Rheological Properties of Industrial Hot Trub
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Experimental Setup
2.2.1. The Determination of Yield Stress and Flow Properties of Hot Trub
2.2.2. The Determination of Rheological Properties of Wort
2.3. Result Analysis
3. Results and Discussion
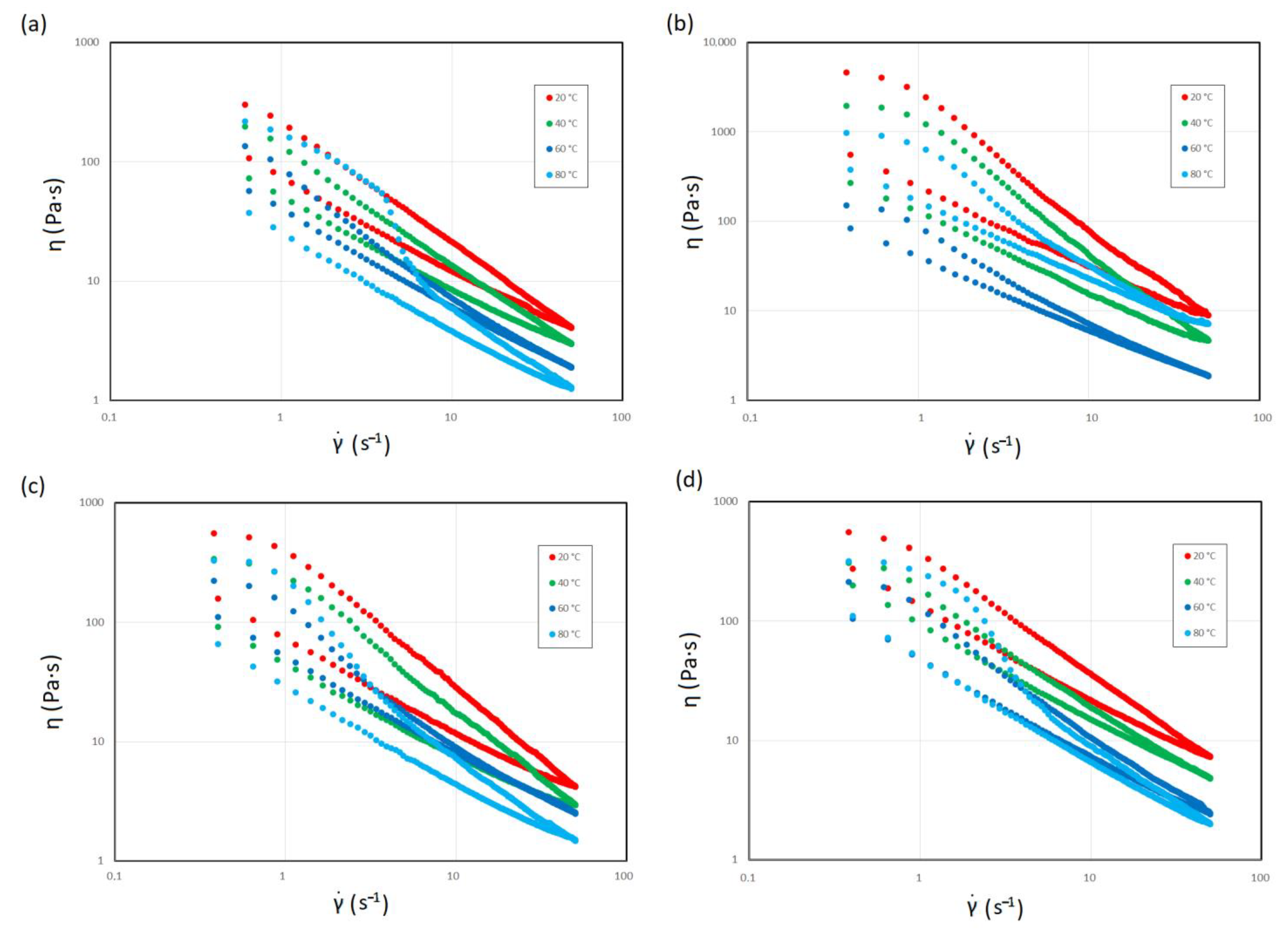
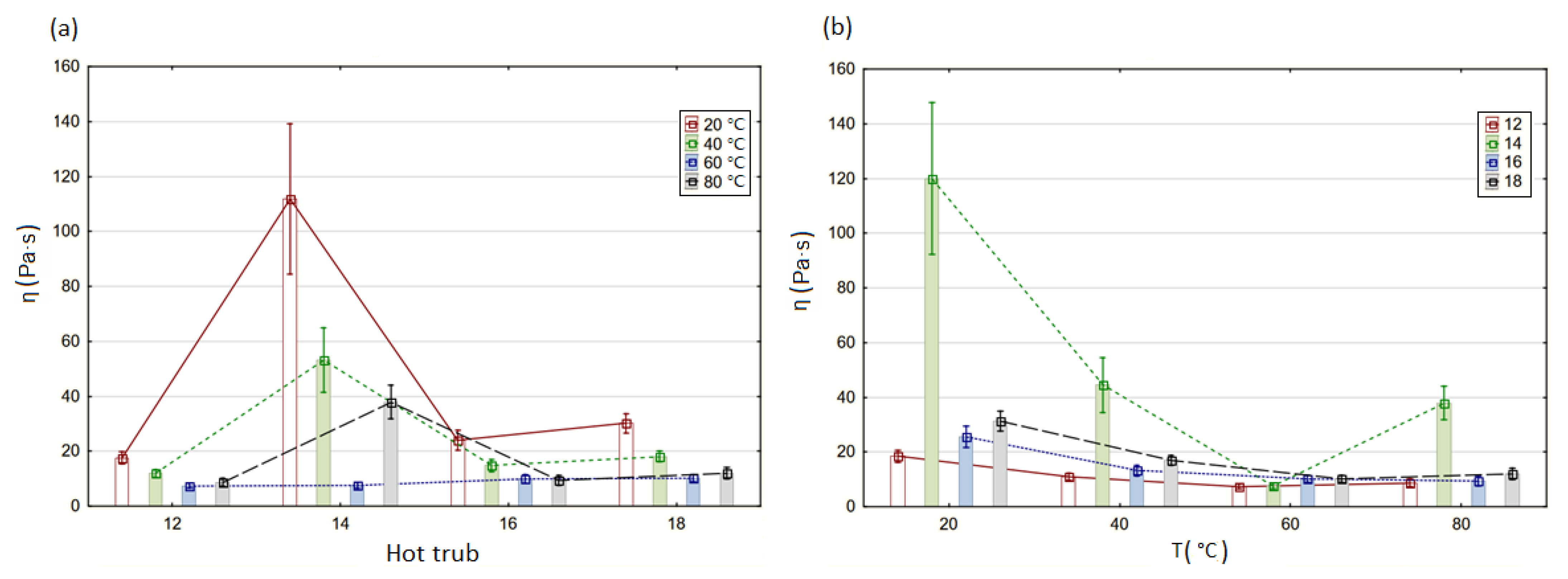
3.1. Non-Newtonian Characteristics
3.2. Time-Dependent Decrease in Viscosity
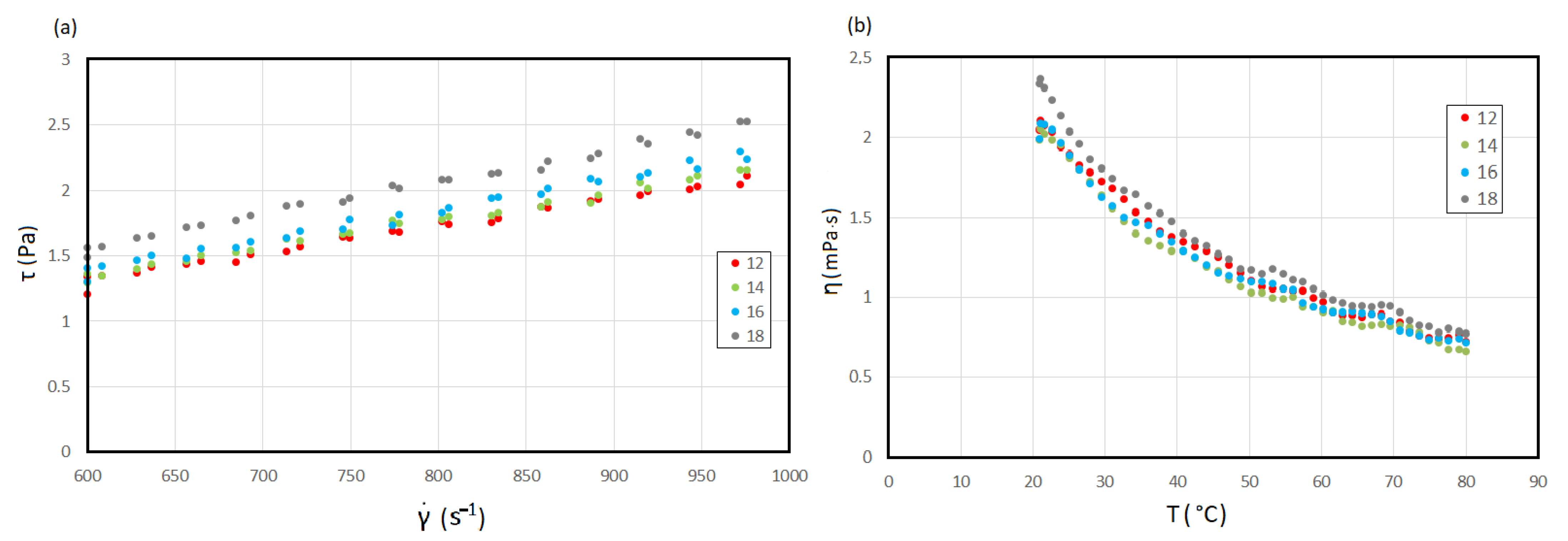
3.3. Wort Viscosity
3.4. Parameter Estimation for Herschel–Bulkley Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| shear rate (s−1) | |
| τ | shear stress (Pa) |
| τ0 | yield stress (Pa) |
| η | viscosity (Pa·s) |
| η∞ | viscosity of infinite shear rate (Pa·s) |
| ηrec | recovery viscosity (Pa·s) |
| ΔE | energy dissipated (mJ) |
| n | flow index (-) |
| k | consistency index (Pa·sn) |
| T | temperature (°C) |
References
- Palmer, J.J. How to Brew: Everything You Need to Know to Brew Beer Right the First Time; Brewers Publications: Boulder, CO, USA, 2006. [Google Scholar]
- Eßlinger, H.M. Handbook of Brewing: Processes, Technology, Markets; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527623488. [Google Scholar] [CrossRef]
- Kühbeck, F.; Schütz, M.; Thiele, F.; Krottenthaler, M.; Back, W. Influence of Lauter Turbidity and Hot Trub on Wort Composition, Fermentation, and Beer Quality. J. Am. Soc. Brew. Chem. 2006, 64, 16–28. [Google Scholar] [CrossRef]
- Kühbeck, F.; Müller, M.; Back, W.; Kurz, T.; Krottenthaler, M. Effect of hot trub and particle addition on fermentation performance of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2007, 41, 711–720. [Google Scholar] [CrossRef]
- Jakubowski, M.; Antonowicz, A.; Janowicz, M.; Sterczyńska, M.; Piepiórka-Stepuk, J.; Poreda, A. An assessment of the potential of Shadow Sizing analysis and Particle Image Velocimetry (PIV) to characterise hot trub morphology. J. Food Eng. 2016, 173, 34–41. [Google Scholar] [CrossRef]
- Lewis, M.J.; Bamforth, C.W. Essays in Brewing Science; Springer Science+Business Media, LLC: Berlin, Germany, 2006; e-ISBN 978-0387-33011-2. [Google Scholar]
