Simulation Study on the Heat Transfer Characteristics of Oil Shale under Different In Situ Pyrolysis Methods Based on CT Digital Rock Cores
Abstract
:1. Introduction
2. Material and Experiment
2.1. Sample Preparation
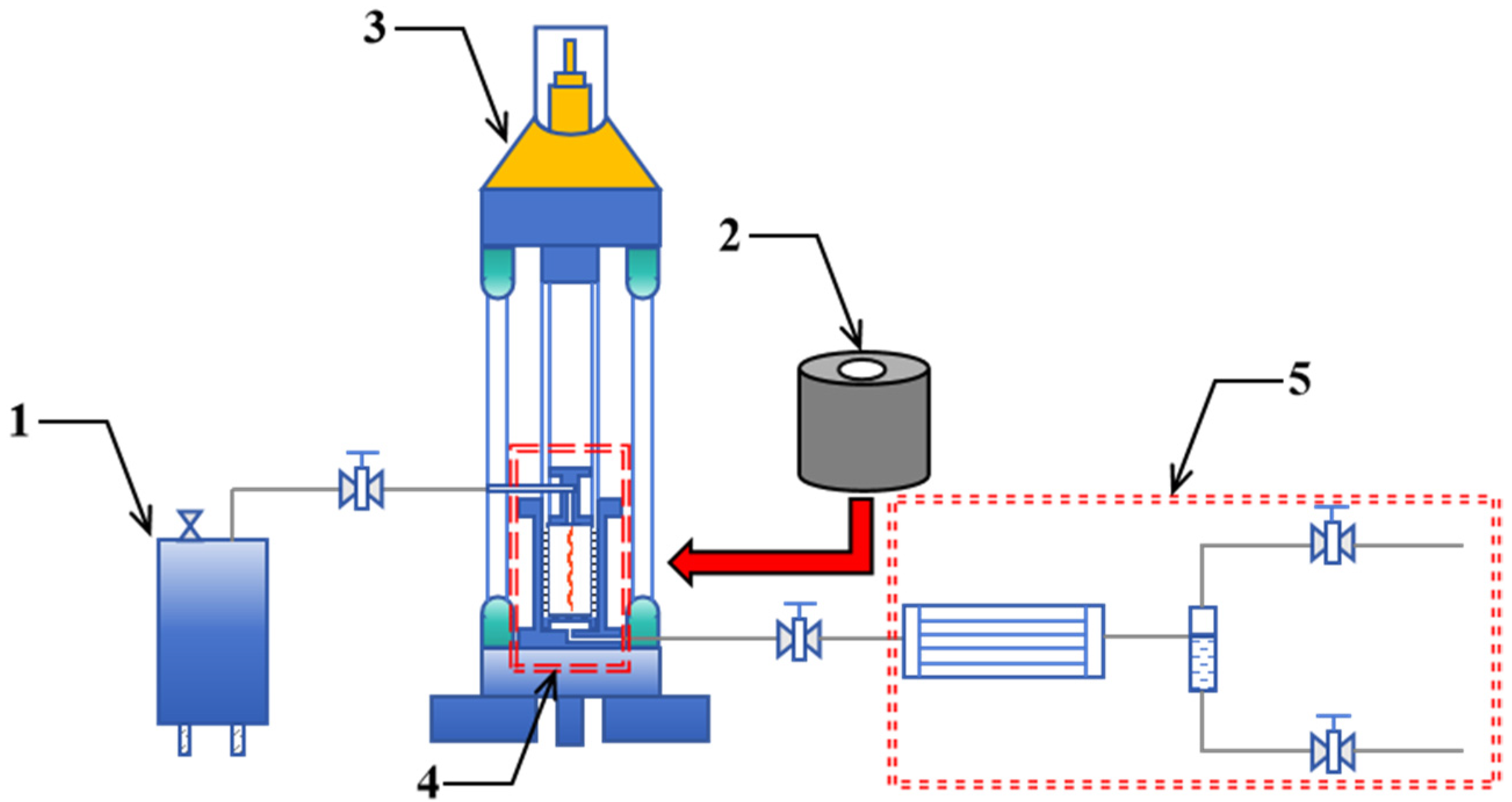
2.2. In Situ Pyrolysis of Oil Shale Using High-Temperature Steam Convective Heating and Electrical Conductive Heating
- Place the pre-prepared split standard samples into the high-temperature in situ pyrolysis reaction vessel. Utilize the servo hydraulic press to apply 5 MPa axial pressure and 6 MPa confining pressure to the samples.
- Close the outlet valve of the high-temperature steam generator. Activate the high-temperature steam generator to prepare 500 °C steam. Once the temperature reaches 500 °C, open the outlet valve to inject the 500 °C high-temperature steam into the high-temperature in situ pyrolysis reaction vessel.
- After the pyrolysis time reaches 3 h, stop injecting high-temperature steam and allow the high-temperature in situ pyrolysis reaction vessel to cool naturally.
- After the reaction vessel cools to room temperature, remove the samples after pyrolysis for micro-CT scanning.
- Place the pre-prepared split standard samples into the high-temperature in situ pyrolysis reaction vessel. Seal the high-temperature steam injection port with a sealed cap. Use the servo hydraulic press to apply 5 MPa axial pressure and 6 MPa confining pressure to the samples.
- Set the temperature of the electric heating mantle to 500 °C, and start the pyrolysis timer once the temperature reaches 500 °C.
- Stop heating after the pyrolysis time reaches 3 h, allowing the high-temperature in situ pyrolysis reaction vessel to cool naturally.
- After the reaction vessel cools to room temperature, remove the samples after pyrolysis for micro-CT scanning.
3. Numerical Simulation Methods
3.1. Basic Assumptions
- Due to the low oil yield of oil shale, the content of oil and gas products is minimal compared to high-temperature steam. Therefore, the flow characteristics of oil and gas products are neglected in the simulation process.
- Neglecting the effect of surface tension between the water and steam phases.
- Assuming the permeability of the solid matrix is zero, with fluids flowing only within the pore structure.
- Fluid flow within the pore structure follows Darcy’s law at a microscale gradient:
- 5.
- The boiling point of water under different pore pressures follows a Gaussian fitting formula [35]:
3.2. Construction of the Physical Model
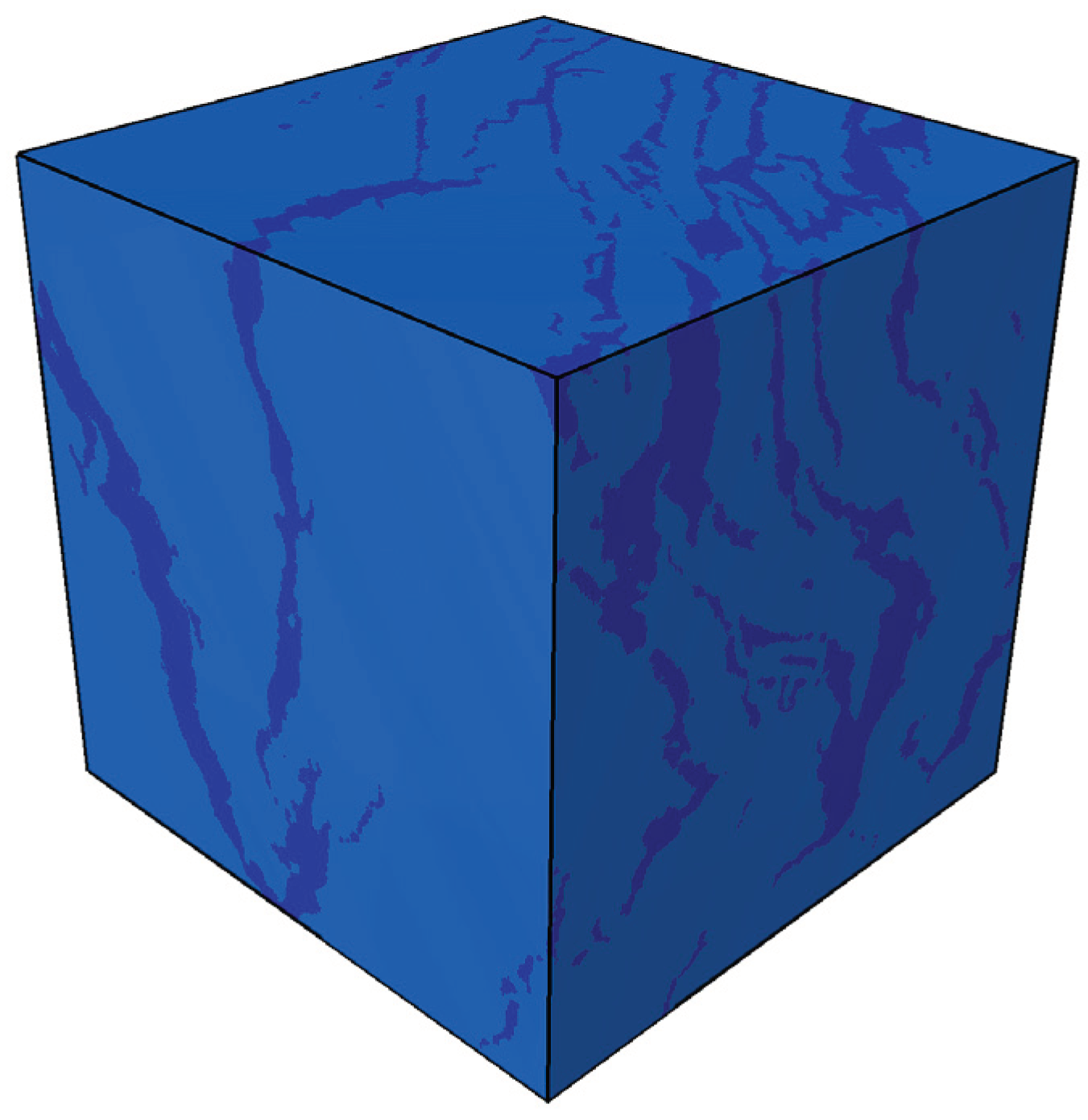
- Following in situ pyrolysis, micro-CT scan data of oil shale are utilized with porosity as a reference indicator to determine the minimum representative elementary volume (REV). Subsequently, threshold segmentation is applied to the REV to achieve the segmentation of solid matrix and pore structures within the oil shale.
