The Role of Solar Concentrators in Photocatalytic Wastewater Treatment
Abstract
1. Introduction
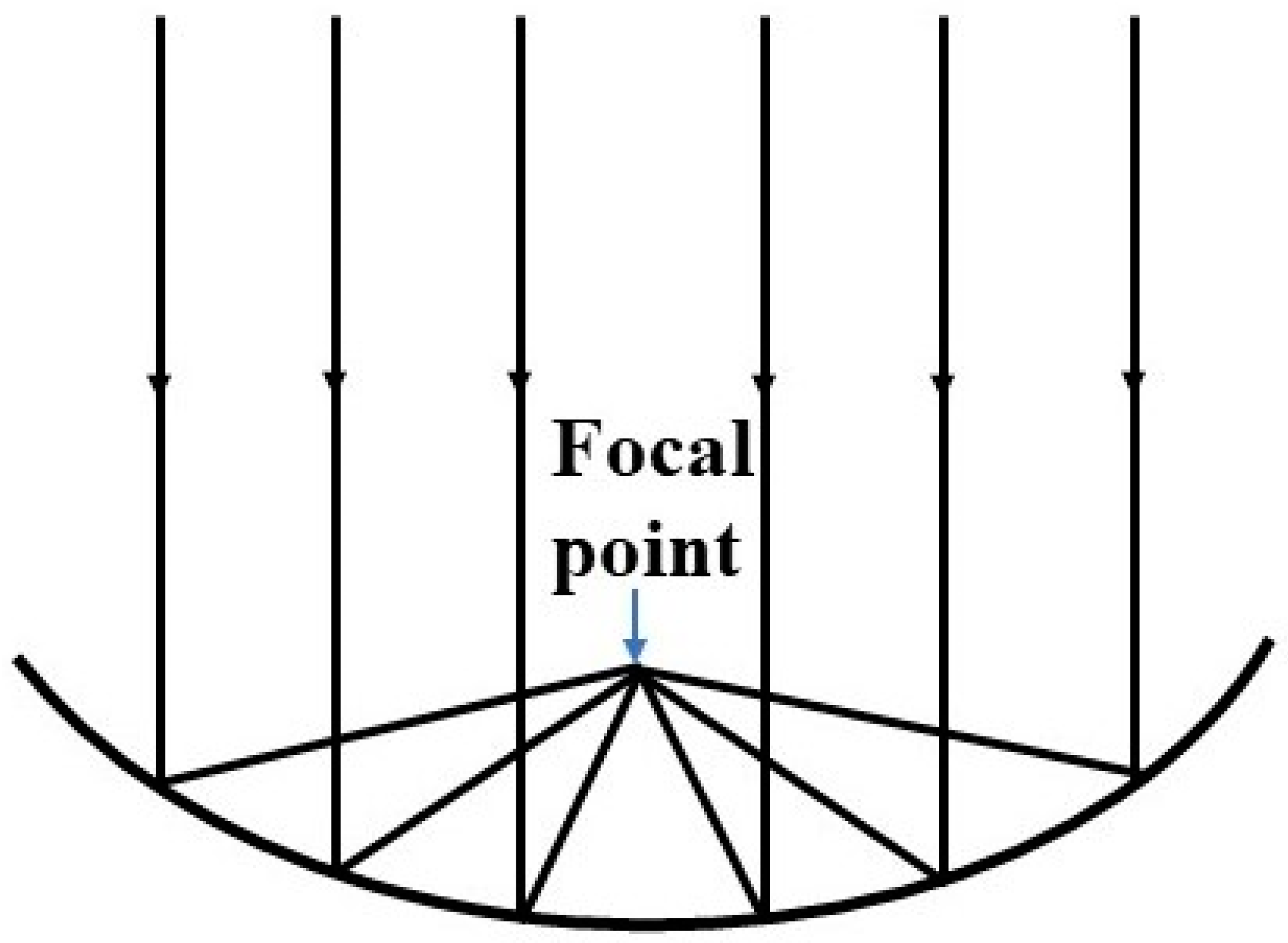
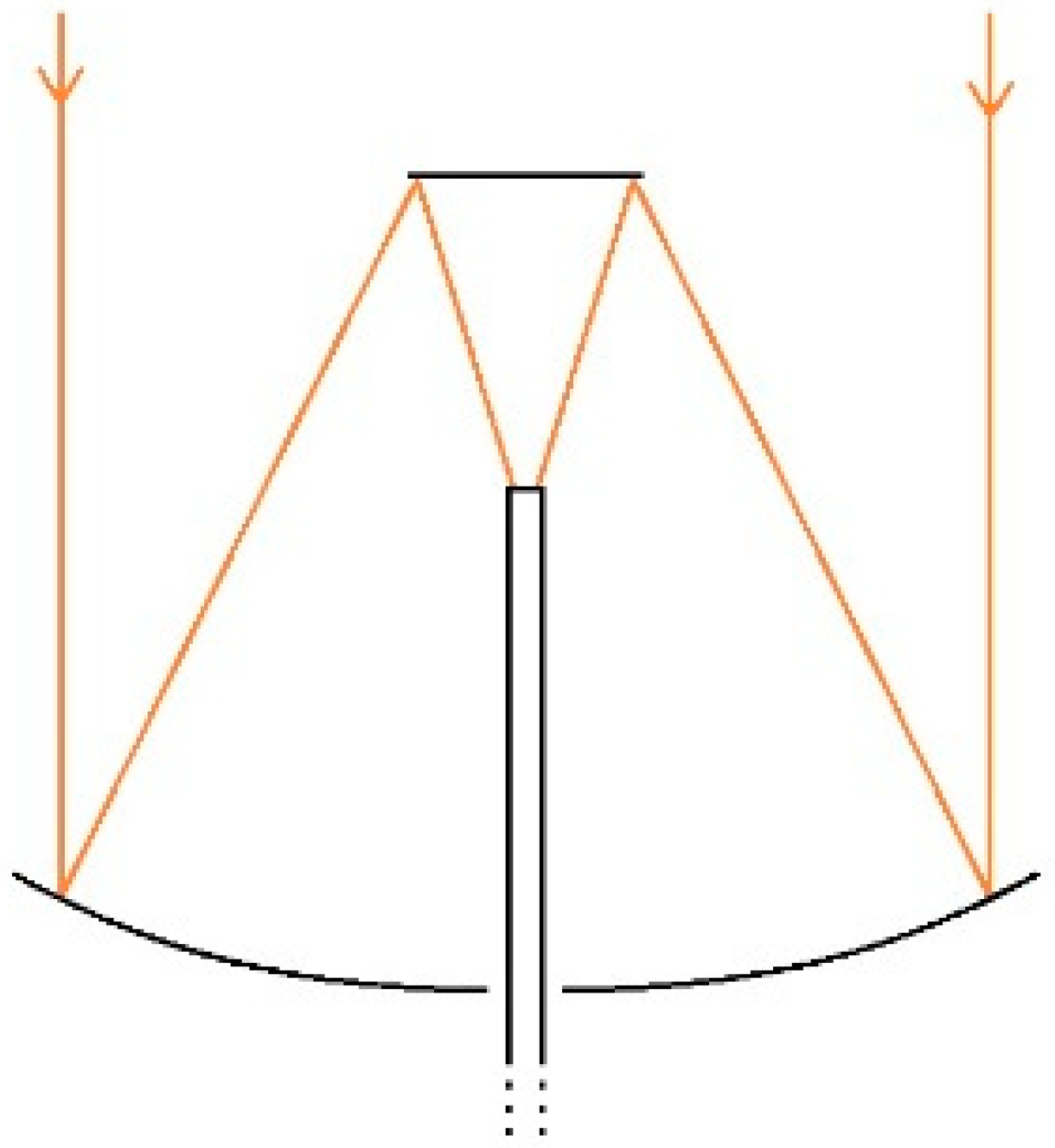
2. Solar Concentrator
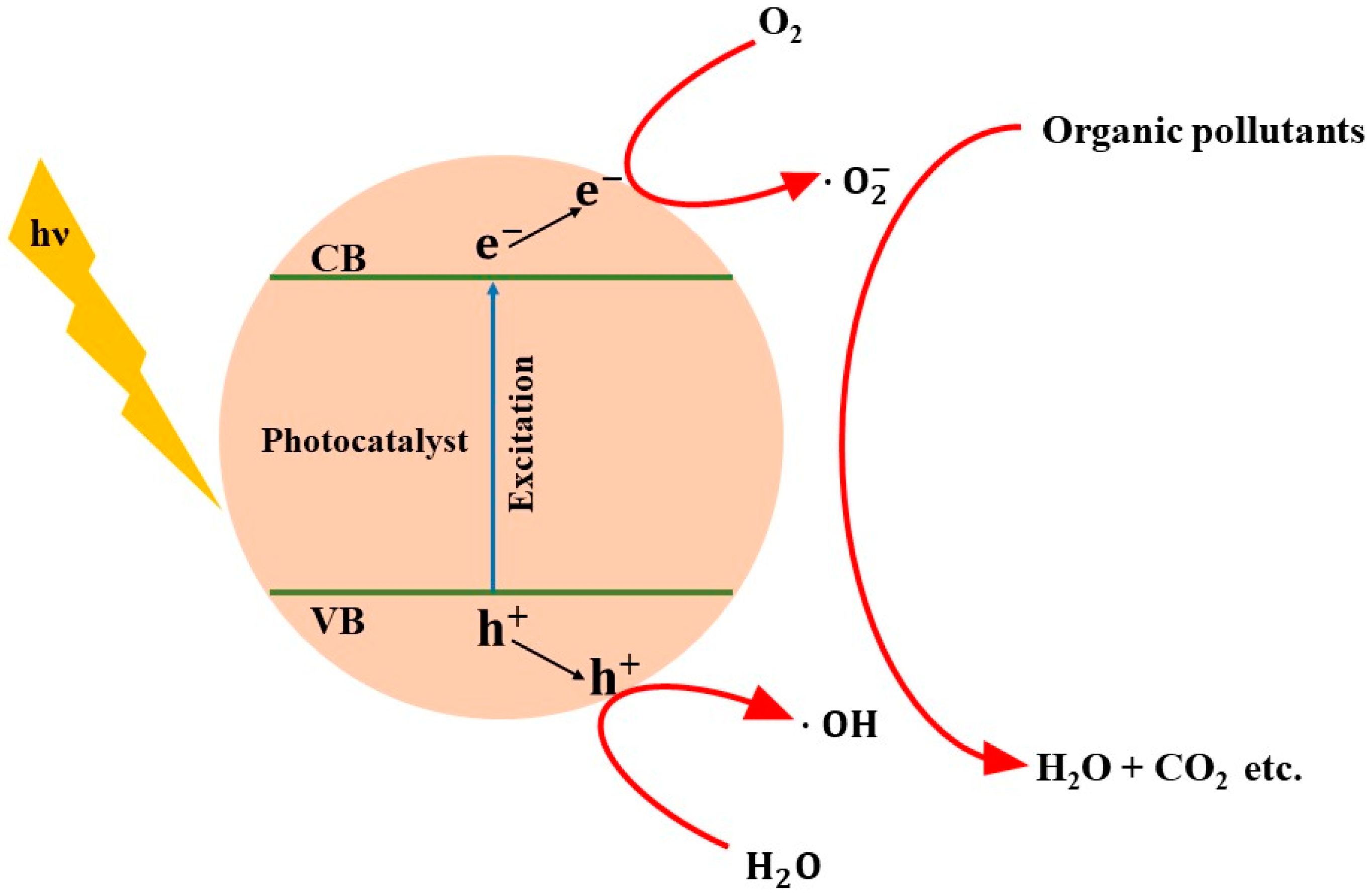
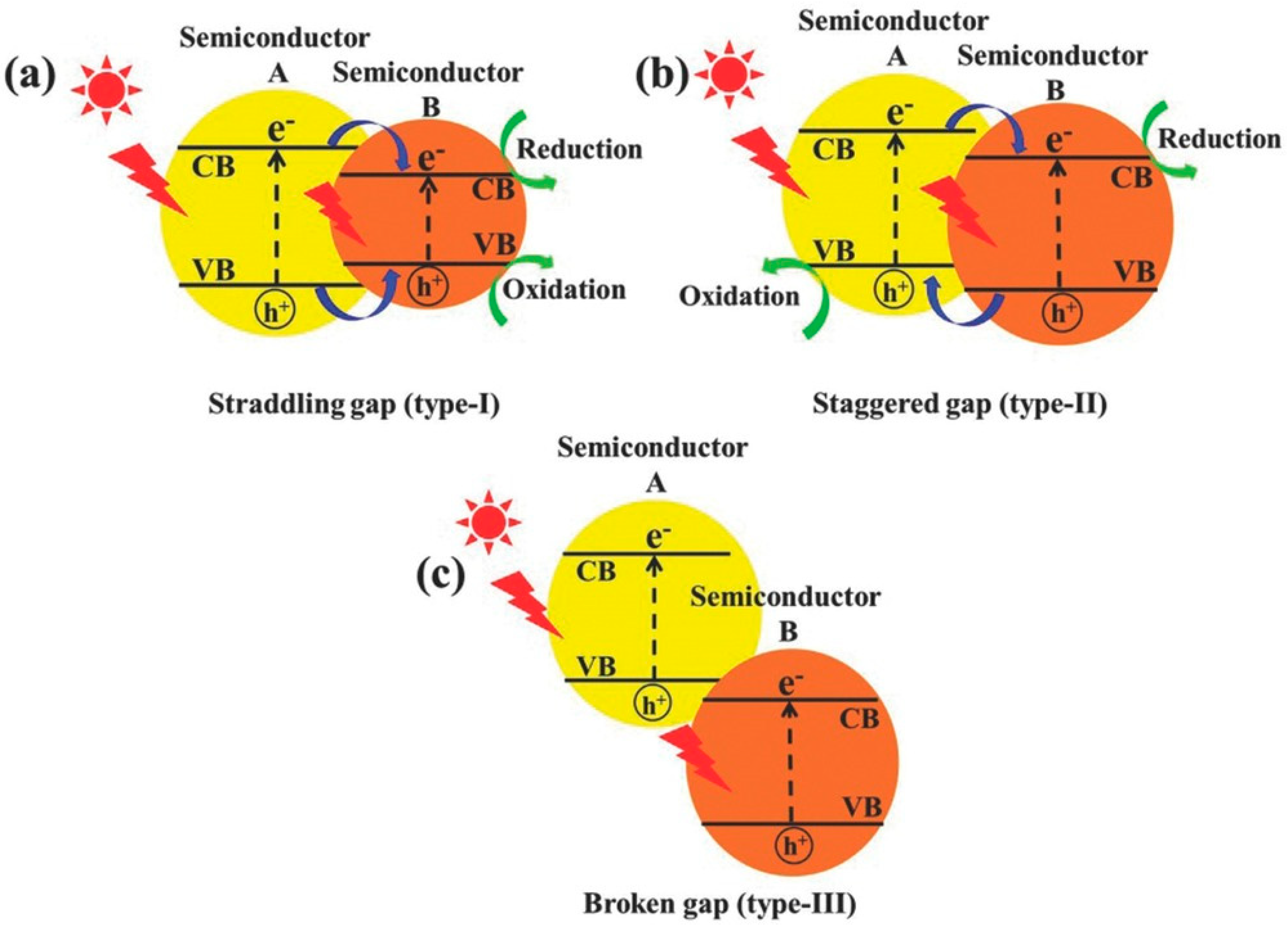
3. Photocatalysis
4. Solar Concentrators for Water Treatment
5. Advantages and Disadvantages of Using a Solar Concentrator
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Roy, J.S.; Bhattacharya, G.; Chauhan, D.; Deshmukh, S.; Upadhyay, R.; Priyadarshini, R.; Sinha Roy, S. Potential use of smartly engineered red mud nanoparticles for removal of arsenate and pathogens from drinking water. SN Appl. Sci. 2020, 2, 796. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef] [PubMed]
- Gitis, V.; Hankins, N. Water treatment chemicals: Trends and challenges. J. Water Process. Eng. 2018, 25, 34–38. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: Kinetic and thermodynamic studies. Desalination 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Roy, J.S.; Morency, S.; Messaddeq, Y. Ultrafast cleaning of methylene blue contaminated water accelarating photocatalytic reaction rate of the BiVO4 nanoflakes under highly intense sunlight irradiation. J. Photochem. Photobiol. 2021, 7, 100037. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.S.; Dugas, G.; Morency, S.; Messaddeq, Y. Rapid degradation of Rhodamine B using enhanced photocatalytic activity of MoS2 nanoflowers under concentrated sunlight irradiation. Phys. E Low-Dimens. Syst. Nanostructures 2020, 120, 114114. [Google Scholar] [CrossRef]