- Briggs, D.E.; Boulton, C.; Brooks, P.; Stevens, R. Brewing Science and Practice; Woodhead Publishing Limited: Sawston, UK, 2004; ISBN 0-8493-2547-1. [Google Scholar]
- Narziß, L. Die Technologie der Würzebereitung; Ferdinand EnkeVerlag: Stuttgart, Germany, 1992; Volume 2, pp. 319–320. [Google Scholar]
- Andrews, J.M.H. Brewing. New Technologies; (Red. Bamforth C.W.); Woodhead Publishing: Sawston, UK, 2006; ISBN 978-1-84569-173-8. [Google Scholar]
- Navarro, S.; Pérez, G.; Navarro, G.; Mena, L.; Vela, N. Influence of Fungicide Residues on the Primary Fermentation of Young Lager Beer. J. Agric. Food Chem. 2007, 55, 1295–1300. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing and Malting, 4th ed.; VLB: Berlin, Germany, 2010; ISBN 978-3-921690-64-2. [Google Scholar]
- Long, D.F.; Perivilli, S.V.; Mauger, J.W. Einstein’s Tea Leaf Paradox and Its Relevance to Dissolution Testing. Dissolution Technol. 2014, 21, 17–18. [Google Scholar] [CrossRef]
- European Commission, European Integrated Pollution Prevention and Control Bureau (EIPPCB). Reference Document on Best Available Techniques (BAT) in the Food, Drink and Milk Industries; EIPPCB: Seville, Spain, 2006. [Google Scholar]
- Olajire, A.A. The brewing industry and environmental challenges. J. Clean. Prod. 2020, 256, 102817. [Google Scholar] [CrossRef]
- World Bank Group. Pollution Prevention and Abatement Handbook, Toward Cleaner Production; The World Bank Group: Washington, DC, USA, 1998. [Google Scholar]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Jarosz, R. Biological activity of composts obtained from hop waste generated during the brewing. Biomass Convers. Bioref. 2020, 1–9. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, J.; Krishnamurthy, N.P.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Ooi, C.W. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: Extraction, characterization and kinetic studies. Food Chem. 2019, 296, 47–55. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.J.; Ibrahim, S.A. Food Ingredients and Active Compounds against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- Navarro, S.; Pérez, G.; Navarro, G.; Mena, L.; Vela, N. Decay of Dinitroaniline Herbicides and Organophosphorus Insecticides during Brewing of Lager Beer. J. Food Prot. 2006, 69, 1699–1706. [Google Scholar] [CrossRef]
