The Feasibility Study of In Situ Conversion of Oil Shale Based on Calcium-Oxide-Based Composite Materia Hydration Exothermic Reaction
Abstract
:1. Introduction
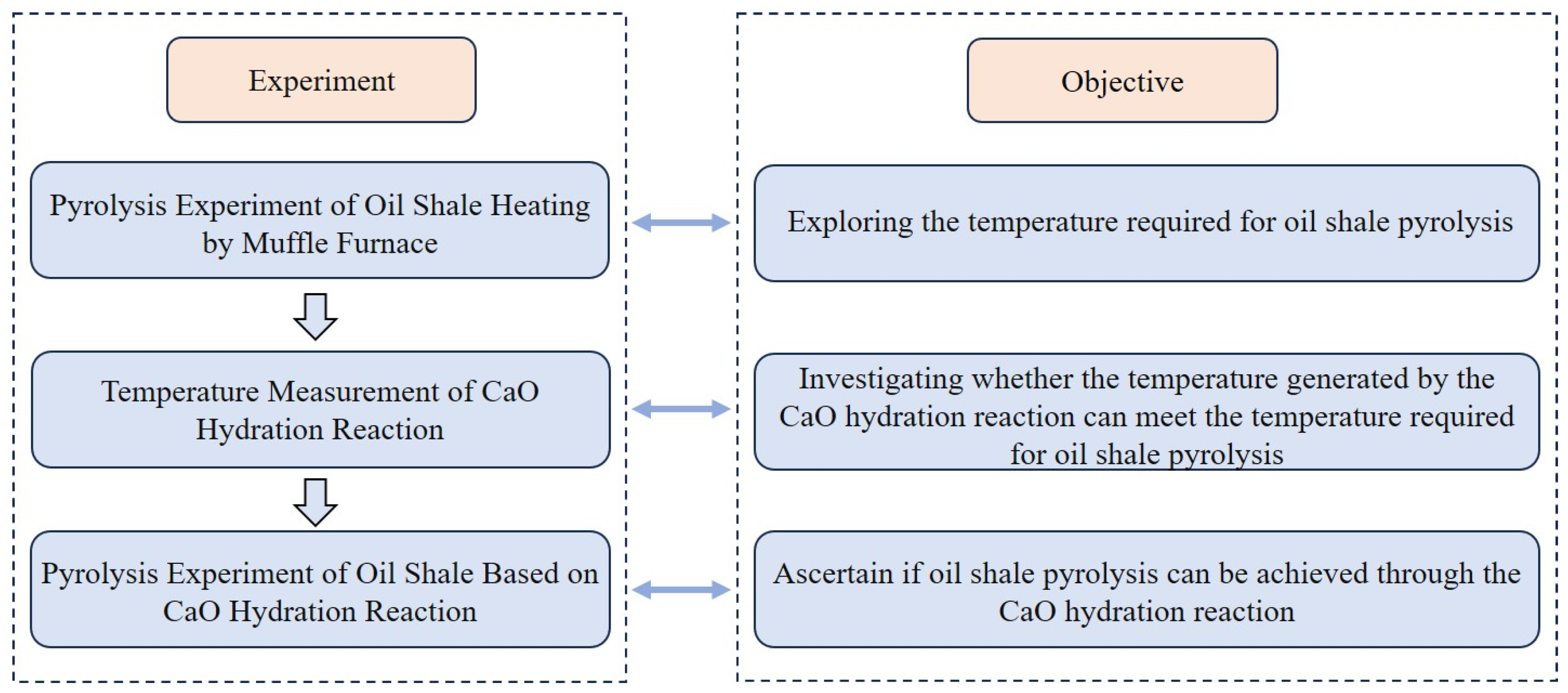
2. Experimental Design and Instrumentation Introduction
2.1. Pyrolysis Experiment of Oil Shale Heating by Muffle Furnace
2.2. Measure the Temperature of the CaO-CM Hydration Reaction
- (1)
- The pressure inside the reaction vessel is set at 0.1 MPa, 2 MPa, 4 MPa, and 6 MPa up to 18 MPa, with the ambient temperature being room temperature.
