Abstract
Mini passive direct methanol fuel cells (mpDMFCs) appear to be a promising alternative for powering portable devices, since they use a liquid fuel, have a fast refuelling time, have a high efficiency and have a low environmental impact. However, some issues need to be solved before their commercialization, such as methanol crossover, short lifetime and high costs. The present work studies the effect of reducing the anode and cathode catalyst loading on the performance of a mpDMFC towards a reduction in the system costs and the characterization of the system losses. The undesirable losses that affect the fuel cell performance were identified and quantified using the electrochemical impedance spectroscopy (EIS) technique. Accordingly, a novel equivalent electric circuit (EEC) was proposed, accurately reproducing the mini pDMFC. In this work, a maximum power density of 7.07 mW cm−2 was obtained, with a methanol concentration of 5 M, using 2 mg cm−2 Pt-RuB and 4 mg cm−2 PtB. The mpDMFC allowed the cell to work with high methanol concentrations and reduced anode catalyst loadings.
1. Introduction
Alternative power sources with low environmental impacts are of vital importance to the planet’s health. Additionally, the growing interest in portable electronic devices motivates research on finding alternative technologies to overcome the limitations of conventional batteries. Mini passive direct methanol fuel cells (mpDMFCs) appear to be a promising solution due to their low environmental impact, high efficiency, quick refuelling and simplicity, since methanol is a liquid. In contrast to rechargeable batteries, mpDMFCs do not require recharging, only refuelling, which is a fast process, and they are able to operate while the fuel is being supplied. Furthermore, the fact that the fuel cell operates on passive mode allows the transport of the reactants to the system by a natural mechanism, such as capillarity, convection and diffusion, not requiring external energy to operate [1,2]. Additionally, in isolated areas and developing countries, where there is limited access to the grid, mpDMFCs allow easier and local electricity production. Nevertheless, these systems present some barriers that need to be overcome before their commercialization, such as slow electrochemical reactions, methanol crossover through the membrane, high costs and short lifetime [3,4].
The mpDMFC catalysts, where the electrochemical reactions occur, are responsible for a major fraction of these system costs, since the most used catalysts are noble metals, like platinum (Pt) and ruthenium (Ru). Based on that, many efforts have been made to improve the activity of the anode catalyst of a DMFC, but Pt-hybrid nanocatalysts are still the most effective for the methanol oxidation reaction (MOR) [5,6,7,8]. As this reaction occurs in an incomplete way on the Pt surface, with the formation of intermediate compounds that poison the catalyst, a second metal is needed to provide the removal of these compounds. Among the different metals studied, Ru is still the most effective. On the cathode catalyst of a DMFC, the oxygen reduction reaction (ORR) and the parasitic methanol oxidation reaction occur due methanol crossover. The competition between these two species for the cathode catalyst active sites is negatively potentialized, decreasing the catalyst availability for the ORR, limiting this reaction rate, increasing the cathode losses and decreasing its performance and consequently the mpDMFC performance. Therefore, noble metals, such as Pt, are used to overcome the sluggish ORR kinetics and circumvent the presence of methanol [8,9,10,11,12,13,14]. Moreover, to overcome the slow kinetics, high catalyst loadings are commonly used at the anode and cathode, namely 4 mg cm−2 Pt-Ru on the anode and 4 mg cm−2 Pt on the cathode, increasing the system costs [15].
The mpDMFC performance is commonly analysed through the polarization and power curves, which allow the key losses that affect the fuel cell efficiency to be recognized, such as activation, ohmic and concentration losses [16]. Nevertheless, since different phenomena occur in these fuel cells and all of them are affected by the operating and configuration conditions employed, the identification and quantification of their impact on the different losses is a difficult task by these techniques. Thus, electrochemical impedance spectroscopy (EIS) emerges as a powerful complementary tool to be used on the pDMFC diagnosis, since it provides useful information to identify and quantify the different phenomena that occur in the system and affect its performance. More specifically, this technique allows the identification and quantification of the main losses that negatively affect the fuel cell [15,17,18]. The EIS measurements can be performed in situ using a two-electrode configuration, allowing the cell to be studied as a whole, or ex situ through a classic three-electrode configuration, allowing the analysis of each fuel cell component [17]. Since ex situ measurements are conducted without the fuel cell normal operation, the in situ tests are the ideal approach to study the pDMFC in a real environment [1,19,20,21,22]. In situ measurements can be conducted with an independent reference electrode, which is very challenging when solid electrolytes are used and requires a modification of the fuel cell system [23]. As an alternative, a dynamic hydrogen electrode (DHE) can be used through replacing one of the reactants (methanol or oxygen) with hydrogen, which acts as the reference electrode. Using this methodology, it is considered that the losses of the DHE are irrelevant, with all the losses being associated with the other electrode, the one under analysis [17]. Different works demonstrate the use of the DHE technique to study the losses that negatively affect the passive DMFCs, through the comparison between the overall fuel cell spectrum (obtained when the system operates in real conditions with methanol and air) with the single-cell spectrum (given by the DHE measurements) [15,24,25,26,27,28,29,30]. Despite being a much-used solution, this method also leads to a change in the fuel cell system and in its behaviour, since one of the reactants is replaced with hydrogen. Siracusano et al. [31] showed how the EIS technique can be applied as a non-invasive diagnostic tool in electrolysis, using lower catalyst loadings on one electrode to suppress one electrode behaviour and obtain useful information from the other. Based on this study and in order to avoid the disadvantages of using a DHE, in this work, it was studied the effect of a reduction in the anode and cathode catalyst loading on the performance of an mpDMFC. This also allowed us to evaluate the possibility of using lower loadings to decrease the fuel cell costs without a significant decrease in the system efficiency.
As the methanol concentration has a clear impact on the pDMFC performance, the effect of the methanol concentration was also evaluated, studying different methanol/water solutions. The methanol concentration that leads to a higher performance results in a balance between the negative effect of an increase in the alcohol concentration on its crossover rate and the positive effect of an increase in its concentration on the anode oxidation reaction rate [32].
Another target of this work was to estimate the major losses that affect the system (activation, ohmic and concentration losses) at the anode and cathode using the EIS technique.
2. Materials and Methods
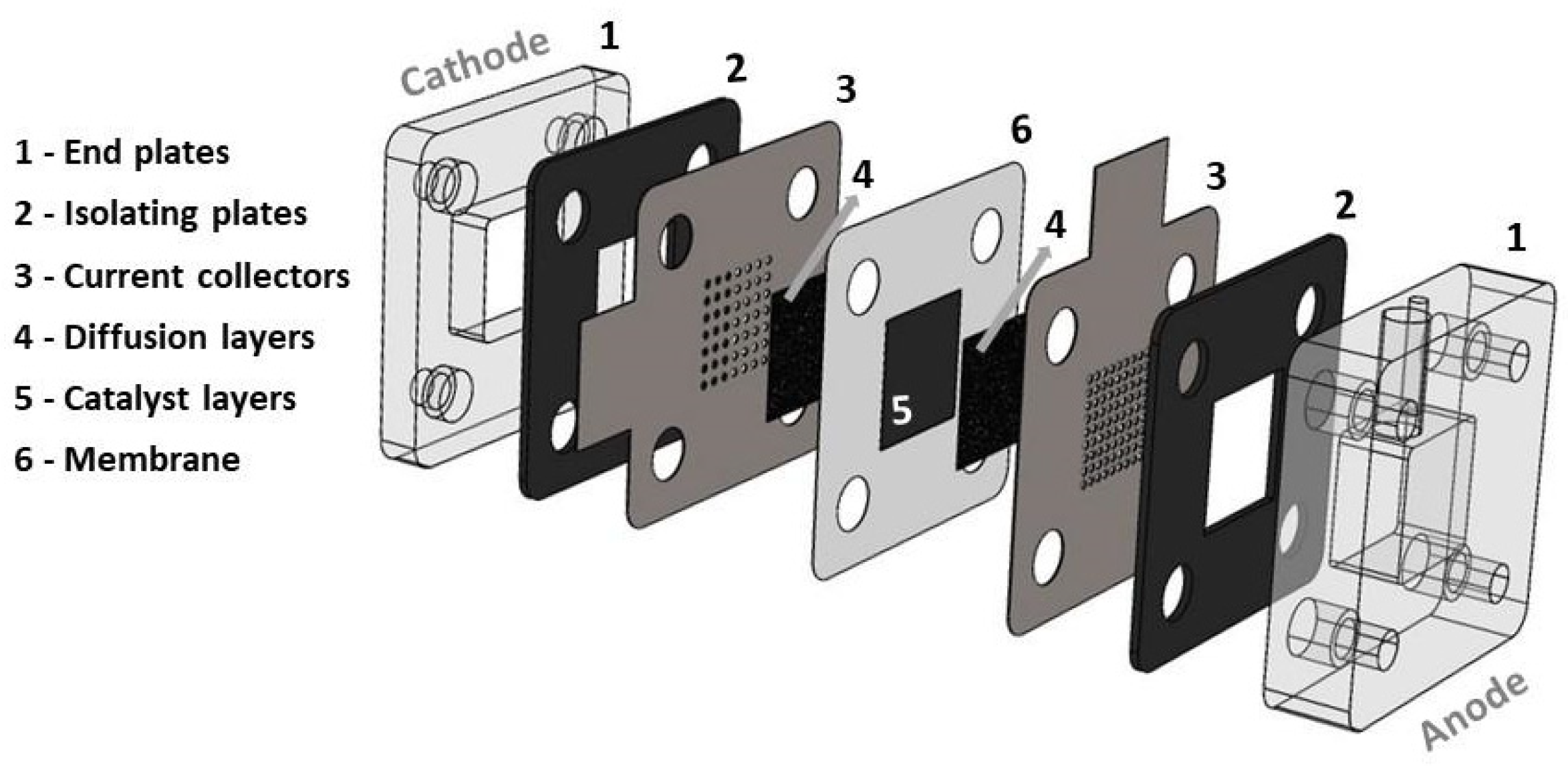
The mpDMFC has an active area of 2.25 cm2, and it is composed of two acrylic end plates (with a fuel reservoir of 1.8 cm3 at the anode and an opening at the cathode), two rubber plates, two stainless-steel current collectors (with an open ratio of 28% at the anode and 17% at the cathode) and a membrane electrode assembly (MEA) (Figure 1).