- Hu, L.; Gastl, M.; Linkmeyer, A.; Hess, M.; Rychlik, M. Fate of enniatins and beauvericin during the malting and brewing process determined by stable isotope dilution assays. LWT Food Sci. Technol. 2014, 56, 469–477. [Google Scholar] [CrossRef]
- Sterczyńska, M.; Stachnik, M.; Kowalczewski, P.; Piepiórka-Stepuk, J. Pesticides as the problem of toxins being found in the food, residue removal with example of beer manufacture. Technol. Prog. Food Process. 2019, 1, 82–88. [Google Scholar]
- McMurrough, I.; Madigan, D.; Kelly, R.J.; O’Rourke, T. Haze formation and shelf-life prediction for lager beer. Food Technol. 1999, 53, 58–62. [Google Scholar]
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of protein–polyphenol haze in beverages. J. Agric. Food Chem. 1996, 44, 1997–2005. [Google Scholar] [CrossRef]
- Siebert, K.J.; Lynn, P.Y. Comparison of polyphenol interactions with polyvinylpolypyrrolidone and haze-active protein. Am. Soc. Brew. Chem. 2006, 56, 24–31. [Google Scholar] [CrossRef]
- Siebert, K.J. Haze formation in beverages. LWT 1998, 39, 987–994. [Google Scholar] [CrossRef]
- Debourg, A. Improvements in organoleptical and physico-chemical stabilities of beer. Wrocław Sch. Ferment. Technol. 1997, 1, 5–29. [Google Scholar]
- Mierczynska-Vasilev, A.; Smith, P.A. Current state of knowledge and challenges in wine clarification. Aust. J. Grape Wine Res. 2015, 21, 615–626. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Okeyinka, O.M.; Oloke, D.A.; Adebisi, W.A.; Ayininuola, G.M. Investigation into the applicability of brewery sludge residue-ash as a base material for geopolymer concrete. Constr. Build. Mater. 2019, 223, 28–32. [Google Scholar] [CrossRef]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Kanagachandran, K.; Jayaratne, R. Utilization potenctial od brewery waste water sediment as an organic fertilizer. J. Inst. Brew. 2006, 112, 92–96. [Google Scholar] [CrossRef]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Suder, A. The Application Potential of Hop Sediments from Beer Production for Composting. Sustainability 2021, 13, 6409. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Mateusz, M.; Zdaniewicz, M. Energy and microbiological evaluation of the effects of adding bulking agents on biodrying of brewery hot trub. Food Bioprod. Process 2021, in press. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Girardi, J.; Cosci, F.; Conti, B. Not just for beer: Evaluation of spent hops (Humulus lupulus L.) as a source of eco-friendly repellents for insect pests of stored foods. J. Pest Sci. 2015, 88, 583–592. [Google Scholar] [CrossRef]
- Tesio, A.Y.; Gómez-Camer, J.L.; Morales, J.; Caballero, A. Simple and Sustainable Preparation of Non-Activated Porous Carbon from Brewing Waste For High-Performance Lithium-Sulfur Batteries. ChemSusChem 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Guidelines on the Use of By-Products and Recommended Waste Management in Agriculture and the Agri-Food Industry; Ministry of Agriculture and Rural Development, Falenty—Warsaw Institute of Technology and Life Sciences: Warsaw, Poland, 2010; p. 103.
- He, R.; Zheng, S.; Chen, H.; Kuang, D. Investigation of the physical and rheological properties of Trinidad lake asphalt modified bitumen. Constr. Build. Mater. 2019, 203, 734–739. [Google Scholar] [CrossRef]
- Kim, M.-G.; Sivagurunathan, P.; Lee, M.-K.; Im, S.; Shin, S.-R.; Choi, C.-S.; Kim, D.-H. Rheological properties of hydrogen fermented food waste. Int. J. Hydrogen Energy 2019, 44, 2239–2245. [Google Scholar] [CrossRef]
- Malczewska, B.; Biczyński, A. Comparison between different models for rheological characterization of sludge from settling tank. J. Water Land Dev. 2017, 34, 191–196. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, Z.; Cui, W.; Wang, Y.; Yang, P. Rheological Properties of Municipal Sewage Sludge: Dependency on Solid Concentration and Temperature. Procedia Environ. Sci. 2016, 31, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-Z.; Wang, R.-K.; Gao, F.-Y.; Zhou, J.; Cen, K.-F. Rheology and thixotropic properties of slurry fuel prepared using municipal wastewater sludge and coal. Chem. Eng. Sci. 2012, 76, 1–8. [Google Scholar] [CrossRef]
- Puplampu, E.; Siebel, M. Minimisation of water use in a Ghanaian brewery: Effects of personnel practices. J. Clean. Prod. 2005, 13, 1139–1143. [Google Scholar] [CrossRef]