- Roy, J.S.; Morency, S.; Dugas, G.; Messaddeq, Y. Development of an extremely concentrated solar energy delivery system using silica optical fiber bundle for deployment of solar energy: Daylighting to photocatalytic wastewater treatment. Sol. Energy 2021, 214, 93–100. [Google Scholar] [CrossRef]
- Pandey, A.K.; Reji Kumar, R.; Kalidasan, B.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V.V. Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manag. 2021, 297, 113300. [Google Scholar] [CrossRef]
- Muscetta, M.; Ganguly, P.; Clarizia, L. Solar-powered photocatalysis in water purification: Applications and commercialization challenges. J. Environ. Chem. Eng. 2024, 12, 113073. [Google Scholar] [CrossRef]
- Ray, S.K.; Cho, J.; Hur, J. A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manag. 2021, 290, 112679. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Cao, S. Advances in designing heterojunction photocatalytic materials. Chin. J. Catal. 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Proc. Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Lovegrove, K.; Burgess, G.; Pye, J. A new 500 m2 paraboloid dish solar concentrator. Sol. Energy 2011, 85, 620–626. [Google Scholar] [CrossRef]
- Baharoon, D.A.; Rahman, H.A.; Omar, W.Z.W.; Fadhl, S.O. Historical development of concentrating solar power technologies to generate clean electricity efficiently—A review. Renew. Sustain. Energy Rev. 2015, 41, 996–1027. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Wang, J.T.; Wang, W.; Song, J. Realization of high flux daylighting via optical fibers using large Fresnel lens. Sol. Energy 2019, 183, 204–211. [Google Scholar] [CrossRef]
- Chafie, M.; Fadhel, M.; Aissa, B.; Guizani, A. Energetic end exergetic performance of a parabolic trough collector receiver: An experimental study. J. Clean. Prod. 2018, 171, 285–296. [Google Scholar] [CrossRef]