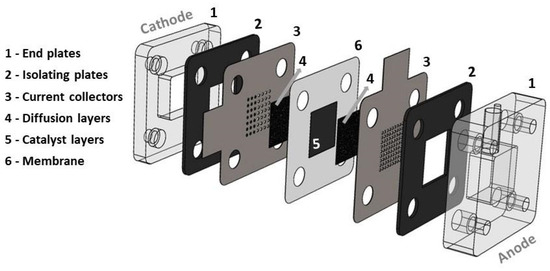
Figure 1.
Schematic representation of the mini passive DMFC.
The MEA was acquired from FuelCellEtc, Bryan, TX, USA, and is composed of carbon cloth with a microporous layer (MPL) as the anode and cathode diffusion layers (with a thickness of 0.410 mm and a porosity of 0.8), Pt-RuB as the anode catalyst, PtB as the cathode catalyst and a Nafion® 117 membrane (Chemours company, Wilmington, DE, USA).
The experimental tests were performed at room temperature and pressure, with different methanol solution concentrations and different anode and cathode catalyst loadings (4, 2 and 0.5 mg cm−2), setting a loading on one side of the cell and using different loadings on the other side.
The mpDMFC behaviour was analysed through polarization and power density curves and EIS data using a commercial fuel cell test station, Zahner Elektrik GmbH & Co. KG (Zahner from Kronach, Germany). The polarization tests were conducted in galvanostatic mode, while the EIS ones were conducted in potentiostatic mode. The impedance tests were performed in situ at three voltages (0.4, 0.3 and 0.2 V), with an amplitude of 10 mV and a frequency range between 0.01 Hz and 100 kHz. The impedance data were fitted to an EEC to quantify the different resistances (losses) that negatively affect the mpDMFC using the Thales Z2.02 software from Zahner.
3. Results and Discussion
In this section, the experimental results are presented and discussed in light of the polarization and power density curves and the EIS data, presenting a selected set of values that show the same patterns and trends as the other results. The EIS results are first interpreted, an EEC is proposed and the quality of the EEC fitting is presented. The effect of a reduction in the catalyst loading on both sides of the mini pDMFC performance is analysed and discussed, identifying and quantifying the different losses/resistances that negatively affect the anode and the cathode of the cell under study. Since the methanol concentration (Cmethanol) has an important impact on the passive DMFC performance, the effect of its concentration on the mini pDMFC behaviour is also presented in this section.
3.1. Electrochemical Characterization of a Mini pDMFC
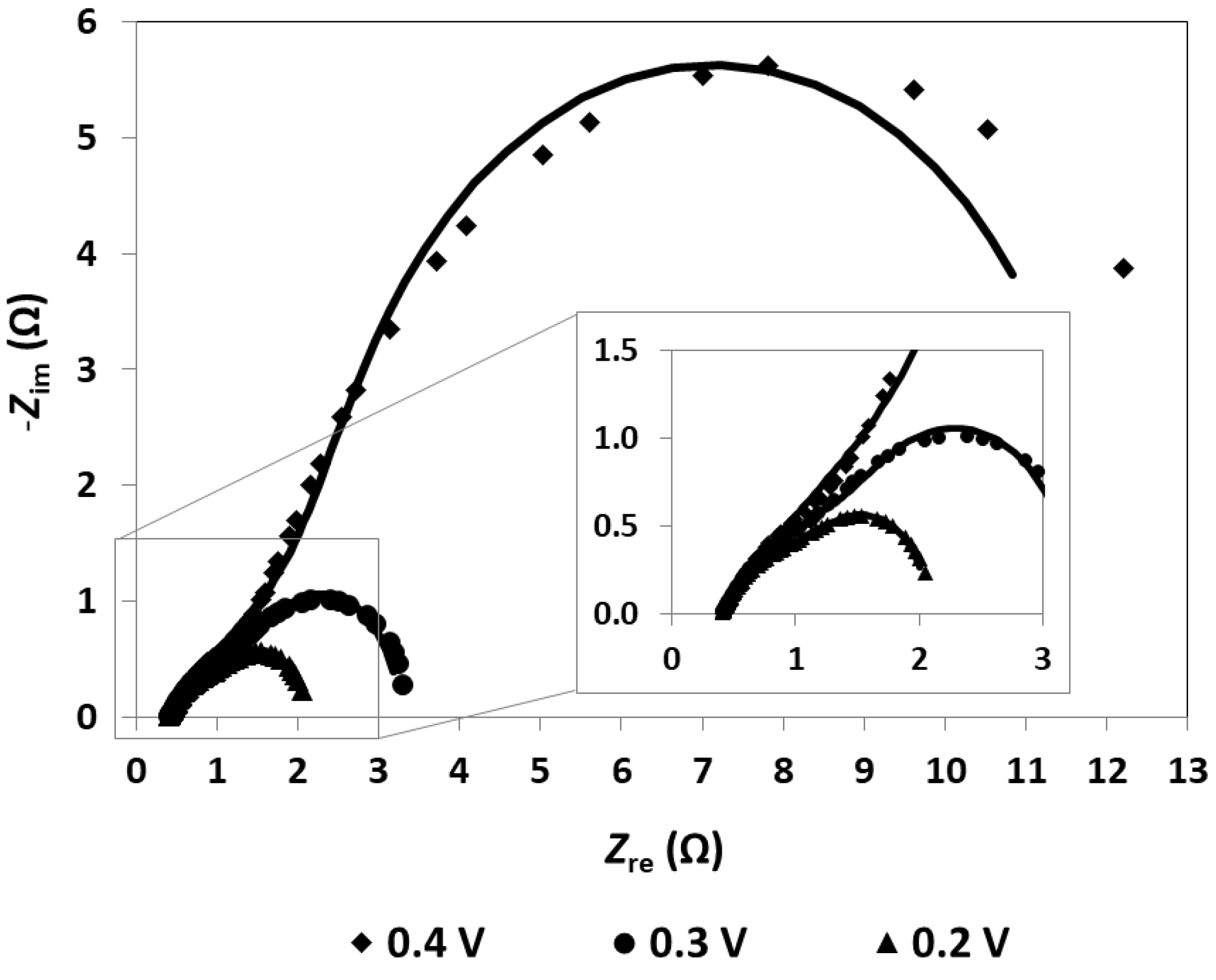
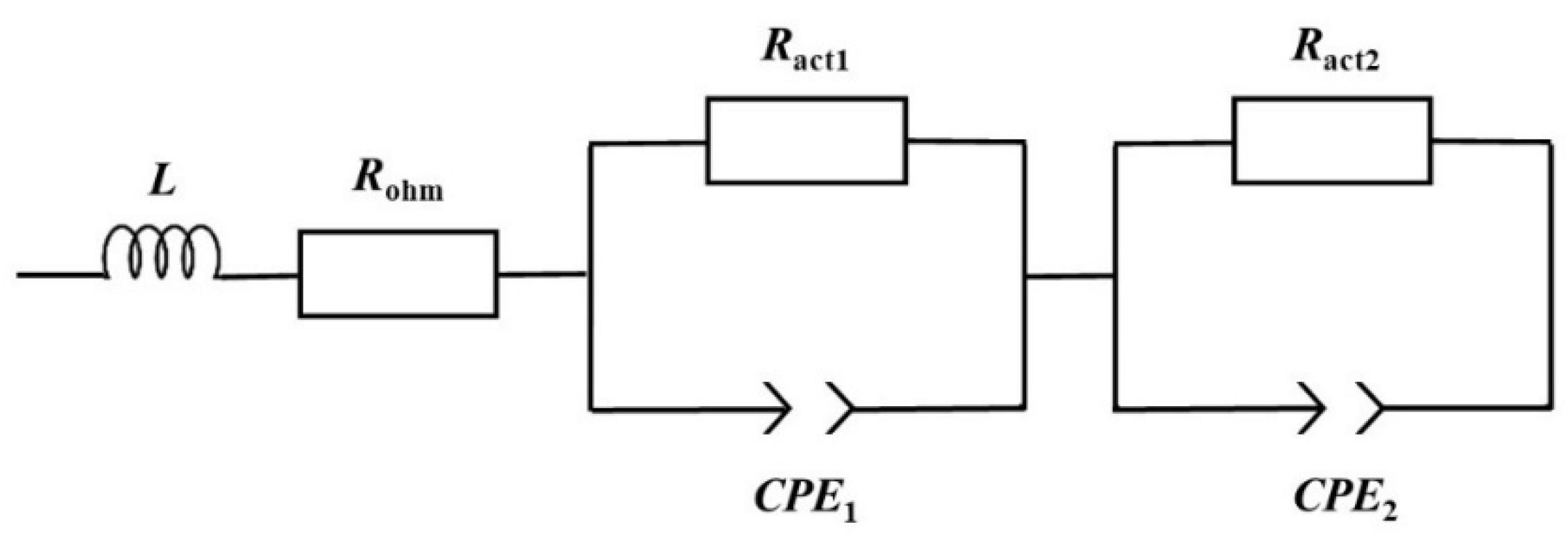
As mentioned, the EIS measurements are realized as a complementary diagnostic technique, allowing the identification and quantification of the undesirable losses that affect the mpDMFC. The EIS data are, usually, represented using a Nyquist plot, where the imaginary impedance (Zim) is represented as a function of the real impedance (Zre) (Figure 2). The different losses are identified based on the characteristics and shape of each plot and quantified by fitting an EEC to the plots (Table 1). Consequently, different combinations and different electrical elements should be used to develop the EEC that will be used to describe the impedance spectrum (Figure 3).