- Yang, X.; Berry, T.K.; Foegeding, E.A. Foams Prepared from Whey Protein Isolate and Egg White Protein: 1. Physical, Microstructural, and Interfacial Properties. J. Food Sci. 2009, 74, E259–E268. [Google Scholar] [CrossRef]
- Yang, X.; Foegeding, E.A. The stability and physical properties of egg white and whey protein foams explained based on microstructure and interfacial properties. Food Hydrocoll. 2011, 25, 1687–1701. [Google Scholar] [CrossRef]
- Berski, W.; Ptaszek, A.; Ziobro, R.; Kowalski, G.; Grzesik, M.; Achremowicz, B. Pasting and rheological properties of oat starch and its derivatives. Carbohydr. Polym. 2011, 83, 665–671. [Google Scholar] [CrossRef]
- Żmudziński, D.; Goik, U.; Ptaszek, P. Functional and Rheological Properties of Vicia faba L. Protein Isolates. Biomolecules 2021, 11, 178. [Google Scholar] [CrossRef]
- Sterczyńska, M.; Zdaniewicz, M.; Wolny-Koładka, K. Rheological and Microbiological Characteristics of Hops and Hot Trub Particles Formed during Beer Production. Molecules 2021, 26, 681. [Google Scholar] [CrossRef]
- Hsu, C.-P.; Ramakrishna, S.N.; Zanini, M.; Spencer, N.D.; Isa, L. Roughness-dependent tribology effects on discontinuous shear thickening. Proc. Natl. Acad. Sci. USA 2018, 115, 5117–5122. [Google Scholar] [CrossRef] [Green Version]
- Goode, D.L.; Wijngaard, H.H.; Arendt, E. Mashing with unmalted barley-impact of malted barley and commercial enzyme (Bacillus spp.) additions. Tech. Q.-Master Brew. Assoc. Am. 2005, 42, 184–198. [Google Scholar]
- Schnitzenbaumer, B.; Arendt, E.K. Brewing with up to 40% unmalted oats (Avena sativa) and sorghum (Sorghum bicolor): A review. J. Inst. Brew. 2014, 120, 315–330. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, M.; Wyczalkowski, W.; Poreda, A. Flow in a symmetrically filled whirlpool: CFD modelling and experimental study based on Particle Image Velocimetry (PIV). J. Food Eng. 2015, 145, 64–72. [Google Scholar] [CrossRef]
- Kunz, T.; Müller, C.; Mato-Gonzales, D.; Methner, F.-J. The influence of unmalted barley on the oxidative stability of wort and beer. J. Inst. Brew. 2012, 118, 32–39. [Google Scholar] [CrossRef]
