Microbial Fuel Cell Technology as a New Strategy for Sustainable Management of Soil-Based Ecosystems
Abstract
:1. Introduction
- (1)
- Soil MFCs—work in different types of soil, e.g., soil from farmland, swamps, meadows, peats, and forests; soil is the supporting matrix, separator between electrodes, and source of microorganisms and organic matter; in this type of MFC, soil is used without growing plants (in laboratory experiments) or the influence of plants growing in the soil is neglected (in field tests).
- (2)
- Sediment MFCs—work in sediments that are comprised of two phases: soil and water; soil function is the same as in the case of soil MFCs and the influence of possible growing plants is neglected.
- (3)
- Plant MFCs—work in the rhizosphere of living plants; soil is mainly a supporting matrix, source of microorganisms, and separator between electrodes.
- (4)
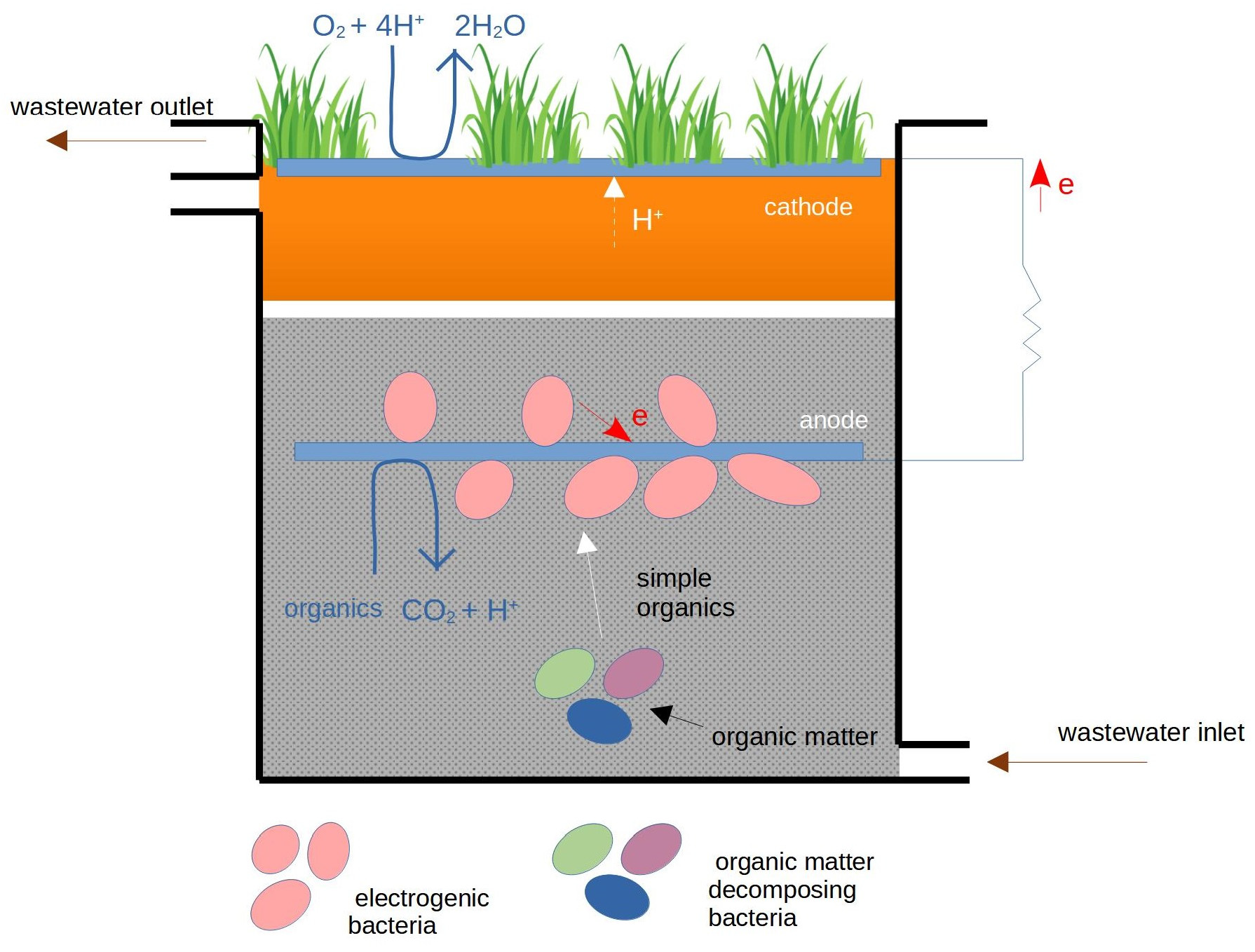
- CW-MFCs—MFC technology coupled with constructed wetlands (CWs), dedicated to wastewater treatment.
- (1)
- Direct production of electric current from different types of soils and sediments realized with the use of mainly soil MFCs, sediment MFCs, and plant MFCs;
- (2)
- Bioremediation from organic and inorganic pollutants contaminating soil-based ecosystems with the use of soil MFCs and sediment MFCs;
- (3)
- Effective wastewater treatment in wetlands realized by coupling constructed wetlands (CW) with MFC technology;
- (4)
- Greenhouse gas mitigation, soil salinity mitigation, and heavy metal accumulation mitigation with the use of soil MFCs and plant MFCs;
- (5)
- Wireless energy-neutral sensing with the use of soil MFCs, sediment MFCs, and plant MFCs.
2. Soil as a Source of Microorganisms and Biomass
3. Production of Electricity from Soil-Based Ecosystems Using MFC Technology
3.1. Soil MFCs
3.2. Plant MFC
3.3. Sediment MFCs
4. MFC Technology as a Tool for Soil Bioremediation
5. MFC Technology Applied for Wastewater Treatment in Soil-Based Ecosystems
6. Soil Ecosystem-Based MFCs as a Strategy for Methane Mitigation, Soil Desalination, and Mitigation of Heavy Metal Accumulation
7. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leifeld, J. Carbon farming: Climate change mitigation via non-permanent carbon sinks. J. Environ. Manag. 2023, 339, 117893. [Google Scholar] [CrossRef]
- Tang, K.; He, C.; Ma, C.; Wang, D. Does carbon farming provide a cost-effective option to mitigate GHG emissions? Evidence from China. Aust. J. Agric. Resour. Econ. 2019, 63, 575–592. [Google Scholar] [CrossRef]
- Chiri, E.; Nauer, P.A.; Henneberger, R.; Zeyer, J.; Schroth, M.H. Soil–methane sink increases with soil age in forefields of Alpine glaciers. Soil. Biol. Biochem. 2015, 84, 83–95. [Google Scholar] [CrossRef]
- Lal, R.; Monger, C.; Nave, L.; Smith, P. The role of soil in regulation of climate. Phil. Trans. R. Soc. B. 2021, 376, 20210084. [Google Scholar] [CrossRef]
- Jones, M.B.; Donnely, A. Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2. New Phytol. 2004, 164, 423–439. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.G.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the carbon cycle: From local processes to global implications—A synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Liu, H.; Price, J.; Rezanezhad, F.; Lennartz, B. Centennial-scale shifts in hydrophysical properties of peat induced by drainage. Water Resour. Res. 2020, 56, e2020WR027538. [Google Scholar] [CrossRef]
- Łachacz, A.; Kalisz, B.; Sowiński, P.; Smreczak, B.; Niedźwiecki, J. Transformation of organic soils due to artificial drainage and agricultural use in Poland. Agriculture 2023, 13, 634. [Google Scholar] [CrossRef]
- Dawson, Q.; Kechavarzi, C.; Leeds-Harrison, P.; Burton, R. Subsidence and degradation of agricultural peatlands in the Fenlands of Norfolk UK. Geoderma 2010, 154, 181–187. [Google Scholar] [CrossRef]
- Krüger, J.P.; Leifeld, J.; Glatzel, S.; Szidat, S.; Alewell, C. Biogeochemical indicators of peatland degradation—A case study of a temperate bog in northern Germany. Biogeosciences 2015, 12, 2861–2871. [Google Scholar] [CrossRef]
- Carroll-Burke, P. Material designs: Engineering cultures and engineering states—Ireland 1650–1900. Theor. Soc. 2002, 31, 75–114. [Google Scholar] [CrossRef]
- Liu, H.; Wrage-Mönnig, N.; Lennartz, B. Rewetting strategies to reduce nitrous oxide emissions from European peatlands. Commun. Earth Environ. 2020, 1, 17. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, G.; Chen, B.; Zhang, K.; Niu, S.; Wang, J.; Ciais, P.; Zuo, H. A globally robust relationship between water table decline, subsidence rate, and carbon release from peatlands. Commun. Earth Environ. 2022, 3, 254. [Google Scholar] [CrossRef]
- Joosten, H.; Tapio-Biström, M.L.; Tol, S. Peatlands: Guidance for Climate Change Mitigation Through Conservation, Rehabilitation and Sustainable Use; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Nascimento, C.M.; de Sousa, M.W.; Quiñonez Silvero, N.E.; Poppiel, R.R.; Sayão, V.M.; Carnieletto, D.A.; Valadares dos Santos, N.; Accorsi Amorim, M.T.; Demattê, J.A.M. Soil degradation index developed by multitemporal remote sensing images, climate variables, terrain and soil atributes. J. Environ. Manag. 2021, 277, 111316. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Majumder, A.; Dhaliwal, S.S.; Toor, A.S.; Mani, P.K.; Naresh, R.K.; Gupta, R.K.; Mitran, T. Impact of agricultural management practices on soil carbon sequestration and its monitoring through simulation models and remote sensing techniques: A review. Crit. Rev. Environ. Sci. Tec. 2022, 52, 1–49. [Google Scholar] [CrossRef]
- Louwagie, G.; Gay, S.H.; Sammeth, F.; Ratinger, T. The potential of European Union policies to address soil degradation in agriculture. Land. Degrad. Dev. 2011, 22, 5–17. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Davis, S.C.; Masters, M.D.; Delucia, E.H. Changes in soil organic carbon under biofuel crops. Glob. Change Biol. Bioenerg. 2009, 1, 75–96. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Choi, S. Electrogenic bacteria promise new opportunities for powering, sensing, and synthesizing. Small 2022, 18, 2107902. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.T.; Ghangrekar, M.M.; Mukherjee, C.K.; Min, B. Biofouling effects on the performance of microbial fuel cells and recent advances in biotechnological and chemical strategies for mitigation. Biotechnol. Adv. 2019, 37, 107420. [Google Scholar] [CrossRef]
- Syed, Z.; Sonu, K.; Sogani, M. Cattle manure management using microbial fuel cells for green energy generation. Biofuel. Bioprod. Bior. 2022, 16, 460–470. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Removal of hepatitis B virus surface HBsAg and core HBcAg antigens using microbial fuel cells producing electricity from human urine. Sci. Rep. 2019, 9, 11787. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Wu, Y.; Cao, D.; Zhao, L.; Sun, Q. Electricity generation and modeling of microbial fuel cell from continuous beer brewery wastewater. Bioresour. Technol. 2009, 100, 4171–4175. [Google Scholar] [CrossRef]
- Durruty, I.; Bonanni, P.S.; González, J.F.; Busalmen, J.P. Evaluation of potato-processing wastewater treatment in a microbial fuel cell. Bioresour. Technol. 2012, 105, 81–87. [Google Scholar] [CrossRef]
- Din, M.I.; Ahmed, M.; Ahmad, M.; Iqbal, M.; Ahmad, Z.; Hussain, Z.; Khalid, R.; Samad, A. Investigating the activity of carbon fiber electrode for electricity generation from waste potatoes in a single-chambered microbial fuel cell. J. Chem. 2023, 2023, 8520657. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R.; Szymona, K.; Kloch, M. Bioelectricity production from wood hydrothermal-treatment wastewater: Enhanced power generation in MFC-fed mixed wastewaters. Sci. Total Environ. 2018, 634, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Toczyłowska-Mamińska, R.; Mamiński, M.Ł. Wastewater as a renewable energy source—Utilisation of microbial fuel cell technology. Energies 2022, 15, 6928. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Shen, Y.; Gao, L.; Liao, Z.H.; Sun, J.Z.; Yong, Y.C. Improving the extracellular electron transfer of: Shewanella oneidensis MR-1 for enhanced bioelectricity production from biomass hydrolysate. RSC Adv. 2017, 7, 30488–30494. [Google Scholar] [CrossRef]
- Nandy, A.; Radović, J.R.; Novotnik, B.; Sharma, M.; Larter, S.R.; Thangadurai, V. Investigation of crude oil degradation using metal oxide anode-based microbial fuel cell. Bioresour. Technol. Rep. 2020, 11, 100449. [Google Scholar]