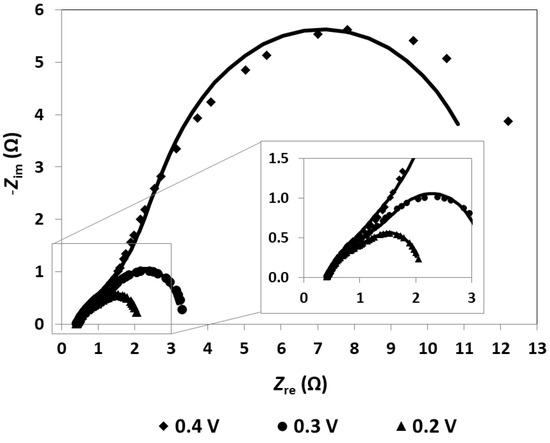
Figure 2.
Impedance spectrum of the mpDMFC for different cell voltages. Lines: EEC fitting; dots: experimental data. Methanol concentration: 3 M; anode catalyst: 4 mg cm−2 Pt-RuB; cathode catalyst: 4 mg cm−2 PtB.

Table 1.
Resistances values obtained through EEC fitting for different cell voltages. Methanol concentration: 3 M; anode catalyst: 4 mg cm−2 Pt-RuB; cathode catalyst: 4 mg cm−2 PtB.
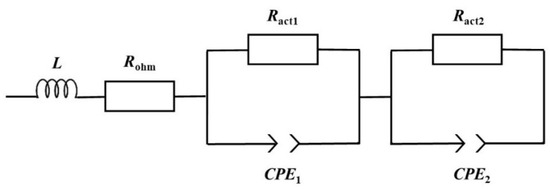
Figure 3.
Equivalent electric circuit applied to characterize the mpDMFC.
The impedance spectrum can be divided in three different regions based on its frequency range: the high-frequency region (lower real impedance values), the medium-frequency region (intermediate values) and the lower-frequency region (higher values), each region being associated with one type of loss [17,33]. In the high-frequency region, the spectrum is described by a point where the plot intercepts the real impedance axis, known as the high-frequency resistance (HFR), representing the ohmic losses/resistance (Figure 2). This time-independent loss is characterized using Ohm’s law and is expressed by a resistor in the EEC, namely ohmic resistance (Rohm)(Figure 3). The medium-frequency region usually resembles an arc, which represents the activation losses/resistances, described by a decrease in its value with a decrease in the cell voltage. The low-frequency region can also resemble an arc, associated with mass transport or concentration losses, where the resistance increases with a decrease in the fuel cell voltage [17].
Based on the plots depicted in Figure 2 representing the mini pDMFC behaviour, and on the values of the resistances that affect the system under study presented in Table 1, it can be concluded that the mini pDMFC is not greatly affected by mass transport losses. This is evidenced by the decrease in the two resistances representing the arcs shown in Figure 2 with a decrease in cell voltage (Table 1). Thus, the two arcs that appear in the Nyquist plot are due to activation losses, characterized in the EEC (Figure 3) by a resistor (Ract) in parallel with a constant-phase element (CPE). The CPE is associated with the double-layer interfaces in the system, describing the capacitance properties. As can be seen in Figure 2, the EEC proposed (lines) accurately reproduces the experimental data (dots).
Besides identifying and quantifying the different losses that adversely affect the system under analysis, it is important to link each loss with the different phenomena that occur in the system, to avoid and/or minimize them. In a working fuel cell, the activation losses are due to the electrochemical reactions taking place on both sides of the cell: the fuel oxidation at the anode side and the oxygen reduction at the cathode side. Nevertheless, as in a direct methanol fuel cell, both electrochemical