Application of a Novel CVD TiN Coating on a Biomedical Co–Cr Alloy: An Evaluation of Coating Layer and Substrate Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
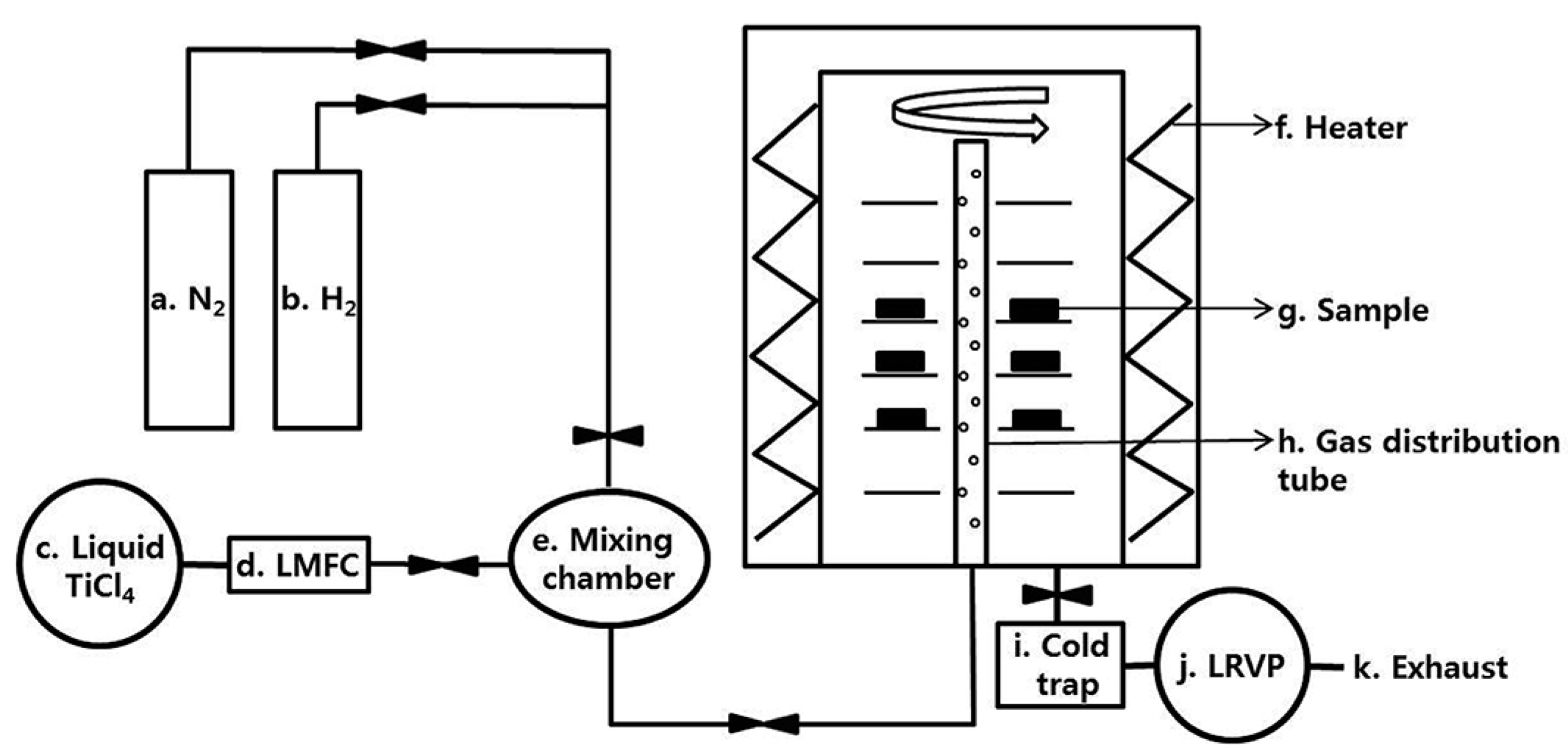
2.2. PVD and CVD Coating
2.3. Characterization
2.4. In Vitro Cytotoxicity Test
3. Results and Discussion
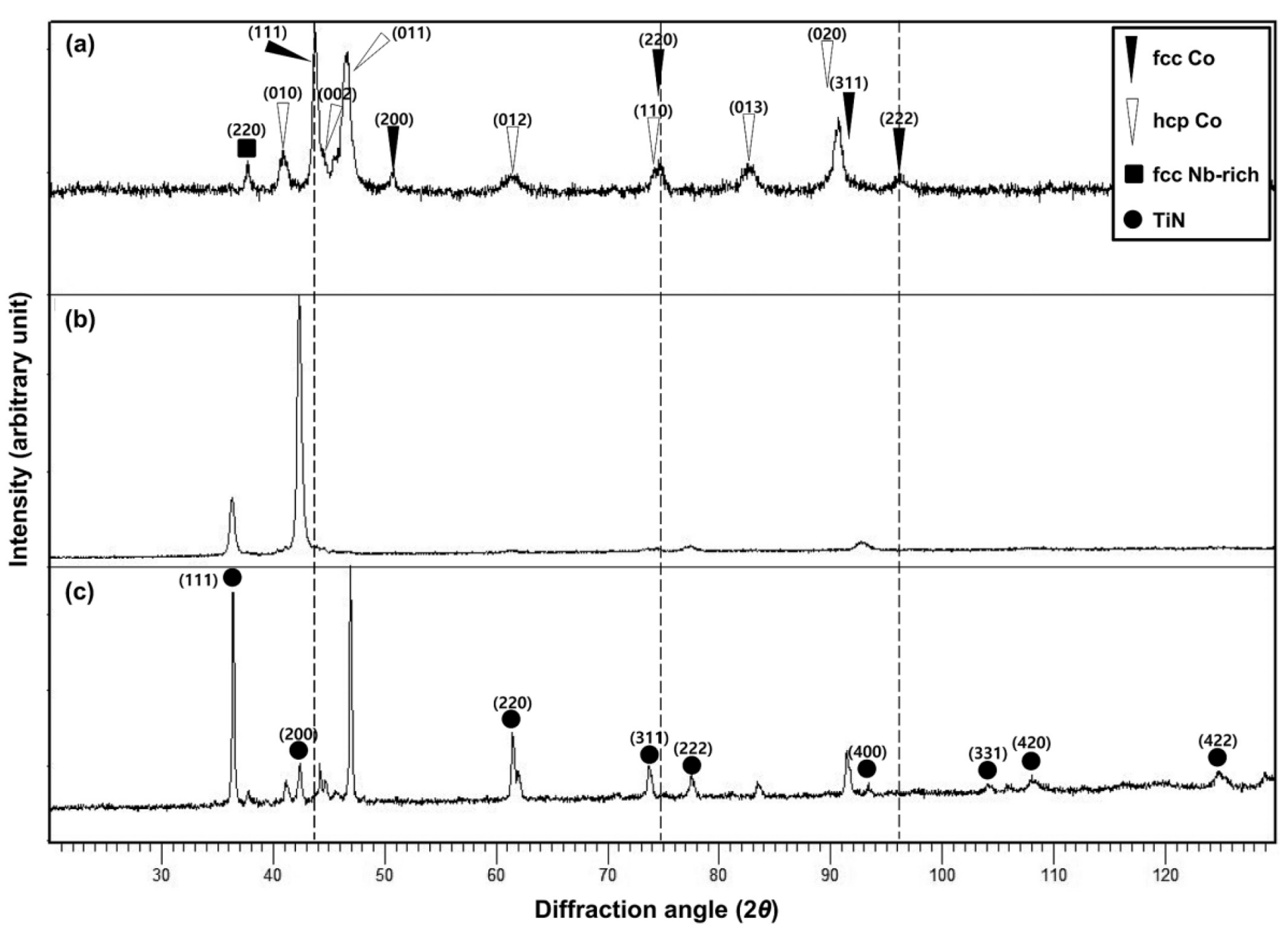
3.1. Phase Identification
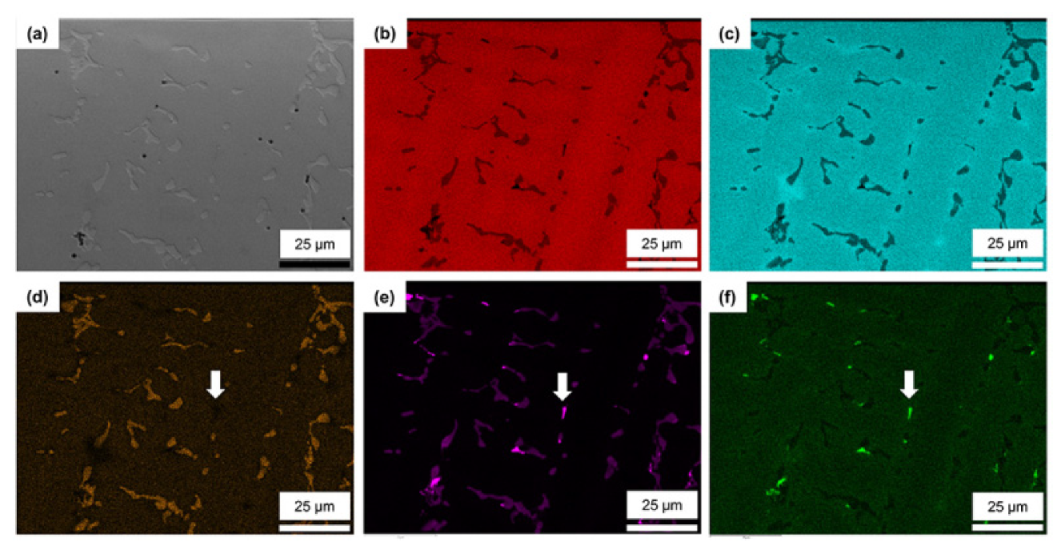
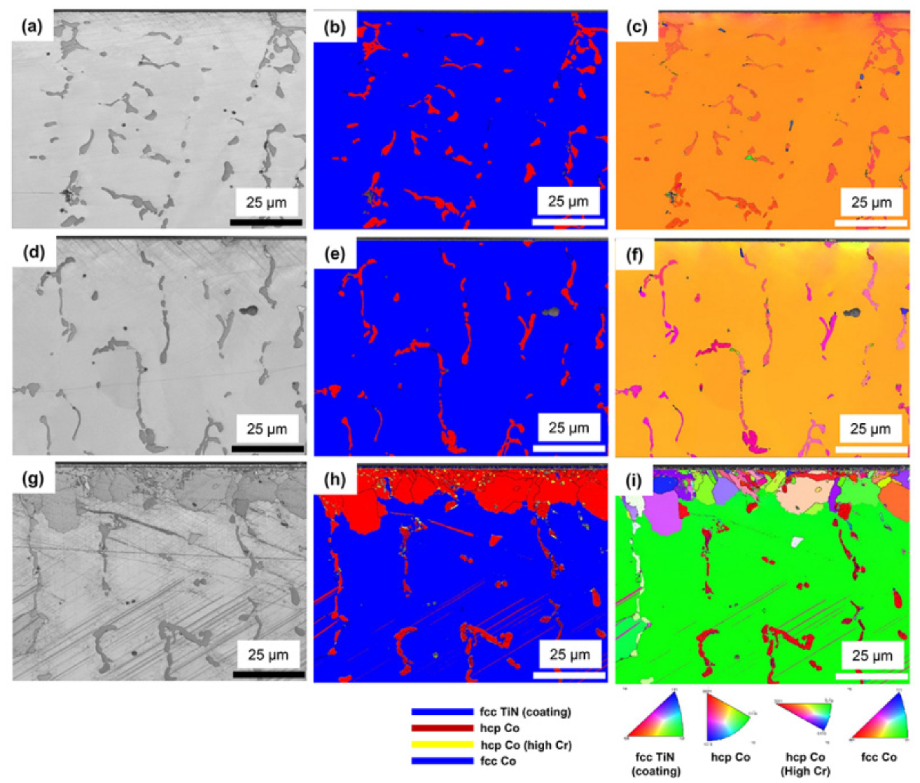
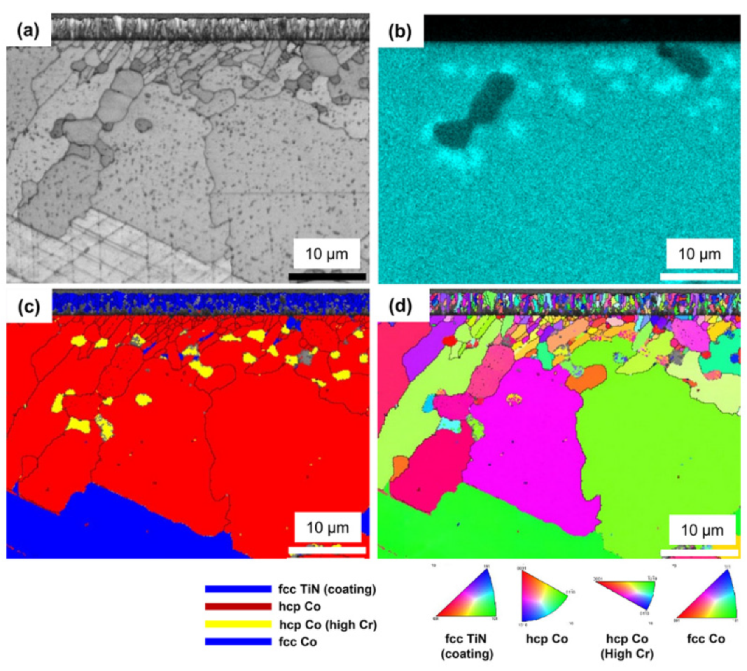
3.2. Microstructure
3.3. Crystallographic Features
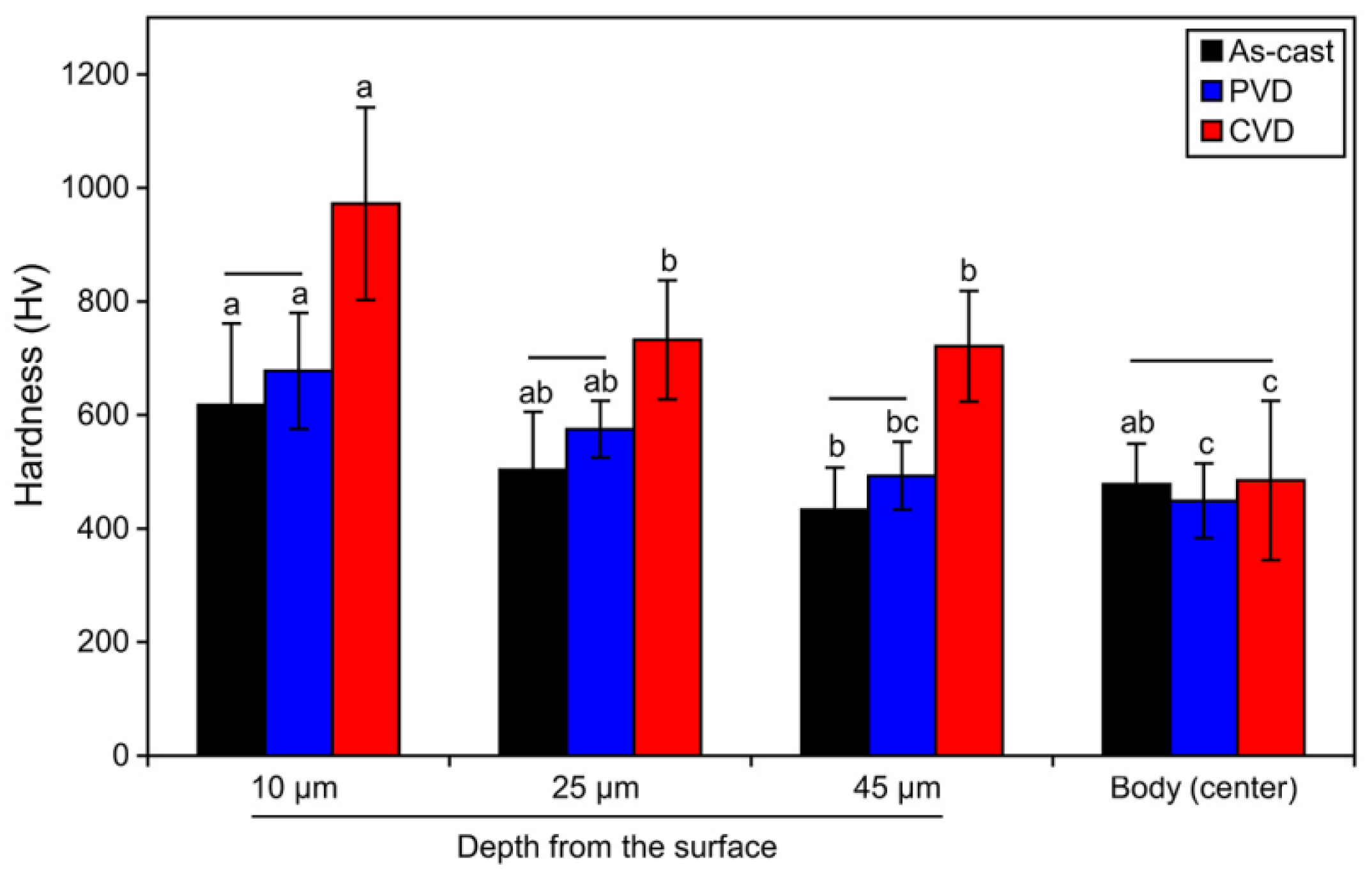
3.4. Vickers Hardness
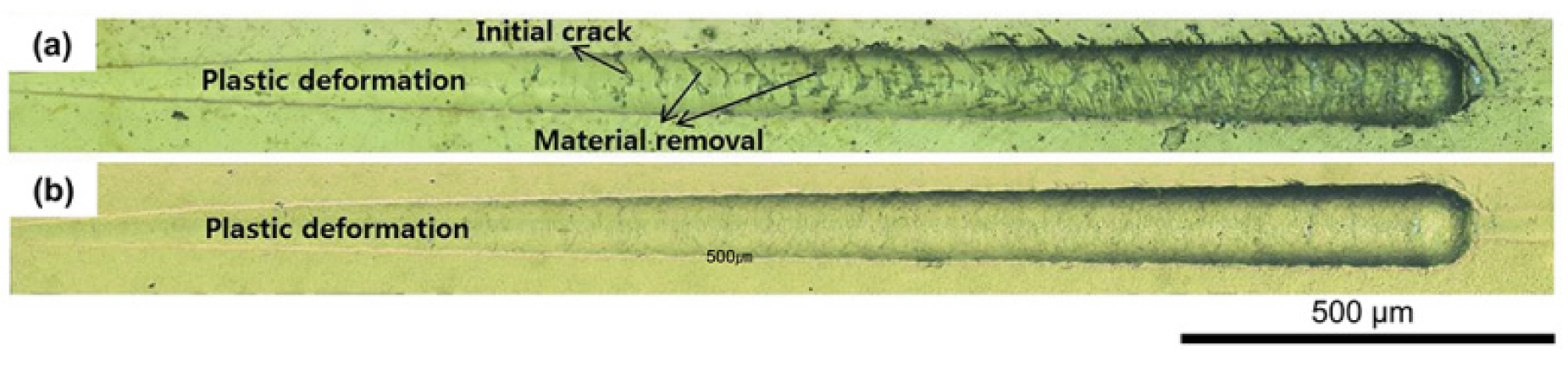
3.5. Adhesion of the Coating
3.6. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co–Cr–Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.O.A.; Mischler, S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, O.; Türkan, U.; Eroğlu, A.E. Metal ion release from nitrogen ion implanted CoCrMo orthopedic implant material. Surf. Coat. Technol. 2006, 200, 5687–5697. [Google Scholar] [CrossRef] [Green Version]
- Gotman, I.; Gutmanas, E.Y. Titanium nitride-based coatings on implantable medical devices. Adv. Biomater. Devices Med. 2014, 1, 53–73. [Google Scholar]