- Bhattacharya, R.; Bose, D.; Yadav, J.; Sharma, B.; Sangli, E.; Patel, A.; Mukherjee, A.; Singh, A.A. Bioremediation and bioelectricity from Himalayan rock soil in sediment-microbial fuel cell using carbon rich substrates. Fuel 2023, 341, 127019. [Google Scholar] [CrossRef]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting energy from the marine sediment--water interface. Environ. Sci. Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, J.J.; Chow, A.T.; Conner, W.H. Electrical energy production form forest detritus in a forested wetland using microbial fuel cells. GCB Bioenergy 2015, 7, 244–252. [Google Scholar] [CrossRef]
- Zaeni, A.; Susilowati, P.E.; Alwahab Ahmad, L.O. Renewable energy from sediment microbial fuel cell technology from Kendari Bay swamp sediments. AIP Conf. Proc. 2020, 2237, 020004. [Google Scholar]
- Wang, H.; Song, H.; Yu, R.; Cao, X.; Fang, Z.; Li, X. New process for copper migration by bioelectricity generation in soil microbial fuel cells. Environ. Sci. Pollut. R. 2016, 23, 13147–13154. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.-Y.; Tseng, Y.-H.; Tsang, D.C.W.; Hu, A.; Yu, C.-P. Wetland plant microbial fuel cells for remediation of hexavalent chromium contaminated soils and electricity production. J. Hazard. Mater. 2019, 365, 137–145. [Google Scholar] [CrossRef]
- Cologgi, D.L.; Speers, A.M.; Bullard, B.A.; Kelly, S.D.; Reguera, G. Enhanced uranium immobilization and reduction by Geobacter sulfurreducens biofilms. Appl. Environ. Microbiol. 2014, 80, 6638–6646. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.; Liu, F.; Chen, Z. Mechanisms and challenges of microbial fuel cells for soil heavy metal(loid)s remediation. Sci. Total Environ. 2021, 756, 143865. [Google Scholar] [CrossRef]
- Gupta, S.; Patro, A.; Mittal, Y.; Dwivedi, S.; Saket, P.; Panja, R.; Saeed, T.; Martinez, F.; Yadav, A.K. The race between classical microbial fuel cells, sediment-microbial fuel cells, plant-microbial fuel cells, and constructed wetlands-microbial fuel cells: Applications and technology readiness level. Sci. Total Environ. 2023, 879, 162757. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhu, H.; Yan, B.; Banuelos, G.; Shutes, B.; Wang, X.; Cao, S.; Cheng, R.; Tian, L. High removal efficiencies of antibiotics and low accumulation of antibiotic resistant genes obtained in microbial fuel cell-constructed wetlands intensified by sponge iron. Sci. Total Environ. 2022, 806, 150220. [Google Scholar] [CrossRef]
- Chesworth, W. (Ed.) Encyclopedia of Soil Science, 1st ed.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Weil, R.; Brady, N. The Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2017. [Google Scholar]
- Pietrzak, S.; Urbaniak, M. The relationship between the granulometric composition of grassland soils and their content of mineral nitrogen and organic carbon. J. Water Land. Dev. 2023, 57, 69–77. [Google Scholar] [CrossRef]
- Hemming, D.; Yakir, D.; Ambus, P.; Aurela, M.; Besson, C.; Black, K.; Buchmann, N.; Burlett, R.; Cescatti, A.; Clement, R.; et al. Pan-European δ13C values of air and organic matter from forest ecosystems. Glob. Change Biol. 2005, 11, 1065–1093. [Google Scholar] [CrossRef]
- Steiner, J.L.; Franzluebbers, A.J. Farming with grass—For people, for profit, for production, for protection. J. Soil. Water Conserv. 2009, 64, 75A–80A. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Grass roots of soil carbon sequestration. Carbon. Manag. 2012, 3, 9–11. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable soil use and management: An interdisciplinary and systematic approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.R.; Lohse, K.A.; Seyfried, M.S.; Godsey, S.E.; Parsons, S.B. Topographic controls of soil organic carbon on soil-mantled landscapes. Sci. Rep. 2019, 9, 6390. [Google Scholar] [CrossRef] [PubMed]
- Black, H.I.J.; Perekh, N.R.; Chaplow, J.S.; Monson, F.; Watkins, J.; Creamer, R.; Potter, E.D.; Poskitt, J.M.; Rowland, P.; Ainsworth, G.; et al. Assessing soil biodiversity across Great Britain: National trends in the occurrence of heterotrophic bacteria and invertebrates in soil. J. Environ. Manag. 2003, 67, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Roesch, L.F.W.; Fulthorpe, R.R.; Riva, A.; Casella, G.; Hadwin, A.K.M.; Kent, A.D.; Daroub, S.H.; Camargo, F.A.O.; Farmerie, W.G.; Tripkett, E.W. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 2007, 1, 283–290. [Google Scholar] [CrossRef] [PubMed]
- McNear, D.H., Jr. The Rhizosphere—Roots, Soil and Everything In Between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, L.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.; Ma, X.; Tsetsegmaa, M.; Zheng, Y.; Qu, H.; Dai, Y.; Li, J.; Bao, Y. Response of soil microbial community composition and diversity at different gradients of grassland degradation in central Mongolia. Agriculture 2022, 12, 1430. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Xu, J.; Brookes, P.C. Soil biochemical properties and bacteria community in a repeatedly fumigated-incubated soil. Biol. Fert. Soils 2020, 56, 619–631. [Google Scholar] [CrossRef]
- Nealson, K.H.; Saffarini, D. Iron and manganese in anaerobic respiration: Environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 1994, 48, 311–343. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef] [PubMed]
- Methe, B.A.; Nelson, K.E.; Eisen, J.A.; Paulsen, I.T.; Nelson, W.; Heilderberg, J.F.; Wu, D.; Wu, M.; Ward, N.; Beanan, M.J.; et al. Genome of Geobacter sulfurreducens: Metal reduction in subsurface environments. Science 2003, 302, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Giovannoni, S.J.; White, D.C.; Champine, J.E.; Phillips, E.J.; Gorby, Y.A.; Goodwin, S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch. Microbiol. 1993, 159, 336–344. [Google Scholar] [CrossRef]
- Rossmann, F.; Bremzinger, S.; Knauer, C.; Dörrich, A.K.; Ruppert, U.; Bange, G.; Thormann, K.M. The role of FlhF and HubP as polar landmark proteins in Shewanella putrefaciens CN-32. Mol. Biol. 2015, 98, 727–742. [Google Scholar] [CrossRef] [PubMed]
- de Celis, M.; Serrano-Aguirre, L.; Belda, I.; Liébana-García, R.; Arroyo, M.; Marquina, D.; de la Mata, I.; Santos, A. Acylase enzymes disrupting quorum sensing alter the transcriptome and phenotype of Pseudomonas aeruginosa, and the composition of bacterial biofilms from wastewater treatment plants. Sci. Total Environ. 2021, 799, 149401. [Google Scholar] [CrossRef] [PubMed]
- Toczyłowska-Mamińska, R.; Pielech-Przybylska, K.; Sekrecka-Belniak, A.; Dziekońska-Kubczak, U. Stimulation of electricity production in microbial fuel cells via regulation of syntrophic consortium development. Appl. Energ. 2020, 271, 115184. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Harden, J.W.; Turetsky, M.R.; Petersen, D.G.; McGuire, A.D.; Briones, M.J.I.; Churchill, A.C.; Doctro, D.H.; Pruett, L.E. Bacterial and enchytraeid abundance accelerate soil carbon turnover along a lowland vegetation gradient in interior Alaska. Soil. Biol. Biochem. 2012, 50, 188–198. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Kokila, V.; Prasanna, R.; Kumar, A.; Nishanth, S.; Shukla, J.; Gulia, U.; Nain, L.; Shivay, J.S.; Singh, A.K. Cyanobacterial inoculation in elevated CO2 environment stimulates soil C enrichment and plant growth of tomato. Environ. Technol. Innovation 2022, 26, 102234. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil. Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Whitman, W.B.; Bowen, T.L.; Boone, D.R. The Methanogenic Bacteria. In The Prokaryotes. A Handbook on the Biology of Bacteria, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H., Stackebrandt, E., Eds.; Archaea. Bacteria: Firmicutes, Actinomycetes; Springer: New York, NY, USA, 2006; Volume 3, pp. 165–207. [Google Scholar]
- Kuleshova, T.; Rao, A.; Bhadra, S.; Garlapati, V.K.; Sharma, S.; Kaushik, A.; Goswami, P.; Sreekirshnan, T.R.; Sevda, S. Plant microbial fuel cells as an innovative, versatile agrotechnology for green energy generation combined with wastewater treatment and food production. Biomass Bioenerg. 2022, 167, 106629. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Kacmaz, G.K.; Eczacioglu, N. The mechanism of bioelectricity generation from organic wastes: Soil/plant microbial fuel cells. Environ. Technol. Rev. 2024, 13, 76–95. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S. Overview of recent advancements in the microbialfuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
- Nitisoravut, R.; Regmi, R. Plant microbial fuel cells: A promising biosystems engineering. Renew. Sustain. Energ. Rev. 2017, 76, 81–89. [Google Scholar] [CrossRef]