reactions have a marked impact on the cell performance, and it is very difficult to link each activation loss/resistance (Ract1 and Ract2) to each electrochemical reaction. Therefore, to address this issue, a study was conducted where the catalyst loading of one side of the cell was reduced to a very low value, 0.5 mg cm−2, to try to distinguish the losses of each electrode. The following section examines the impact of reducing catalyst loading on both the anode and cathode sides on the cell performance and impedance spectrum, in order to identify the activation resistances, Ract1 and Ract2, as anode and cathode resistances.
3.2. Analysis of the Mini pDMFC with Reduced Anode and Cathode Catalyst Loadings
It is known that the methanol concentration has a significant influence on the passive DMFC behaviour, since the optimal concentration is a balance between the positive effect on the MOR rate and the negative effect on the alcohol crossover rate [34]. Therefore, this sub-section presents the results regarding the study of a mini pDMFC using reduced loadings on both the anode and cathode sides for different methanol concentrations (Table 2 and Table 3).

Table 2.
Values of the maximum power density achieved for the different cathode catalyst loadings and different methanol concentrations tested.

Table 3.
Values of the maximum power density achieved for the different anode catalyst loadings and different methanol concentrations tested.
As can be seen in Table 2 and Table 3, the best concentration, the one that leads to a higher performance, was 2 M for the highest loading used, 4 mg cm−2. For concentrations above this value, the negative impact of increased methanol concentrations on methanol crossover outweighs any potential gains in the MOR rate, resulting in decreased cell performances.
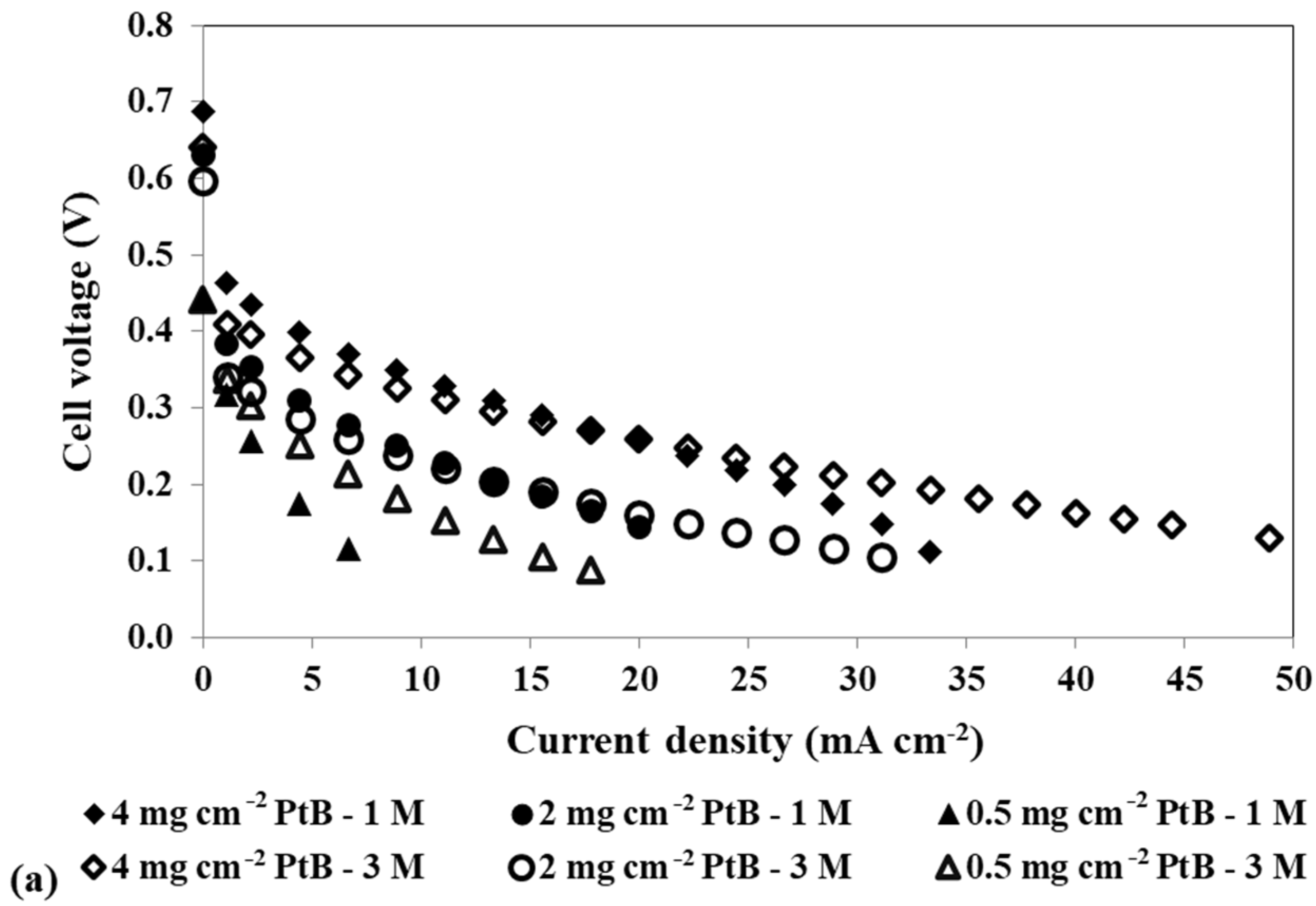
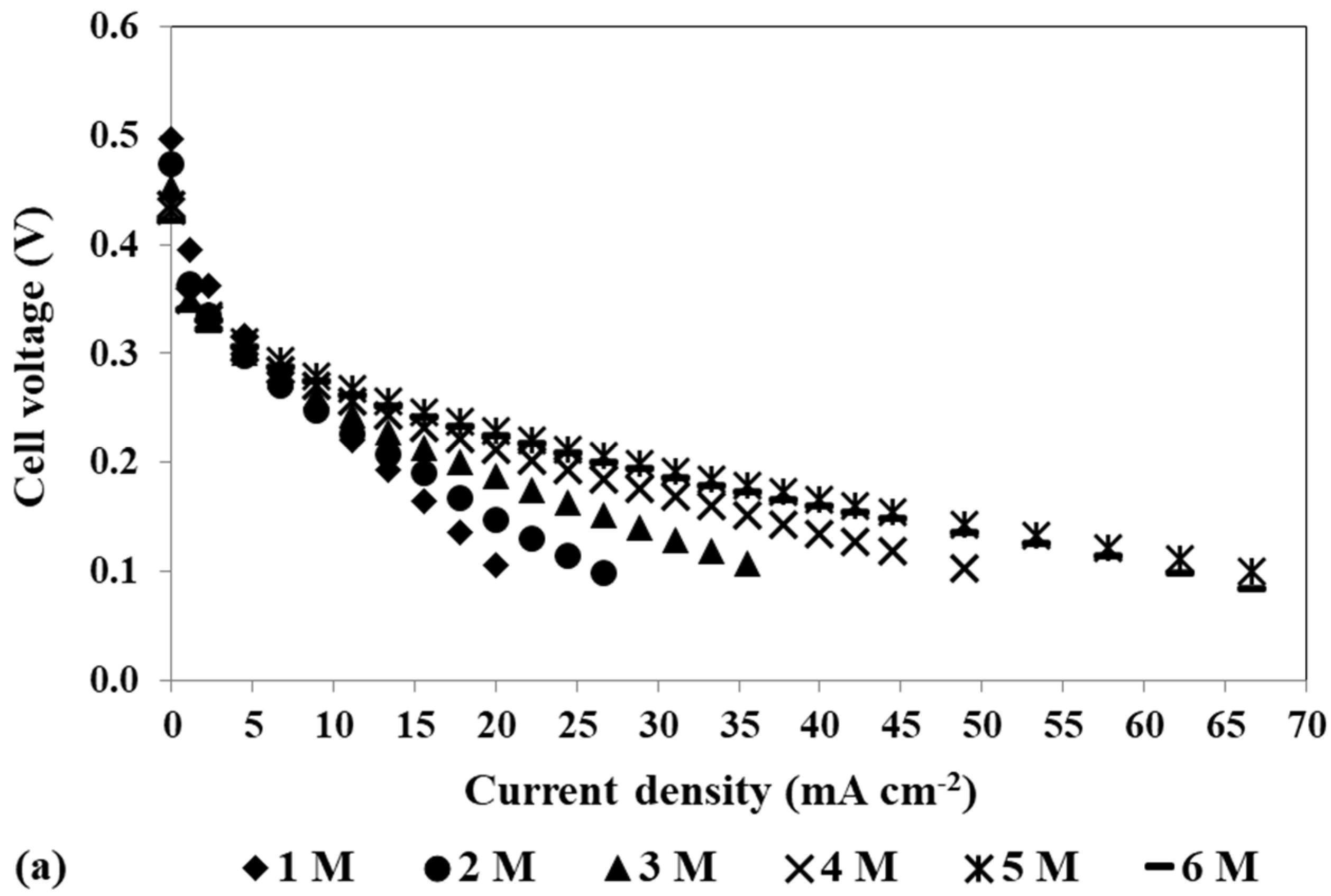
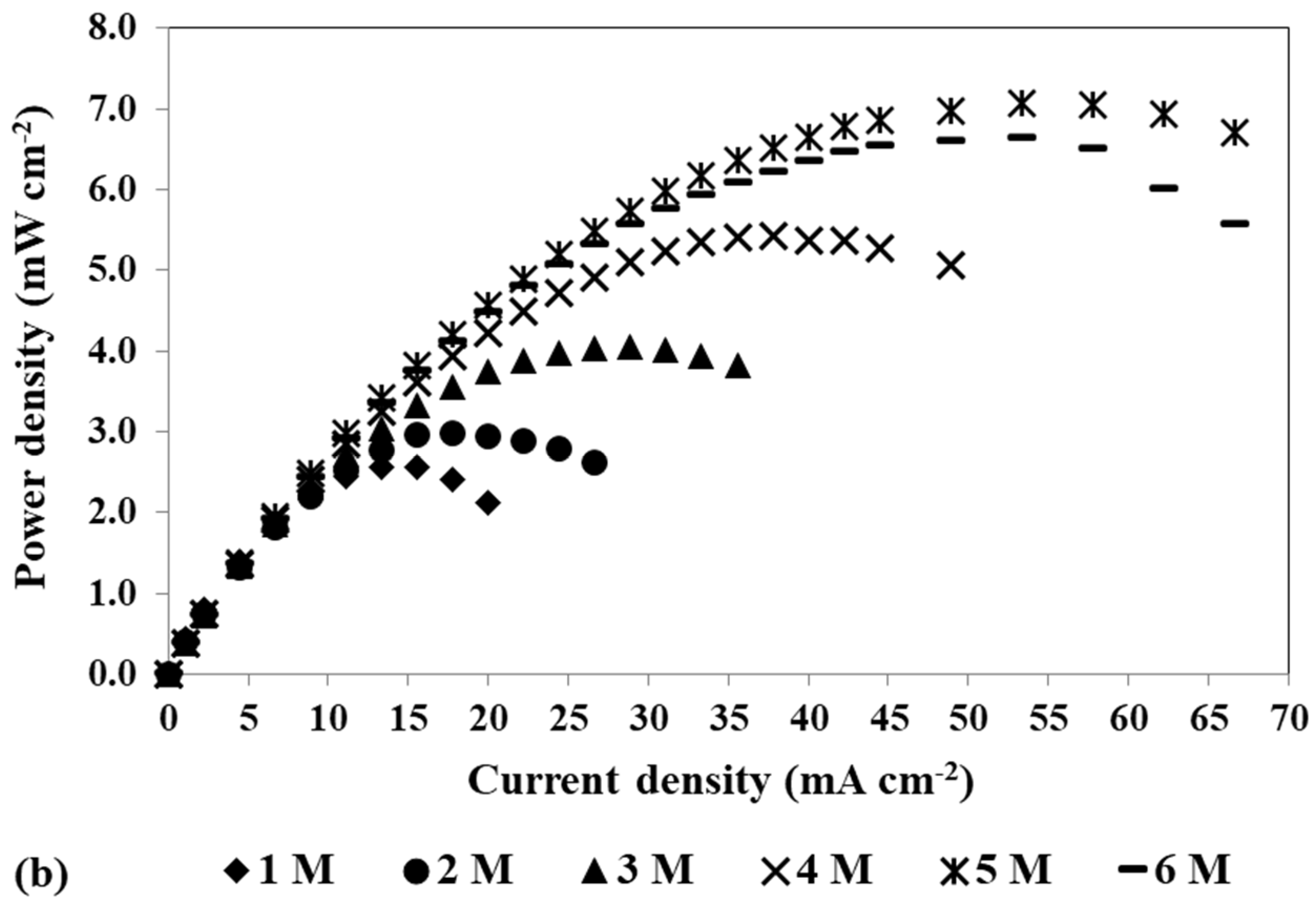
To study the impact of changing the cathode catalyst loading on the mini pDMFC behaviour, three different catalyst loadings, 4, 2 and 0.5 mg cm−2 PtB, were tested, keeping the anode catalyst loading at 4 mg cm−2 Pt-RuB. Figure 4a,b show the polarization and power density curves, respectively, for the different catalyst loadings tested at the cathode side and the two methanol concentrations selected, 1 M and 3 M. These concentrations were chosen as they are common to all the loadings studied and exhibit the same patterns and trends as the other concentrations tested. The values of the different resistances affecting the system for the different voltages tested (0.4, 0.3 and 0.2 V) obtained by fitting the experimental data to the EEC proposed (Figure 3), as well as the maximum power density obtained for each condition, are given in Table 4.
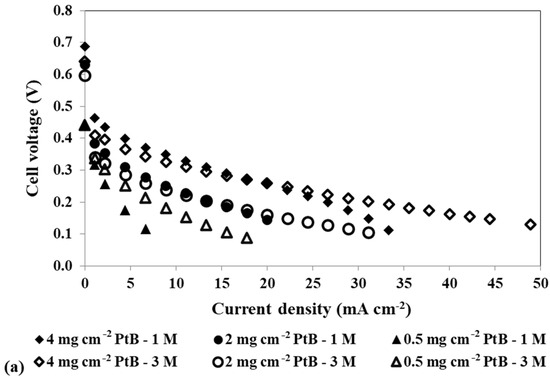
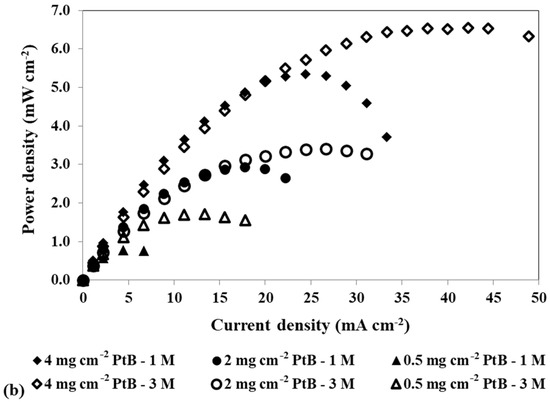
Figure 4.