- Van Hove, R.P.; Sierevelt, I.N.; van Royen, B.J.; Nolte, P.A. Titanium-nitride coating of orthopaedic implants: A review of the literature. Biomed Res. Int. 2015, 2015, 485975. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.H.; Lee, C.H.; Chung, C.H.; Son, M.K.; Choe, H.C. Effects of TiN and WC coating on the fatigue characteristics of dental implant. Surf. Coat. Technol. 2014, 243, 71–81. [Google Scholar] [CrossRef]
- Ching, H.A.; Choudhury, D.; Nine, M.J.; Abu Osman, N.A. Effects of surface coating on reducing friction and wear of orthopaedic implants. Sci. Technol. Adv. Mater. 2014, 15, 014402. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, K.; Mashima, I.; Nakazawa, F.; Nakanishi, Y.; Ochi, M. Application of dental implants coated with titanium nitride: The experimental study with Porphyromonas gingivalis infection. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Das, M.; Balla, V.K.; Bodhak, S.; Murugesan, V.K. Mechanical, wear, corrosion and biological properties of arc deposited titanium nitride coatings. Surf. Coat. Technol. 2018, 344, 214–222. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S.; Fehrman, J.; Barnes, A.C.; Zapf, A.M.; Zinelis, S.; Berzins, D.W. Titanium nitride and nitrogen ion implanted coated dental materials. Coatings 2012, 2, 160–178. [Google Scholar] [CrossRef]
- Pešlová, F.; Koštialiková, D.; Janeková, M.; Puchnin, M. The effect of the primary implant production technology on the occurrence of degradation. Procedia Eng. 2016, 136, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Gotman, I.; Gutmanas, E.Y.; Hunter, G. Wear-resistance ceramic films and coatings. In Comprehensive Biomaterials, 1st ed.; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 1, pp. 127–155. [Google Scholar]
- Kuo, D.H.; Huang, K.W. Kinetics and microstructure of TiN coatings by CVD. Surf. Coat. Technol. 2001, 135, 150–157. [Google Scholar] [CrossRef]
- Wagner, J.; Mitterer, C.; Penoy, M.; Michotte, C.; Wallgram, W.; Kathrein, M. The effect of deposition temperature on microstructure and properties of thermal CVD TiN coatings. Int. J. Refract. Met. Hard Mater. 2008, 26, 120–126. [Google Scholar] [CrossRef]