- (2)
- The ambient temperature is set at an initial reaction temperature of 50 °C (achieved using a water bath to heat the reaction vessel), with the pressure being 0.1 Mpa, 2 MPa, 4 MPa, and 6 MPa up to 16 MPa.
- (3)
- Hydration reactions are conducted using CaO-CM masses of 40 g, 100 g, 250 g, and 20 kg, respectively, under room temperature and standard atmospheric pressure.
2.3. Pyrolysis Experiment of Oil Shale Based on CaO-CM Hydration Reaction
3. Results and Discussion
3.1. Determination of the Oil Shale Pyrolysis Temperature
3.2. The Temperature Properties of CaO-CM Hydration Reaction
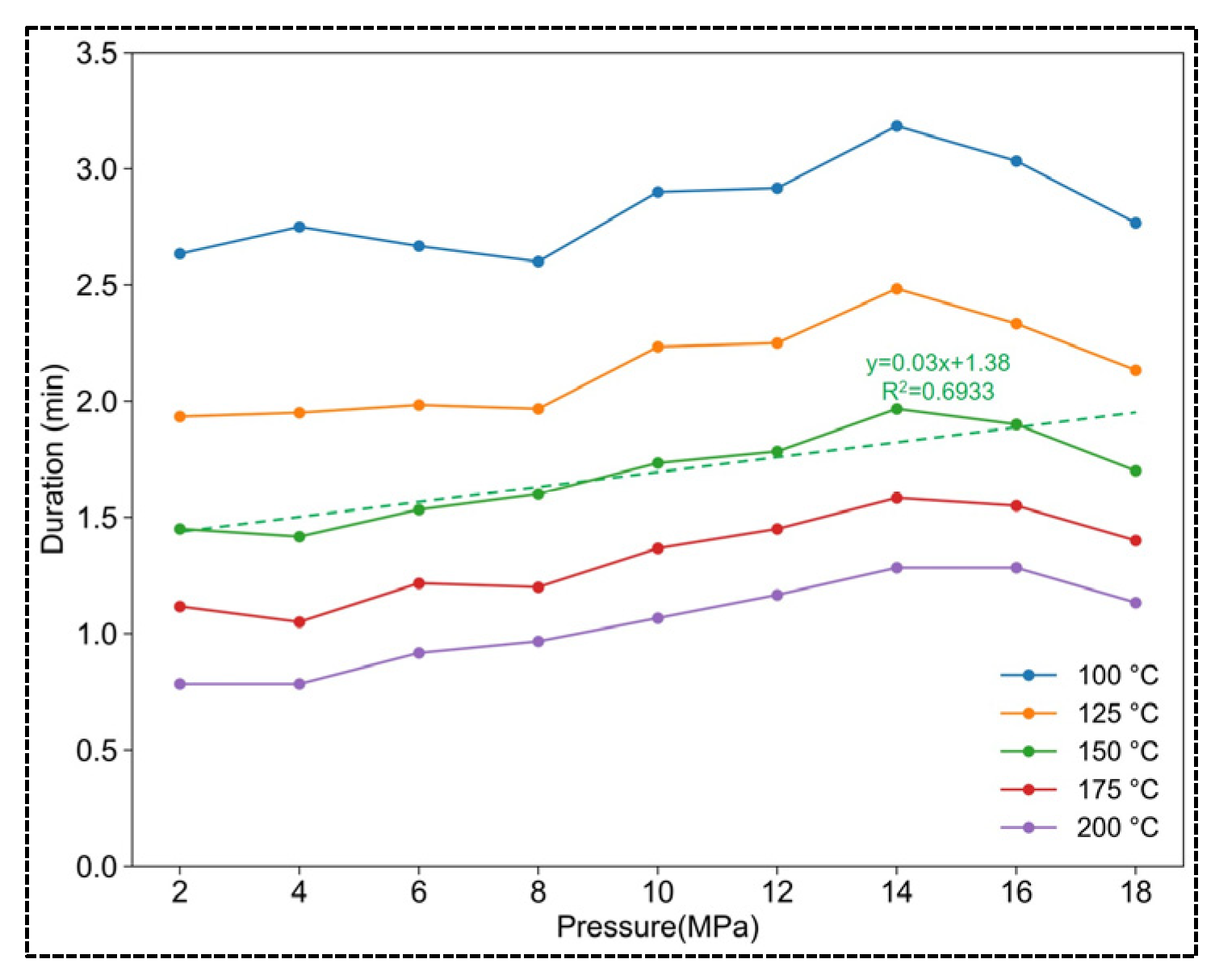
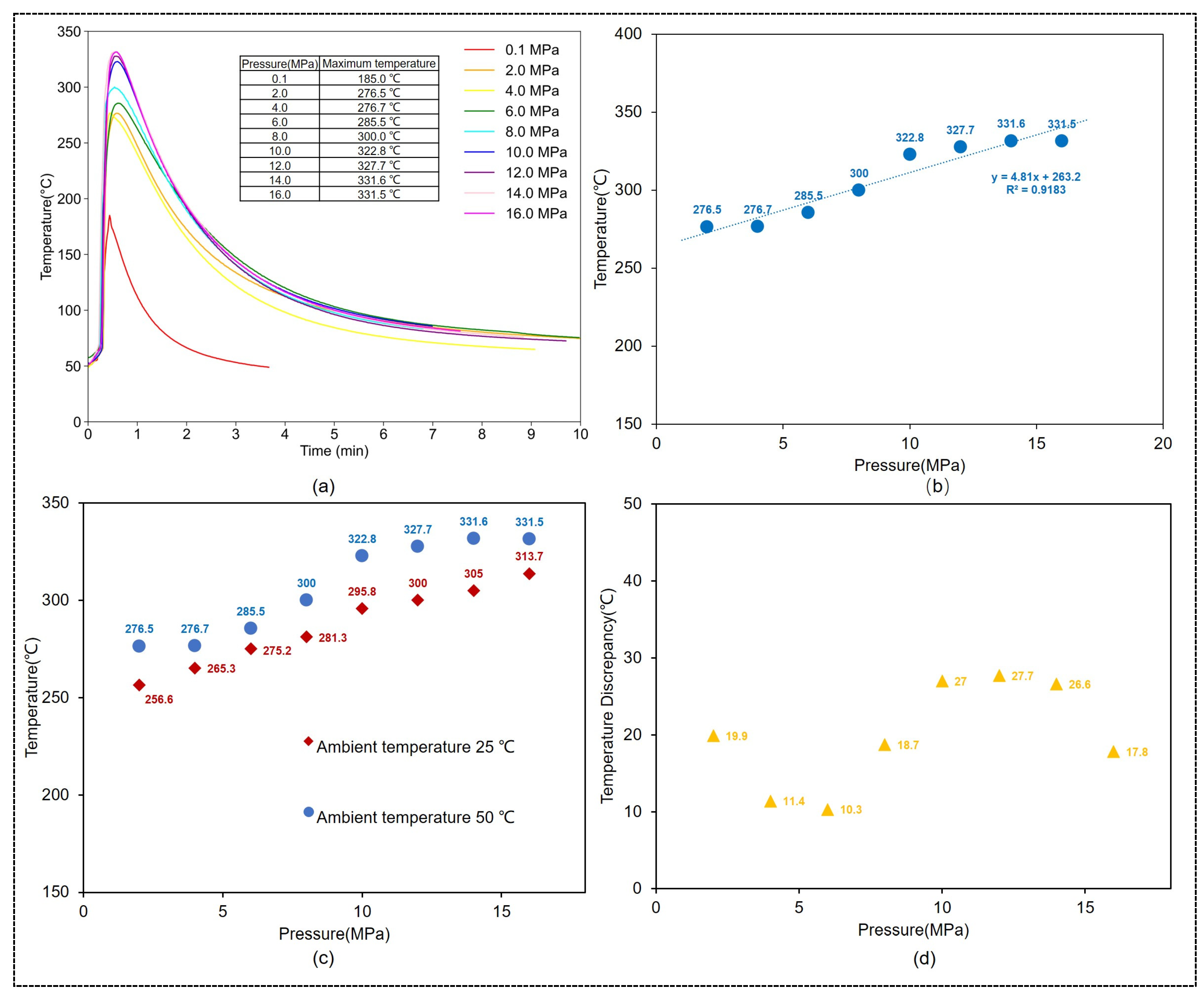
3.2.1. The Impact of Pressure on the Temperature of CaO-CM Hydration Reaction
- (1)
- The effect of pressure on the maximum temperature
- (2)
- The effect of pressure on the duration of temperature
3.2.2. The Impact of Environmental Temperature on the Hydration Reaction Temperature of CaO-CM
- (1)