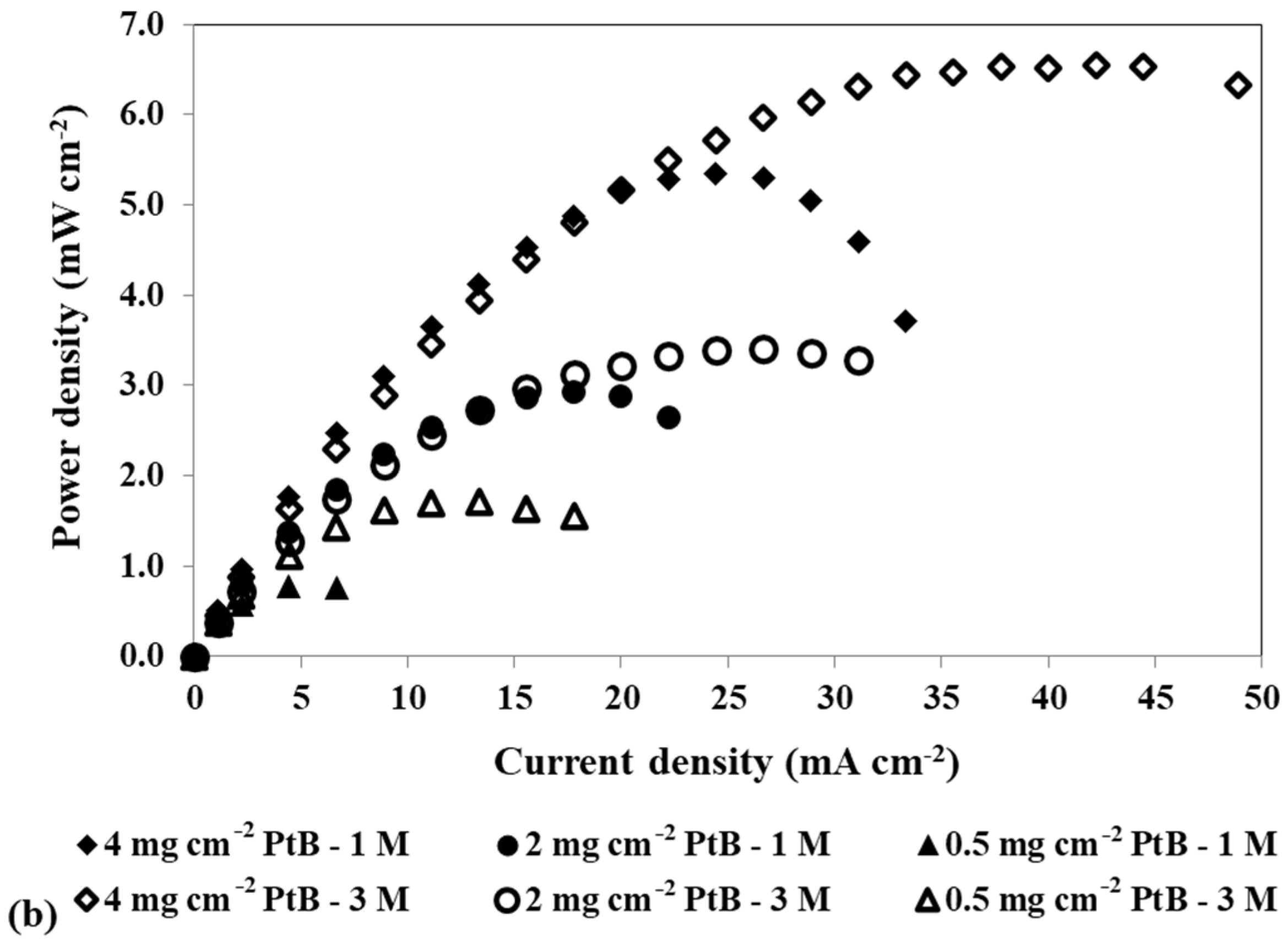
Effect of a loading reduction in the cathode catalyst on the mpDMFC performance for two methanol concentrations, 1 M and 3 M. (a) Polarization and (b) power density curves. Anode catalyst: 4 mg cm−2 Pt-RuB.

Table 4.
Resistances values obtained by the EEC fitting for the different cathode catalyst loadings tested and two methanol concentrations, 1 M and 3 M, and its maximum power density. Anode catalyst: 4 mg cm−2 Pt-RuB.
Analysing Figure 4, despite some overlapping points at the open-circuit voltage (OCV), it is possible to verify that the OCV decreases with increasing methanol concentration. This trend occurs because higher concentrations lead to a larger concentration gradient between the anode and cathode sides, resulting in greater methanol crossover rates through the membrane towards the cathode. This leads to a loss of fuel at the anode side, where it is needed for the fuel oxidation reaction, and to the poisoning of the cathode catalyst through the methanol oxidation reaction that occurs at this side. Based on the plots from Figure 4 and the values of the maximum power density summarized in Table 4, it can also be concluded that lower cathode loadings result in lower performances. Reduced loadings result in fewer active sites available for the oxygen reduction reaction, decreasing the rate of this reaction. Moreover, due to methanol crossover, higher cathode loadings are needed to overcome the competition between the oxygen and the methanol for the catalyst active sites. Therefore, the optimal power outputs were achieved with a cathode catalyst loading of 4 mg cm−2 (Table 2 and Table 4).
As shown in Table 4, the values of Rohm do not significantly change with the methanol concentration and cathode catalyst loading, as these parameters do not significantly affect the contact and electronic transport in the fuel cell.
Examining the two activation resistances values, it is evident that these resistances increase with a decrease in cathode catalyst loading. Lower loadings result in lower reaction rates, thus contributing to the observed trend. Analysis of the values for the different voltages tested reveals that at 0.4 V, where the methanol crossover rate is higher, both activation resistances have the highest values, but Ract1 decreases with an increase in methanol concentration, while Ract2 increases. Furthermore, these values demonstrate a tendency to increase with a decrease in cathode catalyst loading, indicating higher activation losses due to reduced electrochemical reaction rates within the cell. As already mentioned, the methanol that crosses the Nafion® membrane reacts on the cathode electrode, decreasing the catalyst available sites for the oxygen reduction reaction. Therefore, it is expected that lower loadings and higher methanol concentrations result in less available catalyst for the ORR, leading to increased cathode losses and decreased cathode performance, which is in accordance with the obtained Ract2 values. At 0.2 V, where crossover effects are less relevant, a decrease in cathode loading results in an increase in Ract2. However, this increase is lower compared to the one observed at 0.4 V, as the parasitic reaction occurs to a lesser extent, and more catalyst active sites are available for the ORR. As the anode catalyst is the same for all experiments in this study, it is expected that the anode will not suffer to a great extent from the different cathode loadings, being more influenced by voltage and methanol concentration. Therefore, it is expected to have higher losses for higher voltages due to methanol crossover, but an increase in the methanol concentration and a decrease in the cell voltage can lead to lower losses, as more methanol is available for the MOR, increasing the anode performance, which is consistent with the values of Ract1. These findings suggest that Ract2 corresponds to cathode resistance, while Ract1 corresponds to anode resistance.
To confirm this conclusion, a similar study was conducted on the anode side, using three different catalyst loadings (4, 2 and 0.5 mg cm−2) of Pt-RuB, while keeping the cathode catalyst loading at 4 mg cm−2 PtB, the loading that leads to the higher performances. This study was performed for different methanol concentrations to evaluate the impact of the solution concentration on the cell performance and methanol crossover rate (Table 3). The polarization and power density curves for two methanol concentrations, 1 M and 3 M, are shown, respectively, in Figure 5a,b, while the values of the different resistances and the maximum power outputs are displayed in Table 5.
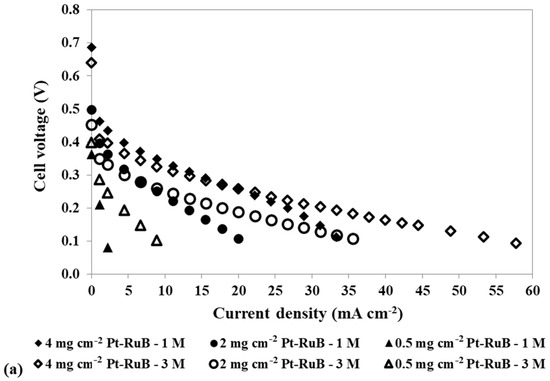
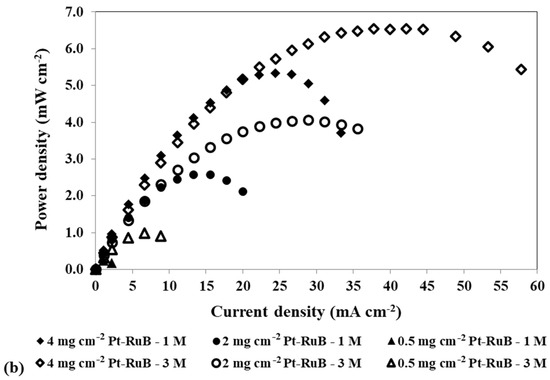
Figure 5.