- Tanveer, M.; Guyer, G.T. Solar assisted photo degradation of wastewater by compound parabolic collectors: Review of design and operational parameters. Renew. Sustain. Energy Rev. 2013, 24, 534–543. [Google Scholar] [CrossRef]
- Bigoni, R.; Kötzsch, S.; Sorlini, S.; Egli, T. Solar water disinfection by a Parabolic Trough Concentrator (PTC): Flow-cytometric analysis of bacterial inactivation. J. Clean. Prod. 2014, 67, 62–71. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Duran, A. Fresnel lens to concentrate solar energy for the photocatalytic decoloration and mineralization of orange II in aqueous solution. Chemosphere 2006, 65, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.S.; Dugas, G.; Morency, S.; Ribeiro, S.J.L.; Messaddeq, Y. Enhanced photocatalytic activity of silver vanadate nanobelts in concentrated sunlight delivered through optical fiber bundle coupled with solar concentrator. SN Appl. Sci. 2020, 2, 185. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Synthesis, characterization and photocatalytic property of novel ZnO/bone char composite. Mater. Res. Bull. 2018, 102, 45–50. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Highly efficient photocatalytic degradation of methylene blue by P2ABSA-modified TiO2 nanocomposite due to the photosensitization synergetic effect of TiO2 and P2ABSA. RSC Adv. 2017, 7, 23699–23708. [Google Scholar] [CrossRef]