- The effect of environmental temperature on the maximum temperature
- (2)
- The effect of ambient temperature on the duration of temperature
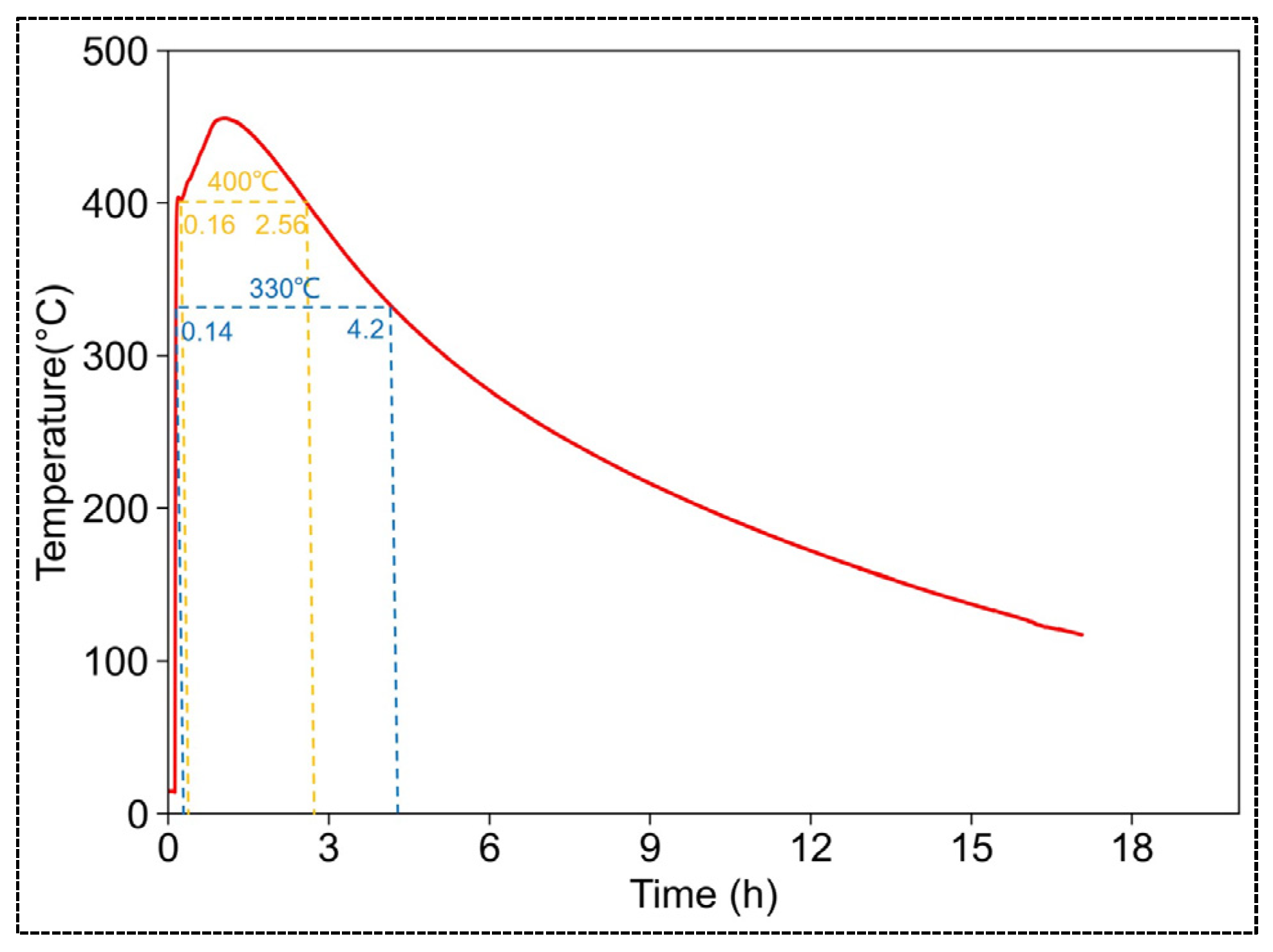
3.2.3. The Impact of the Quantity of CaO-CM Hydration Reaction Mass on the Reaction Temperature
3.3. Thermal Decomposition Experiment of Oil Shale Based on CaO-CM Hydration Reaction
3.4. Discussion
4. Conclusions
- (1)
- The minimum pyrolysis temperature of Xinjiang oil shale is 330 °C, with significant yields observed at a pyrolysis temperature of 400 °C.