- After segmenting the solid matrix and pore structures of the digitized oil shale core, tetrahedral meshing is performed, followed by importation into COMSOL to obtain the required physical model for simulation.
3.3. Control Equations
3.3.1. Fluid Dynamics Equations
3.3.2. Solid Mechanics Equations
3.3.3. Heat Conduction Equation
3.3.4. Coupling Conditions
3.4. Initial and Boundary Condition
- Solid Mechanics:
- Boundary Conditions:
- Stress boundaries at x = 0 µm and x = 1200 µm: σ = 5 MPa;
- Stress boundaries at y = 0 µm, z = 0 µm, y = 1200 µm, and z = 1200 µm: σ = 6 MPa.
- Initial Conditions:
- Initial displacement U = 0 at arbitrary positions.
- 2.
- Non-isothermal Flow (Simulating in situ pyrolysis with high-temperature steam):
- Boundary Conditions:
- Flow inlet boundary at z = 0 µm: Fluid inlet with pin = 2 MPa, Tin = 500 °C
- Flow outlet boundary at z = 1200 µm: Fluid outlet with pout = 0.1 MPa
- Initial Conditions:
- Initial velocity u = 0 at arbitrary positions within the flow domain
- Initial temperature T = 20 °C
- 3.
- Solid Heat Transfer (Simulating electrical conduction heating for in situ pyrolysis):