- Mishra, B.P.; Parida, K. Orienting Z scheme charge transfer in graphitic carbon nitride-based systems for photocatalytic energy and environmental applications. J. Mater. Chem. 2021, 9, 10039–10080. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Jingyu, H.; Ran, Y.; Zhaohui, L.; Yuanqiang, S.; Lingbo, Q.; Nti Kani, A. In-situ growth zinc oxide globular on the graphitic carbon nitride to fabrication binary heterojunctions and their photocatalytic degradation performance for the tetracycline. Solid State Sci. 2019, 92, 60–67. [Google Scholar] [CrossRef]
- Roy, J.S.; Pal Majumder, T.; Dabrowski, R. Photoluminescence behavior of TiO2 nanoparticles doped with liquid crystals. J. Mol. Struc. 2015, 1098, 351–354. [Google Scholar] [CrossRef]
- Baruah, S.; Afre, R.A.; Pugliese, D. Effect of Size and Morphology of Different ZnO Nanostructures on the Performance of Dye-Sensitized Solar Cells. Energies 2024, 17, 2076. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Y.; Liu, X.; Xue, H.; Qian, Q.; Chen, Q. Design of Cu–Ce co-doped TiO2 for improved photocatalysis. J. Mater. Sci. 2017, 52, 1265–1271. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Zhang, W.; Cao, P. Synthesis, characterization, and photocatalytic performance of Cu/Y co-doped TiO2 nanoparticles. Mater. Chem. Phys. 2022, 277, 125558. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar]
- Zhang, Z.; Wang, W.; Gao, E. Polypyrrole/Bi2WO6 composite with high charge separation efficiency and enhanced photocatalytic activity. J. Mater. Sci. 2014, 49, 7325–7332. [Google Scholar] [CrossRef]
- Vu, T.A.; Dao, C.D.; Hoang, T.T.T.; Dang, P.T.; Tran, H.T.K.; Nguyen, K.T.; Le, G.H.; Nguyen, T.V.; Lee, G.D. Synthesis of novel silver vanadates with high photocatalytic and antibacterial activities. Mater. Lett. 2014, 123, 176–180. [Google Scholar] [CrossRef]
