An Experimental Investigation into the Role of an In Situ Microemulsion for Enhancing Oil Recovery in Tight Formations
Abstract
1. Introduction
2. Construction of Microemulsion System
2.1. Materials
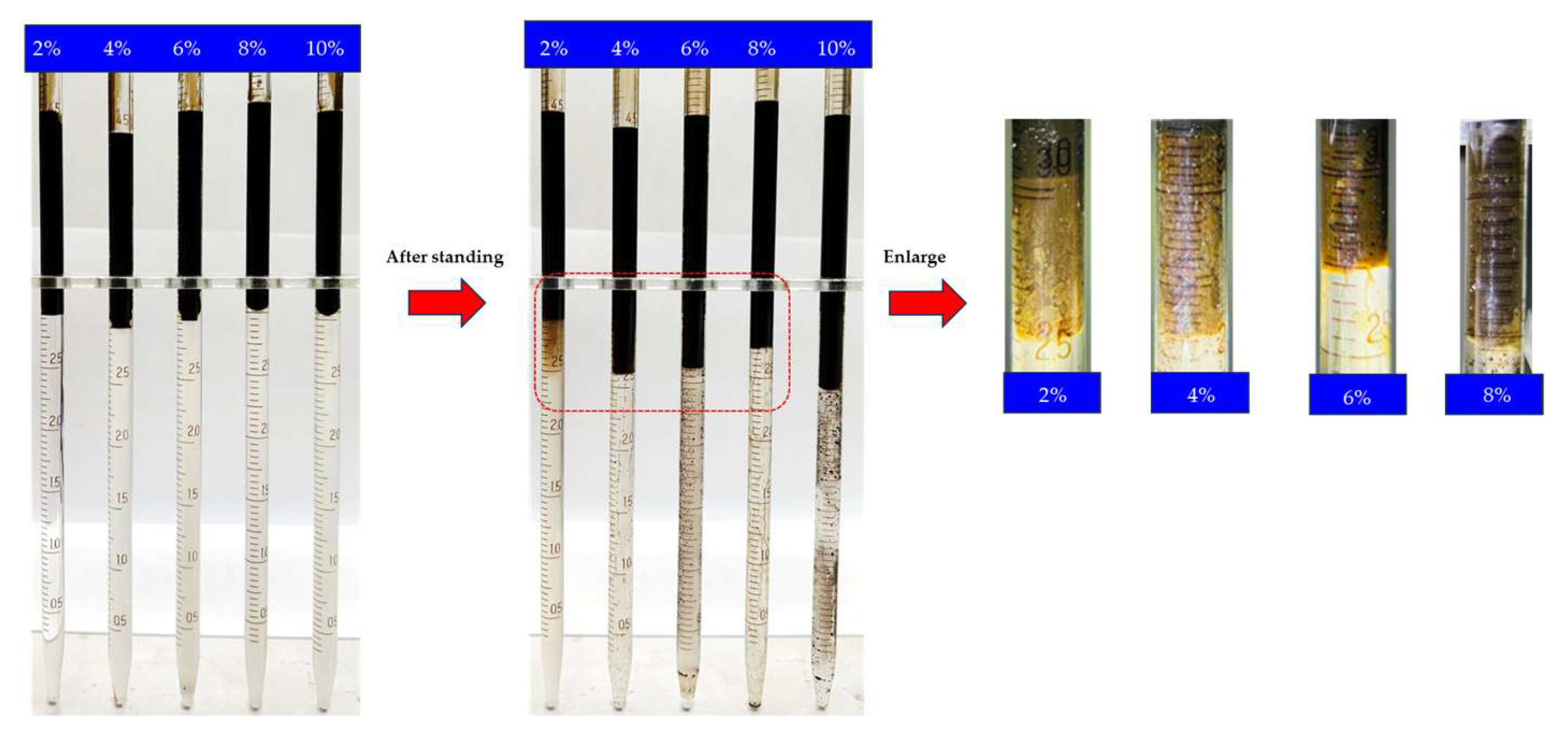
2.2. Salinity Scans Experiment
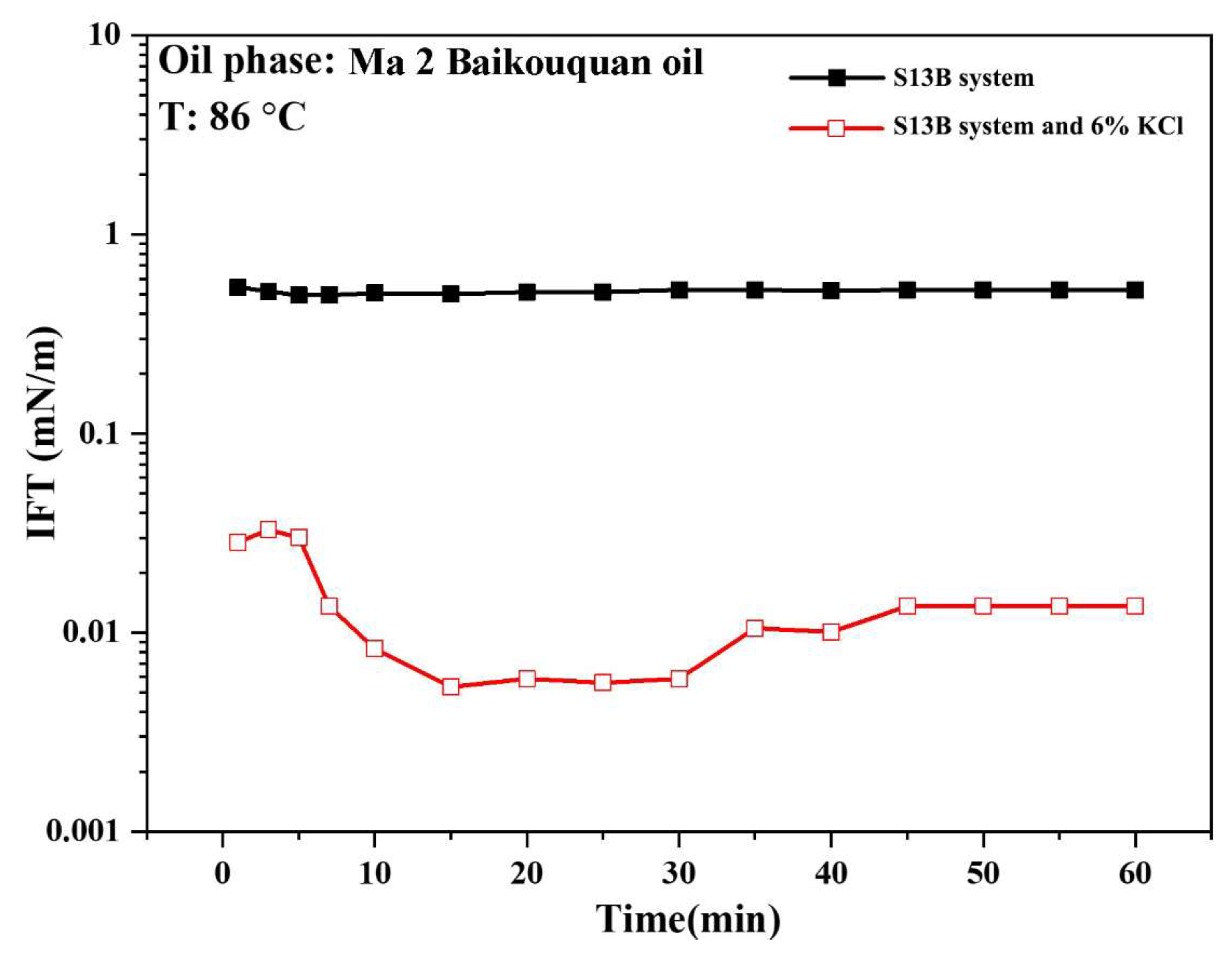
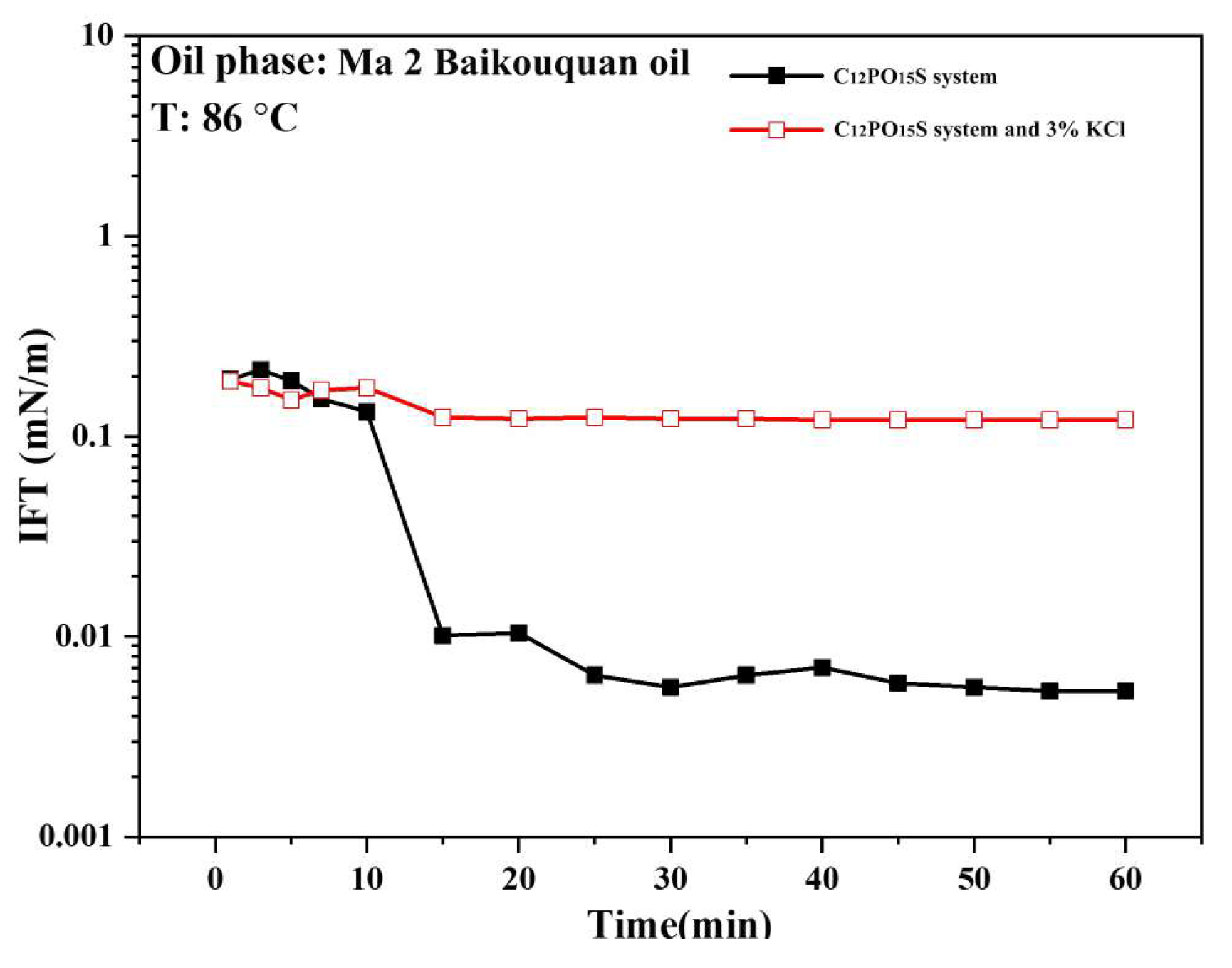
2.3. Interfacial Tension Measurement
2.4. Results and Discussion