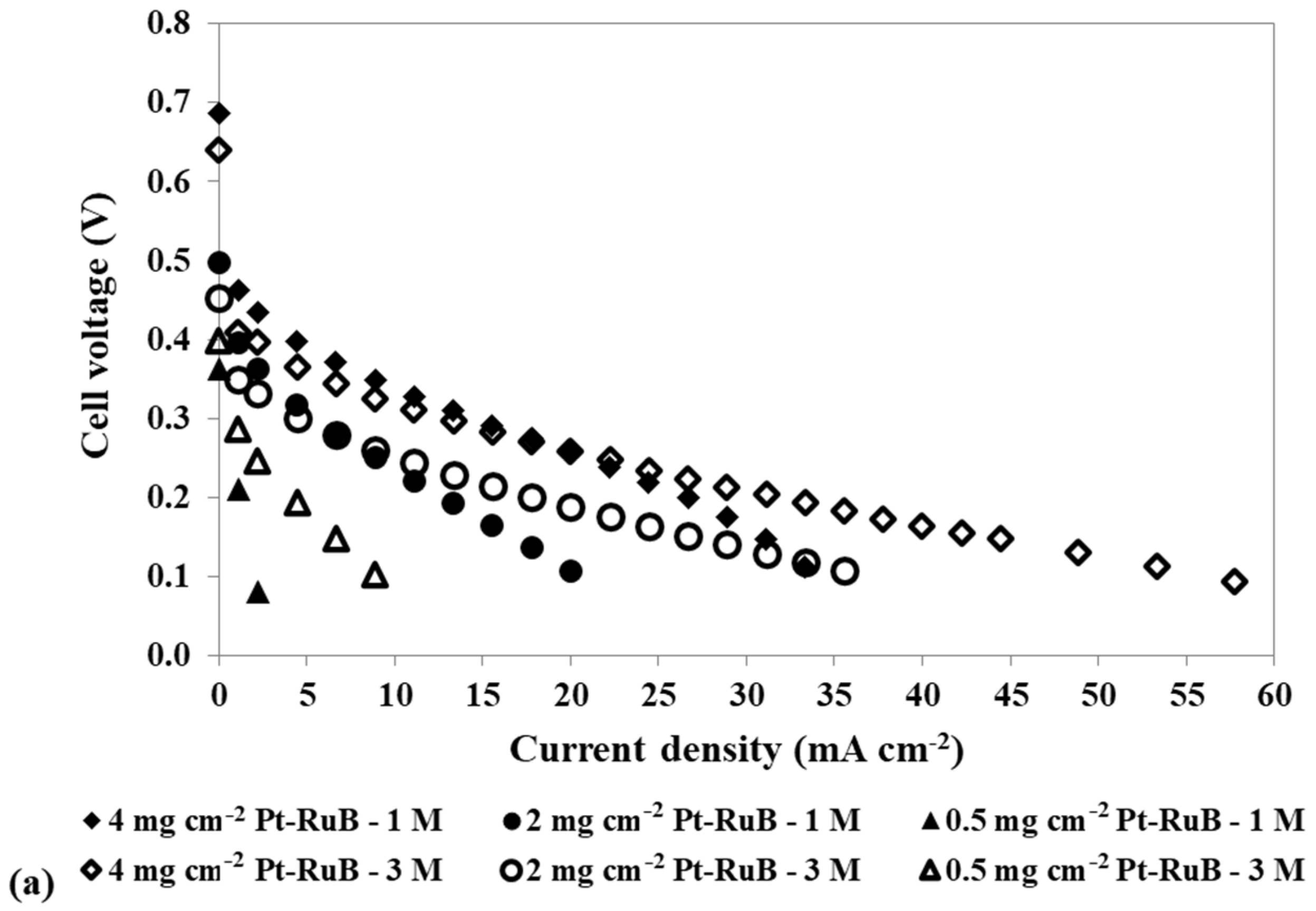
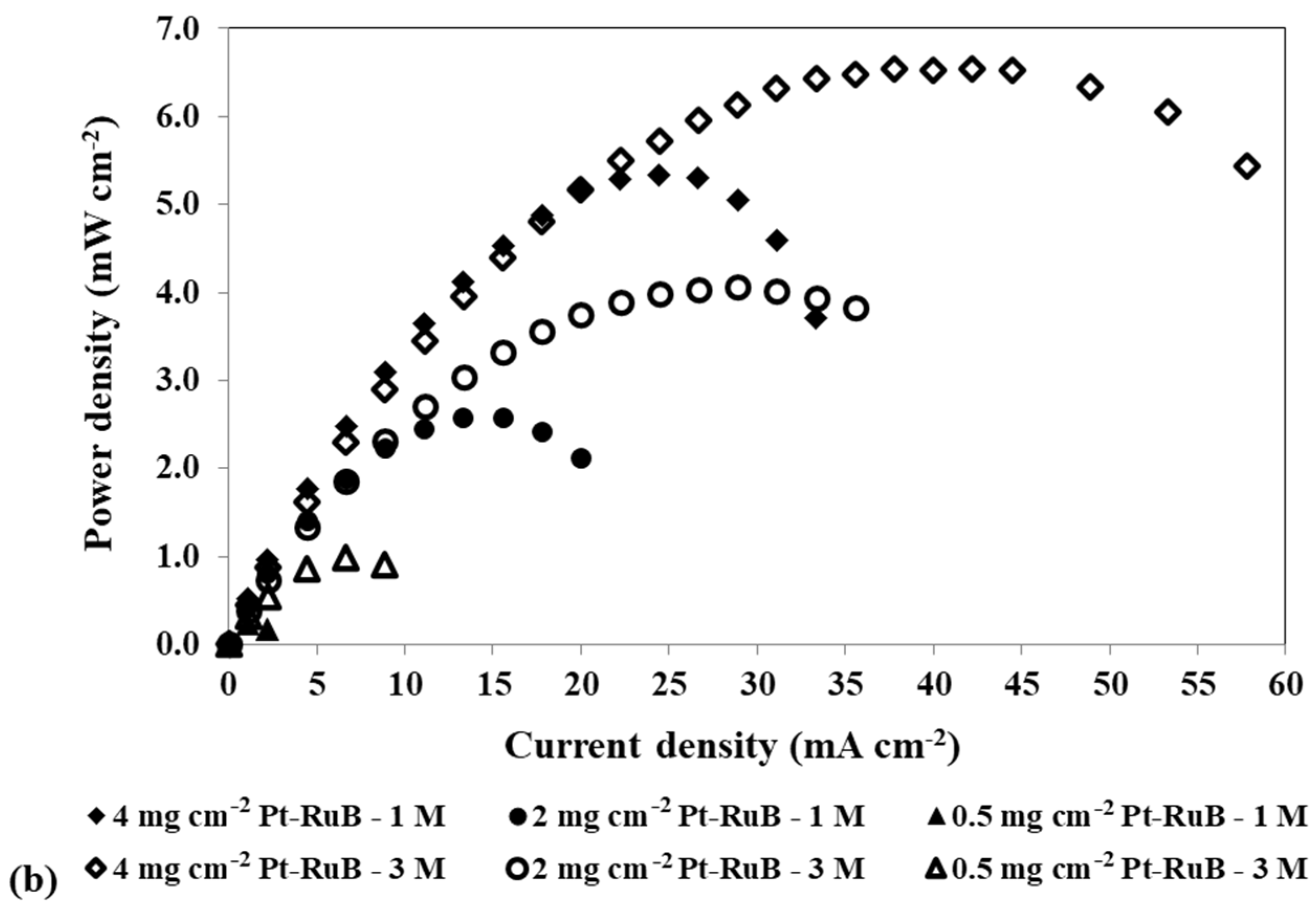
Effect of a loading reduction in the anode catalyst on the mpDMFC performance for two methanol concentrations, 1 M and 3 M. (a) Polarization and (b) power density curves. Cathode catalyst: 4 mg cm−2 PtB.

Table 5.
Resistances values obtained through the EEC fitting for the different anode catalyst loadings tested and two methanol concentrations, 1 M and 3 M, and its maximum power density. Cathode catalyst: 4 mg cm−2 PtB.
Figure 5 shows that reducing the anode catalyst loading and increasing the methanol concentration results in a decrease in OCV, since less catalyst is available for the methanol oxidation reaction, and consequently, more alcohol crosses the membrane to the cathode side. However, for 0.5 mg cm−2, the OCV is lower for the lower concentration tested, showing that using a lower concentration with a lower loading at the anode side has a more relevant impact on the reaction rate of the methanol oxidation reaction, owing to a lack of available catalyst sites for this reaction, than on the methanol crossover rate.
As observed in Table 5, for the lower loading used at the anode side, 0.5 mg cm−2, the losses are so high that the OCV is lower than 0.4 V, not allowing us to estimate the resistance values for this condition. The results in Table 5 demonstrate that reducing the anode catalyst loading leads to increased activation resistances (Ract1 and Ract2). However, the impact on Ract2 is more pronounced, as it exhibits higher values. A reduction in the anode catalyst loading results in a reduction in the active sites for the MOR, decreasing the reaction rate. Additionally, it leads to an increase in the methanol crossover rate, as less methanol reacts at the anode side, allowing more methanol to cross the membrane towards the cathode side. As the alcohol that crosses the membrane has a more relevant impact on the cathode side, due to the competition of the MOR and ORR for the cathode catalyst sites, it is expected that this resistance/loss will be higher for lower loadings (Ract2). Additionally, for 0.4 V, where the methanol crossover is more relevant, an increase in the concentration leads to an increase in the Ract2, indicating that this resistance is associated with the methanol crossover losses. For the other two voltages tested, 0.3 V and 0.2 V, as the methanol crossover effect decreases, an increase in the concentration leads to lower Ract2, as more methanol reacts at the anode and consequently less methanol is available to cross to the cathode side. Therefore, it can be concluded that Ract2 is associated with the cathode losses, being the cathode activation resistance. This conclusion is consistent with the observed Ract1 values, which correspond to anode losses or anode activation resistance. These values decrease with an increase in methanol concentration and a decrease in voltage. An increase in the methanol concentration leads to an increase in the MOR rate and consequently a decrease in the anode activation resistances/losses. For the lower loadings tested, 2 mg cm−2 and 0.5 mg cm−2, this resistance decreases with an increase in methanol concentration. This trend occurs because the deficiency of catalyst available for the MOR is offset by a higher methanol concentration at the anode. Consistently, for both concentrations tested, Ract1 increases with a decrease in the anode catalyst loading due to a decrease in the available catalyst for the MOR.
For the two concentrations displayed in Figure 5, the best performance was achieved for the higher loading used at the anode side, 4 mg cm−2 Pt-RuB. However, after analysing all the concentrations and loadings tested at the anode side, presented in Table 3, it can be concluded that a slightly higher power output was obtained using an anode loading of 2 mg cm−2 Pt-RuB. This is a very important result, since a reduction of 50% in the anode loading allowed the cell to work with higher methanol concentrations without sacrificing performance, thereby reducing costs.