- Choudhary, S.; Bisht, A.; Mohapatra, S. Facile synthesis, morphological, structural, photocatalytic and optical properties of CoFe2O4 nanostructures. SN Appl. Sci. 2019, 1, 1613. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Ahamad, T.; Al-Saeedi, S.I.; Al-Senani, G.M.; Al-kadhi, N.S.; Stadler, F.J. Facile fabrication of Zr2Ni1Cu7 trimetallic nano-alloy and its composite with Si3N4 for visible light assisted photodegradation of methylene blue. J. Mol. Liq. 2018, 272, 170–179. [Google Scholar] [CrossRef]
- Wu, S.Z.; Li, K.; Zhang, W.D. On the heterostructured photocatalysts Ag3VO4/g-C3N4 with enhanced visible light photocatalytic activity. App. Surf. Sci. 2015, 324, 324–331. [Google Scholar] [CrossRef]
- Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation. Energies 2021, 14, 7223. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Singh Bhadwal, A.; Tripathi, R.M.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv. 2014, 4, 9484–9490. [Google Scholar] [CrossRef]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Hossain, S.F.; Mukherjee, S.K. Toxicity of cadmium sulfide (CdS) nanoparticles against Escherichia coli and HeLa cells. J. Hazard. Mater. 2013, 260, 1073–1082. [Google Scholar] [CrossRef]
- Llobet, J.M.; Domingo, J.L. Acute toxicity of vanadium compounds in rats and mice. Toxicol. Lett. 1984, 23, 227–231. [Google Scholar] [CrossRef]
- Wörle-Knirsch, J.M.; Kern, K.; Schleh, C.; Adelhelm, C.; Feldmann, C.; Krug, H.F. Nanoparticulate Vanadium Oxide Potentiated Vanadium Toxicity in Human Lung Cells. Environ. Sci. Technol. 2007, 41, 331–336. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.-C. Solvothermal synthesis of facet-dependent BiVO4 photocatalyst with enhanced visible-light-driven photocatalytic degradation of organic pollutant: Assessment of toxicity by zebrafish embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef]