- Von Fieandt, L.; Larsson, T.; Lindahl, E.; Bäcke, O.; Boman, M. Chemical vapor deposition of TiN on transition metal substrates. Surf. Coat. Technol. 2018, 334, 373–383. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, S.H.; Kim, Y.K.; Son, J.S.; Min, B.K.; Kim, K.H.; Kwon, T.Y. Microstructures and mechanical properties of Co-Cr dental alloys fabricated by three CAD/CAM-based processing techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef]
- Cheng, H.E.; Chiang, M.J.; Hon, M.H. Growth characteristics and properties of TiN coating by chemical vapor deposition. J. Electrochem. Soc. 1995, 142, 1573–1578. [Google Scholar] [CrossRef]
- Hong, M.H.; Hanawa, T.; Song, S.H.; Min, B.K.; Kwon, T.Y. Enhanced biocompatibility of a Ni–Cr alloy prepared by selective laser melting: A preliminary in vitro study. J. Mater. Res. Technol. 2019, 8, 1587–1592. [Google Scholar] [CrossRef]
- Lee, S.H.; Takahashi, E.; Nomura, N.; Chiba, A. Effect of heat treatment on microstructure and mechanical properties of Ni- and C-free Co–Cr–Mo alloys for medical applications. Mater. Trans. 2005, 46, 1790–1793. [Google Scholar] [CrossRef] [Green Version]
