± 0.35 | 0.72 ± 0.25 | 6.46 ± 0.26 | 0.60 ± 0.05 | 4.05 ± 0.25 | 0.64 ± 0.13 |
| Volatile content (db) | 98.27 ± 0.30 | 25.94 ± 0.17 | 96.07 ± 0.89 | 12.90 ± 1.22 | 95.20 ± 0.21 | 23.97 ± 0.11 |
| Ash content (db) | 0.02 ± 0.01 | 1.09 ± 0.18 | 0.02 ± 0.00 | 5.56 ± 0.79 | 0.01 ± 0.00 | 3.00 ± 0.38 |
| Fixed carbon (db) ** | 1.71 | 72.97 | 3.91 | 81.55 | 4.79 | 73.04 |
| Ultimate (%, db) | ||||||
| C # | 60.98 ± 0.16 a | 70.32 ± 0.90 | 63.44 ± 0.05 b | 95.16 ± 0.73 | 69.16 ± 0.20 c | 90.35 ± 0.23 |
| H | 6.66 ± 0.26 a | 3.06 ± 0.01 | 6.44 ± 0.17 a | 3.56 ± 0.07 | 6.64 ± 0.29 a | 8.66 ± 0.30 |
| N | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.17 ± 0.01 | 0.41 ± 0.06 | 0.16 ± 0.04 | 0.18 ± 0.08 |
| S | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.03 | 0.08 ± 0.01 |
| O ** | 32.32 ± 0.42 | 26.57 ± 0.66 | 29.89 ± 0.24 | 0.84 ± 0.73 | 24.00 ± 0.51 | 0.73 ± 0.61 |
| HHV (MJ kg−1) | 22.95 ± 1.00 | 28.43 ± 1.09 | 24.26 ± 0.15 | 32.66 ± 2.06 | 25.32 ± 1.92 | 38.23 ± 0.23 |
| TAN (mg KOH g−1) | 56.37 ± 1.41 | 46.91 ± 1.39 | NA | 35.53 ± 0.96 | NA | |
| Density (g cm−3) | 1.22 | 1.17 | NA | 1.17 | NA | |
| Kinematic viscosity (mm2 s−1) | 123.5 ± 0.71 | 83.3 ± 0.85 | NA | 114 ± 0.71 | NA | |
| Compound Type | Bio-Oil Source (Area%) | ||
|---|---|---|---|
| Douglas Fir | Eucalyptus | Poplar | |
| Carbohydrate-derived | 44.48 | 26.42 | 18.94 |
| Lignin-derived | 42.29 | 60.62 | 67.04 |
| H-lignin | 9.59 | 38.77 | 33.74 |
| G-lignin | 32.70 | 17.38 | 22.83 |
| S-lignin | 0.00 | 4.47 | 10.47 |
| Aliphatic OH | C5-Substituted Guaiacol Phenolic OH | Catechol, Guaiacol, and p-Hydroxy-Phenyl OH | Acid-OH | Total * | |
|---|---|---|---|---|---|
| Integration region (ppm) | 150.0–145.0 | 144.7–140.2 | 140.2–137.3 | 136.6–133.6 | 150.0–136.6 |
| Douglas fir | 2.02 ± 0.32 | 0.20 ± 0.04 | 1.25 ± 0.16 | 0.60 ± 0.03 | 4.06 ± 0.18 a |
| Eucalyptus | 1.37 ± 0.42 | 0.51 ± 0.11 | 1.80 ± 0.01 | 0.67 ± 0.01 | 4.35 ± 0.53 a |
| Poplar | 1.69 ± 0.21 | 0.70 ± 0.19 | 3.24 ± 0.30 | 0.43 ± 0.14 | 6.06 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakhakarmy, M.; Kemp, A.; Biswas, B.; Kafle, S.; Adhikari, S. A Comparative Analysis of Bio-Oil Collected Using an Electrostatic Precipitator from the Pyrolysis of Douglas Fir, Eucalyptus, and Poplar Biomass. Energies 2024, 17, 2800. https://doi.org/10.3390/en17122800
Sakhakarmy M, Kemp A, Biswas B, Kafle S, Adhikari S. A Comparative Analysis of Bio-Oil Collected Using an Electrostatic Precipitator from the Pyrolysis of Douglas Fir, Eucalyptus, and Poplar Biomass. Energies. 2024; 17(12):2800. https://doi.org/10.3390/en17122800
Chicago/Turabian StyleSakhakarmy, Manish, Ayden Kemp, Bijoy Biswas, Sagar Kafle, and Sushil Adhikari. 2024. "A Comparative Analysis of Bio-Oil Collected Using an Electrostatic Precipitator from the Pyrolysis of Douglas Fir, Eucalyptus, and Poplar Biomass" Energies 17, no. 12: 2800. https://doi.org/10.3390/en17122800
APA StyleSakhakarmy, M., Kemp, A., Biswas, B., Kafle, S., & Adhikari, S. (2024). A Comparative Analysis of Bio-Oil Collected Using an Electrostatic Precipitator from the Pyrolysis of Douglas Fir, Eucalyptus, and Poplar Biomass. Energies, 17(12), 2800. https://doi.org/10.3390/en17122800