- Ghaware, R.C.; Birajdar, N.B.; Kamble, G.S.; Kolekar, S.S. Degradation of organic Pollutant by Using of BiVO4–NiFe2O4 Heterostructure Photocatalyst under Visible Light Irradiation: Assessment of Detoxicity Study Using Cirrhinus mrigala. Langmuir 2024, 40, 14426–14439. [Google Scholar] [CrossRef] [PubMed]
- Tojo, F.; Ishizaki, M.; Kubota, S.; Kurihara, M.; Hirose, F.; Ahmmad, B. Histidine Decorated Nanoparticles of CdS for Highly Efficient H2 Production via Water Splitting. Energies 2020, 13, 3738. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Rebekah, A.; Lee, K.S. Two-stage hydrothermal process driven visible light sensitive photocatalytic m-ZnWO4/m-WO3 heterojunction composite materials. APL Mater. 2024, 12, 041122. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Nie, H.; Kong, B. The direct Z-scheme character and roles of S vacancy in BiOCl/Bi2S3-(001) heterostructures for superior photocatalytic activity: A hybrid density functional investigation. Phys. Chem. Chem. Phys. 2024, 26, 10723–10736. [Google Scholar] [CrossRef] [PubMed]
- Guernanou, R.; Sadek, I.; Belgassim, B.; Lamine, A.; Aïcha, S.; Feriel, K.; Fatima, B. Solar wastewater treatment: Advantages and efficiency for reuse in agriculture and industry. In Proceedings of the International Renewable and Sustainable Energy Conference (IRSEC), Agadir, Morocco, 27–30 November 2019. [Google Scholar] [CrossRef]