- Cabrol, E.; Boher, C.; Vidal, V.; Rézaï-Aria, F.; Touratier, F. Plastic strain of cobalt-based hardfacings under friction loading. Wear 2015, 330–331, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Li, K.C.; Ting, S.; Prior, D.J.; Waddell, J.N.; Swain, M.V. Microstructural analysis of Co-Cr dental alloy at the metal-porcelain interface: A pilot study. N. Z. Dent. J. 2014, 110, 138–142. [Google Scholar] [PubMed]
- Karaali, A.; Mirouh, K.; Hamamda, S.; Guiraldenq, P. Microstructural study of tungsten influence on Co–Cr alloys. Mater. Sci. Eng. A 2005, 390, 255–259. [Google Scholar] [CrossRef]
- López, H.F.; Saldivar-Garcia, A.J. Martensitic transformation in a cast Co-CrCo–Cr-Mo-C alloy. Metall. Mater. Trans. A 2008, 39, 8–18. [Google Scholar] [CrossRef]
- Vander Sande, J.B.; Coke, J.R.; Wulff, J. A transmission electron microscopy study of the mechanisms of strengthening in heat-treated Co-Cr-Mo-C alloys. Metall. Trans. A 1976, 7, 389–397. [Google Scholar] [CrossRef]
- Li, K.C.; Tran, L.; Prior, D.J.; Waddell, J.N.; Swain, M.V. Porcelain bonding to novel Co-Cr alloys: Influence of interfacial reactions on phase stability, plasticity and adhesion. Dent. Mater. 2016, 32, 1504–1512. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Adhesion of porcelain to three-dimensionally printed and soft milled cobalt chromium. J. Prosthodont. Res. 2019. [Google Scholar] [CrossRef]