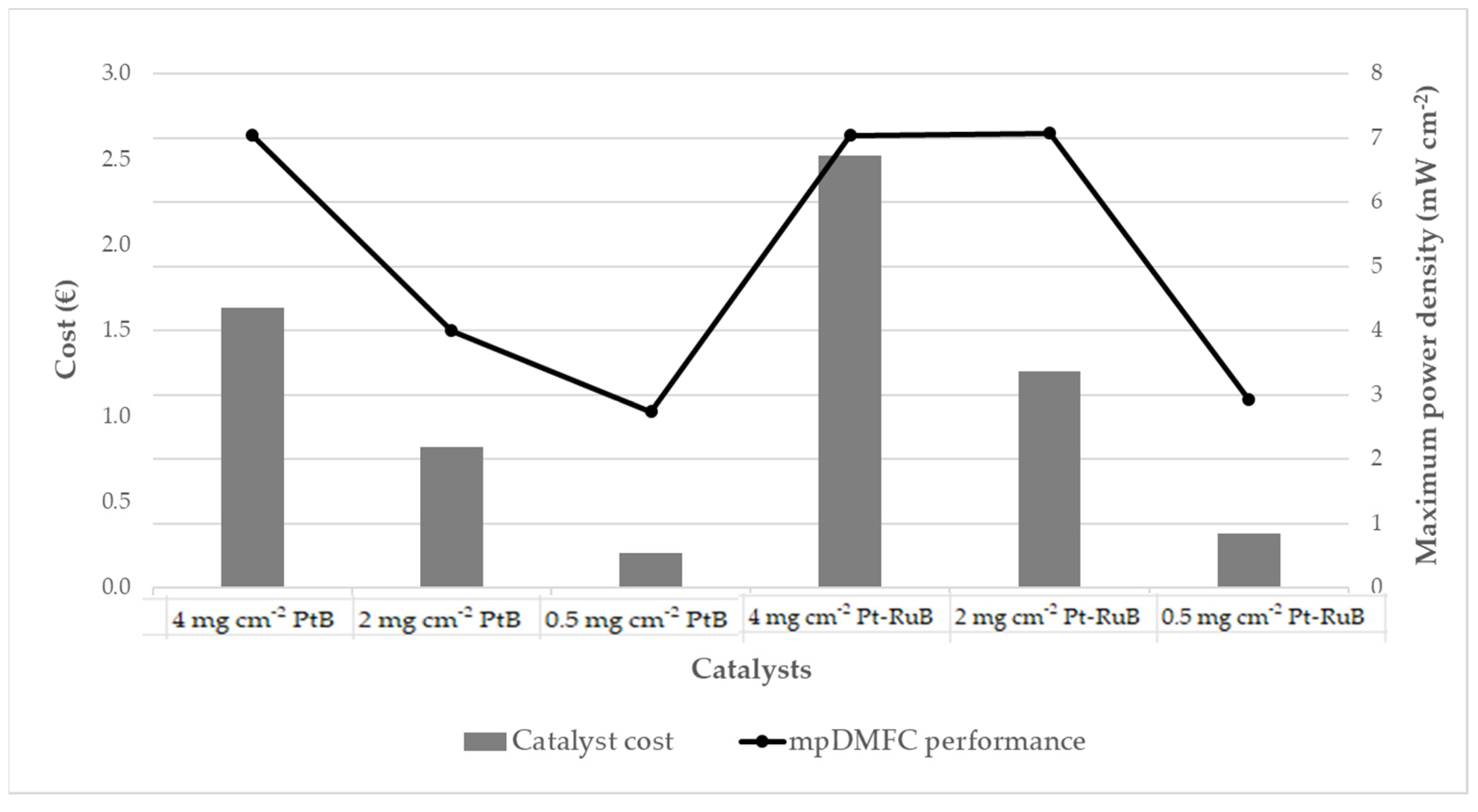
Figure 6 presents the catalyst cost associated with each electrode tested, as well as the maximum power density obtained by the mpDMFC. It is evident that a reduction in metal loading results in a notable decrease in catalyst cost, as a smaller quantity of noble metal is necessary. Furthermore, the mpDMFC performance is also compromised by the reduction in the quantity of metal available for the desired electrochemical reactions. However, this study identified an electrode for the anode side (2 mg cm−2 Pt-RuB) that enabled a reduction in metal loading and costs without a significant decline in the mpDMFC performance. Nevertheless, this analysis was conducted on a limited basis, and further investigation is required to assess additional factors, such as the cost of all cell components, stability and durability, with the objective of advancing the system towards commercialization.
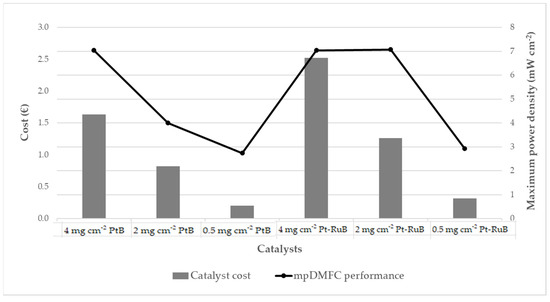
Figure 6.
Catalyst cost and mpDMFC performance achieved for each electrode studied.
3.3. Effect of the Methanol Concentration
Based on the results presented in the previous sub-section, Section 3.2, it was verified that better performances were achieved for 2 mg cm−2 Pt-RuB of anode catalyst loading and 4 mg cm−2 PtB of cathode catalyst loading. Therefore, these two loadings were used to study the methanol concentration effect on the mini pDMFC performance towards a further increase in its power output. The corresponding polarization and power density curves are presented in Figure 7, while the different values of the resistances and the maximum power densities obtained for each concentration tested (1 M to 6 M) are depicted in Table 6.
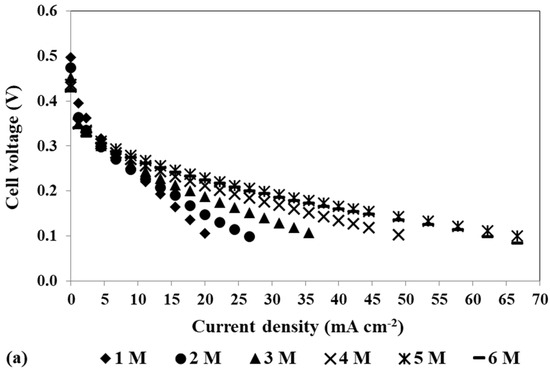
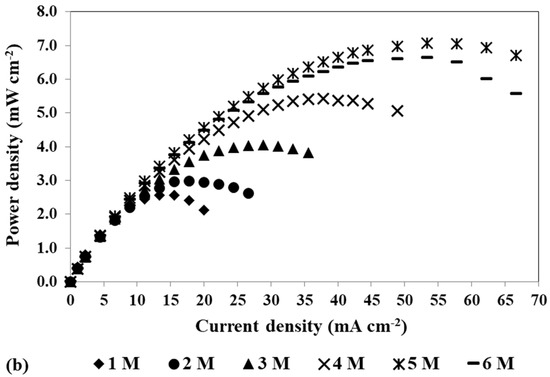
Figure 7.
Effect of the methanol concentration on the mpDMFC performance. (a) Polarization and (b) power density curves. Anode catalyst: 2 mg cm−2 Pt-RuB; cathode catalyst: 4 mg cm−2 PtB.

Table 6.
Resistances values obtained through the EEC fitting for the different methanol concentrations tested and its maximum power density. Anode catalyst: 2 mg cm−2 Pt-RuB; cathode catalyst: 4 mg cm−2 PtB.
Figure 7 shows that for the different solution concentrations tested, the OCV is considerably lower than the ideal value (1.21 V) and decreases with an increase in the methanol concentration, due to methanol crossover through the membrane. As already mentioned, higher methanol concentrations lead to higher concentration gradients between the two electrodes, which is the force of diffusion, increasing the rate of methanol crossing the membrane towards the cathode side and its concentration at the cathode. At this side, the methanol reacts incompletely at the cathode catalyst (PtB) that is not the most suitable one for this reaction, poisoning the catalyst active sites and lowering its activity and availability for the ORR. This leads to a decrease in the cathode performance and an increase in the cathode losses. However, as can be seen in Figure 7 and on the maximum power density values presented in Table 6, the best performance was achieved with a methanol concentration of 5 M, this being the optimal concentration. This concentration allowed an optimal balance between the positive effect of the methanol concentration on the MOR rate and the negative effect of the methanol crossover rate.
Analysing the values of the two activation resistances presented in Table 6 for 0.4 V, where the methanol crossover is dominant, it can be observed that Ract2 increases with the methanol concentration, while Ract1 decreases. Additionally, as the voltage decreases, the impact of the methanol concentration on Ract2 becomes less relevant as the amount of methanol available at the anode side is lower, due to its oxidation at the anode side, and less methanol crosses to the cathode side. This behaviour is in line with the cathode losses. Therefore, as concluded in the previous sub-section, Ract2 represents the cathode activation losses. It can also be seen that Ract1 decreases with a decrease in the cell voltage and an increase in the methanol concentration, which is consistent with the anode activation losses. An increase in the alcohol concentration leads to a higher methanol diffusion rate at this side, increasing the amount of methanol that reaches the anode catalyst active sites, increasing the methanol oxidation rate and decreasing the activation losses associated with this reaction.
The maximum power density obtained (7.07 mW cm−2) is compared with other similar works [35,36], as shown in Table 7. Nevertheless, it should be noted that Zuo et al. [36] demonstrated a higher power density, although this was achieved through the utilization of higher catalyst loadings. In contrast, Abdullah et al. [35] employed lower catalyst loadings, resulting in considerably inferior performance. Therefore, the results obtained in the present work demonstrate a notable accomplishment, with a reduction in the anode catalyst loading, operation at elevated methanol concentrations and a decreased active area.

Table 7.
Comparison of the performance of the presented mpDMFC with similar research works.
4. Conclusions
Costs, efficiency and lifetime are key challenges to overcome in order to introduce the mini pDMFC into the market, aiming towards the replacement of lithium batteries in portable electronic applications. Based on that, this work presents the effect of a reduction in the anode and cathode catalyst loading on the performance of a mini pDMFC with an active area of 2.25 cm2. This study was performed to identify and quantify in detail the different losses that negatively affect the system using the EIS technique. Additionally, we explored the possibility of reducing the system costs by reducing the catalyst loading, without a significant loss in the cell efficiency. Despite the initial anticipation that the results might fall below the desired values, a low loading of 0.5 mg cm−2 was tested at both the anode and cathode sides, since the losses of the electrode using this loading overlap with the losses of the other electrode, allowing its characterization.
The cell behaviour was analysed through polarization curves, power density curves and EIS data by fitting it with an electric equivalent circuit proposed in this work, which showed good agreement with the experimental data. The electrochemical characterization of the mini pDMFC allowed us to verify that, besides the ohmic losses, the cell major losses are activation ones, which decrease with a decrease in the cell voltage.
The study regarding the analysis of the mini pDMFC with reduced anode and cathode catalyst loadings allowed the identification of Ract2 as the cathode activation resistance and Ract1 as the anode activation resistance and the quantification and contribution of these two losses for the overall cell performance. It was observed that for the different conditions tested, the cathode activation resistance tends to be higher than that of the anode, showing that the cathode losses contribute to a great extent to the overall cell losses. The results demonstrated that the cathode losses decrease with a decrease in the cell voltage, as under these conditions, the cell suffers to a lesser extent from methanol crossover, and more active sites are available for the ORR. Furthermore, as the voltage decreases, the impact of the higher methanol concentrations on the cathode losses becomes less relevant, as more methanol reacts at the anode side, decreasing the amount of methanol that reaches and crosses the membrane. Despite this, due to the methanol that reaches the cathode side, it is necessary to work the cell with higher cathode catalyst loadings to ensure a proper amount of catalyst for the ORR. Therefore, it was verified that the best power outputs were achieved for a cathode catalyst loading of 4 mg cm−2. The lower loadings tested at the cathode side lead to similar power outputs.