- Golli, A.E.; Fendrich, M.; Bazzanella, N.; Dridi, C.; Miotello, A.; Orlandi, M. Wastewater remediation with ZnO photocatalysts: Green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 2021, 286, 112226. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Fernández, G.; Klamerth, N.; Maldonado, M.I.; Álvarez, P.M.; Malato, S. Efficiency of different solar advanced oxidation processes on the oxidation of bisphenol A in water. Appl. Catal. B Environ. 2010, 95, 228–237. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martínez, C.M.; Flores, P.; Navarro, S. Semiconductor oxides-sensitized photodegradation of fenamiphos in leaching water under natural sunlight. Appl. Catal. B Environ. 2012, 115–116, 31–37. [Google Scholar] [CrossRef]
- Barwal, A.; Chaudhary, R. Feasibility study for the treatment of municipal wastewater by using a hybrid bio-solar process. J. Environ. Manag. 2016, 177, 271–277. [Google Scholar] [CrossRef]
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar photocatalytic disinfection of agricultural pathogenic fungi (Curvularia sp.) in real urban wastewater. Sci. Total. Environ. 2017, 607–608, 1213–1224. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Polo-López, M.I.; Mosteo, R.; Ormad, M.P.; Fernández-Ibáñez, P. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2014, 150–151, 619–629. [Google Scholar] [CrossRef]
- Onotri, L.; Race, M.; Clarizia, L.; Guida, M.; Alfè, M.; Andreozzi, R.; Marotta, R. Solar photocatalytic processes for treatment of soil washing wastewater. Chem. Eng. J. 2017, 318, 10–18. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar reclamation of wastewater effluent polluted with bisphenols, phthalates and parabens by photocatalytic treatment with TiO2/Na2S2O8 at pilot plant scale. Chemosphere 2018, 212, 95. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.; Monteagudo, J.M.; San Martín, I. Photocatalytic treatment of an industrial effluent using artificial and solar UV radiation: An operational cost study on a pilot plant scale. J. Environ. Manag. 