- (2)
- The temperature of the hydration reaction of CaO-CM is influenced by environmental temperature, pressure, and the quantity of reactants. For the influence on the maximum temperature achievable by the reaction, environmental temperature factors have a relatively minor impact, while pressure and the mass of reactants have a significant impact. As for the influence on the duration of temperature sustenance, pressure and temperature factors have a relatively minor impact, while the mass of reactants has a significant impact. When the mass of CaO-CM is 20 kg, the hydration reaction can reach a maximum temperature of 455.5 °C, sustaining temperatures above 400 °C for 2.4 h and above 330 °C for 4.06 h. The temperature conditions generated by the hydration reaction of CaO-CM meet the temperature requirements for oil shale pyrolysis.
- (3)
- Through an oil shale pyrolysis experiment based on the CaO-CM hydration reaction, it was found that there was a significant decrease in pyrolysis hydrocarbons after the experiments. This indicates that the temperature generated by the hydration reaction of CaO-CM induced the pyrolysis of organic matter in oil shale, ultimately resulting in a reduction in the pyrolysis hydrocarbon content in the oil shale. This finding substantiates the feasibility of oil shale pyrolysis based on the hydration reaction of CaO-CM.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, L.; Luo, X.; Lin, S.; Li, Y.; Zhang, L.; Ma, W. Assessment of recoverable oil and gas resources by in-situ conversion of shale—Case study of extracting the Chang 73 shale in the Ordos Basin Petrol. Pet. Sci. 2022, 19, 441–458. [Google Scholar] [CrossRef]
- Jin, Z.; Bai, Z.; Gao, B.; Li, M. Has China ushered in the shale oil and gas revolution? Oil Gas Geol. 2019, 40, 451–458. (In Chinese) [Google Scholar] [CrossRef]
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development potential and technical strategy of continental shale oil in China. Pet. Explor. Dev. 2020, 47, 819–828. [Google Scholar] [CrossRef]
- Zou, C.; Pan, S.; Jing, Z.; Gao, J.; Yang, Z.; Wu, S.; Zhao, Q. Shale oil and gas revolution and its impact. Acta Pet. Sin. 2020, 41, 1–12. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, R.; Hu, S.; Hou, L.; Wu, S. Accumulation contribution differences between lacustrine organic-rich shales and mudstones and their significance in shale oil evaluation. Pet. Explor. Dev. 2020, 47, 1079–1089. [Google Scholar] [CrossRef]
- Aurela, M.; Mylläri, F.; Konist, A.; Saarikoski, S.; Olin, M.; Simonen, P.; Bloss, M.; Nešumajev, D.; Salo, L.; Maasikmets, M.; et al. Chemical and physical characterization of oil shale combustion emissions in Estonia Atmos. Atmos. Environ. 2021, 12, 100139. [Google Scholar]
- Kalu, I.E.; Jossou, E.; Arthur, E.K.; Ja’afaru, S.; Ishidi, E.Y. Characterization and Mechanical Property Measurements by Instrumented Indentation Testing of Niger Delta Oil Shale Cuttings. Int. J. Eng. Res. Afr. 2022, 59, 89–100. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, W.; Deng, S. The status and development trend of in-situ conversion and drilling exploitation technology for oil shale. Drill. Eng. 2021, 48, 57–67. (In Chinese) [Google Scholar]
- Burwell, E.L.; Sterner, T.E.; Carpenter, H.C. Shale Oil Recovery by In-Situ Retorting—A Pilot Study. J. Pet. Technol. 1970, 22, 1520–1524. [Google Scholar] [CrossRef]