- He, Z.; Shao, H.; Angenent, L.T. Increased power production from a sediment microbial fuel cell with a rotating cathode. Biosens. Bioelectron. 2007, 22, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Simeon, M.I.; Asoiro, F.U.; Aliyu, M.; Raji, O.A.; Freitag, R. Polarization and power density trends of a soil-based microbial fuel cell treated with human urine. Int. J. Energ. Res. 2020, 44, 5968–5976. [Google Scholar] [CrossRef]
- Simeon, I.M.; Weig, A.; Freitag, R. Optimization of soil microbial fuel cell for sustainable bio-electricity production: Combined effects of electrode material, electrode spacing, and substrate feeding frequency on power generation and microbial community diversity. Biotechnol. Biofuels. Bioprod. 2022, 15, 124. [Google Scholar] [CrossRef]
- Lu, L.; Huggins, T.; Jin, S.; Zuo, Y.; Ren, Z.J. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ. Sci. Technol. 2014, 48, 4021–4029. [Google Scholar] [CrossRef]
- Afsham, N.; Roshandel, R.; Yaghmaei, S.; Vajihinejad, V.; Sherafatmand, M. Bioelectricity generation in a soil microbial fuel cell with biocathode denitrification. Energ. Source Part A 2015, 37, 2092–2098. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z.; Bielecka, A.; Ciepielski, J. Bioelectricity production from soil using microbial fuel cells. Appl. Biochem. Biotech. 2014, 173, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Dunaj, S.J.; Vallino, J.J.; Hines, M.E.; Gay, M.; Kobyljanec, C.; Rooney-Varga, J.N. Relationships between soil organic matter, nutrients, bacterial community structure, and the performance of microbial fuel cells. Environ. Sci. Technol. 2012, 46, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Dziegielowski, J.A.; Mascia, M.; Metcalfe, B.; Di Lorenzo, M. Voltage evolution and electrochemical behaviour of soil microbial fuel cells operated in different quality soils. Sustain. Energ. Technol. Assess. 2023, 56, 103071. [Google Scholar] [CrossRef]
- Dziegielowski, J.A.; Bregu, G.; Hulse, L.; Di Lorenzo, M. Assessing the effect of the electrode orientation on the performance of soil microbial fuel cells. E3S Web Conf. 2022, 334, 08003. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Yong, Y.C.; Chang, F.X. Anode materials for soil microbial fuel cells: Recent advances and future perspectives. Int. J. Energy Res. 2022, 46, 712–725. [Google Scholar] [CrossRef]
- Sharafat, I.; Ali, J.; Hussain, A.; Torres, C.I.; Ali, N. Trivalent iron shaped the microbial community structure to enhance the electrochemical performance of microbial fuel cells inoculated with soil and sediment. J. Environ. Chem. Eng. 2022, 10, 107790. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Nguyen, H.U.D.; Taguchi, K. Portable membrane-less soil microbial fuel cell: Using multiwalled CNT paper electrodes. J. Electron. Mater. 2022, 51, 5946–5955. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Taguchi, K. A compact, membrane-less, easy-to-use soil microbial fuel cell: Generating electricity from household rice washing wastewater. Biochem. Eng. J. 2022, 179, 108338. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Zhang, H.; Cao, X.; Wang, H.; Li, X. Effects of cathode/anode electron accumulation on soil microbial fuel cell power generation and heavy metal removal. Environ. Res. 2021, 198, 111217. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Santra, M.; Sanka, R.V.S.P.; Krishnakumar, B. Bioremediation analysis of sediment-microbial fuel cells for energy recovery from microbial activity in soil. Int. J. Energy Res. 2021, 45, 6436–6445. [Google Scholar] [CrossRef]
- Li, C.; Omine, K.; Zhang, Z.; Sivasankar, V.; Sano, H.; Chicas, S.D. Development of peat microbial fuel cells (Peat MFCs)—The green and sustainable generators of electricity. Energ. Convers. Manag. 2023, 279, 116771. [Google Scholar] [CrossRef]
- Dziegielowski, J.; Metcalfe, B.; Villegas-Guzman, P.; Martínez-Huitle, C.A.; Goraye, A.; Wenk, J.; Di Lorenzo, M. Development of a functional stack of soil microbial fuel cells to power a water treatment reactor: From the lab to field trials in North East Brazil. Appl. Energ. 2020, 278, 115680. [Google Scholar] [CrossRef]
- Dziegielowski, J.; Metcalfe, B.; Di Lorenzo, M. Towards effecting Energy harvesting from stacks of soil microbial fuel cells. J. Power Sources 2021, 515, 230591. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Taguchi, K. A portable soil microbial fuel cell for sensing soil water content. Meas. Sens. 2021, 18, 100231. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, J.W.; Zhao, L.T.; Abbas, S.Z.; Yang, Z.; Yong, Y.C. Soil microbial fuel cell based self-powered cathodic biosensor for sensitive detection of heavy metals. Biosensors 2023, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Olias, L.G.; Otero, A.R.; Cameron, P.J.; Di Lorenzo, M. A soil microbial fuel cell-based biosensor for dissolved oxygen monitoring in water. Electrochim. Acta 2020, 362, 137108. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil. Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Maddalwar, S.; Nayak, K.K.; Kumar, M.; Singh, L. Plant microbial fuel cell: Opportunities, challenges, and prospects. Bioresour. Technol. 2021, 341, 125772. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Greenman, J.; Thorn, R.; Willey, N.; Ieropoulos, I. Energy harvesting from plants using hybrid microbial fuel cells; potential applications and future exploitation. Front. Bioeng. Biotechnol. 2024, 12, 1276176. [Google Scholar] [CrossRef]
- Yamamoto, S.; Okazaki, S.; Monica, N.D.; Ontsu, N.O.; Tanaka, H.; Sugihara, S. Effect of rhizobium inoculation on rhizosphere phosphorous dynamics and fertilised phosphorous use efficiency in a maize–pigeon pea intercropping system in weathered tropical soil. J. Sustain. Agric. Environ. 2023, 2, 357–368. [Google Scholar] [CrossRef]
- Van Limbergen, T.; Bonné, R.; Hustings, J.; Valcke, R.; Thijs, S.; Vangronsveld, J.; Manca, J.V. Plant microbial fuel cells from the perspective of photovoltaics: Efficiency, power, and applications. Renew. Sustain. Energy Rev. 2022, 169, 112953. [Google Scholar] [CrossRef]
- Gulamhussein, M.; Randall, D.G. Design and operation of plant microbial fuel cells using municipal sludge. J. Water Process. Engineer. 2020, 38, 101653. [Google Scholar] [CrossRef]
- Sophia, A.C.; Sreeja, S. Green energy generation from plant microbial fuel cells (PMFC) using compost and a novel clay separator. Sustain. Energy Technol. Assess. 2017, 21, 59–66. [Google Scholar] [CrossRef]
- Kuleshova, T.E.; Gall, N.R.; Galushko, A.S.; Panova, G.G. Electrogenesis in plant–microbial fuel cells in parallel and series connections. Tech. Phys. 2021, 66, 496–504. [Google Scholar] [CrossRef]
- Bombelli, P.; Dennis, R.J.; Felder, F.; Cooper, M.B.; Madras Rajaraman Iyer, D.; Royles, J.; Harrison, S.T.L.; Smith, A.G.; Harrison, C.J.; Howe, C.J. Electrical output of bryophyte microbial fuel cell systems is sufficient to power a radio or an environmental sensor. R. Soc. Open Sci. 2016, 3, 160249. [Google Scholar] [CrossRef] [PubMed]
- Apollon, W.; Kamaraj, S.-K.; Silos-Espino, H.; Perales-Segovia, C.; Valera-Montero, L.L.; Maldonado-Ruelas, V.A.; Vásques-Gutiérrez, M.A.; Ortiz-Medina, R.A.; Flores-Benírez, S.; Gómez-Leyva, J.F. Impact of Opuntia species plant biobattery in a semi-arid environment: Demonstration of their applications. Appl. Energy 2020, 279, 115788. [Google Scholar] [CrossRef]
- Schievano, A.; Colombo, A.; Grattieri, M.; Trasatti, S.; Liberale, A.; Tremolada, P.; Pino, C.; Cristiani, P. Floating microbial fuel cells as energy harvesters for signal transmission from natural water bodies. J. Power Sources 2017, 340, 80–88. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, H.; Chow, A.T.; Chen, P.; Wang, Y.; Xu, X.; Gong, Z.; Huang, S. Evaluation of organic matter and nitrogen removals, lectricity generation and bacterial community responses in sediment microbial fuel cell coupled with vallisneria natans. J. Environ. Chem. Eng. 2023, 11, 110058. [Google Scholar] [CrossRef]
- Timmers, R.A.; Strik, D.P.B.T.B.; Hamelers, H.V.M.; Buisman, C.J.M. Electricity generation by a novel design tubular plant microbial fuel cell. Biomass Bioenerg. 2013, 51, 60–67. [Google Scholar] [CrossRef]
- Sonu, K.; Sogani, M.; Syed, Z.; Rajvanshi, J.; Pandey, S.C. Performance evaluation of epipremnum aureum plant-based microbial fuel cel using composite anode made up of carbonized corncob and carbon rod. Biomass Convers. Biorefin. 2022, 14, 5149–5156. [Google Scholar] [CrossRef]