2012, 98, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, J.; Bayarri, B.; Gonzalez, O.; Malato, S.; Peral, J.; Esplugas, S. Advanced Oxidation Processes at Laboratory Scale: Environmental and Economic Impacts. ACS Sustain. Chem. Eng. 2015, 3, 3188–3196. [Google Scholar] [CrossRef]




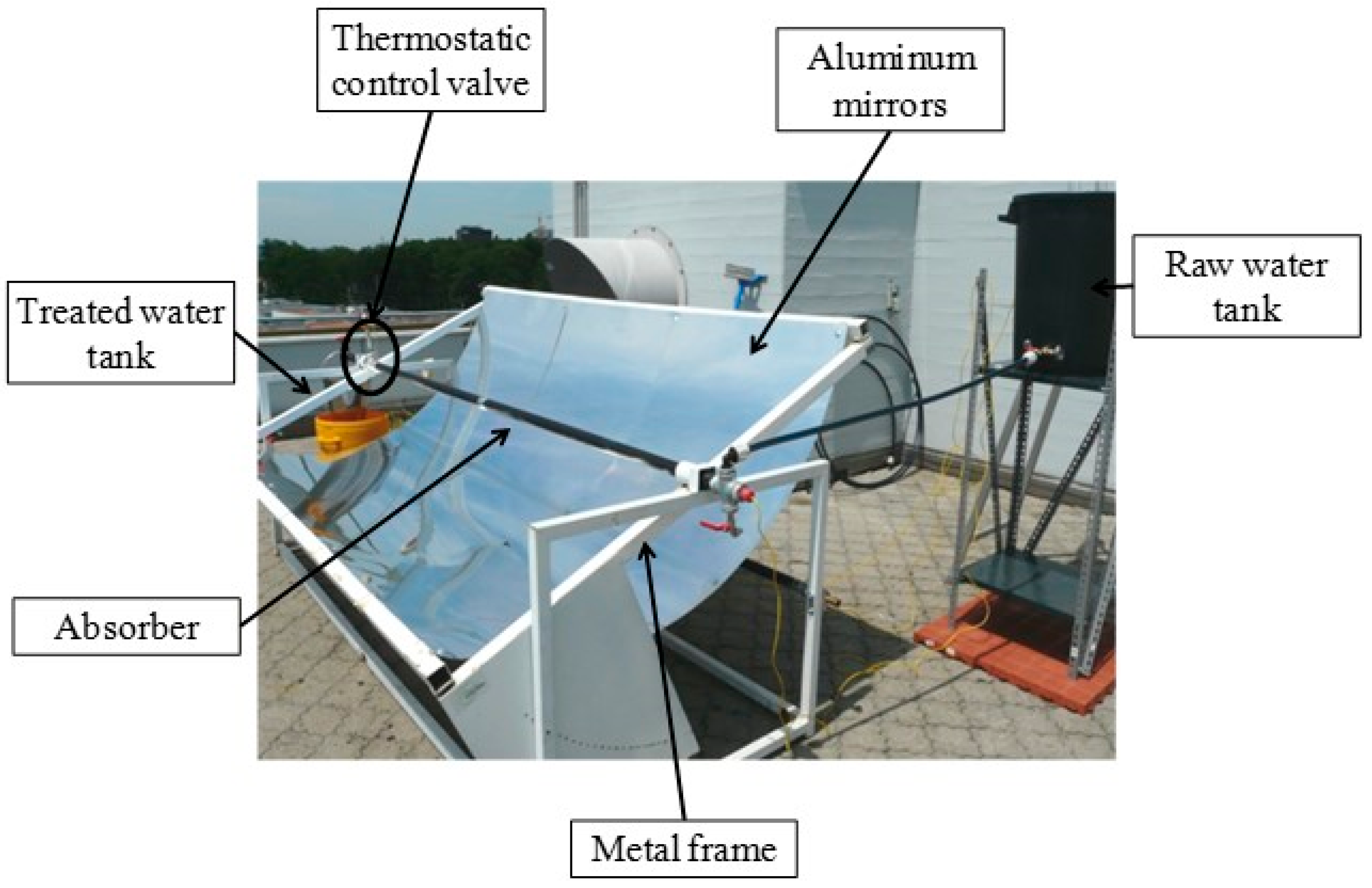
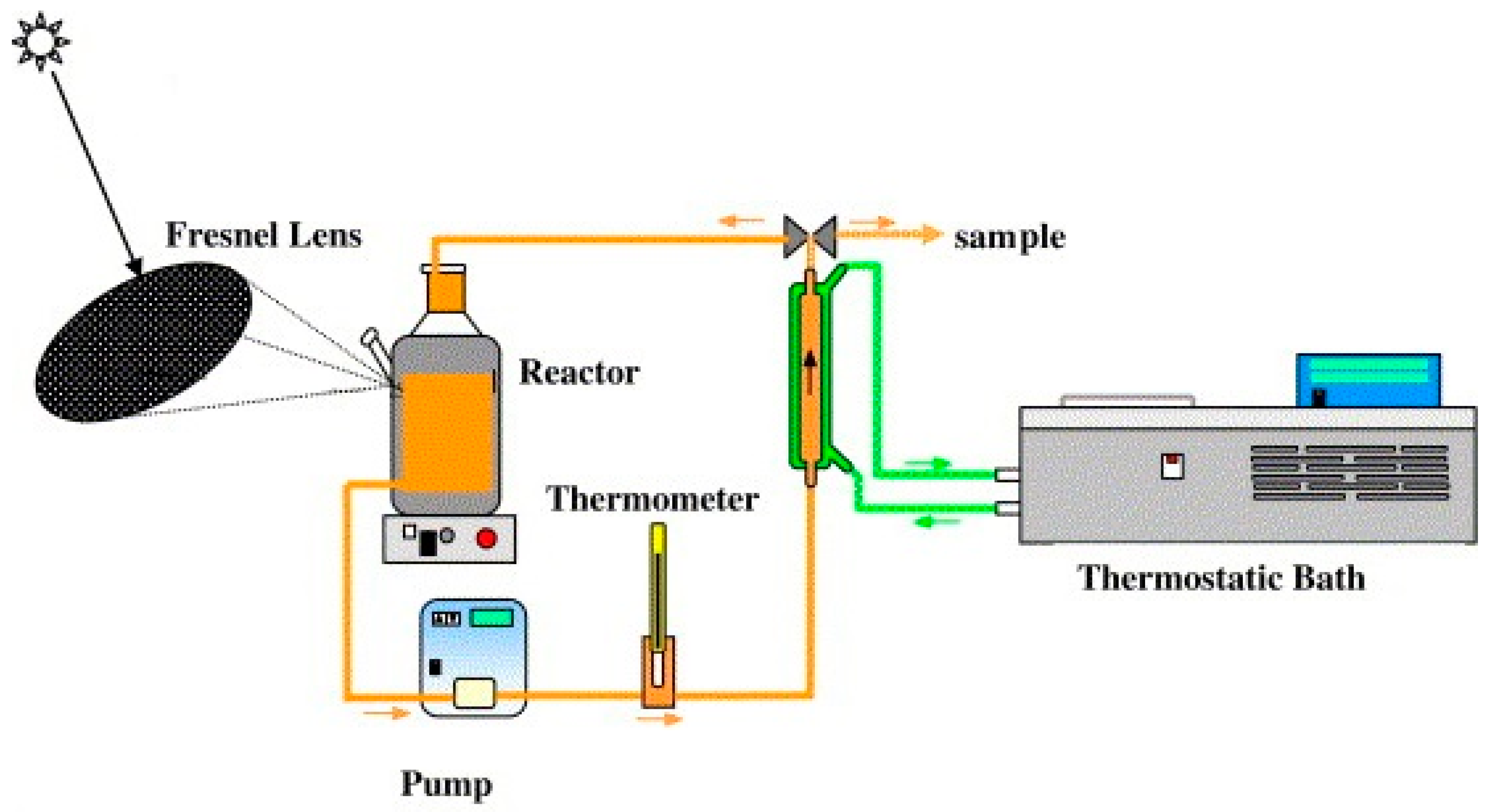




| Solar Collector | Photocatalyst | Pollutant | Reference |
|---|---|---|---|
| CPC | TiO2 | Bisphenol A | Rodriguez et al. [57] |
| CPC | TiO2, ZnO | Fenamiphos pesticide | Fenoll et al. [58] |
| PTC | TiO2 | Urea, ammonium chloride, peptone | Barwal et al. [59] |
| CPC | Ferrous sulfate heptahydrate | Agricultural pathogenic fungi | Aguas et al. [60] |
| CPC | ferrous sulfate in slurry | E. coli | Rodríguez-Chueca et al. [61] |
| CPC | TiO2 | copper, iron, zinc | Onotri et al. [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, J.S.; Messaddeq, Y. The Role of Solar Concentrators in Photocatalytic Wastewater Treatment. Energies 2024, 17, 4001. https://doi.org/10.3390/en17164001
Roy JS, Messaddeq Y. The Role of Solar Concentrators in Photocatalytic Wastewater Treatment. Energies. 2024; 17(16):4001. https://doi.org/10.3390/en17164001
Chicago/Turabian StyleRoy, Joy Sankar, and Younès Messaddeq. 2024. "The Role of Solar Concentrators in Photocatalytic Wastewater Treatment" Energies 17, no. 16: 4001. https://doi.org/10.3390/en17164001
APA StyleRoy, J. S., & Messaddeq, Y. (2024). The Role of Solar Concentrators in Photocatalytic Wastewater Treatment. Energies, 17(16), 4001. https://doi.org/10.3390/en17164001