- Carpenter, H.C.; Burwell, E.L.; Sohns, H.W. Evaluation of an In-Situ Retorting Experiment in Green River Oil Shale. J. Pet. Technol. 1972, 24, 21–26. [Google Scholar] [CrossRef]
- Miller, J.S.; Howell, W.D. Explosive fracturing tested in oil shale. Colo. Sch. Min. Q. 1967, 62, 63–73. [Google Scholar]
- Ryan, R.C.; Fowler, T.D.; Beer, G.L.; Nair, V. Oil Shale: A Solution to the Liquid Fuel Dilemma; ACS Publications: Washington, DC, USA, 2010; pp. 161–183. [Google Scholar]
- Yang, H.; Gao, X.; Xiong, f.; Zhang, J.; Li, Y. Temperature distribution simulation and optimization design of electric heater for in-situ oil shale heating. Oil Shale 2014, 31, 105. [Google Scholar]
- Vinegar, H. Shell’s in-situ conversion process. In Proceedings of the 26th Oil Shale Symposium, Golden, CO, USA, 16–20 October 2006. [Google Scholar]
- Bunger, J.W.; Crawford, P.M.; Johnson, H.R. Is oil shale America’s answer to peak-oil challenge? Oil Gas J. 2004, 8, 16–24. [Google Scholar]
- Tanaka, P.L.; Yeakel, J.D.; Symington, W.A.; Spiecker, P.M.; Del Pico, M.; Thomas, M.M.; Sullivan, K.B.; Stone, M.T. Plan to test ExxonMobil’s in situ oil shale technology on a proposed RD&D lease. In Proceedings of the 31st Annual Oil Shale Symposium, Golden, CO, USA, 17–21 October 2011. [Google Scholar]
- Melton, N.M.; Cross, T.S. Fracturing Oil Shale with Electricity. J. Pet. Technol. 1968, 20, 37–41. [Google Scholar] [CrossRef]
- Grant, B.F. Retorting oil shale underground—Problems and possibilities. Q. Colo. Sch. Mines 1964, 59, 39–46. [Google Scholar]
- Knaus, E.; Killen, J.; Biglarbigi, K.; Crawford, P. An overview of oil shale resources. Oil Shale Solut. Liq. Fuel Dilemma 2010, 1032, 3–20. [Google Scholar]
- Boak, J. Oil Shale: Is Now the Time? Garfield County Energy Advisory Board: Denver, CO, USA, 2011. [Google Scholar]
- Crawford, P.M.; Killen, J.C. Oil Shale: A Solution to the Liquid Fuel Dilemma; ACS Publications: Washington, DC, USA, 2010; pp. 21–60. [Google Scholar]
- Jaber, J.O.; Probert, S.D. Non-isothermal thermogravimetry and decomposition kinetics of two Jordanian oil shales under different processing conditions. Fuel Process. Technol. 2000, 63, 57–70. [Google Scholar] [CrossRef]
- Crawford, P.M.; Biglarbigi, K.; Dammer, A.R.; Knaus, E. Advances in world oil shale production technologies. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Allix, P.; Burnham, A.; Fowler, T.; Herron, M.; Kleinberg, R.; Symington, B. Coaxing oil from shale. Oilfield Rev. 2010, 22, 4–15. [Google Scholar]
- Sun, Y.; Deng, S.; Wang, H. Advances in the exploitation technologies and researches of oil shale in the world: Report on 33rd oil shale symposium in US. J. Jilin Univ. (Earth Sci. Ed.) 2015, 4, 1052–1059. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Meng, X.; Su, J.; Li, Z. Enlinhtenment of American’s oil shale in-suit retorting technology. Oil Drill. Prod. Technol. 2013, 35, 55–59. (In Chinese) [Google Scholar] [CrossRef]
- Kang, Z.; Zhao, Y.; Yang, D. Review of oil shale in-situ conversion technology. Appl. Energy 2020, 269, 115121. [Google Scholar] [CrossRef]
- Wang, H.; Deng, S.; Li, J. A Method for the In-Situ Extraction of Hydrocarbon Compounds from Oil Shale. CN101871339A, 15 May 2020. [Google Scholar]
- States United Congress. Office of Technology Assessment. An Assessment of Oil Shale Technologies; Congress, Office of Technology Assessment: Washington, DC, USA, 1980.