- Khudzari, M.; Gariépy, Y.J.; Kurian, J.; Tartakovsky, B.; Raghavan, G.S.V. Effects of biochar anodes in rice plantmicrobial fuel cells on the production of bioelectricity, biomass, and methane. Biochem. Eng. J. 2019, 141, 190–199. [Google Scholar] [CrossRef]
- Khudzari, M.; Kurian, J.; Gariépy, Y.; Tartakovsky, B.; Raghavan, G.S.V. Effects of salinity, growing media, and photoperiod on bioelectricity production in plant microbial fuel cells with weeping alkaligrass. Biomass Bioenerg. 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Helder, M.; Strik, D.P.B.T.B.; Hamelers, H.V.M.; Kuhn, A.J.; Blok, C.; Buisman, C.J.N. Concurrent bio-electricity and biomass production in three plant-microbial fuel cells using Spartina anglica, Arundinella anomala and Arundo donax. Bioresour. Technol. 2010, 101, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.N.; Strik, D.P.B.T.B. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energ. 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Guadarrama-Pérez, O.; Bahena-Rabadan, K.Y.; Dehesa-Carrasco, U.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Bioelectricity production using shade macrophytes in constructed wetlands-microbial fuel cells. Environ. Technol. 2020, 43, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, R.; Pearman, J.K.; Atalah, J.; Waters, S.; Vandergoes, M.J.; Howarth, J.D.; Thomson-Laing, G.; Thompson, L.; Wood, S.A. A taxonomy-free diatom eDNA-based technique for assessing lake trophic level using lake sediments. J. Environ. Manag. 2023, 345, 118885. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Gao, Q.; Dong, S.; Shi, S. Deep-sea cage culture altered microbial community composition in the sediments of the Yellow Sea Cold Water Mass. Mar. Pollut. Bull. 2022, 183, 114081. [Google Scholar] [CrossRef]
- Matiasek, S.J.; Pellerin, B.A.; Spencer, R.G.M.; Bergamaschi, B.A.; Hernes, P.J. Water-soluble organic carbon release from mineral soils and sediments in an irrigated agricultural system. J. Environ. Manag. 2023, 343, 118184. [Google Scholar] [CrossRef] [PubMed]
- Fussmann, D.; Hoyningen-Huene, A.J.E.V.; Reimer, A.; Schneider, D.; Babkov’a, H.; Peticzka, R.; Maier, A.; Arp, G.; Daniel, R.; Meister, P. Authigenic formation of Ca–Mg carbonates in the shallow alkaline Lake Neusiedl, Austria. Biogeosciences 2020, 17, 2085–2106. [Google Scholar] [CrossRef]
- Fagbayigbo, B.; Opeolu, B.; Fatoki, O.; Olatunji, O.; Akharame, M.; Human, I. Sorption and partitioning of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) onto sediments of Diep and Plankenburg river systems Western Cape, South Africa. Environ. Technol. Innov. 2022, 25, 102110. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef] [PubMed]
- Pulley, S.; Collins, A.L. Using the colour of recent overbank sediment deposits in two large catchments to determine sediment sources for targeting mitigation of catchment-specific management issues. J. Environ. Manag. 2023, 336, 117657. [Google Scholar] [CrossRef] [PubMed]
- Baragaño, D.; Berrezueta, E.; Komárekm, M.; Menéndez Aguado, J.M. Magnetic separation for arsenic and metal recovery from polluted sediments within a circular economy. J. Environ. Manag. 2023, 339, 117884. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Bond, D.R.; O’Neil, R.A.; Reimers, C.E.; Tender, L.R.; Lovely, D.R. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 2004, 48, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Yu, H.Q. Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol. Adv. 2015, 33, 1–12. [Google Scholar] [CrossRef]
- De Schamphelaire, L.; Rabaey, K.; Boeckx, P.; Boon, N.; Verstaeta, W. Outlook for benefits of sediment microbial fuel cells with two bi-electrodes. Microb. Biotechnol. 2008, 1, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovely, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Tender, L.M.; Gray, S.A.; Groveman, E.; Lowy, D.A.; Kauffman, P.; Melhado, J.; Tyce, R.C.; Flynn, D.; Petrecca, R.; Dobarro, J. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J. Power Sources 2008, 179, 571–575. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: Mechanisms and future prospective. Int. J. Energy Res. 2017, 41, 1242–1264. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, L.; He, Z. Powering a wireless temperature sensor using sediment microbial fuel cells with vertical arrangement of electrodes. J. Power Sources 2011, 196, 9568–9573. [Google Scholar] [CrossRef]
- Alipanahi, R.; Rahimnejad, M.; Najafpour, G. Improvement of sediment microbial fuel cell performances by design and application of power management systems. Int. J. Hydrogen Energy 2019, 44, 16965–16975. [Google Scholar] [CrossRef]
- Tominaga, M.; Ohmura, K.; Ototani, S.; Darmawan, R. Accelerating electricity power generation and shortening incubation period of microbial fuel cell operated in tidal flat sediment by artificial surfactant anode modification. Biochem. Eng. J. 2022, 185, 108536. [Google Scholar] [CrossRef]
- Lowy, D.A.; Tender, L.M.; Zeikus, J.G.; Park, D.H.; Lovley, D.R. Harvesting Energy from the marine sediment-water interfaceII—Kinetic activity of anode materials. Biosens. Bioelectron. 2006, 21, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Rivas, M.; Ramirez-Pereda, B.; Estrada-Godoy, F.; Cuesta-Zede, L.F.; Rochín-Medina, J.J.; Bustos-Terrones, Y.A.; Gonzalez-Huitron, V.A. Performance of a sediment microbial fuel cell for bioenergy production: Comparison of fluvial and marine sediments. Biomass Bioenerg. 2023, 168, 106657. [Google Scholar] [CrossRef]
- Huang, Y.; He, Z.; Kan, J.; Manohar, A.K.; Nealson, K.H.; Mansfeld, F. Electricity generation from a floating microbial fuel cell. Bioresour. Technol. 2012, 114, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Erable, B.; Lacroix, R.; Etcheverry, L.; Feron, D.; Delia, M.L.; Bergel, A. Marine floating microbial fuel cell involving aerobic biofilm on stainless steel cathodes. Bioresour. Technol. 2013, 142, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Zai, X.; Guo, M.; Hao, Y.; Hou, S.; Yang, Z.; Li, J.; Li, Y.; Ji, H.; Fu, Y. 3-aminopropyltriethoxysilane complexation with iron ion modified anode in marine sediment microbial fuel cells with enhanced electrochemical performance. J. Ocean Univ. China 2021, 20, 581–589. [Google Scholar] [CrossRef]
- Nielsen, M.E.; Reimers, C.E.; Stecher, H.A. Enhanced power from chambered benthic microbial fuel cells. Environ. Sci. Technol. 2007, 41, 7895–7900. [Google Scholar] [CrossRef]
- Reimers, C.E.; Girguis, P.; Stecher, H.A.; Ender, L.M.; Ryckelynck, N.; Whaling, P. Microbial fuel cell energy from an ocean cold seep. Geobiology 2006, 4, 123–136. [Google Scholar] [CrossRef]
- Tender, L.M.; Reimers, C.E.; Stecher, H.A.; Holmes, D.E.; Bond, D.R.; Lowy, D.A.; Pilobello, K.; Fertig, S.J.; Lovley, D.R. Harnessing microbially generated power on the seafloor. Nat. Biotechnol. 2002, 20, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, Y.; Zhang, J.; Ning, H.; Xiang, Y.; Song, X.; Zhao, W.; Guo, F. Power performance improvement in sediment microbial fuel cells: Recent advances and future challenges. Int. J. Hydrogen Energy 2023, 48, 24426–24446. [Google Scholar] [CrossRef]
- Massaglia, G.; Margaria, V.; Sacco, A.; Tommasi, T.; Pentassuglia, S.; Ahmed, D.; Mo, R.; Pirri, C.F.; Quaglio, M. In situ continuous current production from marine floating microbial fuel cells. Appl. Energ. 2018, 230, 78–85. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Huang, X.; Li, Y.; Zhang, H.; Zai, X. Humic acid and iron chelation modified anode improves the electrochemical performance of marine sediment microbial fuel cell. J. Ocean Univ. China 2022, 21, 388–394. [Google Scholar] [CrossRef]
- Bergel, A.; Feron, D.; Mollica, A. Catalysis of oxygen reduction in PEM fuel cel by seawater biofilm. Electrochem. Commun. 2005, 7, 900–904. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Brennan, R.; Logan, B.E. Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ. Sci. Technol. 2007, 41, 4053–4058. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xue, H.; Wang, M.; Feng, X.; Lee, H.S. The role of microbial electrogenesis in regulating methane and nitrous oxide emissions from constructed wetland-microbial fuel cel. Int. J. Hydrogen Energy 2022, 47, 27279–27292. [Google Scholar] [CrossRef]
- Bourguet, D.; Guillemaud, T. The Hidden and External Costs of Pesticide Use. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 19. [Google Scholar]
- World Bank Group; Cassou, E. Agricultural Pollution. Pesticides. 2018. Available online: https://documents1.worldbank.org/curated/en/689281521218090562/pdf/124345-BRI-p153343-PUBLIC-march-22-9-pm-WB-Knowledge-Pesticides.pdf/ (accessed on 30 September 2023).