The anode losses decrease with a decrease in the fuel cell voltage due to a decrease in the loss of fuel due to crossover. For the lower loadings tested, an increase in the methanol concentration leads to a decrease in the anode losses since the lack of catalyst for the MOR is offset by the higher concentrations. The results showed that better overall performances were achieved using an anode catalyst loading of 2 mg cm−2 Pt-RuB.
For the conditions tested and this specific cell design, the maximum power density, 7.07 mW cm−2, was obtained using 2 mg cm−2 Pt-RuB of anode catalyst loading and 4 mg cm−2 PtB of cathode catalyst loading with a methanol concentration of 5 M. Although this power output is not very high, it can be enough for some specific applications and was obtained with an anode catalyst loading 50% lower than the most common one used on this type of fuel cell, which is 4 mg cm−2 Pt-Ru, allowing a decrease in the system costs.
Author Contributions
Investigation, C.S.M.; Writing—original draft, C.S.M.; Writing—review & editing, A.M.F.R.P. and V.B.O.; Supervision, A.M.F.R.P. and V.B.O.; Funding acquisition, V.B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES, Project PTDC/NewPortCell-POCI-01-0145-FEDER-032116.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
This work was supported by LA/P/0045/2020 (ALiCE), UIDB/00532/2020 and UIDP/00532/2020 (CEFT), funded by national funds through FCT/MCTES (PIDDAC).

Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, W.; Zhang, X.; Hou, C.; Zhang, Y.; Wang, H.; Liu, X. Enhanced Water Management via the Optimization of Cathode Microporous Layer Using 3D Graphene Frameworks for Direct Methanol Fuel Cell. J. Power Sources 2020, 451, 227800. [Google Scholar] [CrossRef]
- Abraham, B.G.; Chetty, R. Design and Fabrication of a Quick-Fit Architecture Air Breathing Direct Methanol Fuel Cell. Int. J. Hydrogen Energy 2021, 46, 6845–6856. [Google Scholar] [CrossRef]
- Baruah, B.; Deb, P. Performance and Application of Carbon-Based Electrocatalysts in Direct Methanol Fuel Cell. Mater. Adv. 2021, 2, 5344–5364. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent Advances in Multi-Scale Design and Construction of Materials for Direct Methanol Fuel Cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Deng, P.; et al. Recent Advances in Two-Dimensional Pt Based Electrocatalysts for Methanol Oxidation Reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Mansor, M.; Timmiati, S.N.; Lim, K.L.; Wong, W.Y.; Kamarudin, S.K.; Nazirah Kamarudin, N.H. Recent Progress of Anode Catalysts and Their Support Materials for Methanol Electrooxidation Reaction. Int. J. Hydrogen Energy 2019, 44, 14744–14769. [Google Scholar] [CrossRef]
- Yang, L.; Ge, J.; Liu, C.; Wang, G.; Xing, W. Approaches to Improve the Performance of Anode Methanol Oxidation Reaction—A Short Review. Curr. Opin. Electrochem. 2017, 4, 83–88. [Google Scholar] [CrossRef]
- de Sá, M.H.; Moreira, C.S.; Pinto, A.M.F.R.; Oliveira, V.B. Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells. Energies 2022, 15, 6335. [Google Scholar] [CrossRef]
- Wang, L.; Holewinski, A.; Wang, C. Prospects of Platinum-Based Nanostructures for the Electrocatalytic Reduction of Oxygen. ACS Catal. 2018, 8, 9388–9398. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A Review of Pt-Based Electrocatalysts for Oxygen Reduction Reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Stacy, J.; Regmi, Y.N.; Leonard, B.; Fan, M. The Recent Progress and Future of Oxygen Reduction Reaction Catalysis: A Review. Renew. Sustain. Energy Rev. 2017, 69, 401–414. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, H.J.; Chung, Y.H. Recent Developments of Nano-Structured Materials as the Catalysts for Oxygen Reduction Reaction. Nano Converg. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct Methanol Fuel Cells System–A Review of Dual-Role Electrocatalysts for Oxygen Reduction and Methanol Oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Braz, B.A.; Moreira, C.S.; Oliveira, V.B.; Pinto, A. Effect of the Current Collector Design on the Performance of a Passive Direct Methanol Fuel Cell. Electrochim. Acta 2019, 300, 306–315. [Google Scholar] [CrossRef]
- Ismail, A.; Kee, Y.W. Investigation on Voltage Loss Mechanism for Direct Methanol Fuel Cell. Energy Rep. 2023, 10, 535–543. [Google Scholar] [CrossRef]
- Braz, B.A.; Moreira, C.S.; Oliveira, V.B.; Pinto, A. Electrochemical Impedance Spectroscopy as a Diagnostic Tool for Passive Direct Methanol Fuel Cells. Energy Rep. 2022, 8, 7964–7975. [Google Scholar] [CrossRef]
- Pivac, I.; Barbir, F. Inductive Phenomena at Low Frequencies in Impedance Spectra of Proton Exchange Membrane Fuel Cells—A Review. J. Power Sources 2016, 326, 112–119. [Google Scholar] [CrossRef]
- Mallick, R.K.; Thombre, S.B.; Motghare, R.V.; Chillawar, R.R. Analysis of the Clamping Effects on the Passive Direct Methanol Fuel Cell Performance Using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2016, 215, 150–161. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Sayed, E.T.; Nakagawa, N. Significance of Diffusion Layers on the Performance of Liquid and Vapor Feed Passive Direct Methanol Fuel Cells. Energy 2020, 209, 118492. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B. Effect of Current Collector Roughness on Performance of Passive Direct Methanol Fuel Cell. Renew. Energy 2019, 138, 272–283. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, Y.; Li, X.; Liu, X. Performance Investigation and Effect of Temperature on a Passive ΜDMFC with Stainless Steel Mesh. Appl. Therm. Eng. 2018, 141, 642–647. [Google Scholar] [CrossRef]
- Gago, A.S.; Esquivel, J.-P.; Sabaté, N.; Santander, J.; Alonso-Vante, N. Comprehensive Characterization and Understanding of Micro-Fuel Cells Operating at High Methanol Concentrations. Beilstein J. Nanotechnol. 2015, 6, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yuan, T.; Huang, Q.; Zhang, H.; Zou, Z.; Zheng, J.; Yang, H. Polypyrrole Nanowire Networks as Anodic Micro-Porous Layer for Passive Direct Methanol Fuel Cells. Electrochim. Acta 2014, 141, 1–5. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Structural Mechanism Investigation on Methanol Crossover and Stability of a Passive Direct Methanol Fuel Cell Performance via Modified Micro-Porous Layer. Int. J. Energy Res. 2021, 45, 12928–12943. [Google Scholar] [CrossRef]
- Pu, L.; Jiang, J.; Yuan, T.; Chai, J.; Zhang, H.; Zou, Z.; Li, X.M.; Yang, H. Performance Improvement of Passive Direct Methanol Fuel Cells with Surface-Patterned Nafion® Membranes. Appl. Surf. Sci. 2015, 327, 205–212. [Google Scholar] [CrossRef]
- Braz, B.A.; Oliveira, V.B.; Pinto, A.M.F.R. Optimization of a Passive Direct Methanol Fuel Cell with Different Current Collector Materials. Energy 2020, 208, 118394. [Google Scholar] [CrossRef]
- Braz, B.A.; Oliveira, V.B.; Pinto, A. Experimental Studies of the Effect of Cathode Diffusion Layer Properties on a Passive Direct Methanol Fuel Cell Power Output. Int. J. Hydrogen Energy 2019, 44, 19334–19343. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, J.; Chai, J.; Yuan, T.; Zhang, H.; Zou, Z.; Zhang, X.; Yang, H. Construction of Porous Anode by Sacrificial Template for a Passive Direct Methanol Fuel Cell. J. Power Sources 2014, 262, 213–218. [Google Scholar] [CrossRef]
- Chen, P.; Wu, H.; Yuan, T.; Zou, Z.; Zhang, H.; Zheng, J.; Yang, H. Electronspun Nanofiber Network Anode for a Passive Direct Methanol Fuel Cell. J. Power Sources 2014, 255, 70–75. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Baglio, V.; Aricò, A.S. Electrochemical Impedance Spectroscopy as a Diagnostic Tool in Polymer Electrolyte Membrane Electrolysis. Materials 2018, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. Approaches to Overcome the Barrier Issues of Passive Direct Methanol Fuel Cell—Review. Renew. Sustain. Energy Rev. 2017, 67, 1087–1104. [Google Scholar] [CrossRef]
- Shou-Shing, H.; Ling-Ching, H.; Ching-Chi, L.; Ching-Feng, H. Analyses of Electrochemical Impedance Spectroscopy and Cyclic Voltammetry in Micro-Direct Methanol Fuel Cell Stacks. Energy Res. 2016, 40, 2162–2175. [Google Scholar] [CrossRef]
- Shrivastava, N.K.; Thombre, S.B.; Chadge, R.B. Liquid Feed Passive Direct Methanol Fuel Cell: Challenges and Recent Advances. Ionics 2016, 22, 1–23. [Google Scholar] [CrossRef]
- Abdullah, N.; Kamarudin, S.K.; Shyuan, L.K. Novel Anodic Catalyst Support for Direct Methanol Fuel Cell: Characterizations and Single-Cell Performances. Nanoscale Res. Lett. 2018, 13, 90. [Google Scholar] [CrossRef]
- Zuo, K.; Yuan, Z.; Cao, C.; Hao, Y. The Mass Transport Based on Convection Effects in a Passive DMFC under Open-Circuit Conditions. Int. J. Hydrogen Energy 2018, 43, 23463–23474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).