- Boundary Conditions:
- Thermal source boundaries at z = 0 µm and z = 1200 µm: T = 500 °C
- Initial Conditions:
- Initial temperature T = 20 °C at arbitrary positions within the solid domain
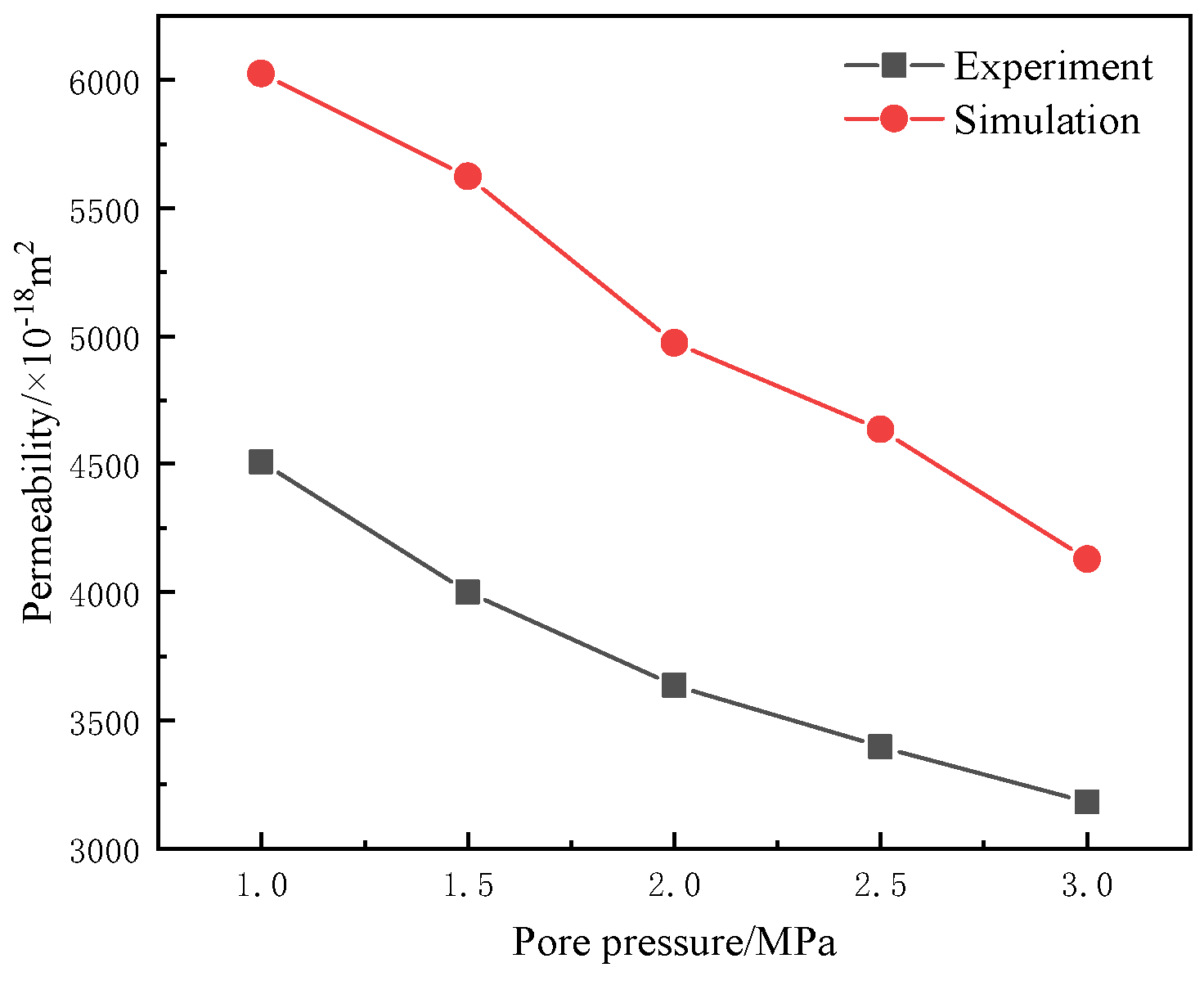
3.5. Simulation Validation
4. Result
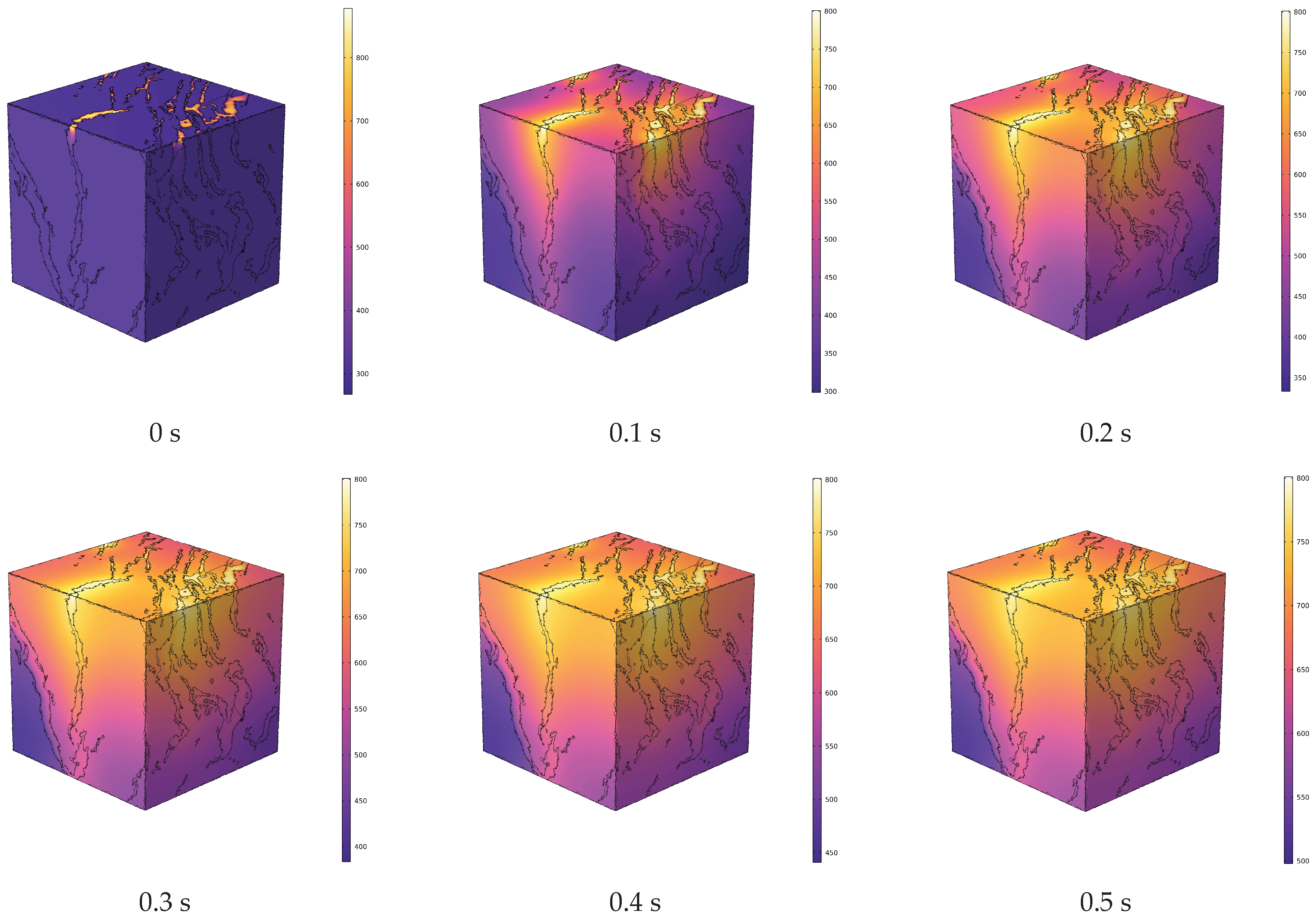
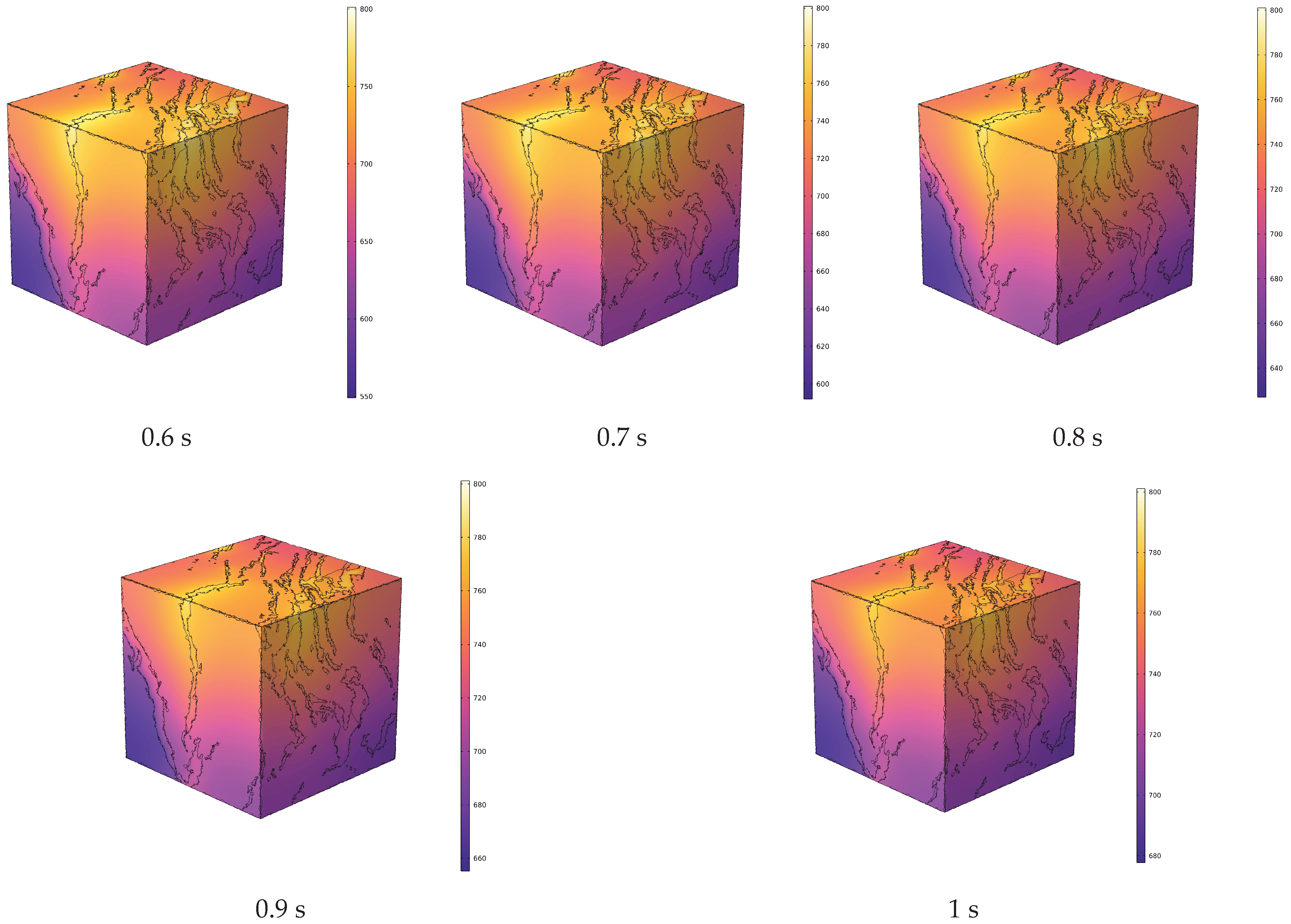
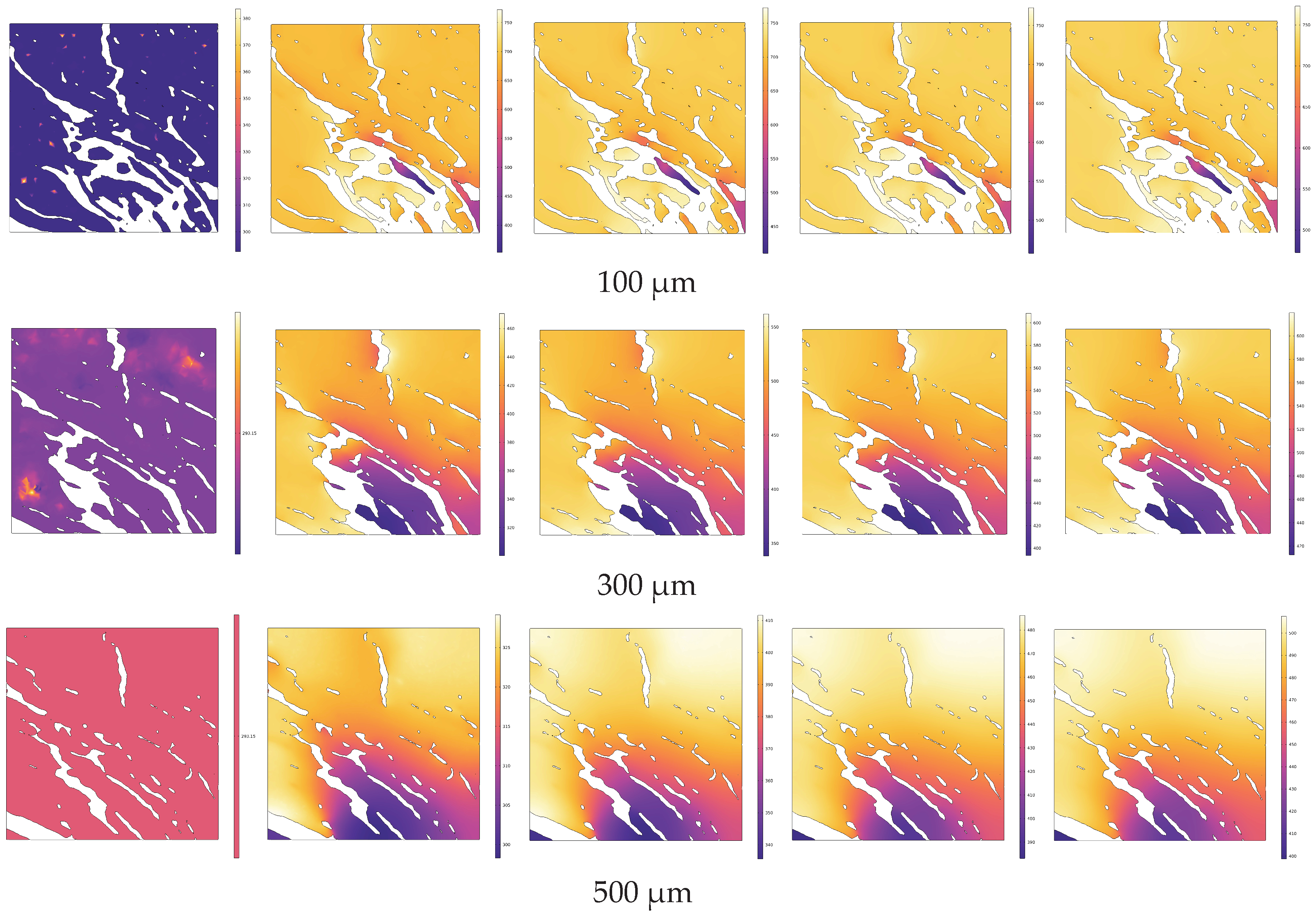
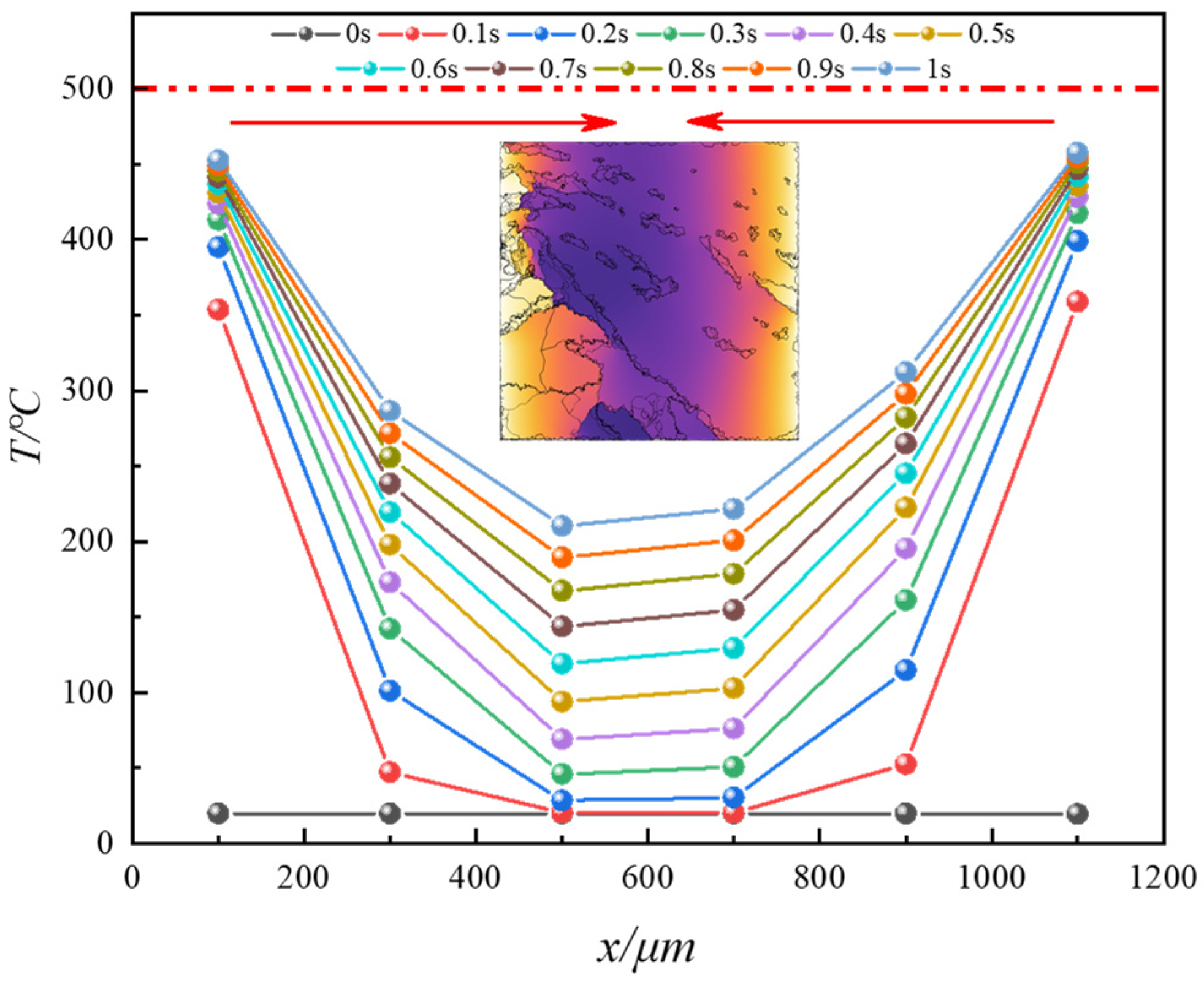
4.1. Analysis of Temperature Field Evolution during In Situ Pyrolysis of Oil Shale with High-Temperature Steam