- Latino, D.A.R.S.; Wicker, J.; Gütlein, M.; Schmid, E.; Kramer, S.; Fenner, K. Eawag-Soil in enviPath: A new resource for exploring regulatory pesticide soil biodegradation pathways and half-life data. Environ. Sci. Proc. Imp. 2017, 19, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Dubey, A.; Malla, M.A.; Kumar, A. Pesticide pestilence: Global scenario and recent advances in detection and degradation methods. J. Environ. Manag. 2023, 338, 117680. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.F.; Haus, N.W.; Sukkariyah, B.F.; Haering, K.C.; Daniels, W.L. Remediation of PAH-Contaminated Soils and Sediments: A Literature Review; Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2011. [Google Scholar]
- Alexander, M. Biodegradation and Bioremediation, 2nd ed.; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Hass, A.; Fine, P. Sequential selective extraction procedures for the study of heavy metals in soils, sediments, and waste materials—A critical review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 365–399. [Google Scholar] [CrossRef]
- Hewage, S.A.; Batagoda, J.H.; Meegoda, J.N. Remediation of contaminated sediments containing both organic and inorganic chemicals using ultrasound and ozone nanobubbles. Environ. Pollut. 2021, 274, 116538. [Google Scholar] [CrossRef]
- Varma, S.; Gupta, A.; Ghosal, P.; Majumder, A. A review on performance of constructed wetlands in tropical and cold climate: Insights of mechanism, role of influencing factors, and system modification in low temperature. Sci. Total Environ. 2021, 755, 142540. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Q.; Chen, F.; Lu, S.; Wang, Y.; Liang, H. Simultaneous removal of copper and biodegradation of BDE-209 with soil microbial fuel cells. J. Environ. Chem. Eng. 2021, 9, 105593. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, Y.; Chen, L.; Yang, Y.; Song, L. Remediation of chromium-contaminated soil in semi-arid areas by combined chemical reduction and stabilization. Environ. Pollut. Bioavail. 2023, 35, 2157332. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Weng, L.; Zhou, Q.; Li, Y. Microbial fuel cells for organic-contaminated soil remedial applications: A review. Energ. Technol. 2017, 5, 1156–1164. [Google Scholar] [CrossRef]
- Liapun, V.; Motola, M. Current overview and future perspective in fungal biorecovery of metals from secondary sources. J. Environ. Manag. 2023, 332, 117345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiao, W.; Huang, S.; Wang, H.; Cao, X.; Li, X.; Samaki, T. Application of microbial fuel cell technology to the remediation of compound heavy metal contamination in soil. J. Environ. Manag. 2022, 320, 115670. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.; Chen, J.; Wang, W.; Luo, H.; Chen, W.; Ma, D.; An, X.; Wei, Z. The role and related microbial processes of Mn-dependent anaerobic methane oxidation in reducing methane emissions from constructed wetland-microbial fuel cel. J. Environ. Manag. 2021, 294, 112935. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.; Kim, B.; Chesson, H.; Di Lorenzo, M.; Mascia, M. Modelling the influence of soil properties on performance and bioremediation ability of a pile of soil microbial fuel cells. Electrochim. Acta 2012, 368, 137568. [Google Scholar] [CrossRef]
- Wu, Q.; Jiao, S.; Ma, M.; Peng, S. Microbial fuel cell system: A promising technology for pollutant removal and environmental remediation. Environ. Sci. Pollut. R. 2020, 27, 6749–6764. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Zhou, S.G.; Chen, Q.; Zhao, B.; Yuan, Y.; Zhuang, L. Enhanced anaerobic degradation of organic pollutants in a soil microbial fuel cell. Chem. Eng. J. 2011, 172, 647–653. [Google Scholar] [CrossRef]
- Cao, M.; Yin, J.; Song, T.; Xie, J. Effects of the presence of phosphate buffer solution on removal efficiency of Pb and Zn in soil by solid phase microbial fuel cells. Biotechnol. Lett. 2022, 44, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, K.; Cao, Y.; Zhu, Y.; Liu, X.; Sun, J. Synergistic remediation of lead contaminated soil by microbial fuel cell and composite remediation agent. Energy Rep. 2022, 8, 388–397. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Wang, H.; Long, X.; Li, X. Simultaneous enhancement of heavy metal removal and electricity generation in soil microbial fuel cell. Ecotox. Environ. Saf. 2020, 192, 110314. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, Y.; Tang, J.; Du, Y. Remediation of cd contaminated soil in microbial fuel cells: Effects of cd concentration and electrode spacing. J. Environ. Eng. 2020, 146, 04020050. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, X.; Li, Q.; Cheng, C.; Shen, H.; Zhang, Z. Bioelectrochemical remediation of Cr (VI)/Cd (II)-contaminated soil in bipolar membrane microbial fuel cells. Environ. Res. 2020, 186, 109582. [Google Scholar] [CrossRef]
- Li, C.; Mei, T.; Song, T.S.; Xie, J. Removal of petroleum hydrocarbon-contaminated soil using a solid-phase microbial fuel cell with a 3D corn stem carbon electrode modified with carbon nanotubes. Bioprocess Biosyst. Eng. 2022, 45, 1137–1147. [Google Scholar] [CrossRef]
- Zhang, H.; Chao, B.; Gao, X.; Cao, X.; Li, X. Effect of starch-derived organic acids on the removal of polycyclic aromatic hydrocarbons in an aquaculture-sediment microbial fuel cell. J. Environ. Manag. 2022, 311, 114783. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Cao, X.; Long, X.; Li, X. Enhanced degradation of atrazine by soil microbial fuel cells and analysis of bacterial community structure. Water Air Soil Pollut. 2017, 228, 308. [Google Scholar] [CrossRef]
