Preparation and Properties of Modified Phenylethynyl Terminated Polyimide with Neodymium Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
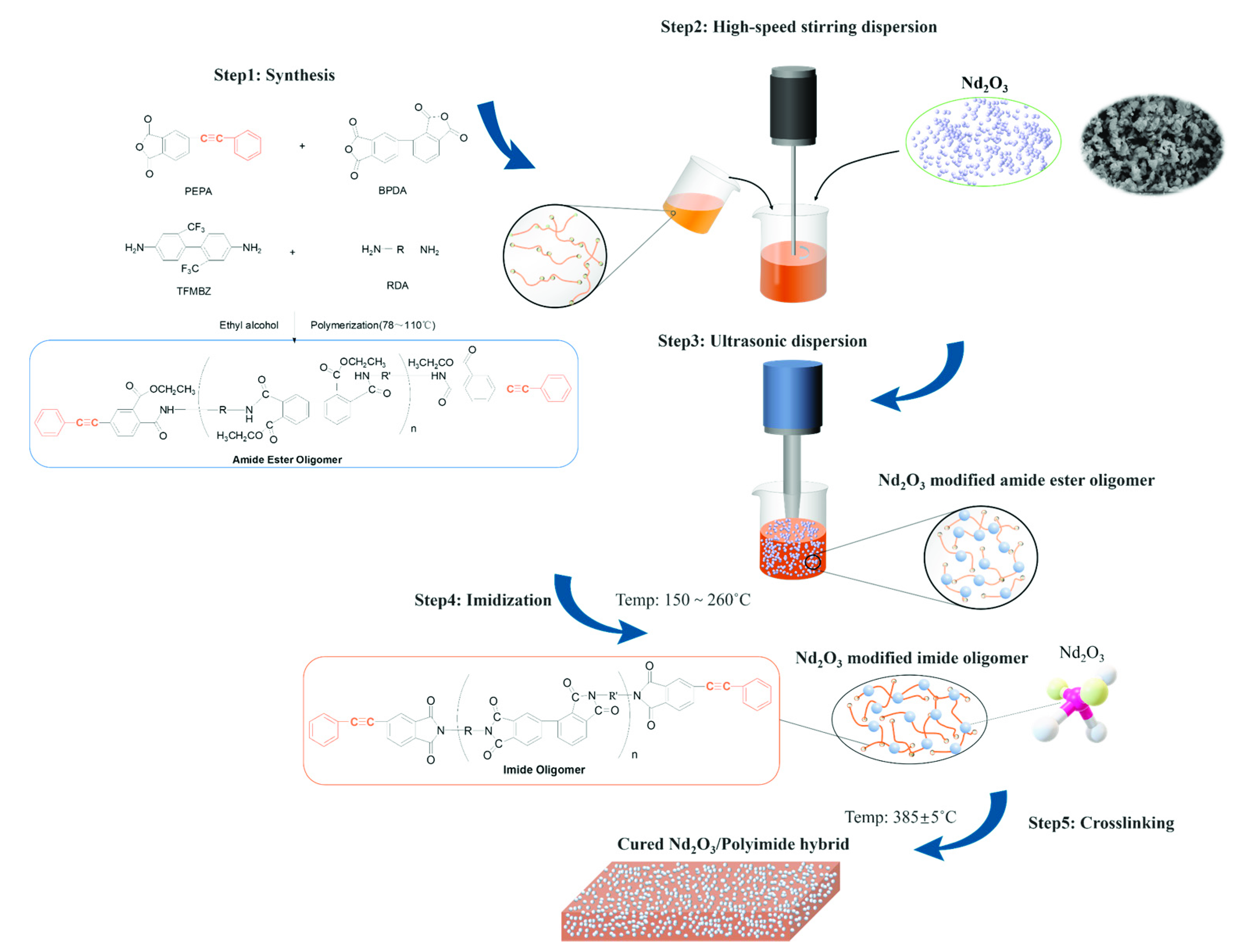
2.2. Preparation of