- Bai, F. Theoretical and Laboratory Study of Localized Chemical Method for Pyrolysis of Oil Shale. Doctoral Thesis, Jilin University, Changchun, China, 2015. [Google Scholar]
- Zhu, J.; Yang, Z.; Li, X.; Qi, S.; Jia, M. Application of microwave heating with iron oxide nanoparticles in the in-situ exploitation of oil shale. Energy Sci. Eng. 2018, 6, 548–562. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, J.; Ma, S.; Sun, Y. Theory and method of in situ conversion of shale oil by chemical thermal. J. Eng. Geol. 2022, 30, 127–143. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Yang, D.; Kang, Z. Characteristics of oil and gas production of oil shale pyrolysis by water vapor injection. Oil Shale 2022, 39, 153–168. [Google Scholar]









| Muffle Furnace | Reaction Vessel | Condensation Collection Device | |||
|---|---|---|---|---|---|
| Brand | Shang hai Sager (Shanghai Sager Furnace Co., Limited; Shanghai, China) | Material | GH4132 | Brand | Zhongke Refrigeration (Tianjin Zhongke Electronic Refrigeration Co., Ltd.; Tianjin, China) |
| Model | SG-XS-1700-3 | Maximum Pressure Resistance | 50 MPa | Model | NGB2-1 |
| Set maximum Temperature | 1700 °C | Maximum Temperature Resistance | 600 °C | Temperature Control Range | Room temperature to −50° |
| Furnace chamber Dimensions | 300 × 300 × 300 mm | Capacity | 100 mL | Temperature Control Accuracy | 0.5 °C |
| Temperature control Accuracy | ±1 °C | Internal Diameter | 7.2 cm | Collection Container Volume | 400 mL |
| Heating element | Molybdenum disilicide rod | Internal Height | 4.2 cm | - | - |
| Heating rate ranging | 0~30 °C/min | - | - | - | - |
| Furnace chamber material | Lightweight alumina ceramic fiber | - | - | - | - |
| The Initial Oil Shale | After Hydration Reaction (Pressure) | After Pyrolysis in the Muffle Furnace | |||
|---|---|---|---|---|---|
| 0.1 MPa | 5 MPa | 10 MPa | |||
| The content of pyrolysis hydrocarbons (mg/g) | 40.96 | 40.6 | 0.88 | 0.08 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Li, S.; Zhang, Z.; Xu, T.; Zheng, B.; Hu, Y.; Li, G.; Li, X. The Feasibility Study of In Situ Conversion of Oil Shale Based on Calcium-Oxide-Based Composite Materia Hydration Exothermic Reaction. Energies 2024, 17, 1798. https://doi.org/10.3390/en17081798
Ma S, Li S, Zhang Z, Xu T, Zheng B, Hu Y, Li G, Li X. The Feasibility Study of In Situ Conversion of Oil Shale Based on Calcium-Oxide-Based Composite Materia Hydration Exothermic Reaction. Energies. 2024; 17(8):1798. https://doi.org/10.3390/en17081798
Chicago/Turabian StyleMa, Shiwei, Shouding Li, Zhaobin Zhang, Tao Xu, Bo Zheng, Yanzhi Hu, Guanfang Li, and Xiao Li. 2024. "The Feasibility Study of In Situ Conversion of Oil Shale Based on Calcium-Oxide-Based Composite Materia Hydration Exothermic Reaction" Energies 17, no. 8: 1798. https://doi.org/10.3390/en17081798