- Baroutian, S.; Eshtiaghi, N.; Gapes, D.J. Rheology of a primary and secondary sewage sludge mixture: Dependency on temperature and solid concentration. Bioresour. Technol. 2013, 140, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Baroutian, S.; Munir, M.T.; Sun, J.; Eshtiaghi, N.; Young, B.R. Rheological characterisation of biologically treated and non-treated putrescible food waste. Waste Manag. 2018, 71, 494–501. [Google Scholar] [CrossRef]
- Senapati, P.K.; Mishra, B.K.; Parida, A. Modeling of viscosity for power plant ash slurry at higher concentrations: Effect of solids volume fraction, particle size and hydrodynamic interactions. Powder Technol. 2010, 197, 1–8. [Google Scholar] [CrossRef]
- Konijn, B.J.; Sanderink, O.B.J.; Kruyt, N.P. Experimental study of the viscosity of suspensions: Effect of solid fraction, particle size and suspending liquid. Powder Technol. 2014, 266, 61–69. [Google Scholar] [CrossRef]
- Houška, M.; Žitný, R. Dynamics of Thixotropic Liquids and Time Dependency; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–63. [Google Scholar] [CrossRef]
- Coussot, P. Rheometry of Pastes, Suspensions and Granular Materials; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Grillet, A.M.; Rao, R.R.; Adolf, D.B.; Kawaguchi, S.; Mondy, L.A. Practical application of thixotropic suspension models. J. Rheol. 2009, 53, 169–189. [Google Scholar] [CrossRef]
- Ovarlez, G.; Mahaut, F.; Deboeuf, S.; Lenoir, N.; Hormozi, S.; Chateau, X. Flows of suspensions of particles in yield stress fluids. J. Rheol. 2015, 59, 1449–1486. [Google Scholar] [CrossRef] [Green Version]
- Dzuy, N.Q.; Boger, D.V. Yield Stress Measurement for Concentrated Suspensions. J. Rheol. 1983, 27, 321–349. [Google Scholar] [CrossRef]
- Zhuang, S.; Shetty, R.; Hansen, M.; Fromberg, A.; Hansen, P.B.; Hobley, T.J. Brewing with 100 % unmalted grains: Barley, wheat, oat and rye. Eur. Food Res. Technol. 2017, 243, 447–454. [Google Scholar] [CrossRef]
- Ramsey, M.S. Rheology, Viscosity, and Fluid Types. In Practical Wellbore Hydraulics and Hole Cleaning; Gulf Publishing Company: Houston, TX, USA, 2019; pp. 217–237. [Google Scholar] [CrossRef]
- Jakubowski, M.; Sterczyska, M.; Matysko, R.; Poreda, A. Simulation and experimental research on the flow inside a whirlpool separator. J. Food Eng. 2014, 133, 9–15. [Google Scholar] [CrossRef]