- Liang, Y.; Ji, M.; Zhai, H.; Zhao, J. Organic matter composition, BaP biodegradation and microbial communities at sites near and far from the bioanode in a soil microbial fuel cell. Sci. Total Environ. 2021, 772, 144919. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, Y.; Cheng, L.; Liu, J.; Li, F.; Gao, B.; Zhou, Q. Extended petroleum hydrocarbon bioremediation in saline soil using Pt-free multianodes microbial fuel cells. RSC Adv. 2014, 4, 59803–59808. [Google Scholar] [CrossRef]
- Cao, X.; Song, H.; Yu, C.; Li, X. Simultaneous degradation of toxic refractory organic pesticide and bioelectricity generation using a soil microbial fuel cell. Bioresour. Technol. 2015, 189, 87–93. [Google Scholar] [CrossRef]
- Cui, H.; Wang, J.; Feng, K.; Xing, D. Digestate of fecal sludge enhances the tetracycline removal in soil microbial fuel cells. Water 2022, 14, 2752. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.; Zhou, Q.; Zhang, Z.; Chen, C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol. Bioeng. 2012, 109, 426–433. [Google Scholar] [CrossRef]
- Rodrigo, J.; Boltes, K.; Esteve-Nuñez, A. Microbial-electrochemical bioremediation and detoxification of dibenzothiophene-polluted soil. Chemosphere 2014, 101, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yazdi, H.; Jin, S.; Zuo, Y.; Fallgren, P.H.; Ren, Z.J. Enhanced bioremediation of hydrocarbon-contaminated soil using pilot-scale bioelectrochemical systems. J. Hazard. Mater. 2014, 274, 8–15. [Google Scholar] [CrossRef]
- Kalathil, S.; Pant, D. Nanotechnology to rescue bacterial bidirectional extracellular electron transfer in bioelectrochemical systems. RSC Adv. 2016, 6, 30582–30597. [Google Scholar] [CrossRef]
- Were, D.; Kansiime, F.; Fetahi, T.; Cooper, A.; Jjuuko, C. Carbon sequestration by wetlands: A critical review of enhancement measures for climate change mitigation. Earth Syst. Environ. 2019, 3, 327–340. [Google Scholar] [CrossRef]
- Xiong, Y.; Mo, S.; Wu, X.; Qu, X.; Liu, Y.; Zhou, L. Influence of human activities and climate change on wetland landscape pattern—A review. Sci. Total Environ. 2023, 879, 163112. [Google Scholar] [CrossRef]
- Paucar, E.; Sato, C. An Overview of microbial fuel cells within constructed wetland for simultaneous nutrient removal and power generation. Energies 2022, 15, 6841. [Google Scholar] [CrossRef]
- Kabutey, F.T.; Zhao, Q.; Wei, L.; Ding, J.; Antwi, P.; Quashie, F.K.; Wang, W. An overview of plant microbial fuel cells (PMFCs): Configurations and applications. Renew. Sustain. Energy Rev. 2019, 110, 402–414. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Mishra, B.K. The effects of microbial fuel cell integration into constructed wetland on the performance of constructed wetland. Bioresour. Technol. 2015, 195, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Gupta, D.K. Constructed wetland, an eco-technology for wastewater treatment: A review on various aspects of microbial fuel cell integration, low temperature strategies and life cycle impact of the technology. Renew. Sustain. Energy Rev. 2021, 148, 111261. [Google Scholar] [CrossRef]
- Saz, Ç.; Türe, C.; Türker, O.C.; Yakar, A. Effect of vegetation type on treatment performance and bioelectric production of constructed wetland modules combined with microbial fuel cell (CW-MFC) treating synthetic wastewater. Environ. Sci. Pollut. R. 2018, 25, 8777–8792. [Google Scholar] [CrossRef] [PubMed]
- Oodally, A.; Gulamhussein, M.; Randall, D.G. Investigating the performance of constructed wetland microbial fuel cells using three indigenous South African wetland plants. J. Water Proc. Eng. 2019, 32, 100930. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Muyeed, A.; Yadav, A.K.; Miah, M.J.; Hasan, M.R.; Zaman, T.; Hasan, M.; Ahmed, T. Influence of aeration, plants, electrodes, and pollutant loads on treatment performance of constructed wetlands: A comprehensive study with septage. Sci. Total Environ. 2023, 892, 164558. [Google Scholar] [CrossRef] [PubMed]
- Yakar, A.; Türe, C.; Türker, O.C.; Vymazal, J.; Saz, Ç. Impacts of various filtration media on wastewater treatment and bioelectric production in upflow constructed wetland combined with microbial fuel cell (UCW-MFC). Ecol. Eng. 2018, 117, 120–132. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Cai, J.; Wen, H.; Mao, Z.; Zhang, H.; Wang, X.; Ma, L.; Zhu, M. Electricity production and the analysis of the anode microbial community in a constructed wetland-microbial fuel cell. RSC Adv. 2019, 9, 21460–21472. [Google Scholar] [CrossRef] [PubMed]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agr. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Takahashi, J.; Mwenya, B.; Snatoso, B.; Sar, C.; Umetsu, K.; Kishimoto, T.; Nishizaki, K.; Kimura, K.; Hamamoto, O. Mitigation of methane emission and energy recycling in animal agricultural systems. Asian-Aust. J. Anim. Sci. 2005, 18, 1199. [Google Scholar]
- Horz, H.P.; Rich, V.; Avarhami, S.; Bohannan, B.J.M. Methane-oxidizing bacteria in a California upland grassland soil: Diversity and response to simulated global change. Appl. Microbiol. 2005, 71, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Crutzen, P.J.; Dentener, F.J. Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B Chem. Phys. Meteorol. 1998, 50B, 128. [Google Scholar] [CrossRef]