Nd2O3 Modified Polyimide Resin
2.3. Characterization
3. Results and Discussion
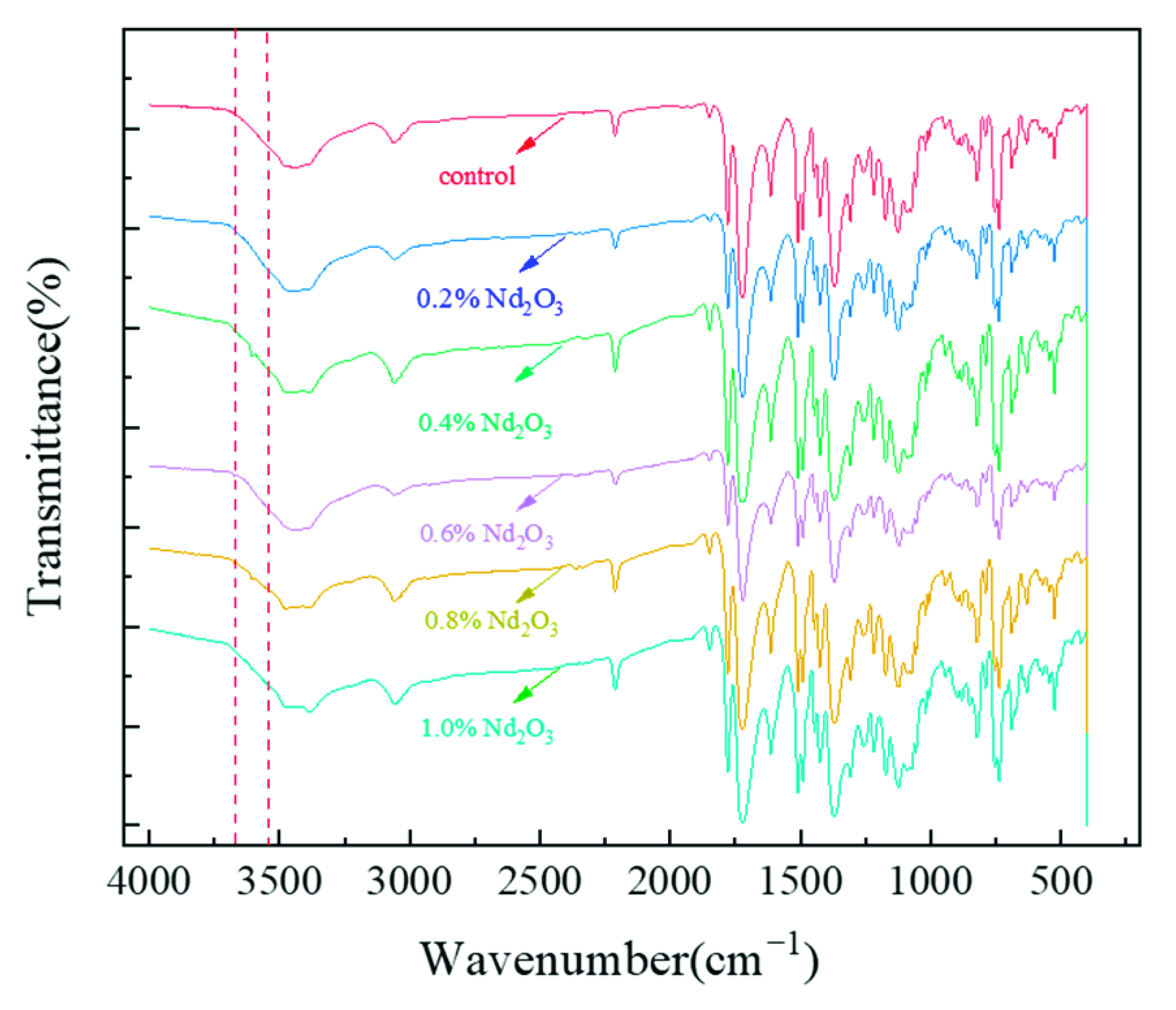