2.4.1. Construction of In Situ Microemulsion System
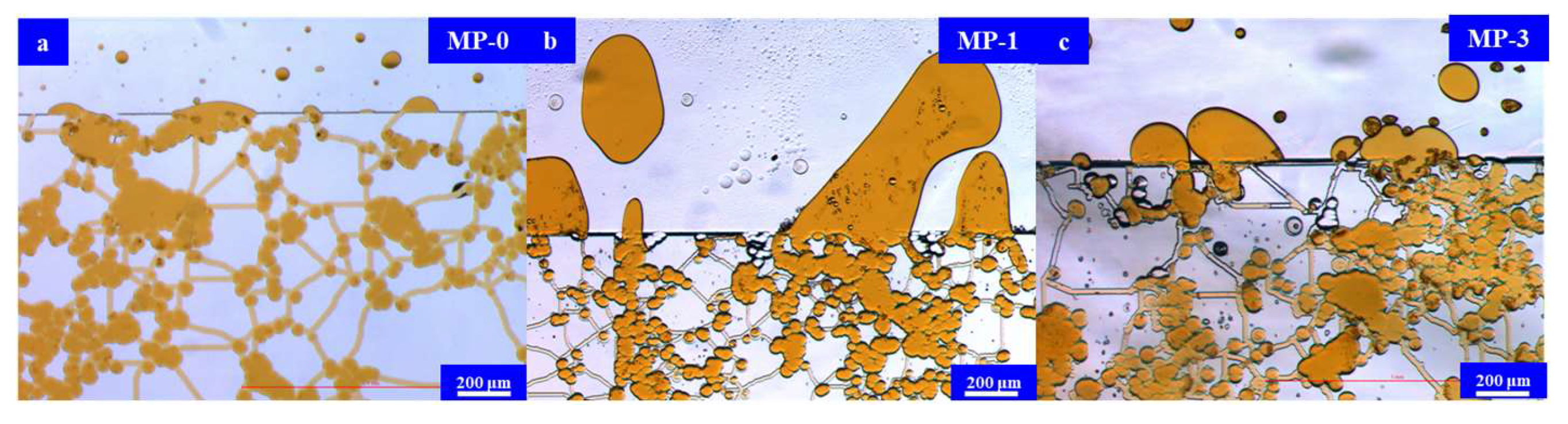
2.4.2. Interfacial Tension Measurement Results
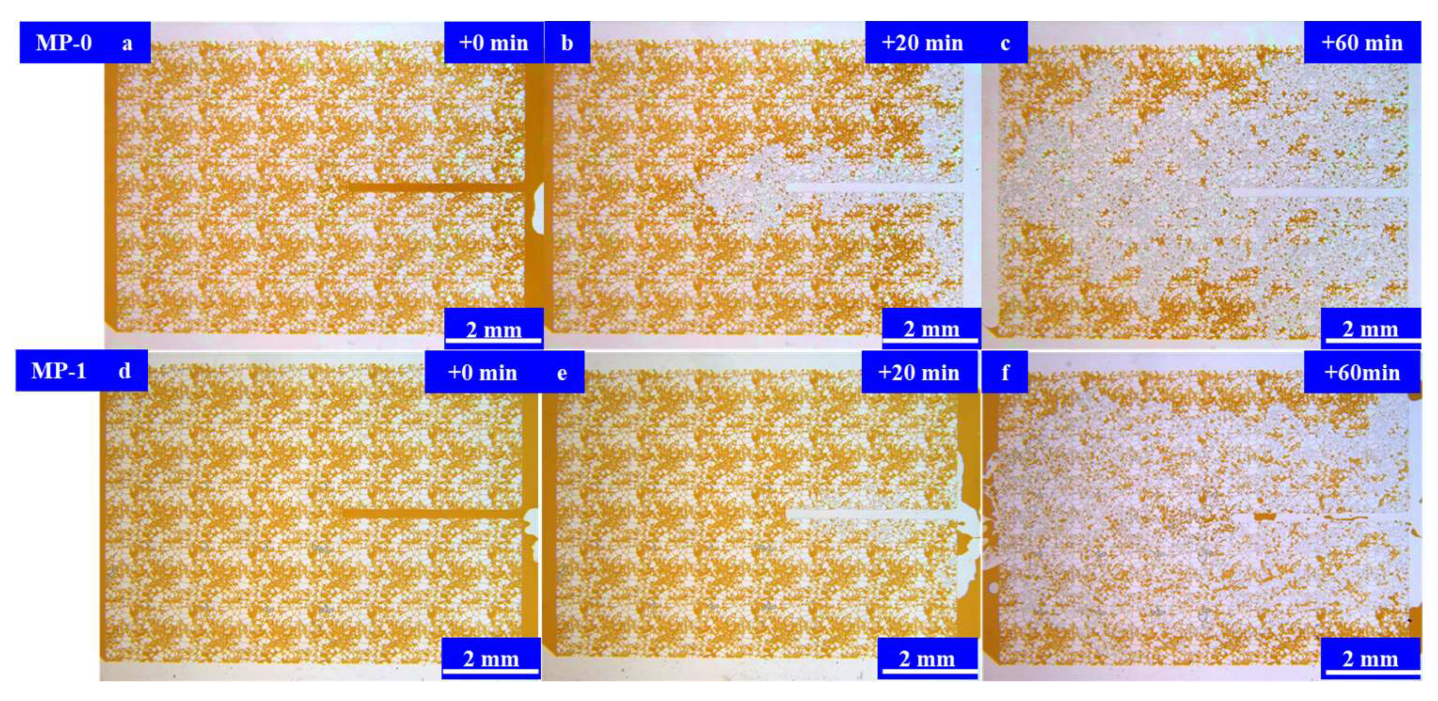
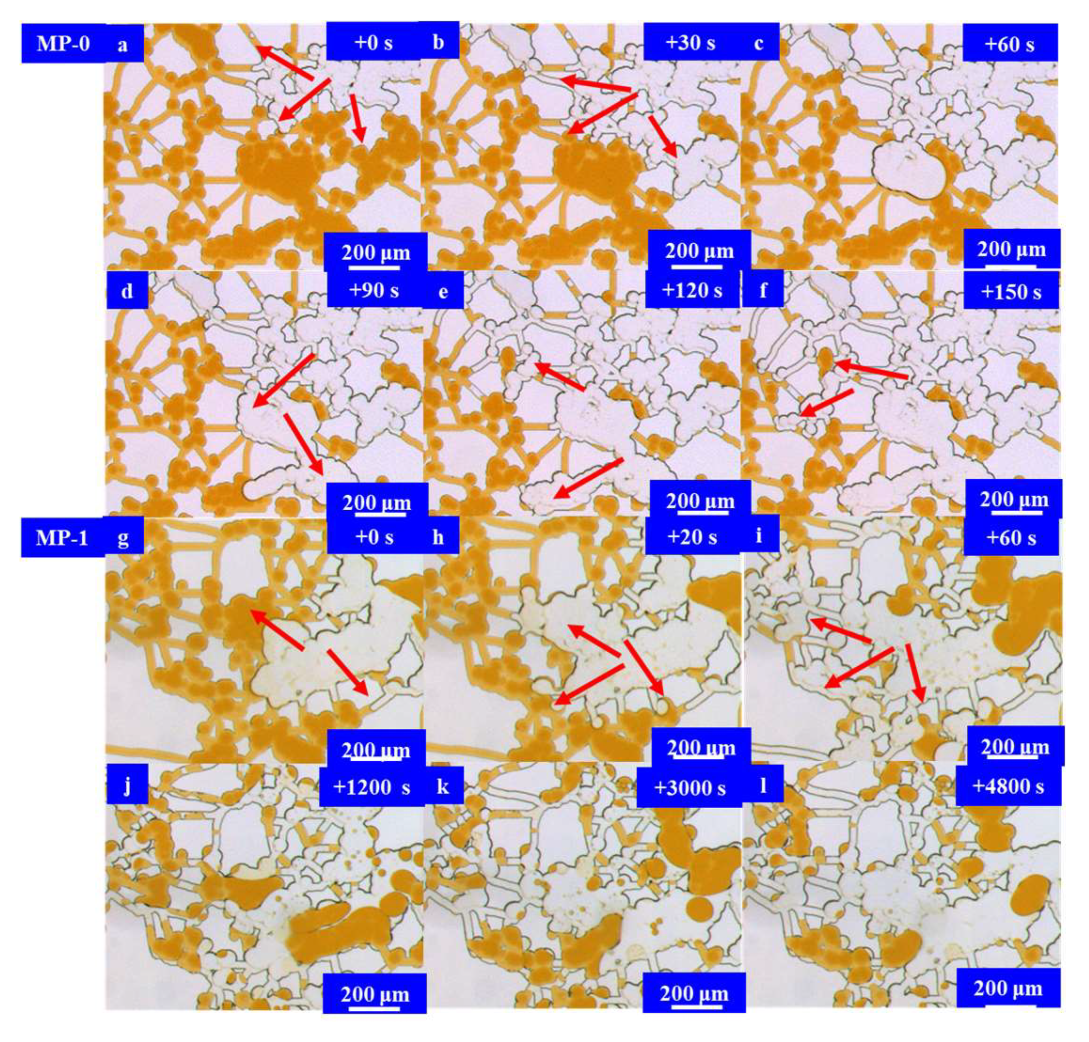
3. Imbibition Behavior of In Situ Microemulsion System
3.1. Materials
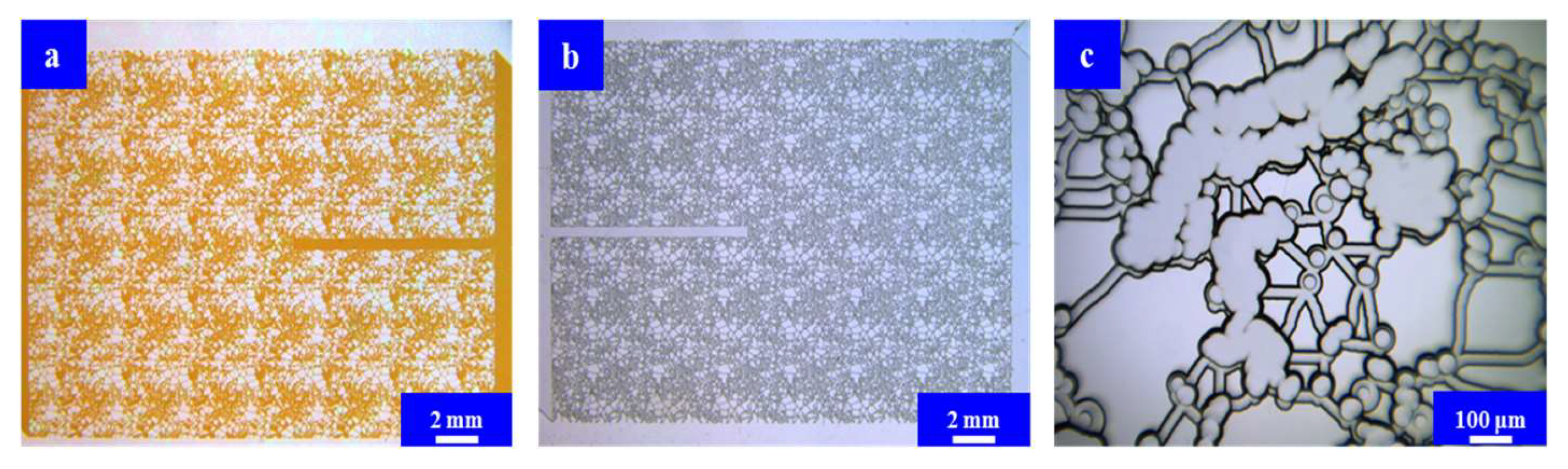
3.2. Microfluidic Imbibition Experiment
- (1)
- The experimental procedure commenced by employing a constant-pressure pump to inject crude oil into the micromodel at a pressure of 3000 mbar. Once the crude oil had completely discharged from the model outlet, the injection pressure was increased to 5000 mbar to eliminate any residual bubbles within the model. Subsequently, the model was saturated with oil and immersed in crude oil, undergoing aging at a temperature of 86 °C for a duration of 48 h.