- Ishii, S.; Hotta, Y.; Watanabe, K. Methanogenesis versus electrogenesis: Morphological and phylogenetic comparisons of microbial communities. Biosci. Biotechnol. Biochem. 2008, 72, 286–294. [Google Scholar] [CrossRef]
- Chen, M.; Chang, L.; Zhang, J.; Guo, F.; Vymazal, J.; He, Q.; Chen, Y. Global nitrogen input on wetland ecosystem: The driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 2020, 4, 100063. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Hafez, H.; Nakhla, G. Enhancement of biohydrogen producing using ultrasonication. Int. J. Hydrogen Energy 2010, 35, 6184. [Google Scholar] [CrossRef]
- Ali, M.A.; Lee, C.H.; Kim, S.Y.; Kim, P.J. Effect of industrial by-products containing electron acceptors on mitigating methane emission during rice cultivation. Waste Manag. 2009, 29, 2759. [Google Scholar] [CrossRef]
- Yagi, K.; Tsuruta, H.; Minami, K. Possible options for mitigating methane emission from rice cultivation. Nutr. Cycl. Agroecosyst. 1997, 49, 213–220. [Google Scholar] [CrossRef]
- Jeon, H.J.; Choi, Y.K.; Kumaran, R.S.; Kim, S.; Song, K.G.; Hong, S.W.; Kim, M.; Kim, H.J. Electrochemical control of methane emission from lake sediment using microbial fuel cells. Bull. Korean Chem. Soc. 2012, 33, 2401–2404. [Google Scholar] [CrossRef]
- De Schamphelaire, L.; Ven den Bossche, L.; Dang, H.S.; Höfte, M.; Boon, N.; Rabaey, K.; Verstraete, W. Microbial fuel cells generating electricity from rhizodeposits of rice plants. Environ. Sci. Technol. 2008, 42, 3053–3058. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, Z.; Zhao, F. Energy from plants and microorganisms: Progress in plant-microbial fuel cells. Chem. Sus. Chem. 2012, 5, 1006–1011. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, K.; Ouyang, H.; Li, M.K.K.; Luo, Z.; Li, Y.; Chen, C.; Yang, X.; Shao, Z.; Yan, D.Y.S. Simultaneous PAHs degradation, odour mitigation and energy harvesting by sediment microbial fuel cell coupled with nitrate-induced biostimulation. J. Environ. Manag. 2021, 284, 112045. [Google Scholar] [CrossRef]
- Rizzo, A.; Boano, F.; Revelli, R.; Ridolfi, L. Can microbial fuel cells be an effective mitigation strategy for methane emissions from paddy fields? Ecol. Eng. 2013, 60, 167–171. [Google Scholar] [CrossRef]
- Kiran Kumar, V.; Man Mohan, K.; Manju, P.; Gajalakshm, S. Harnessing plant microbial fuel cells for resource recovery and methane emission reduction in paddy cultivation. Energy Convers. Manag. 2023, 294, 117545. [Google Scholar]
- Haj-Amor, Z.; Araya, T.; Kim, D.G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Grattieri, M.; Minteer, S. Microbial fuel cells in saline and hypersaline environments: Advancements, challenges and future perspectives. Bioelectrochemistry 2018, 120, 127–137. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef]
- Han, X.; Qu, Y.; Li, D.; Qiu, Y.; Yu, Y.; Feng, Y. Remediation of saline-sodic soil by plant microbial desalination cell. Chemosphere 2021, 277, 130275. [Google Scholar] [CrossRef] [PubMed]
- Gustave, W.; Yuan, Z.; Li, X.; Ren, J.X.; Feng, W.J.; Shen, H.; Chen, Z. Mitigation effects of the microbial fuel cells on heavy metal accumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113989. [Google Scholar] [CrossRef] [PubMed]
- Gustave, W.; Yuan, Z.F.; Sekar, S.; Ren, Y.X.; Liu, J.Y.; Zhang, J.; Chen, Z. Soil organic matter amount determines the behavior of iron and arsenic in paddy soil with microbial fuel cells. Chemosphere 2019, 237, 124459. [Google Scholar] [CrossRef] [PubMed]
- Gustave, W.; Yuan, Z.F.; Sekar, S.; Chang, H.C.; Zhang, J.; Wells, M.; Ren, Y.X.; Chen, Z. Arsenic mitigation in paddy soils by using microbial fuel cells. Environ. Pollut. 2018, 238, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Fraunhofer Institute for Solar Energy Systems ISE. 3D—PV Modules with Contour for Integrated Photovoltaics. 2024. Available online: https://www.ise.fraunhofer.de/en/research-projects/3d.html (accessed on 26 March 2024).

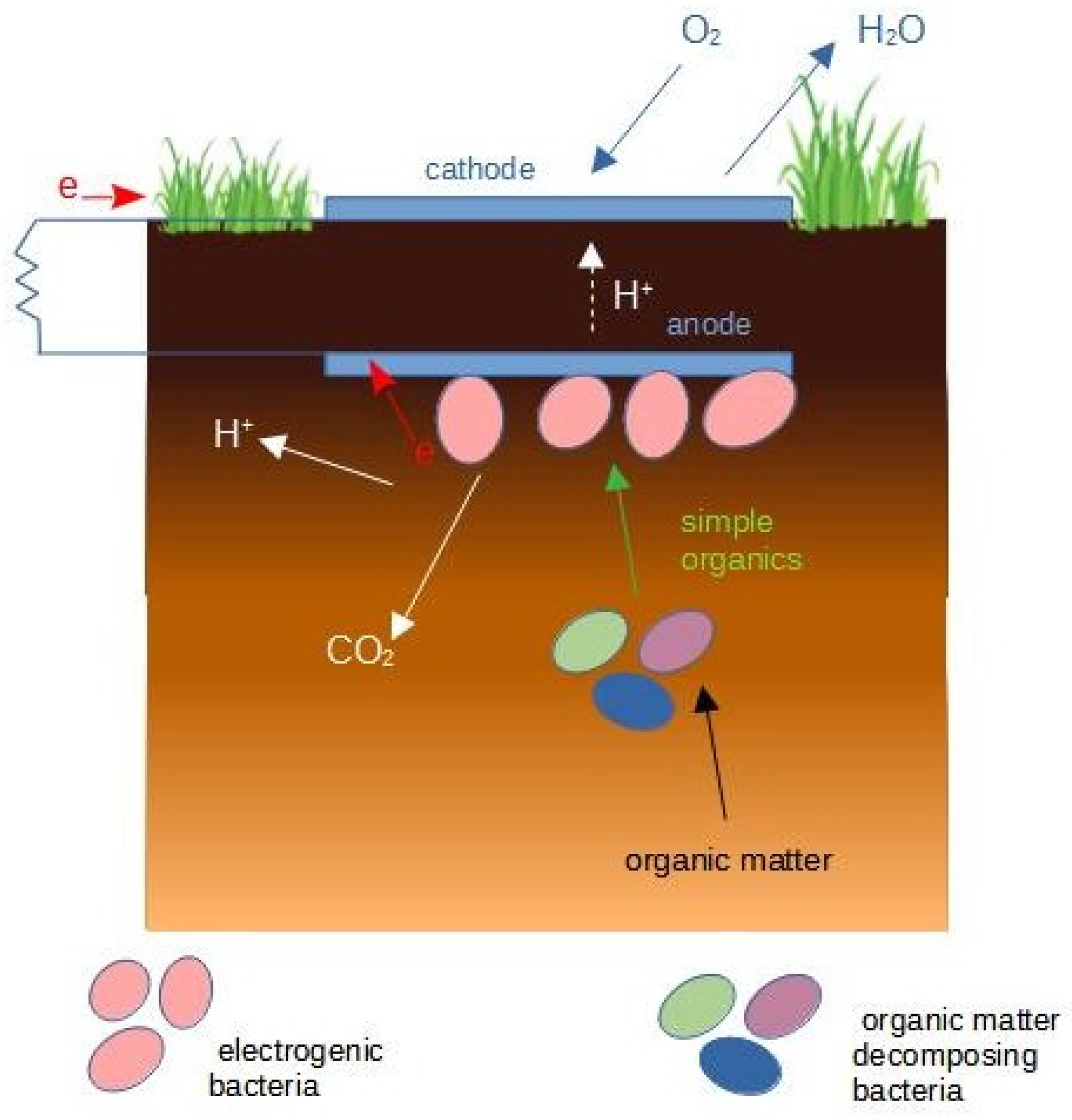
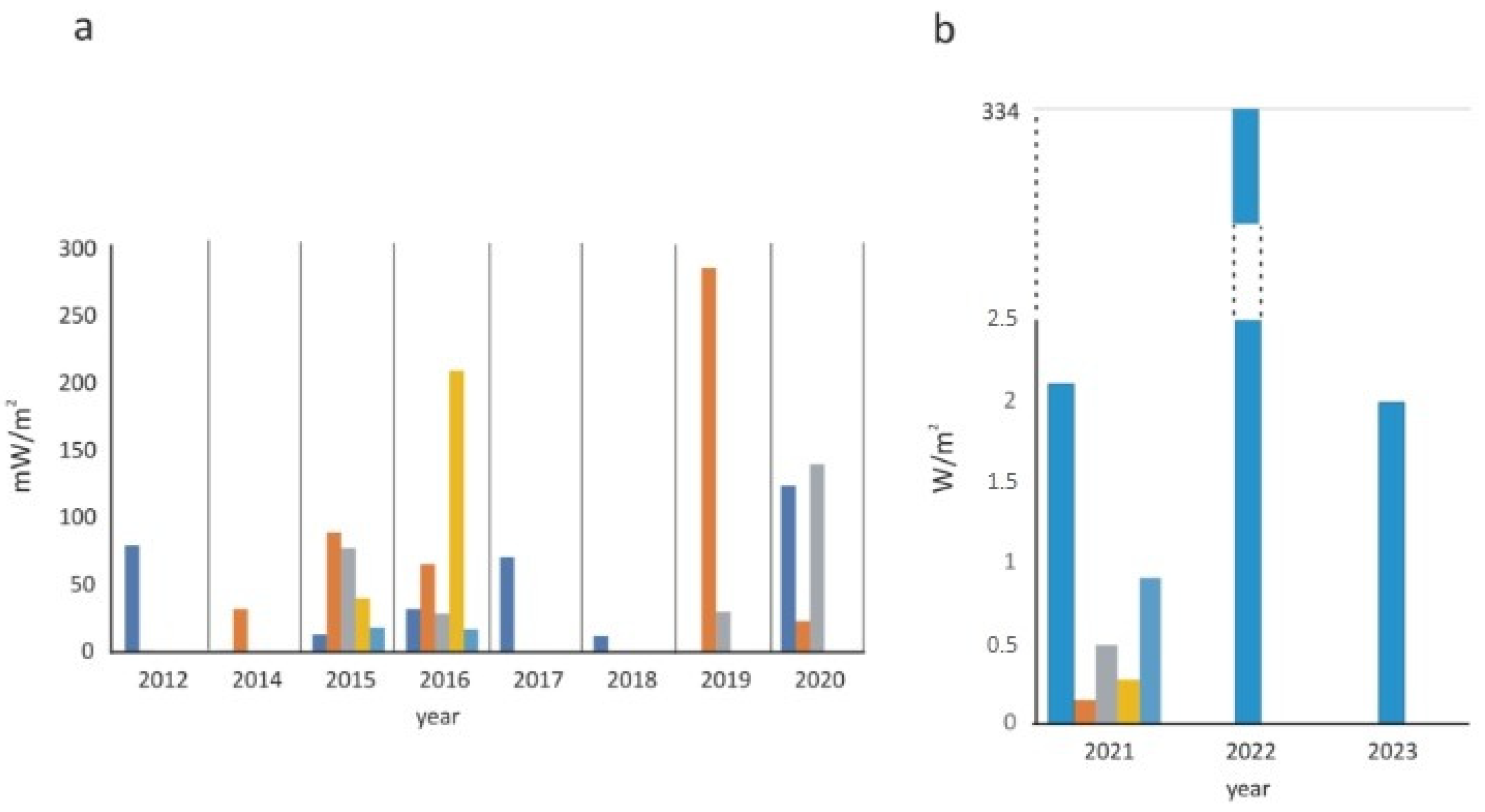
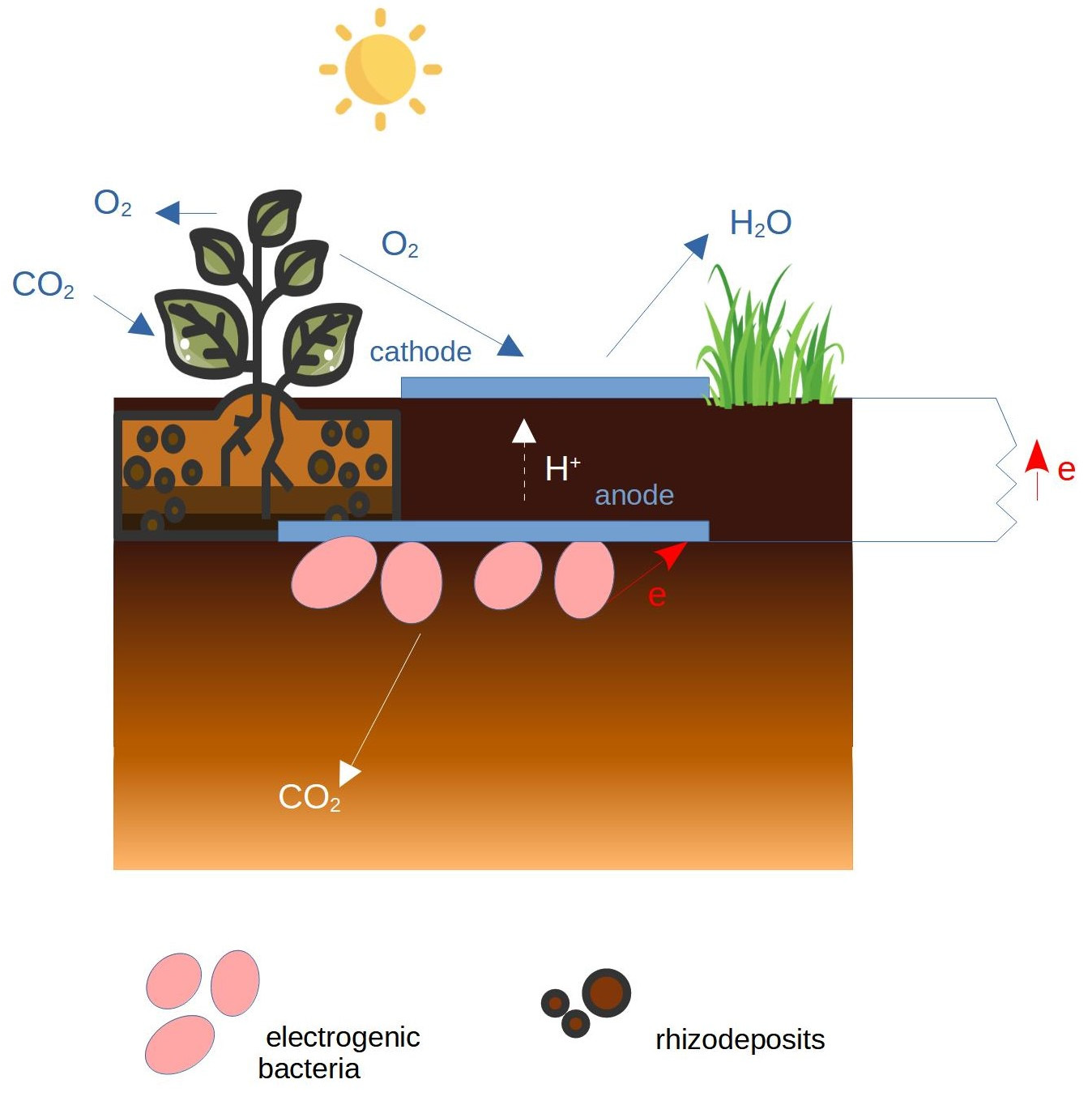
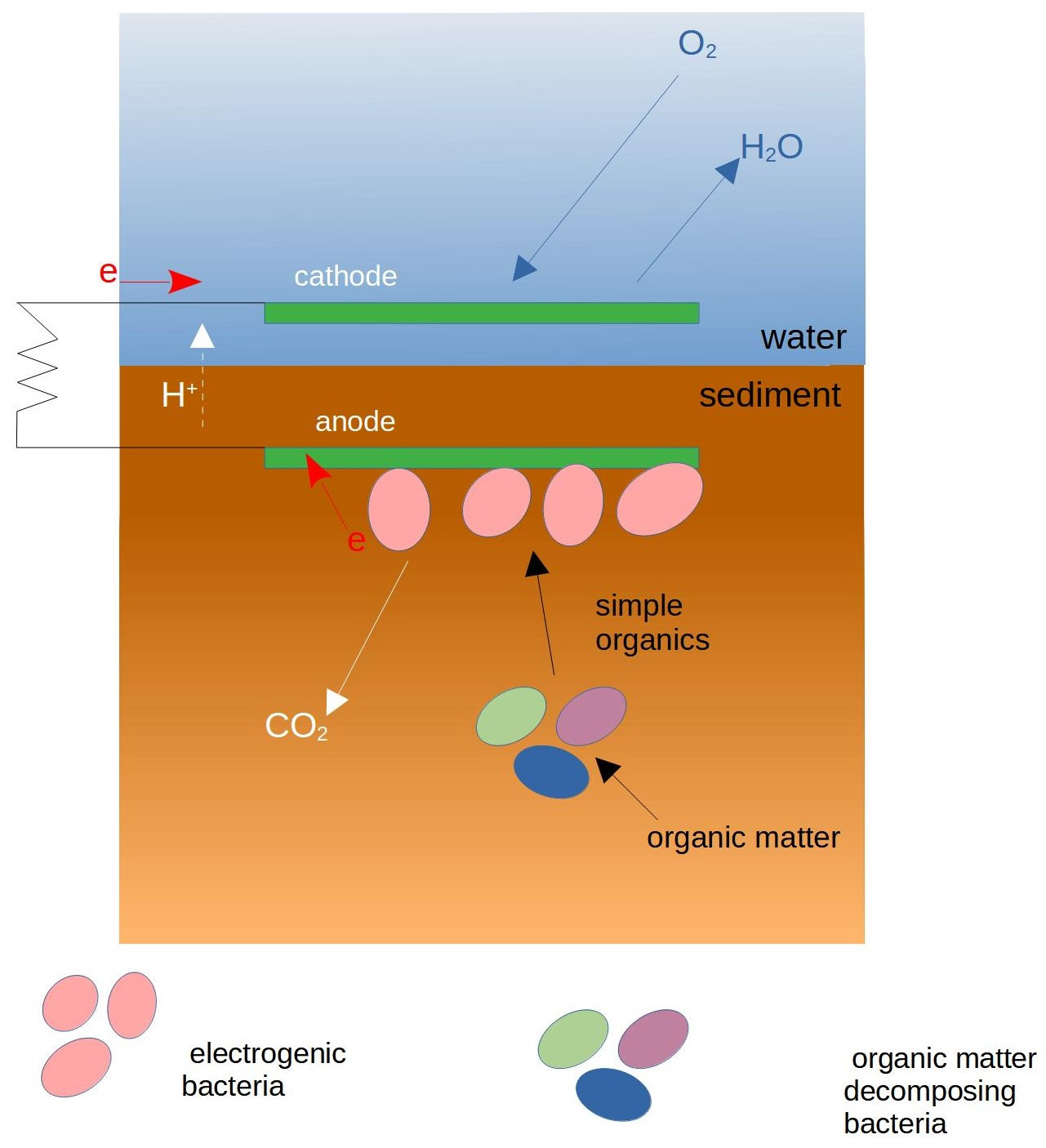
| MFC Configuration | Soil Type | Additional Substrate | Max Power | References |
|---|---|---|---|---|
| Portable, with carbon nanotube-based electrodes and floating air cathode | Rice paddy soil | no | 70 mW/m2 | [86] |
| Dual chamber | Farmland soil and sediment | Fe3+ | 334 W/m2 | [85] |
| Single chamber, carbon black electrodes with PTFE, PVA, epoxy, and PVDF * binders | Topsoil with garden compost | no | 500 mW/m2 | [77] |
| Stainless steel mesh/carbon black electrodes | Soil | Synthetic urine medium | 271 mW/m2 | [77] |
| Membraneless MFC with air cathode, carbon felt electrodes | Muddy soil, sandy soil | Household rice washing wastewater | 485.2 mW/m2 112 mW/m2 | [87] |
| Dual and three-chamber MFC, carbon felt anode, stainless steel cathode | Contaminated soil | Citric acid | 40 mW/m2 | [88] |
| porous carbon electrodes | Farmland soil | Urine | 85 mW/m2 | [76] |
| Stainless steel/epoxy/carbon black composite | Synthetic soil | Synthetic urine | 251.5 mW/m2 | [77] |
| Graphite fiber felt electrodes | Soil from ground level 3 m | Glucose, yeast extract | 2122 mW/m2 | [89] |
| Bamboo charcoal anode, activated carbon cathode | Peat soil | Bamboo waste, fluvic acid | 2011.9 mW/m2 | [90] |