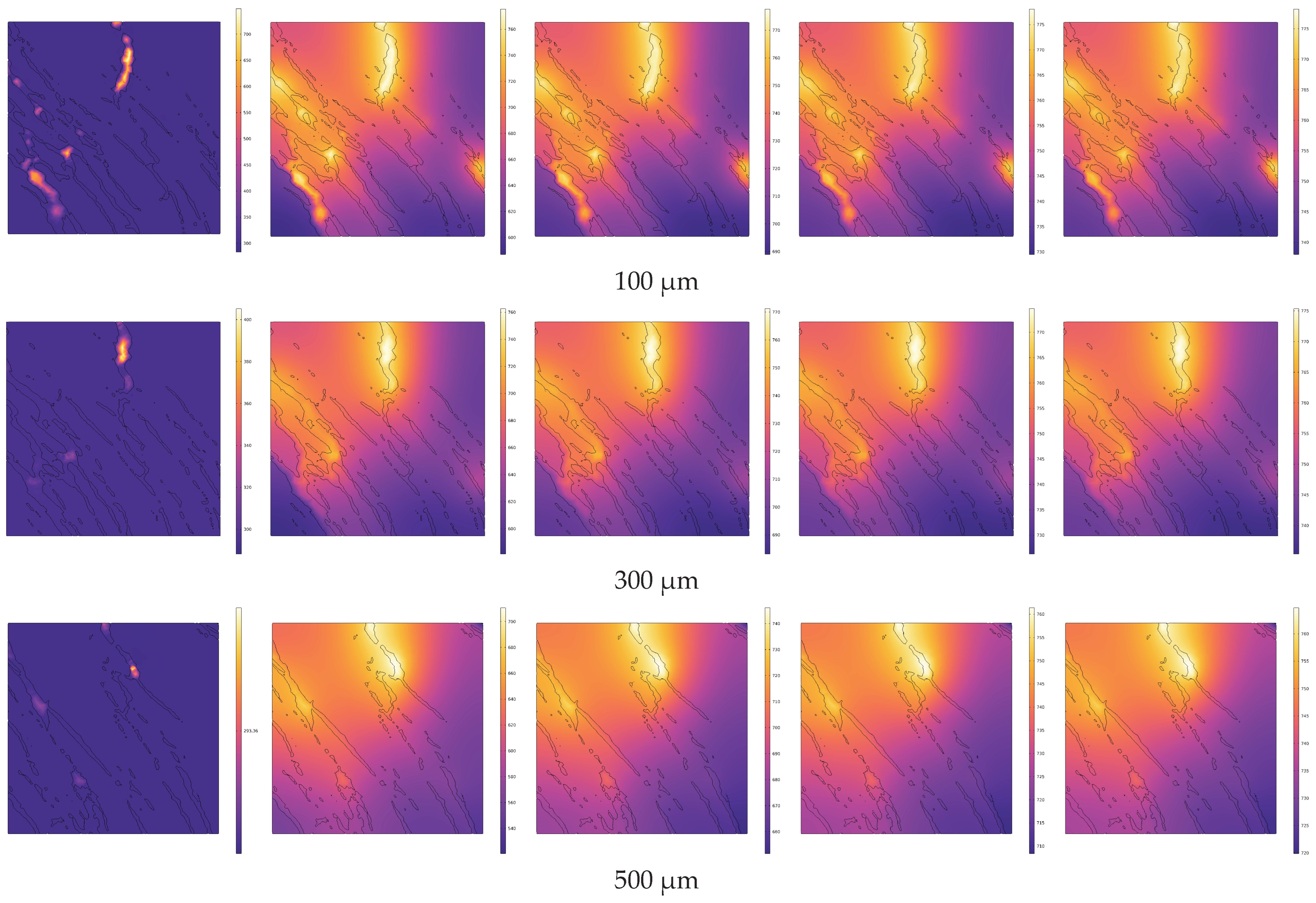
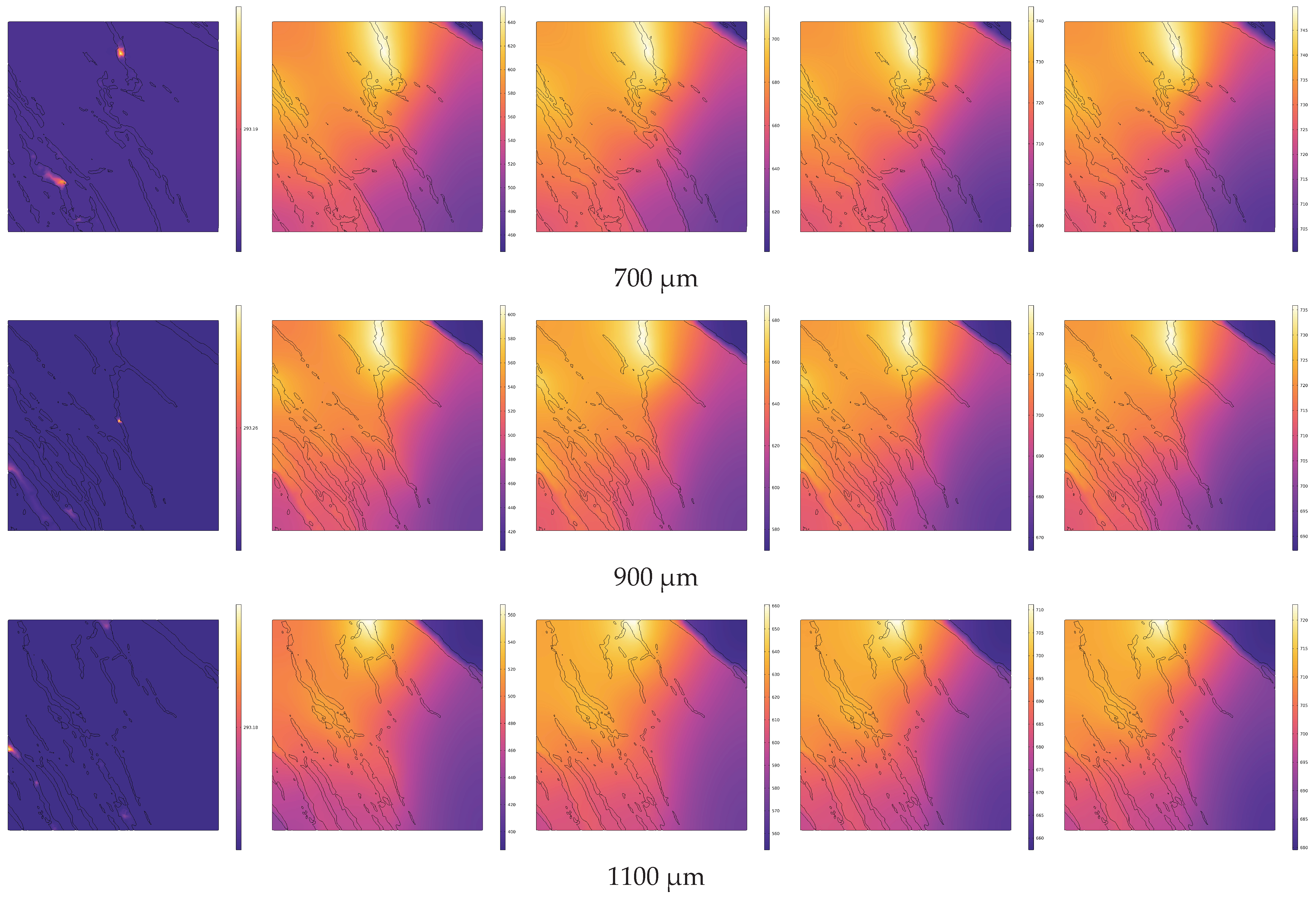
4.2. Analysis of Temperature Field Evolution during Electrical In Situ Pyrolysis of Oil Shale
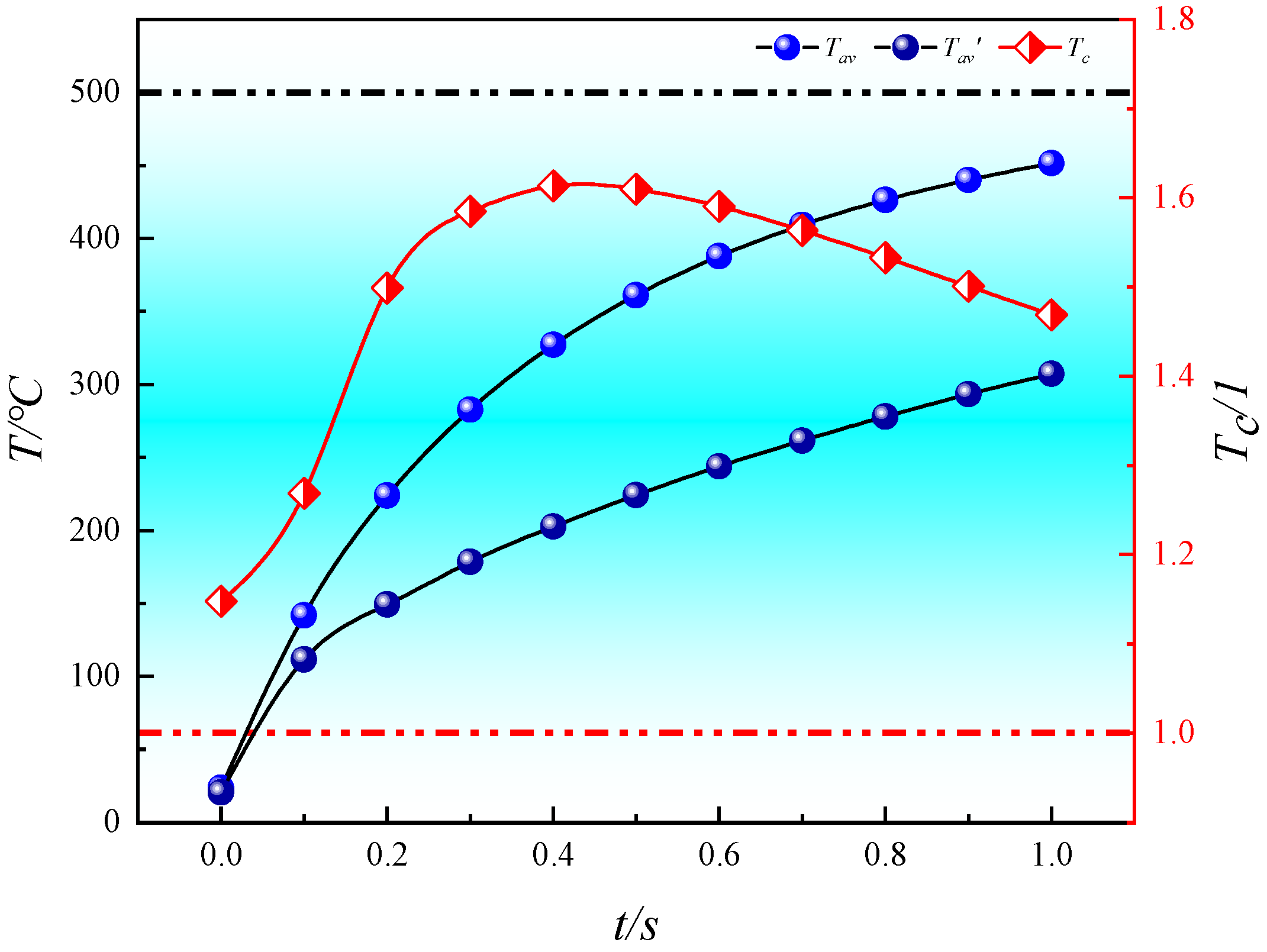
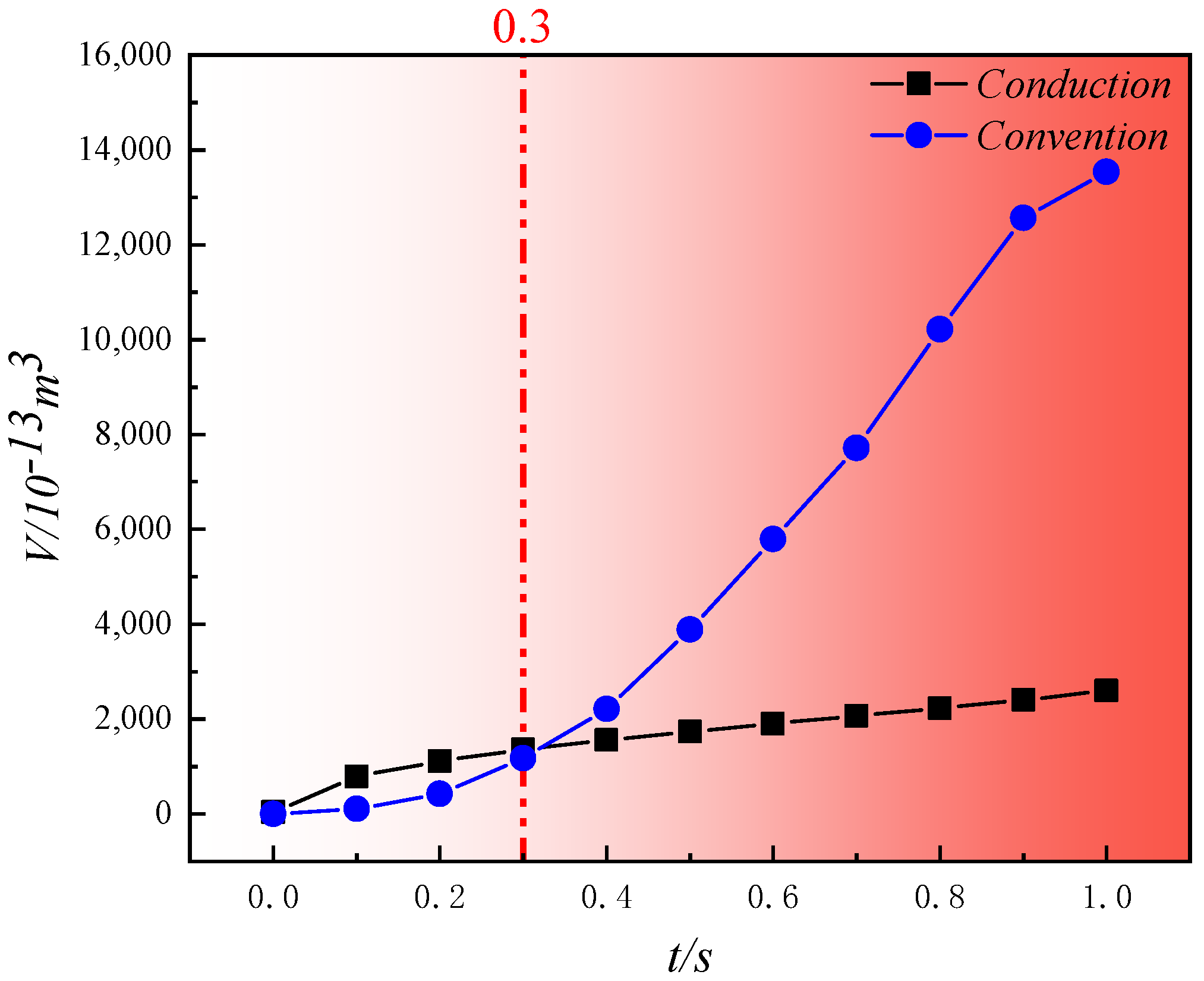
4.3. Comparison of Heat Transfer Efficiency between Convective Heating and Conductive Heating
4.4. Reflection and Outlook
5. Conclusions
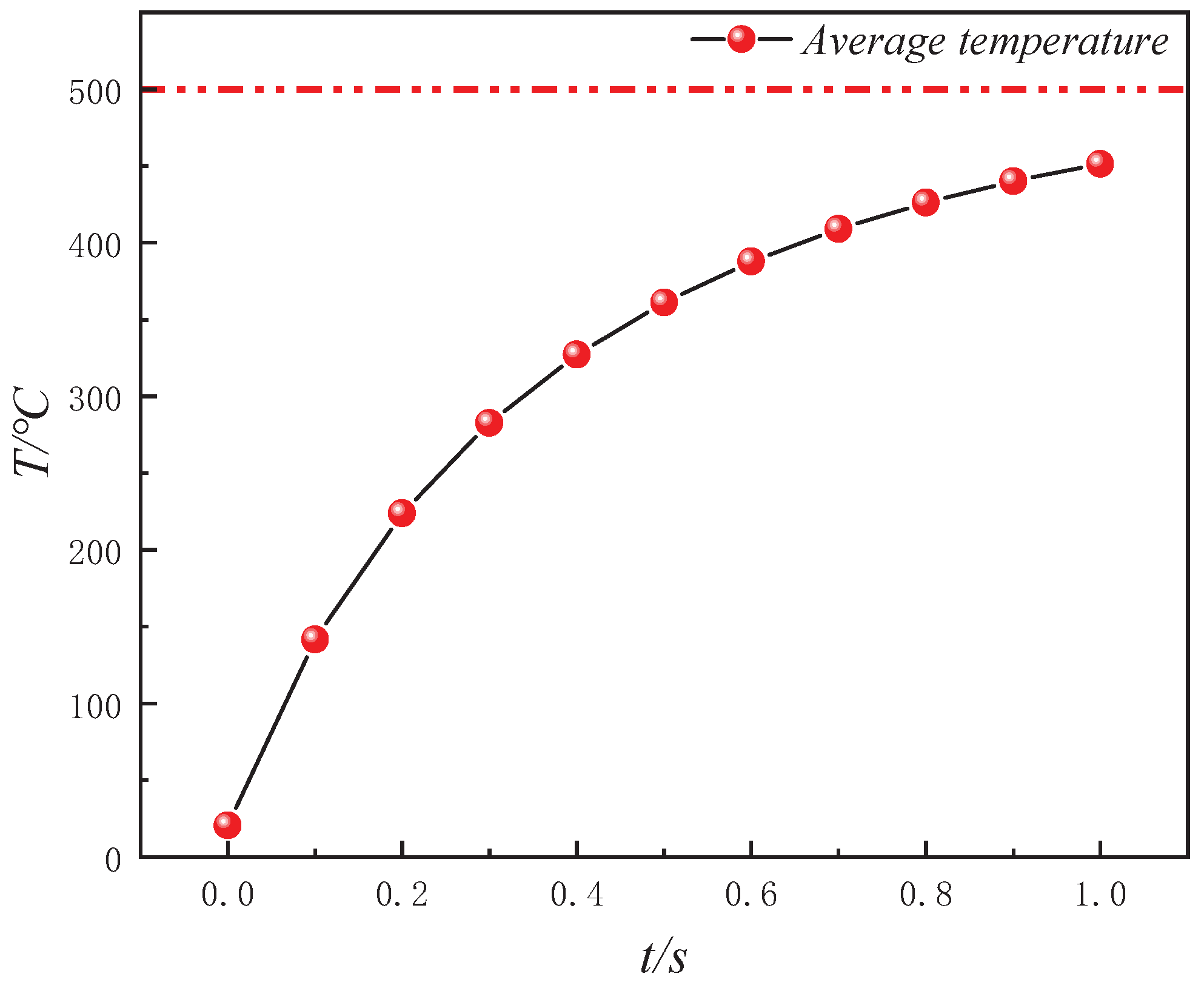
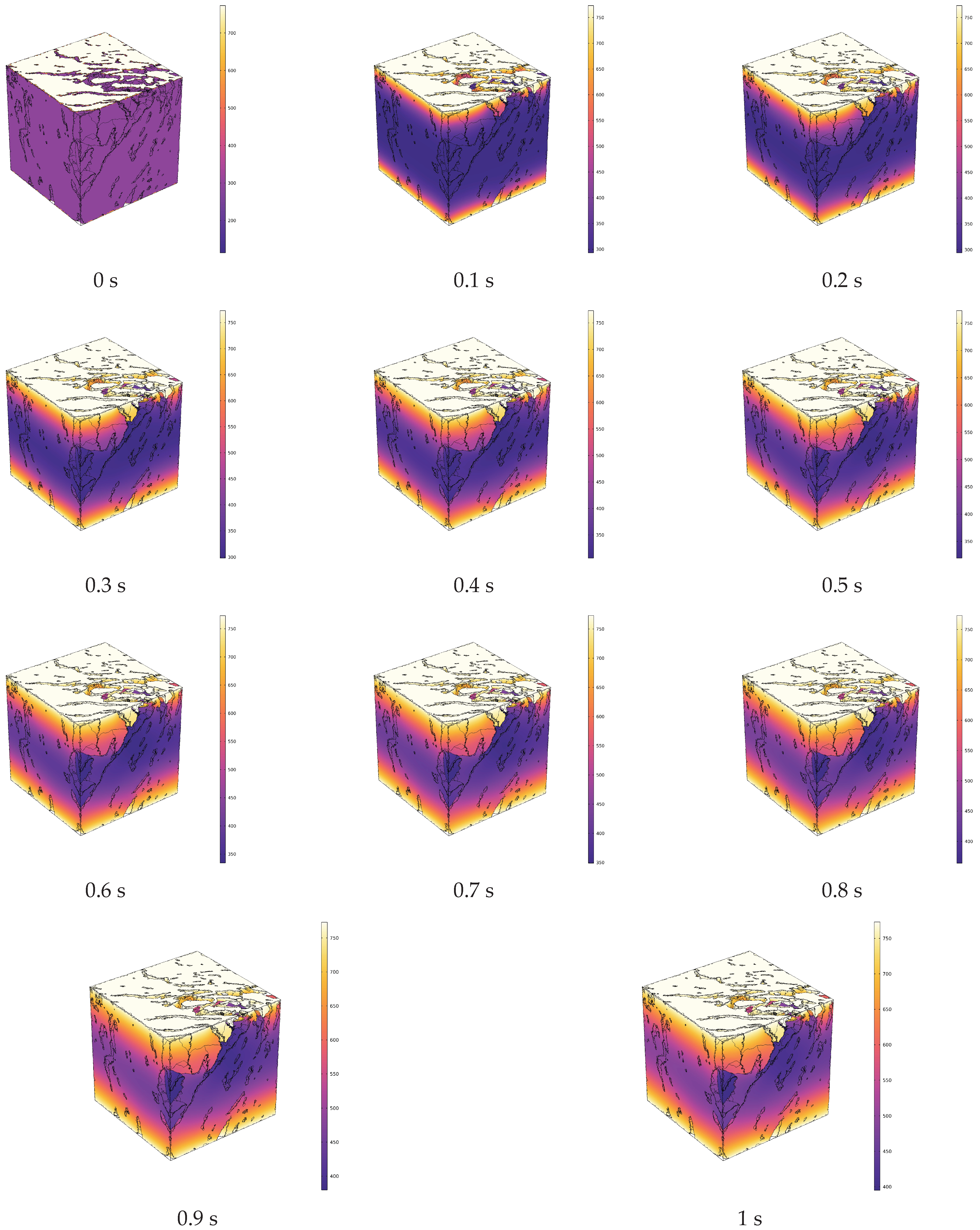
- During the in situ pyrolysis of oil shale using convective heating with high-temperature steam, the overall temperature rise is uniform and orderly. Heat gradually transfers from pore networks to the matrix, with the uneven distribution of pores causing variations in the temperature field. However, no local overheating areas appear, which effectively enhances pyrolysis efficiency. As high-temperature steam continues to be injected, the temperature difference between different cross-sections decreases gradually. By 1 s, the temperature at 1100 μm has reached 426.88 °C, differing from the temperature at 100 μm by only 55.31 °C.