- (2)
- A constant-speed pump was employed to introduce fluid through the injection port, ensuring a controlled injection rate of 0.1 µL/min. Injection was ceased after displacing 10 pore volumes (PV).
- (3)
- The model was placed in a vertical position within a designated chemical system while maintaining a consistent temperature of 86 °C for the imbibition phase. The model was periodically removed for systematic observation of the imbibition dynamics, and images and videos were captured to document the process.
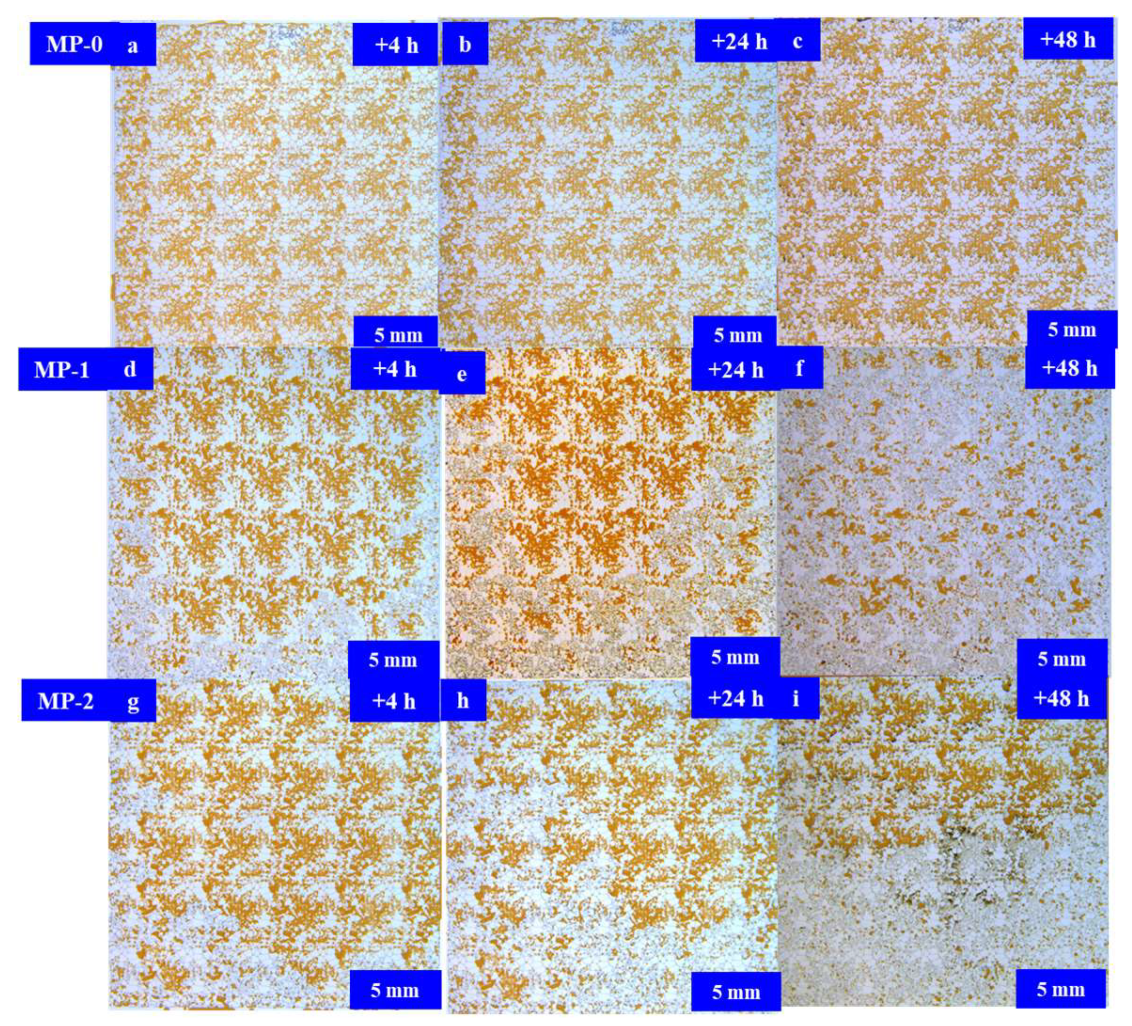
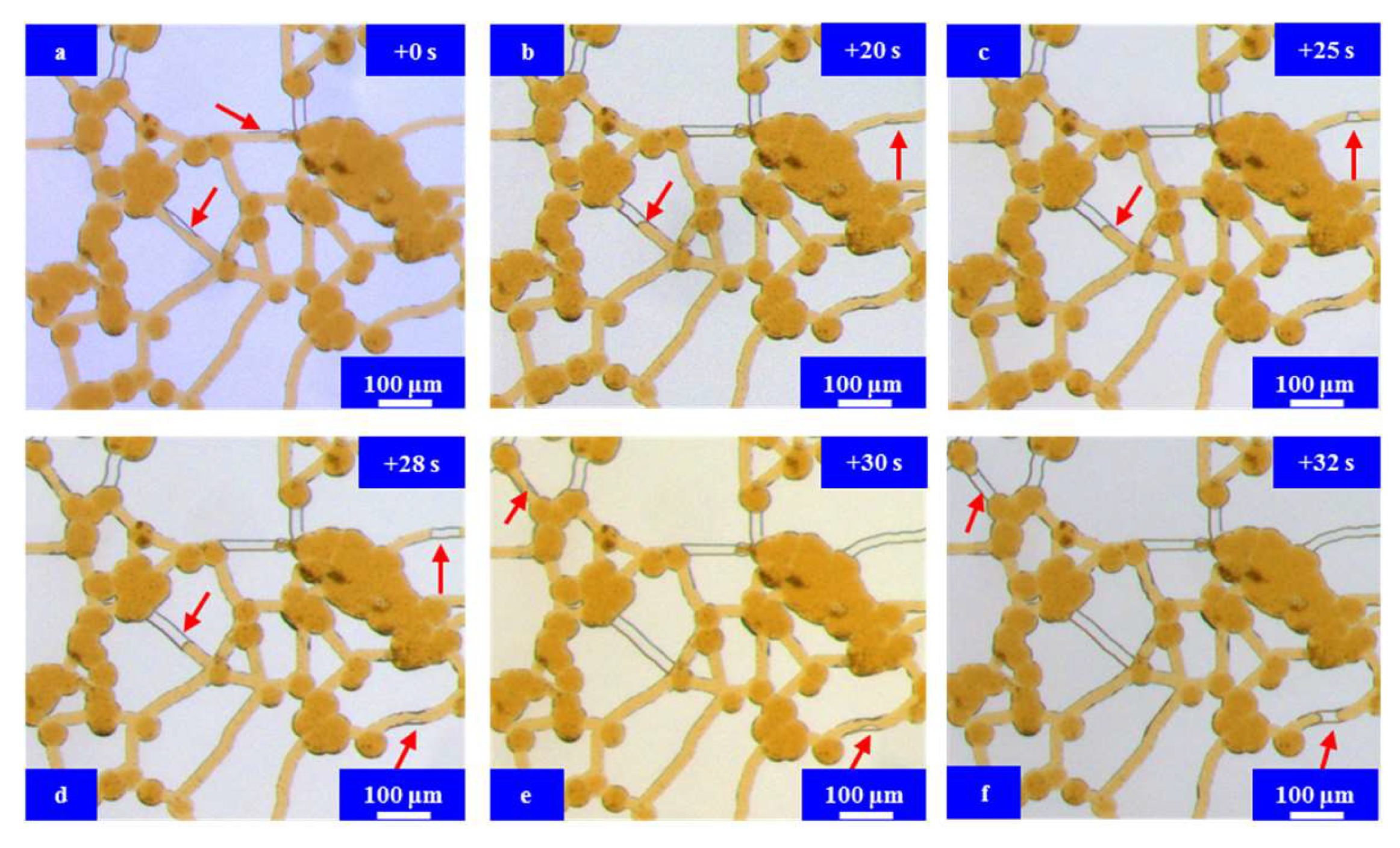
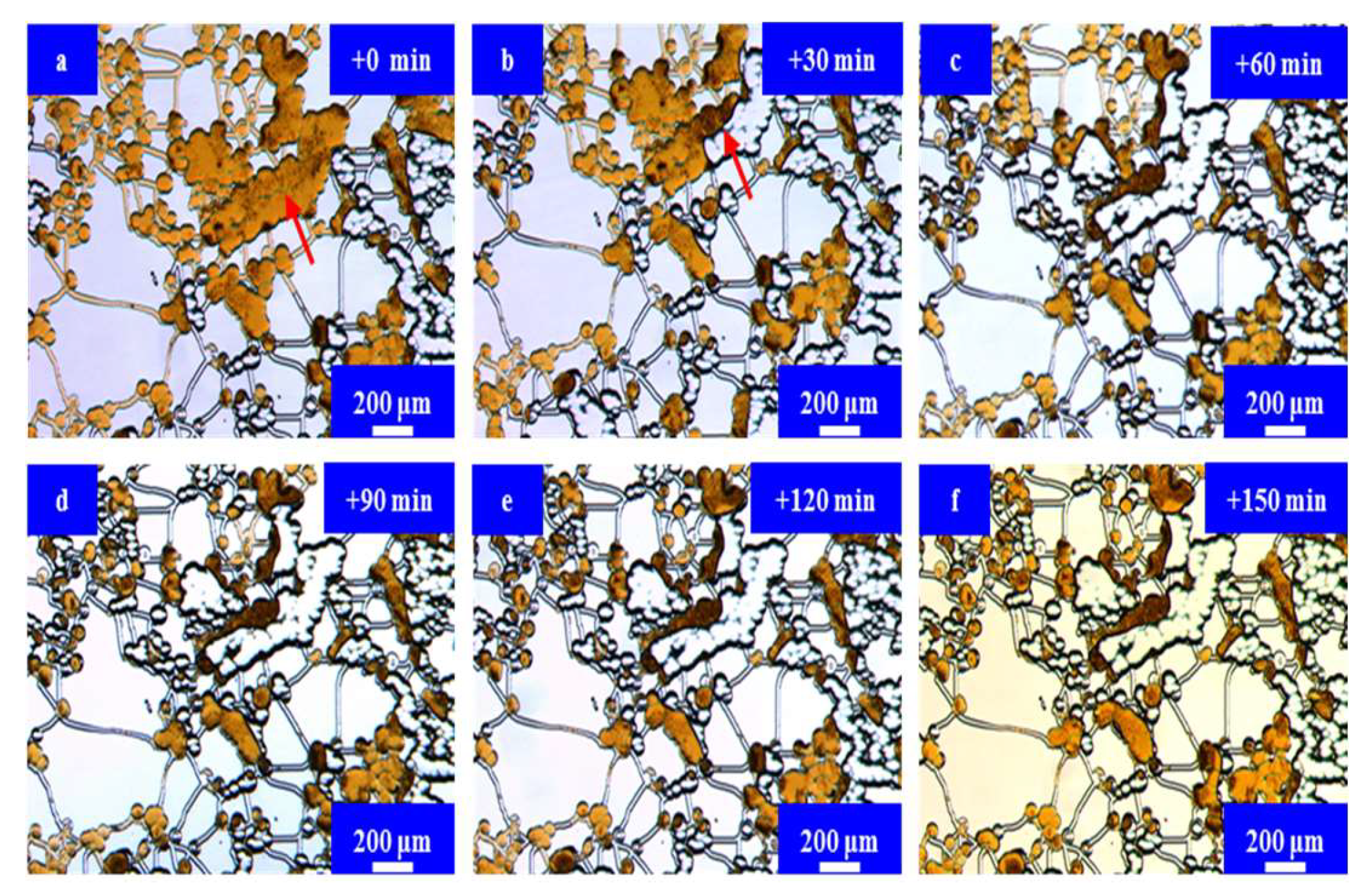
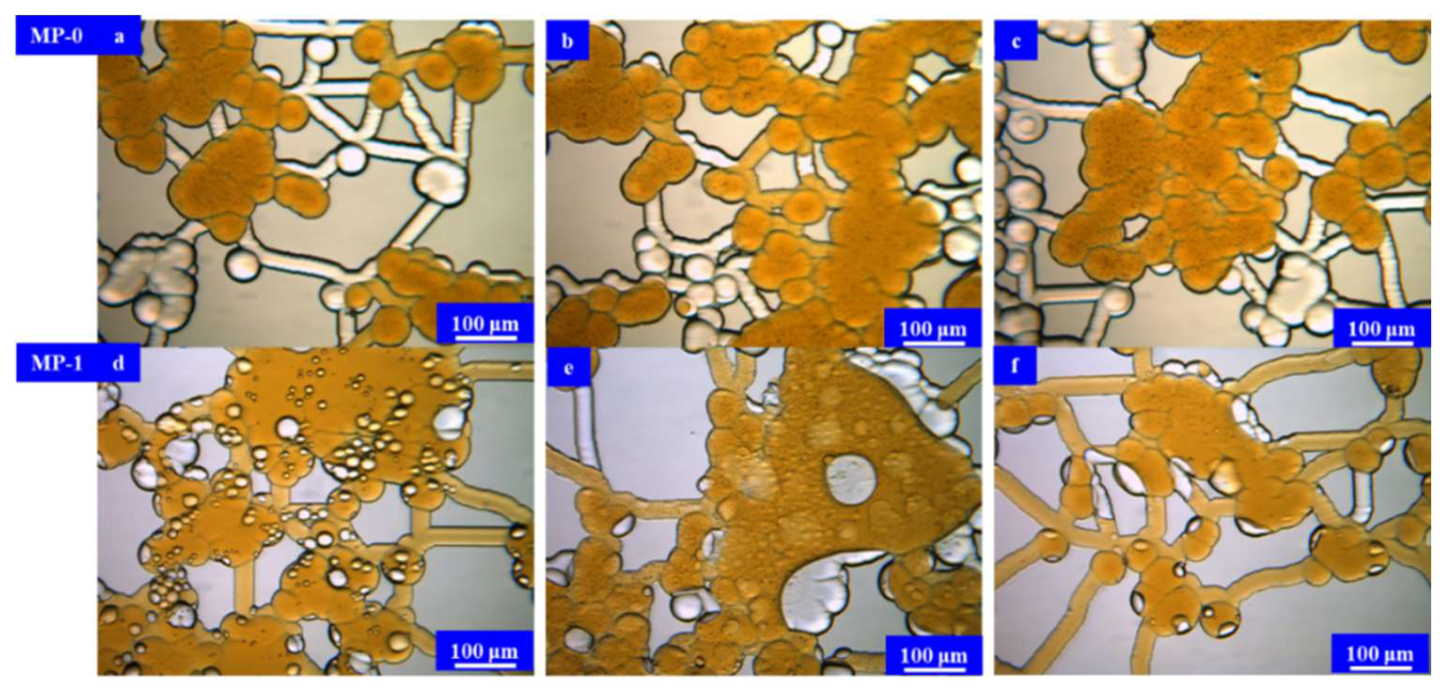
3.3. Results and Discussion
Microfluidic Imbibition Mechanism
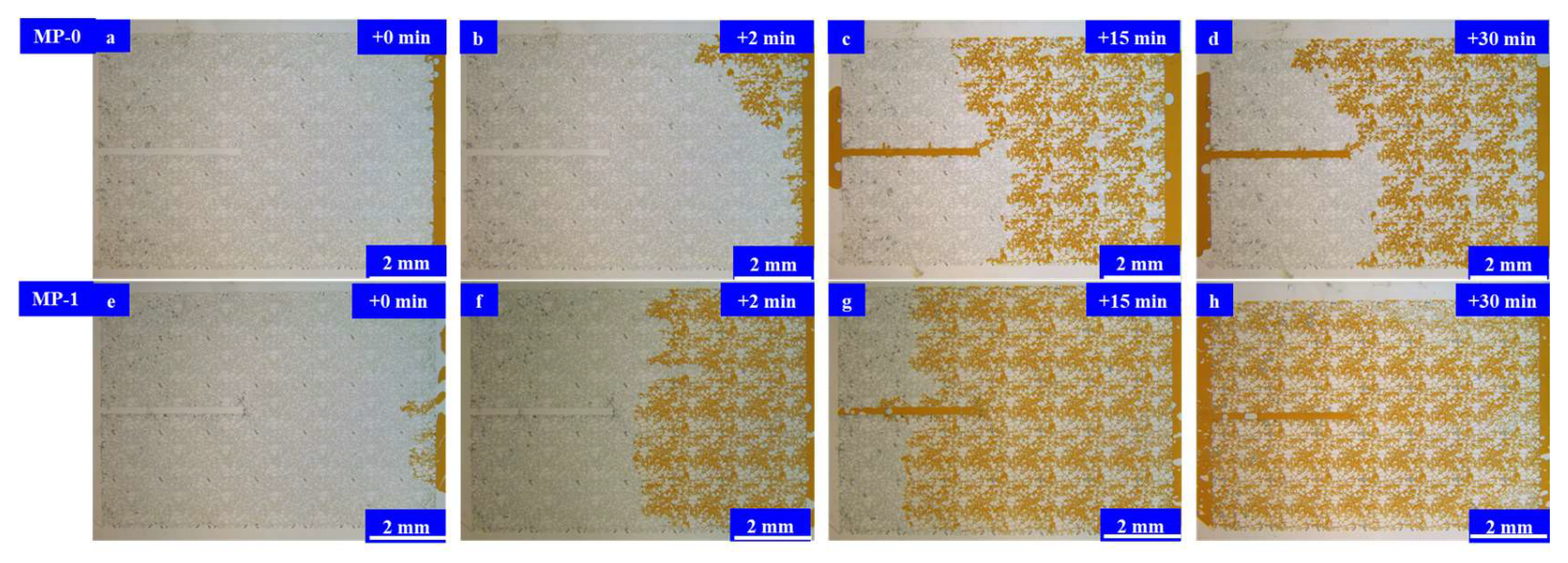
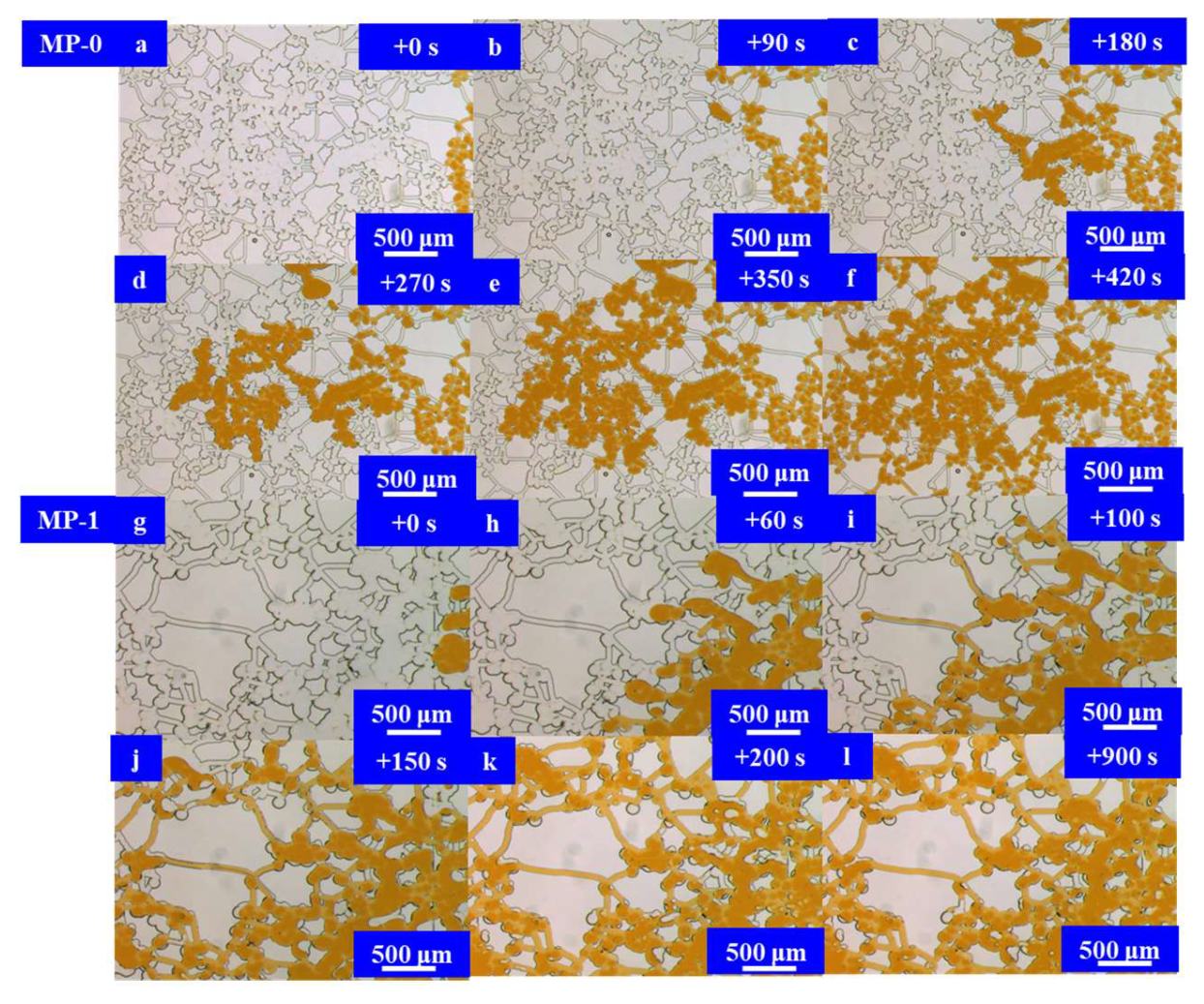
4. Investigation into the Injection and Flowback Mechanism of the Microemulsion System
4.1. Materials
4.2. Microscopic Injection Experiment
- (1)
- Saturated oil. Initially, a constant-pressure pump was used to introduce crude oil into the previously dried model, starting at an initial pressure of 0.3 MPa. The injection pressure was gradually increased to 0.7 MPa until all the air bubbles within the model were expelled.