- Jakubowski, M.; Stachnik, M.; Sterczyńska, M.; Matysko, R.; Piepiórka-Stepuk, J.; Dowgiałło, A.; Ageev, O.V.; Knitter, R. CFD analysis of primary and secondary flows and PIV measurements in whirlpool and whirlpool kettle with pulsatile filling: Analysis of the flow in a swirl separator. J. Food Eng. 2019, 258, 27–33. [Google Scholar] [CrossRef]
- Stachnik, M.; Jakubowski, M. Multiphase model of flow and separation phases in a whirlpool: Advanced simulation and phenomena visualization approach. J. Food Eng. 2020, 274, 109846. [Google Scholar] [CrossRef]
- Askew, K. The Food Navigator Podcast: What Does Coronavirus Mean for the Future of Food? 2020. Available online: https://www.foodnavigator.com/Article/2020/05/15/The-FoodNavigator-Podcast-What-does-Coronavirus-mean-for-the-future-of-food (accessed on 6 June 2021).
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Erzinger, G.S.; Lopes, P.C.; Del Ciampo, L.F.; Zimath, S.C.; Vicente, D.; De Albuquerque, F.M.; Prates, R.C. Bioactive compounds of hops resulting from the discarding of the beer industry in the control of pathogenic bacteria. In Natural Bioactive Compounds; Academic Press: London, UK, 2021; pp. 41–55. [Google Scholar] [CrossRef]
- Omidiji, O.; Okpuzor, J. Enzymic recovery of extract from cold trub derived from brewing with un-malted sorghum (Sorghum bicolor). Process Biochem. 2002, 37, 787–792. [Google Scholar] [CrossRef]


| Extract (°Plato) | Composition of Raw Materials | Code |
|---|---|---|
| 12.5 | 70% malted + 30% unmalted barley | 12 |
| 14.1 | 100% malted barley | 14 |
| 16.1 | 55% malted + 45% unmalted barley | 16 |
| 18.2 | 55% malted + 45% unmalted barley | 18 |
| ηmax (Pa·s) | ||||
| Hot trub | 20 (°C) | 40 (°C) | 60 (°C) | 80 (°C) |
| 12 | 340.8 | 213.9 | 136.7 | 219.6 |
| 14 | 4630.0 | 1970.1 | 919.6 | 979.5 |
| 16 | 559.2 | 341.3 | 223.5 | 330.2 |
| 18 | 557.6 | 310.8 | 215.5 | 320.1 |
| η∞(Pa·s) | ||||
| 12 | 4.1 | 2.9 | 1.9 | 1.3 |
| 14 | 9.1 | 4.8 | 9.8 | 7.3 |
| 16 | 4.2 | 3.0 | 2.4 | 1.5 |
| 18 | 7.4 | 4.0 | 2.5 | 2.0 |
| ηrec (Pa·s) | ||||
| 12 | 157.2 | 103.6 | 84.57 | 62.17 |
| 14 | 557.4 | 271.2 | 490.5 | 383.2 |
| 16 | 158.7 | 92.55 | 110.2 | 66.38 |
| 18 | 277.7 | 200.6 | 111.3 | 105.6 |