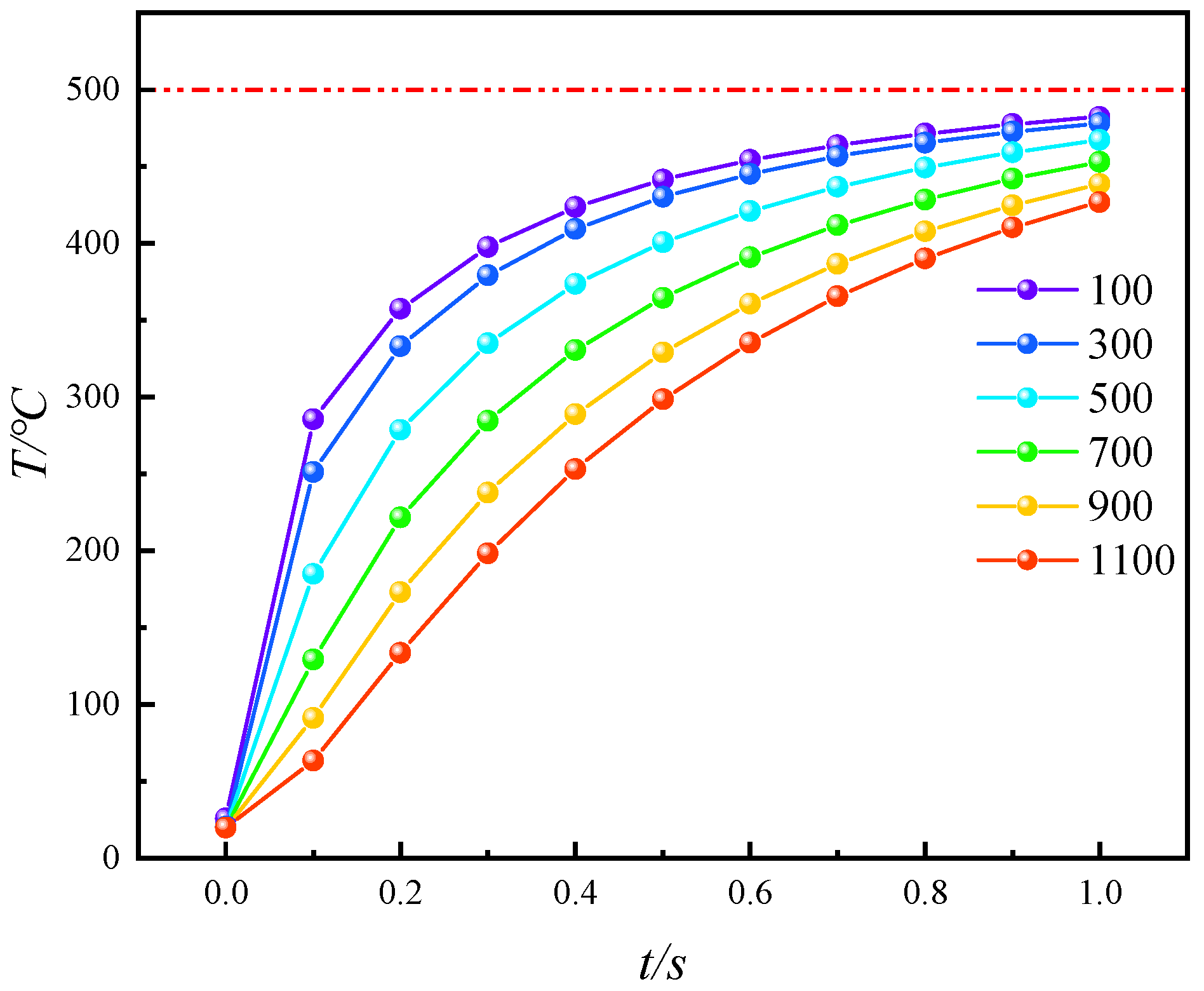
- During conductive heating with electrical methods, heat transfer primarily occurs inward along the normal direction of the heat source surface. The rate of temperature increase is greater in cross-sections closer to the heat source surface. Within 1 s, the temperature increase at 100 μm (near the heat source surface) is 2.27 times greater than that at 500 μm (the cross-section farthest from the heat source surface).
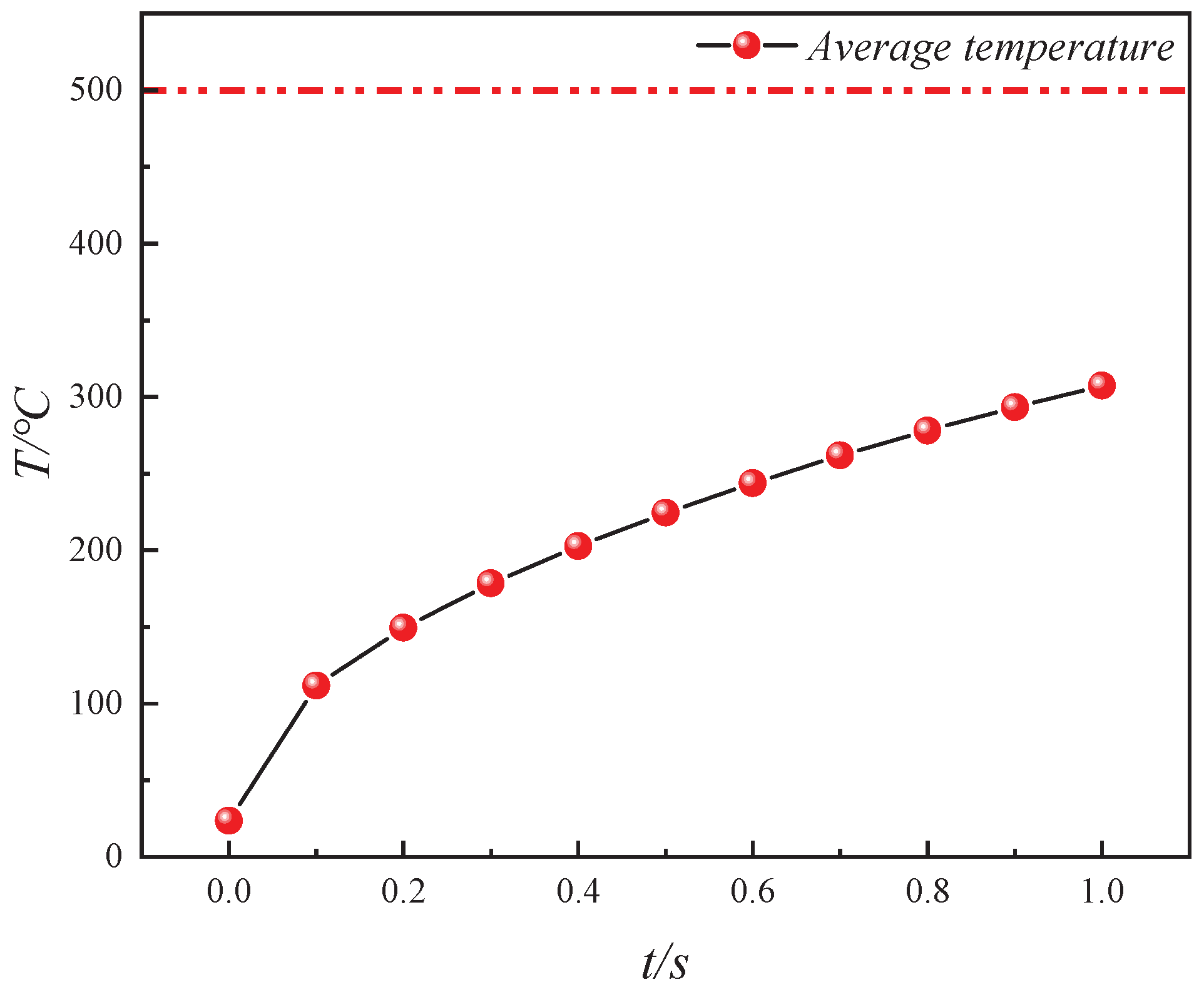
- The heat transfer efficiency of convective heating with high-temperature steam is consistently higher than that of conductive heating with electrical methods. At every moment during in situ pyrolysis with high-temperature steam, the Tav is higher than that at the corresponding moment during in situ pyrolysis with conductive heating using electrical methods (Tav′). Additionally, Tc, which represents the ratio of the average temperatures between the two methods, initially increases and then decreases as pyrolysis progresses, but Tc remains greater than 1 throughout. At 0.4 s, Tc reaches its peak value of 1.61331.
- In the initial stages of pyrolysis, the high-temperature regions formed by conductive heating outperform those formed by convective heating. However, as the heat-carrying fluid stabilizes, the volume of high-temperature regions generated by convective heating increases rapidly compared to those generated by conductive heating. During the initial phase of pyrolysis (0 s~0.3 s), convective heating initially lags behind conductive heating in terms of the growth of high-temperature regions, because high-temperature steam first needs to permeate and then conduct heat towards the solid matrix through the pore structure. In contrast, conductive heating directly transfers heat through the solid matrix. After 0.3 s, the volume of the high-temperature regions rapidly increases with convective heating, while with conductive heating, it continues to grow at a slower pace. By 1 s, the volume of the high-temperature regions formed by convective heating is 5.22 times that of those formed by conductive heating.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Yang, D.; Zhang, Y.; Li, W.; Kang, Z.; Zhao, Y. Research on the reaction mechanism and modification distance of oil shale during high-temperature water vapor pyrolysis. Energy 2022, 261, 125213. [Google Scholar] [CrossRef]
- Guo, W.; Deng, S.; Sun, Y. Recent advances on shale oil and gas exploration and development technologies. Adv. Geo-Energy Res. 2024, 11, 81–87. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Pan, J.; Zhu, C.; Deng, S. In-situ pyrolysis of oil shale in pressured semi-closed system: Insights into products characteristics and pyrolysis mechanism. Energy 2024, 286, 129608. [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Deng, S.; Sun, Y.; Han, J.; Bai, F.; Kang, S.; He, W. Enhanced pyrolysis of Huadian oil shale at high temperature in the presence of water and air atmosphere. J. Pet. Sci. Eng. 2022, 215, 110623. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Zhao, Y.; Kang, Z. Investigating pilot test of oil shale pyrolysis and oil and gas upgrading by water vapor injection. J. Pet. Sci. Eng. 2021, 196, 108101. [Google Scholar] [CrossRef]
- Xu, S.; Sun, Y.; Guo, W.; Yang, Q.; Li, Q.; Guo, M.; Bai, F.; Zhu, C.; Deng, S. Regulating the oxidative assisted pyrolysis of Huadian oil shale by preheating temperature and oxygen flow rate. Energy 2023, 262, 125602. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Kang, Z.; Zhao, J.; Meng, Q. Experimental study on the effects of steam temperature on the pore-fracture evolution of oil shale exposed to the convection heating. J. Anal. Appl. Pyrolysis 2022, 164, 105533. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Zhao, Y.; Kang, Z.; Zhao, J.; Huang, X. Experimental investigation on anisotropic permeability and its relationship with anisotropic thermal cracking of oil shale under high temperature and triaxial stress. Appl. Therm. Eng. 2019, 146, 718–725. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, S.; Gu, Q.; Zhang, Y.; Cui, X.; Wang, H. Pyrolysis kinetic study of Huadian oil shale, spent oil shale and their mixtures by thermogravimetric analysis. Fuel Process. Technol. 2013, 110, 103–108. [Google Scholar] [CrossRef]
- Burger, J.W.; Crawford, P.M.; Johnson, H.R. Is oil shale America answer to peak-oil challenge. Oil Gas J. 2004, 102, 16. [Google Scholar]
- Tanaka, P.L.; Yeakel, J.D.; Symington, W.A.; Spiecker, P.M.; Del Pico, M.; Thomas, M.M.; Sullivan, K.B.; Stone, M.T. Plan to test ExxonMobil’s in situ oil shale technology on a proposed RD&D lease. In Proceedings of the 31st Oil Shale Symposium, Golden, CO, USA, 17–21 October 2011. [Google Scholar]
- Jaber, J.O.; Probert, S.D. Non-isothermal thermogravimetry and decomposition kinetics of two Jordanian oil shales under different processing conditions. Fuel Process. Technol. 2000, 63, 57–70. [Google Scholar] [CrossRef]
- Deng, S.; Wang, Z.; Gu, Q.; Meng, F.; Li, J.; Wang, H. Extracting hydrocarbons from Huadian oil shale by sub-critical water. Fuel Process. Technol. 2011, 92, 1062–1067. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Donzé, F.; Renard, F.; Panahi, H.; Papachristos, E.; Scholtès, L. Microfracturing during primary migration in shales. Tectonophysics 2017, 694, 268–279. [Google Scholar] [CrossRef]
- Geng, Y.; Liang, W.; Liu, J.; Cao, M.; Kang, Z. Evolution of pore and fracture structure of oil shale under high temperature and high pressure. Energy Fuels 2017, 31, 10404–10413. [Google Scholar] [CrossRef]
- Rabbani, A.; Baychev, T.G.; Ayatollahi, S.; Jivkov, A.P. Evolution of pore-scale morphology of oil shale during pyrolysis: A quantitative analysis. Transp. Porous Media 2017, 119, 143–162. [Google Scholar] [CrossRef]
- Huang, X.; Kang, Z.; Zhao, J.; Wang, G.; Zhang, H.; Yang, D. Experimental investigation on micro-fracture evolution and fracture permeability of oil shale heated by water vapor. Energy 2023, 277, 127677. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, Y.; Kang, Z. Numerical simulation of in situ exploitation of oil shale by injecting high-temperature steam. Oil Shale 2019, 36, 483–500. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Kang, Z. Evolution of permeability and mesostructure of oil shale exposed to high-temperature water vapor. Fuel 2021, 290, 119786. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Li, W.; Huang, X.; Zhang, Y.; Yang, D. Study on the pyrolysis behavior and product characteristics of Balikun oil shale with different water pressures in sub-and supercritical states. Fuel 2024, 369, 131701. [Google Scholar] [CrossRef]