- (2)
- Injection. A constant-speed pump was utilized to inject either stratum water or the respective chemical system through the inlet at a controlled injection rate of 0.1 µL/min. The injection was ceased once 2 PV were displaced. Images and videos were periodically captured throughout this injection phase for subsequent analysis.
4.3. Microscopic Flowback Experiment
- (1)
- Saturated fluid. A constant-pressure pump was used to inject different types of fluids into the dried model at a pressure of 0.3 MPa, and the injection pressure was gradually increased to 0.7 MPa until all the bubbles in the model were discharged.
- (2)
- Crude oil flowback. A constant-speed pump was used to inject crude oil from the outlet at an injection rate of 0.1 µL/min. The injection was stopped after displacing 2 PV, and images and videos were collected regularly during this period.
4.4. Results and Discussion
4.4.1. Experimental Study on Injection
4.4.2. Experimental Study on Flowback
5. Conclusions
- (1)
- The results from the salinity scan experiments revealed that only two surfactants, namely, S13B and C12PO15S, possessed the capability of generating a substantial middle-phase microemulsion when combined with Mahu crude oil.
- (2)
- A micromodel was constructed, incorporating a multi-level pore–throat system that encompassed millimeter-scale fractures, micron-scale pores, and nano-scale throats to elucidate the imbibition dynamics of the in situ microemulsion system. The imbibition recovery rates, ranked from highest to lowest, followed the order of the MP-1 solution, the MP-2 solution, and stratum water. The microemulsion system solutions exhibited bottom-up forward imbibition modes facilitated by low interfacial tension. The primary factor contributing to the disparity in the recovery rates lay in the varying degrees of crude oil utilization within the pores. The reduction in interfacial tension within the microemulsion system and the miscibility of the microemulsion formed through emulsification were the principal mechanisms responsible for enhancing the imbibition efficiency.
- (3)
- Experimental investigations were conducted on the injection and flowback within a micromodel representing a multi-stage pore–throat system. This elucidated the pressure reduction, injection mechanism, and flowback dynamics of the microemulsion system. In comparison to stratum water, the microemulsion system displayed more intricate seepage dynamics during injection. It encompassed not only piston flow at the displacement front but also laminar flow involving the microemulsion, crude oil, and water within the affected area. Additionally, free oil droplets were observed in the flow. This unique flow pattern is crucial for achieving depressurization and enhanced injection. The microemulsion system solution effectively mitigated micro-pore throat heterogeneity during the injection process, avoiding crossflow along the throats and enhancing the utilization of the oil phase within the pore body. Within the matrix areas invaded by stratum water, crude oil primarily occupied the central regions of the pores after flowback, with numerous throats containing bound water. This led to a notable reduction in the flow channels available for the oil phase, resulting in a significant decrease in the oil’s relative permeability. However, the microemulsion system effectively addressed the channeling issues caused by disparities in size among the fractures, pores, and throats. It achieved a uniform progression of the oil–water interface during the crude oil flowback process. Bound water primarily existed in the form of small droplets within the pores and water droplets adhering to the walls. Water had minimal impact on the crude oil flow channels, allowing for the restoration of a substantial portion of the oil’s relative permeability.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HnP | Huff-n-puff |
| EOR | Enhanced oil recovery |
| PH | Hydrogen ion concentration |
| rpm | Revolutions per minute |
| PV | Pore volume |
References
- Dou, H.; Ma, S. Lessons learned from oil production of tight oil reservoirs in Bakken play. J. Oil Drill. Prod. Technol. 2012, 34, 120–124. [Google Scholar]
- Capuano, L. Annual Energy Outlook 2019; US Energy Information Administration: Washington, DC, USA, 2019. [Google Scholar]
- Zheng, Z.; Li, D.; Wang, Z.; Jia, J.; Gao, X.; Liu, Z.; Zan, X.; Guo, J. Assessment of the potential of tight oil and gas in major basins in China. J. China Min. Mag. 2017, 26, 22–29. [Google Scholar]
- Wan, T.; Tan, J.; Zhang, J. Practices of Zipper Fracturing for Tight Reservoir Development with Small Well Spacing. Xinjiang Oil Gas 2022, 18, 26–32. [Google Scholar] [CrossRef]
- Wang, M.; Fan, Z.; Luo, W.; Ding, J.; Tian, Q.; Yao, M.; Bu, X. Feasibility Analysis of Water Flooding with Multi-fractured Horizontal Wells in Ultra-low Permeability Reservoirs. J. Geosci. 2016, 30, 1361–1369. [Google Scholar]
- Cui, C.; Chen, H.; Guo, Y.; Wang, Q.; Zhang, S. Reasonable Water-injecting Development Methods of Multiple-fracturing Horizontal Well in Ultra-Low Permeability Glutenite Reservoir. J. Geosci. 2018, 32, 198–204. [Google Scholar]
- Zhu, W.; Yue, M.; Liu, Y.; Liu, K.; Song, Z. Research progress on tight oil exploration in China. J. Chin. J. Eng. 2019, 41, 1103–1114. [Google Scholar]
- Liu, X.; Gu, W. Status and prospect of water injection in the development of oil reservoirs with extra/ultra-low permeability. J. Petrochem. Ind. Appl. 2017, 36, 4–8. [Google Scholar]
- Pu, C.; Jing, C.; He, Y.; Gu, X.; Zhang, Z.; Wei, J. Multistage interwell chemical tracing for step-by-step profile control of water channeling and flooding of fractured ultra-low permeability reservoirs. J. Pet. Explor. Dev. 2016, 43, 621–629. [Google Scholar] [CrossRef]