| ΔE (mJ) | ||||
| 12 | 20,410.4 | 12,284 | 2735.2 | 7469.6 |
| 14 | 140,960.8 | 76,675.2 | 25,230.4 | 28,922.4 |
| 16 | 39,473.6 | 6729.6 | 1802.4 | 6317.6 |
| 18 | 33,344.8 | 9292.8 | 8056.8 | 10,033.6 |
| τ0 (Pa) | ||||
| 12 | 116.6 | 78.7 | 55.1 | 67.4 |
| 14 | 975.6 | 960.1 | 758.2 | 878.1 |
| 16 | 137.4 | 88.7 | 62.5 | 73.7 |
| 18 | 148.6 | 107.4 | 66.2 | 81.2 |
| Hot Trub | T (°C) | Herschel–Bulkley | ||||
|---|---|---|---|---|---|---|
| Parameters | ||||||
| τ₀ (Pa) | k (Pa·sn) | n (-) | χ2 | R2 | ||
| 12 | 20 | 112.3 | 14.5 | 0.5 | 391.4 | 0.99 |
| 40 | 76.7 | 34.0 | 0.5 | 259.3 | 0.99 | |
| 60 | 54.4 | 8.9 | 0.5 | 71.4 | 0.99 | |
| 80 | 66.6 | 13.4 | 0.6 | 243 | 0.99 | |
| 14 | 20 | 982.3 | 322.9 | 0.7 | 645.4 | 0.99 |
| 40 | 967.2 | 108.5 | 0.6 | 560.1 | 0.99 | |
| 60 | 767.5 | 72.8 | 0.4 | 717.2 | 0.99 | |
| 80 | 877.9 | 132.6 | 0.5 | 327.8 | 0.99 | |
| 16 | 20 | 135.6 | 18.5 | 0.7 | 114.3 | 0.99 |
| 40 | 89.5 | 19.2 | 0.6 | 528.2 | 0.99 | |
| 60 | 62.2 | 8.8 | 0.5 | 88.8 | 0.99 | |
| 80 | 73.3 | 18.2 | 0.6 | 118.7 | 0.99 | |
| 18 | 20 | 146.8 | 40.7 | 0.7 | 126.1 | 0.99 |
| 40 | 107.1 | 16.7 | 0.6 | 479.1 | 0.99 | |
| 60 | 66.06 | 12.0 | 0.5 | 67.3 | 0.99 | |
| 80 | 82.4 | 14.6 | 0.6 | 379.4 | 0.99 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachnik, M.; Sterczyńska, M.; Smarzewska, E.; Ptaszek, A.; Piepiórka-Stepuk, J.; Ageev, O.; Jakubowski, M. Rheological Properties of Industrial Hot Trub. Materials 2021, 14, 7162. https://doi.org/10.3390/ma14237162
Stachnik M, Sterczyńska M, Smarzewska E, Ptaszek A, Piepiórka-Stepuk J, Ageev O, Jakubowski M. Rheological Properties of Industrial Hot Trub. Materials. 2021; 14(23):7162. https://doi.org/10.3390/ma14237162
Chicago/Turabian StyleStachnik, Marta, Monika Sterczyńska, Emilia Smarzewska, Anna Ptaszek, Joanna Piepiórka-Stepuk, Oleg Ageev, and Marek Jakubowski. 2021. "Rheological Properties of Industrial Hot Trub" Materials 14, no. 23: 7162. https://doi.org/10.3390/ma14237162
APA StyleStachnik, M., Sterczyńska, M., Smarzewska, E., Ptaszek, A., Piepiórka-Stepuk, J., Ageev, O., & Jakubowski, M. (2021). Rheological Properties of Industrial Hot Trub. Materials, 14(23), 7162. https://doi.org/10.3390/ma14237162