| Plant | Anode | Cathode | Power Production | References |
|---|---|---|---|---|
| Vallisneria natans | Carbon felt | Carbon felt | 45.3 mW/m2 | [108] |
| Glyceria maxima | Graphite felt | Graphite felt | 12 mW/m2 | [109] |
| Epipremnum aureum | Carbon rod | Stainless steel | 73.7 mW/m2 | [110] |
| Oryza sativa | Carbon felt, maple wood biochar granules | Air cathode | 41.4 mW/m2 | [111] |
| Oryza sativa | Carbon cloth | Carbon cloth | 28 mW/m2 | [107] |
| Puccinellia distans | Carbon felt | Air cathode | 83.7 mW/m2 | [112] |
| Cyperus papyrus nanus Wachendorfia thyrsiflora Control (no plant) | Granular activated carbon | Carbon paper | 510 ± 92 mW/m3 1036 ± 59 mW/m3 392 ± 67 mW/m3 | [102] |
| Brassica juncea Trigonella foenum-graecum Canna stuttgart | Carbon brush | Carbon brush | 69.3 mW/m2 80.2 mW/m2 222.5 mW/m2 | [103] |
| Spartina anglica | Graphite rod | Graphite felt | 222 mW/m2 of plant growth area | [113] |
| Spartina anglica | Graphite felt | Graphite felt | 679 mW/m2 of plant growth area | [114] |
| Aglaonema commutatum Epipremnum aureum Dranacaena braunni Philodendron cordatum | Carbon felt | Carbon felt | 12.5 mW/m2 | [115] |
| MFC Configuration | Substrate | Maximum Power mW/m2 | Volume L | References |
|---|---|---|---|---|
| Laboratory experiments | ||||
| Carbon brush electrodes | Lake sediment | 2 | 121 | [130] |
| Graphite fiber felt electrodes, | Sediment enriched with glucose | 2.1 | n/a | [89] |
| Graphite electrodes | Marine sediment | 8 | 0.55 | [131] |
| Carbon felt anode modified with surfactants, Ti cathode | tidal flat sediment | 600 | 0.6 | [132] |
| Carbon felt anode modified with humic acid | Marine sediment | 165 | 0.7 | [133] |
| Single-chamber MFC with carbon fiber electrodes | Marine sediment Fluvial sediment | 1400 700 | 2 2 | [134] |
| Field tests | ||||
| Circular tube from PEM, granular graphite anode | Marine sediment | 4 | 4.5 | [135] |
| Ti/Ir/Ta anode and stainless steel cathode | Marine sediment with acetate | 20 | 0.6 | [136] |
| Carbon felt anode modified with 3-aminopropyl-triethoxysilan or composite with Fe3+ | Marine sediment | 203.8 | n/a | [137] |
| Pt mesh or graphite fiber electrodes | Marine sediment | 10 | n/a | [34] |
| Fine carbon fibers | Marine sediment | 380 | 9.6 | [138] |
| Modified graphite anodes, ceramic-graphite composite anodes with Mn2+ and Ni2+ | Marine sediment | 105 | n/a | [133] |
| Graphite rod anode, graphite plate cathode | Marine sediment | 34 | n/a | [139] |
| MFC Configuration | Soil Ecosystem Type | Removal of Soil Contamination | Power Production During Bioremediation | References |
|---|---|---|---|---|
| Cathode bioremediation | ||||
| Graphite felt electrodes | Farmland soil | Total Pb 14.7% Total Zn 22.3% | 21.7 mW/m2 | [165] |
| Three-chamber MFC | Soil | Total Cu 36.7% Total Cr 52.3% Total Pb 19.6% | n/a | [160] |
| Wetland plant MFC | Constructed wetland | Cr6+ 99% | n/a | [38] |
| Graphite brush anode, graphite felt-activated carbon cathode | Soil from university campus | Pb2+ 21% | n/a | [166] |
| Granular activated carbon electrodes | Farmland soil | Cu2+ 36.9% | 65.7 mW/m2 | [37] |
| Carbon felt electrodes | Soil | Cu2+ 94.7% | n/a | [167] |
| Graphite felt electrodes | Sandy loam soil | Total Cd 130% | 22.7 mW/m2 | [168] |
| Carbon felt electrodes | Soil | Total Cu 69.2% | n/a | [156] |
| Carbon brush anode, carbon cloth cathode | Soil | Cr 36% | 200–300 mW/m2 | [169] |
| Anode bioremediation | ||||
| Single-chamber MFC with graphite felt electrodes | Soil from petrochemical industrial area | Anthracene 61.6% Pyrene 55.9% Total petroleum hydrocarbon 59.1% | 24 mW/m2 | [166] |
| Anode: macroporous corn stem modified with carbon nanotubes; cathode: carbon felt | Soil from university campus | Petroleum hydrocarbon 42.2% | n/a | [170] |
| Dual-chamber MFC | Sediment | PAH (naphthalene 69.9%, acenaphthene 55.6%, pyrene 46.8%) | 25 mW/m2 | [171] |
| Granular activated carbon electrodes | Soil | Herbicide atrazine 91.7% | n/a | [172] |
| Graphite felt electrodes | Soil | Benzo[a]pyrene 72.5% | n/a | [173] |
| Carbon electrodes | Waterlogged soil from paddy field | Phenol 90.1% | 29.45 mW/m2 | [164] |
| Carbon electrodes | Aged saline soil | n-Alkanes (C8–C40) 29% | n/a | [174] |
| Granular activated carbon electrodes | Farmland topsoil | Pesticide hexachlorobenzene 71.1% | 77.5 mW/m2 | [175] |
| Graphite felt anode, active carbon cathode | Soil from university campus | Tetracycline 64.5% | 8.8 W/m3 | [176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toczyłowska-Mamińska, R.; Mamiński, M.Ł.; Kwasowski, W. Microbial Fuel Cell Technology as a New Strategy for Sustainable Management of Soil-Based Ecosystems. Energies 2025, 18, 970. https://doi.org/10.3390/en18040970
Toczyłowska-Mamińska R, Mamiński MŁ, Kwasowski W. Microbial Fuel Cell Technology as a New Strategy for Sustainable Management of Soil-Based Ecosystems. Energies. 2025; 18(4):970. https://doi.org/10.3390/en18040970
Chicago/Turabian StyleToczyłowska-Mamińska, Renata, Mariusz Ł. Mamiński, and Wojciech Kwasowski. 2025. "Microbial Fuel Cell Technology as a New Strategy for Sustainable Management of Soil-Based Ecosystems" Energies 18, no. 4: 970. https://doi.org/10.3390/en18040970
APA StyleToczyłowska-Mamińska, R., Mamiński, M. Ł., & Kwasowski, W. (2025). Microbial Fuel Cell Technology as a New Strategy for Sustainable Management of Soil-Based Ecosystems. Energies, 18(4), 970. https://doi.org/10.3390/en18040970