- Li, E.; Li, T.; Yu, W.; Downhole Operation company of XDEC. Analysis of Fracturing Technology in Ma2 Well Area of Mabei Oilfield. J. Xinjiang Oil Gas 2015, 11, 80–84. [Google Scholar]
- Zheng, C.; Zhang, L.; Xu, J.; Zeng, M.; Fu, G.; Fu, Y. Construction and Performance Evaluation of Nano Microemulsion Oil Displacement System. J. Xinjiang Oil Gas 2023, 19, 89–94. [Google Scholar]
- Al-Mishaal, O.F.; Suwaid, M.A.; Al-Muntaser, A.A.; Khelkhal, M.A.; Varfolomeev, M.A.; Djimasbe, R.; Zairov, R.R.; Saeed, S.A.; Vorotnikova, N.A.; Shestopalov, M.A.; et al. Octahedral Cluster Complex of Molybdenum as Oil-Soluble Catalyst for Improving In Situ Upgrading of Heavy Crude Oil: Synthesis and Application. Catalysts 2022, 12, 1125. [Google Scholar] [CrossRef]
- Tajik, A.; Farhadian, A.; Khelkhal, M.A.; Rezaeisadat, M.; Petrov, S.M.; Eskin, A.A.; Vakhin, A.V.; Babapour Golafshani, M.; Lapuk, S.E.; Buzurov, A.E.; et al. Sunflower oil as renewable biomass source to develop highly effective oil-soluble catalysts for in-situ combustion of heavy oil. Chem. Eng. J. 2023, 453, 139813. [Google Scholar] [CrossRef]
- Chandio, T.A.; Manan, M.A.; Memon, K.R.; Abbas, G.; Abbasi, G.R. Enhanced Oil Recovery by Hydrophilic Silica Nanofluid: Experimental Evaluation of the Impact of Parameters and Mechanisms on Recovery Potential. Energies 2021, 14, 5767. [Google Scholar] [CrossRef]
- Bello, A.; Ivanova, A.; Cheremisin, A. A Comprehensive Review of the Role of CO2 Foam EOR in the Reduction of Carbon Footprint in the Petroleum Industry. Energies 2023, 16, 1167. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, W.; Liu, J.; Cao, B.; Hao, J.; Lu, X.; Zheng, K.; Cui, L.; Cui, T.; Sun, H. Integration of Profile Control and Thermal Recovery to Enhance Heavy Oil Recovery. Energies 2022, 15, 7346. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, F.; Xu, J.; Liu, P.; Chen, S.; Wang, Y. Spontaneous Imbibition and Core Flooding Experiments of Enhanced Oil Recovery in Tight Reservoirs with Surfactants. Energies 2023, 16, 1815. [Google Scholar] [CrossRef]
- Zhao, P.; Jackson, A.C.; Britton, C.; Kim, D.H.; Britton, L.N.; Levitt, D.B.; Pope, G.A. Development of High-Performance Surfactants for Difficult Oil. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008; p. SPE-113432-MS. [Google Scholar]
- Yin, D.; Xu, W.; Zhou, Y. Optimization and Performance Research of Microemulsion Applied on Low Permeability Reservoir. J. Petrochem. Univ. 2017, 30, 20–25. [Google Scholar]
- Xiao, L.; Hou, J.; Wen, Y.; Qu, M.; Wang, W.; Wu, W.; Liang, T. Imbibition mechanisms of high temperature resistant microemulsion system in ultra-low permeability and tight reservoirs. J. Pet. Explor. Dev. 2022, 49, 1398–1410. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Xu, Y.; Zang, W.; Zhu, J.; Bao, X. Development of in-situ microemulsion surfactant system and its application in pressure decrease and injection increase. J. Pet. Geol. Oilfield Dev. Daqing 2021, 40, 116–122. [Google Scholar]
- Kathel, P.; Mohanty, K.K. Wettability Alteration in a Tight Oil Reservoir. J. Energy Fuels 2013, 27, 6460–6468. [Google Scholar] [CrossRef]
- Chen, P.; Mohanty, K.K. Surfactant-Mediated Spontaneous Imbibition in Carbonate Rocks at Harsh Reservoir Conditions. J. SPE J. 2013, 18, 124–133. [Google Scholar] [CrossRef]
- Yu, F.; Jiang, H.; Ma, M.; Xu, F.; Su, H.; Jia, J. Visualization the Surfactant Imbibition at Pore Scale by Using of Fractured Micromodels. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020; p. SPE-200349-MS. [Google Scholar]
- Li, J.; Liu, B.; Guo, C.; Hang, S.; Yu, F.; Ma, M.; Wang, L.; Jiang, H. Mechanisms of water block removal by surfactant micellar solutions in low permeability reservoirs. J. Pet. Explor. Dev. 2022, 49, 348–357. [Google Scholar] [CrossRef]




| Density g/cm3 | HCO3− mg/L | Cl− mg/L | SO42− mg/L | Ca2+ mg/L | Mineralization mg/L | pH |
|---|---|---|---|---|---|---|
| 1.0128 | 3043.07 | 4188.24 | 249.78 | 446.49 | 9848.14 | 6.1 |
| No. | Surfactant Type | Surfactant Concentration | N-Butanol Concentration | Salt | Whether Intermediate Phase Appeared |
|---|---|---|---|---|---|
| ME-1 | S13B | 1% | 2% | KCl | √ |
| ME-2 | C12PO15S | 1% | 2% | KCl | √ |
| ME-3 | AEO-10PS | 1% | 2% | KCl | × |
| ME-4 | AEO-15PS | 1% | 2% | KCl | × |
| ME-5 | C13PO10S | 1% | 2% | KCl | × |
| ME-6 | C8P10E5C | 1% | 2% | KCl | × |
| Formula | Surfactant Type | Surfactant Concentration | N-Butanol Concentration | Salinity |
|---|---|---|---|---|
| MP-0 | / | / | / | stratum water |
| MP-1 | S13B | 1% | 2% | 6% KCl |
| MP-2 | C12PO15S | 1% | 2% | 0.4% KCl |
| Formula | Surfactant Type | Surfactant Concentration | N-Butanol Concentration | Salinity |
|---|---|---|---|---|
| MP-0 | / | / | / | stratum water |
| MP-1 | S13B | 1% | 2% | 6% KCl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Zang, C.; Li, J.; Mou, X.; Wang, R.; Li, H.; Li, J. An Experimental Investigation into the Role of an In Situ Microemulsion for Enhancing Oil Recovery in Tight Formations. Energies 2024, 17, 1879. https://doi.org/10.3390/en17081879
Zeng M, Zang C, Li J, Mou X, Wang R, Li H, Li J. An Experimental Investigation into the Role of an In Situ Microemulsion for Enhancing Oil Recovery in Tight Formations. Energies. 2024; 17(8):1879. https://doi.org/10.3390/en17081879
Chicago/Turabian StyleZeng, Meiting, Chuanzhen Zang, Jie Li, Xiangyu Mou, Rui Wang, Haifu Li, and Junjian Li. 2024. "An Experimental Investigation into the Role of an In Situ Microemulsion for Enhancing Oil Recovery in Tight Formations" Energies 17, no. 8: 1879. https://doi.org/10.3390/en17081879
APA StyleZeng, M., Zang, C., Li, J., Mou, X., Wang, R., Li, H., & Li, J. (2024). An Experimental Investigation into the Role of an In Situ Microemulsion for Enhancing Oil Recovery in Tight Formations. Energies, 17(8), 1879. https://doi.org/10.3390/en17081879