| Coating | Temperature (°C) | Coating Time (min) | Pressure (mbar) | Bias Voltage (V) | Target | Gas |
|---|---|---|---|---|---|---|
| PVD | 550 | 30 | 0.025 | 60 | Ti | N2 100% |
| CVD | 850 | 400 | 150 | N/A 1 | N/A | H2 59.5%; N2 39.0%; TiCl4 1.5% |
| Image | EDS Composition (at%) | Remark | ||||||
|---|---|---|---|---|---|---|---|---|
| Point | Co | Cr | W | Mo | Nb | V | ||
| (a) As-cast | 1 | 63.6 | 29.0 | 3.9 | 0.6 | 0.5 | 2.4 | Co–Cr (matrix) |
| 2 | 49.6 | 20.5 | 12.9 | 2.6 | 12.7 | 1.7 | W-Mo-Nb-rich | |
| 3 | 64.6 | 28.5 | 3.7 | 0.6 | 0.4 | 2.2 | Co–Cr (matrix white line) | |
| 4 | 5.0 | 9.3 | N/D 1 | N/D | 58.9 | 26.8 | Nb-V-rich | |
| (e) PVD-coated | 1 | 62.9 | 29.4 | 3.9 | 0.7 | 0.7 | 2.4 | Co–Cr (matrix) |
| 2 | 50.4 | 20.6 | 12.4 | 2.5 | 12.5 | 1.6 | W-Mo-Nb-rich | |
| 3 | 64.4 | 28.7 | 3.7 | 0.5 | 0.5 | 2.3 | Co–Cr (matrix white line) | |
| 4 | 7.2 | 9.7 | 0.8 | N/D | 56.4 | 25.8 | Nb-V-rich | |
| (i) CVD-coated | 1 | 65.7 | 28.2 | 3.3 | 0.5 | 0.2 | 2.1 | Co–Cr (matrix) |