- Kang, Z.; Jiang, X.; Wang, L.; Yang, D.; Ma, Y.; Zhao, Y. Comparative investigation of in situ hydraulic fracturing and high-temperature steam fracturing tests for meter-scale oil shale. Energy 2023, 281, 128343. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Gao, Y.; Al-Khulaifi, Y.; Marone, F.; Hollis, D.; Blunt, M.J.; Bijeljic, B. 4D in situ synchrotron X-ray tomographic microscopy and laser-based heating study of oil shale pyrolysis. Appl. Energy 2019, 235, 1468–1475. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, D.; Kang, Z.; Feng, Z. A micro-CT study of changes in the internal structure of Daqing and Yan’an oil shales at high temperatures. Oil Shale 2012, 29, 357. [Google Scholar] [CrossRef]
- Cai, F.; Xue, S.; Zhang, M.; Zhang, J.; Chen, X.; Bao, Y.; Li, Y. Assessing reliability and validity of the Chinese version of Crown–Crisp experience index and its application in coal miners. Int. J. Coal Sci. Technol. 2023, 10, 90. [Google Scholar] [CrossRef]
- Yang, S.; Yang, D.; Kang, Z. Experimental investigation of the anisotropic evolution of tensile strength of oil shale under real-time high-temperature conditions. Nat. Resour. Res. 2021, 30, 2513–2528. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, J.; Song, J.; Chang, J.; Shu, C.-M. Apparent activation energy of mineral in open pit mine based upon the evolution of active functional groups. Int. J. Coal Sci. Technol. 2023, 10, 75. [Google Scholar] [CrossRef]
- Wang, C.; Shi, X.; Zhang, W.; Elsworth, D.; Cui, G.; Liu, S.; Wang, H.; Song, W.; Hu, S.; Zheng, P. Dynamic analysis of heat extraction rate by supercritical carbon dioxide in fractured rock mass based on a thermal-hydraulic-mechanics coupled model. Int. J. Min. Sci. Technol. 2022, 32, 225–236. [Google Scholar] [CrossRef]
- Qin, S.; Lin, H.; Yang, S.; Wei, Z. A mathematical model for parameter setting in discrete element numerical simulation. Int. J. Coal Sci. Technol. 2023, 10, 87. [Google Scholar] [CrossRef]
- Yang, S. Study on Microstructure Evolution and Mechanical Response of Oil Shale under Real Time High Temperature. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China. (In Chinese). [CrossRef]
- Liu, F.; Lin, P.; Xu, Z.; Shao, R.; Han, T. Extraction and imaging of indicator elements for non-destructive, in-situ, fast identification of adverse geology in tunnels. Int. J. Min. Sci. Technol. 2023, 33, 1437–1449. [Google Scholar] [CrossRef]
- Zhu, D.; Yu, B.; Wang, D.; Zhang, Y. Fusion of finite element and machine learning methods to predict rock shear strength parameters. J. Geophys. Eng. 2024, 21, gxae064. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, B.; Zhang, L.; Lei, J.; Fan, Y.; Ruhan, A.; Yan, D.; Zhao, Y. A study on water saturation predictions in igneous reservoirs based on the relationship between the transverse relaxation time and the resistivity index. J. Pet. Sci. Eng. 2022, 208, 109519. [Google Scholar] [CrossRef]
- Chen, J.-H.; Althaus, S.M.; Liu, H.-H.; Zhang, J.; Eppler, G.; Duncan, J.C.; Sun, Q. Electromagnetic-heating enhancement of source rock permeability for high recovery. Fuel 2020, 283, 118976. [Google Scholar] [CrossRef]
- Gu, J.; Deng, S.; Sun, Y.; Guo, W.; Chen, H.; Shi, B. Pyrolysis behavior and pyrolysate characteristics of Huadian oil shale kerogen catalyzed by nickel-modified montmorillonite. Adv. Geo-Energy Res. 2024, 11, 168–180. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; Yang, D.; Fu, M. Numerical study on the in-situ pyrolysis process of steeply dipping oil shale deposits by injecting superheated water steam: A case study on Jimsar oil shale in Xinjiang, China. Energy 2022, 239, 718–725. [Google Scholar] [CrossRef]
- Wang, G.; Yang, D.; Kang, Z.; Zhao, J.; Lv, Y. Numerical investigation of the in situ oil shale pyrolysis process by superheated steam considering the anisotropy of the thermal, hydraulic, and mechanical characteristics of oil shale. Energy Fuels 2019, 33, 12236–12250. [Google Scholar] [CrossRef]
- Sun, T.; Liu, H.; Zhang, Y.; Li, Y. Numerical simulation and optimization study of In-Situ Heating for three-dimensional oil shale exploitation with different well patterns. Case Stud. Therm. Eng. 2024, 55, 104089. [Google Scholar] [CrossRef]
- Zhu, J.; Li, F.; Wang, H.; Yang, Z.; Chen, H.; Zhu, H. Numerical analysis of microwave-enhanced oil shale pyrolysis by rotation turntable based on the Arbitrary Lagrangian-Eulerian method. Fuel 2024, 371, 131925. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, C.-Y.; Pu, W.-F.; Chen, Q.-Y.; Yuan, C.-D.; Varfolomeev, M.A.; Sudakov, V. Heat front propagation in shale oil reservoirs during air injection: Experimental and numerical studies. Pet. Sci. 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Yang, D.; Kang, Z.; Zhao, J.; Zhao, Y. Study on the permeability and meso-structure evolution of organic-rich shale with single fracture during in-situ pyrolysis by high-temperature water vapor injection. J. China Coal Soc. 2024, 1–15. (In Chinese) [Google Scholar] [CrossRef]


| TOC (%) | Rock Pyrolysis Parameters | ||||
|---|---|---|---|---|---|
| Tmax (°C) | S1 (mg/g) | S2 (mg/g) | S3 (mg/g) | HI (mg/g TOC) | |
| 20.55 | 451 | 0.36 | 118.21 | 0.01 | 575.12 |
| Solid Matrix Parameters | Mathematical Expression |
|---|---|
| ρs | |
| λper | |
| λpar | |
| βper | |
| βpar | |
| Eper | |
| Epar | |
| vs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, D. Simulation Study on the Heat Transfer Characteristics of Oil Shale under Different In Situ Pyrolysis Methods Based on CT Digital Rock Cores. Energies 2024, 17, 4169. https://doi.org/10.3390/en17164169
Zhang Y, Yang D. Simulation Study on the Heat Transfer Characteristics of Oil Shale under Different In Situ Pyrolysis Methods Based on CT Digital Rock Cores. Energies. 2024; 17(16):4169. https://doi.org/10.3390/en17164169
Chicago/Turabian StyleZhang, Yuxing, and Dong Yang. 2024. "Simulation Study on the Heat Transfer Characteristics of Oil Shale under Different In Situ Pyrolysis Methods Based on CT Digital Rock Cores" Energies 17, no. 16: 4169. https://doi.org/10.3390/en17164169