| 2 | 51.5 | 19.4 | 11.1 | 2.8 | 13.7 | 1.5 | W-Mo-Nb-rich | |
| 3 | 64.8 | 29.3 | 2.9 | 0.5 | 0.3 | 2.2 | Co–Cr (matrix small grain) | |
| 4 | 8.4 | 10.4 | 0.3 | N/D | 56.1 | 24.9 | Nb-V-rich | |
| 5 | 48.3 | 43.2 | 3.5 | 1.2 | 0.8 | 3.0 | Cr-rich | |
| 6 | 59.9 | 27.2 | 7.5 | 1.4 | 1.6 | 2.6 | White pit | |
| 7 | 58.7 | 29.1 | 3.9 | 0.7 | 1.8 | 5.9 | Black pit | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.H.; Min, B.K.; Hong, M.-H.; Kwon, T.-Y. Application of a Novel CVD TiN Coating on a Biomedical Co–Cr Alloy: An Evaluation of Coating Layer and Substrate Characteristics. Materials 2020, 13, 1145. https://doi.org/10.3390/ma13051145
Song SH, Min BK, Hong M-H, Kwon T-Y. Application of a Novel CVD TiN Coating on a Biomedical Co–Cr Alloy: An Evaluation of Coating Layer and Substrate Characteristics. Materials. 2020; 13(5):1145. https://doi.org/10.3390/ma13051145
Chicago/Turabian StyleSong, Si Hoon, Bong Ki Min, Min-Ho Hong, and Tae-Yub Kwon. 2020. "Application of a Novel CVD TiN Coating on a Biomedical Co–Cr Alloy: An Evaluation of Coating Layer and Substrate Characteristics" Materials 13, no. 5: 1145. https://doi.org/10.3390/ma13051145