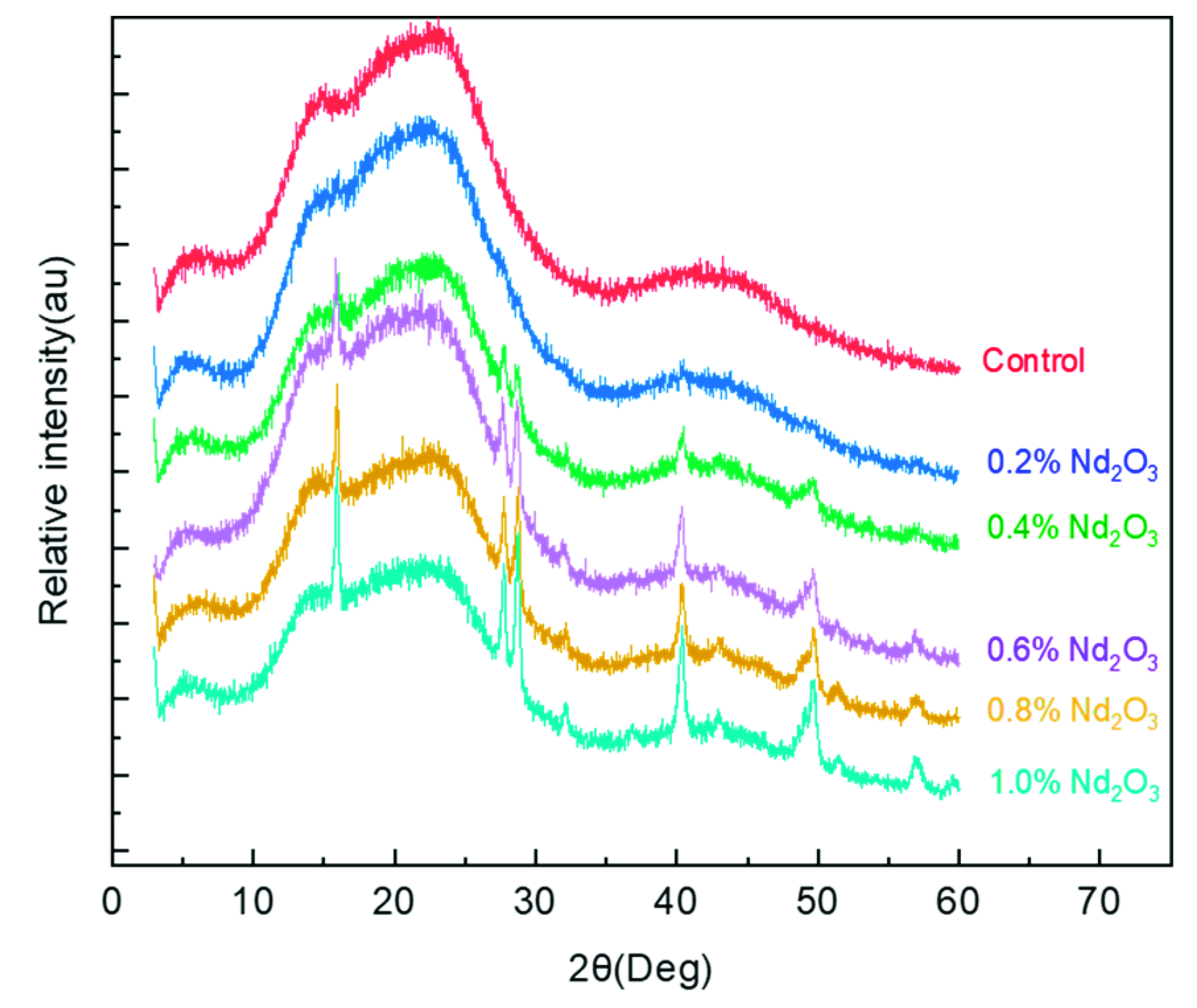
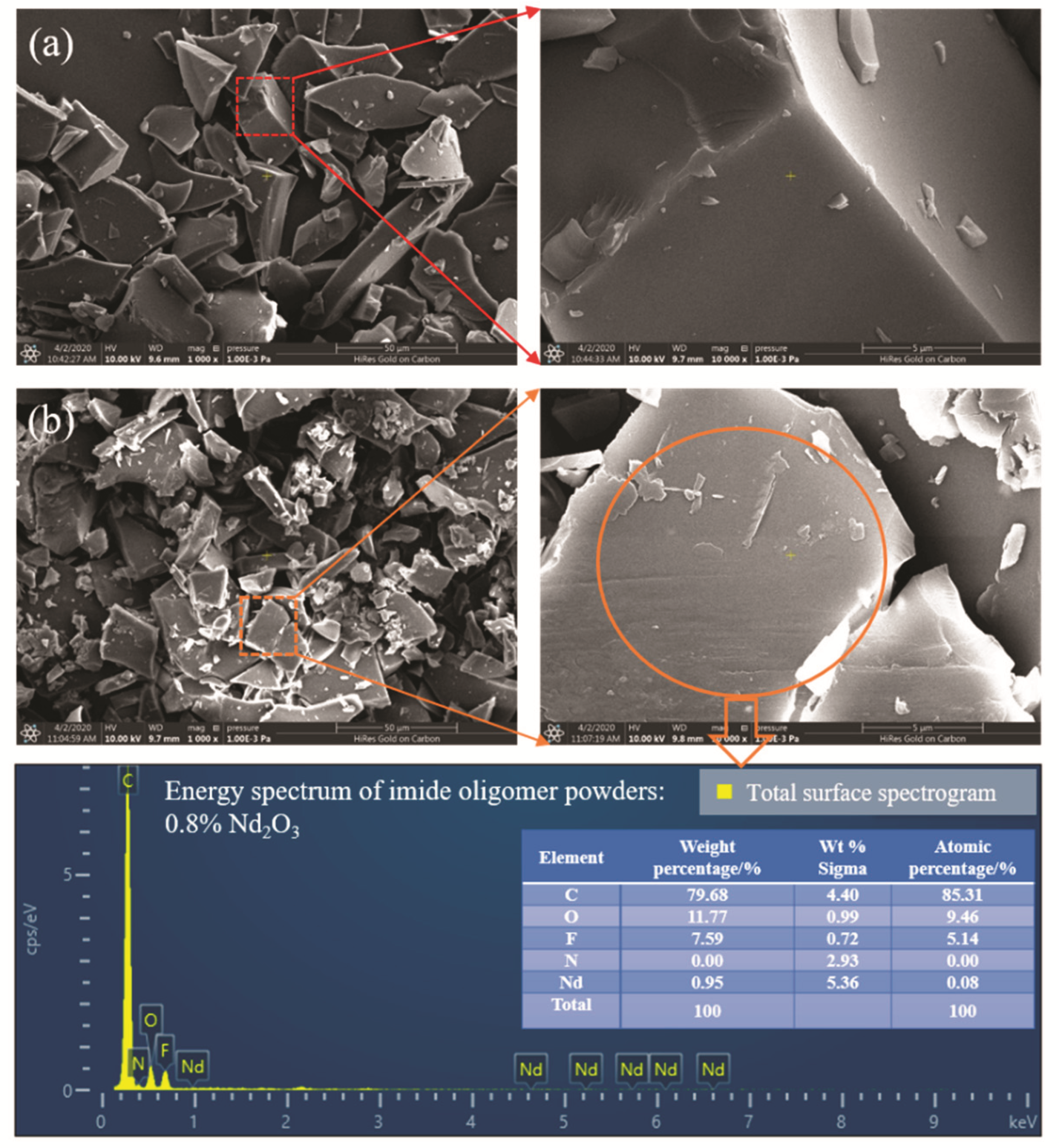
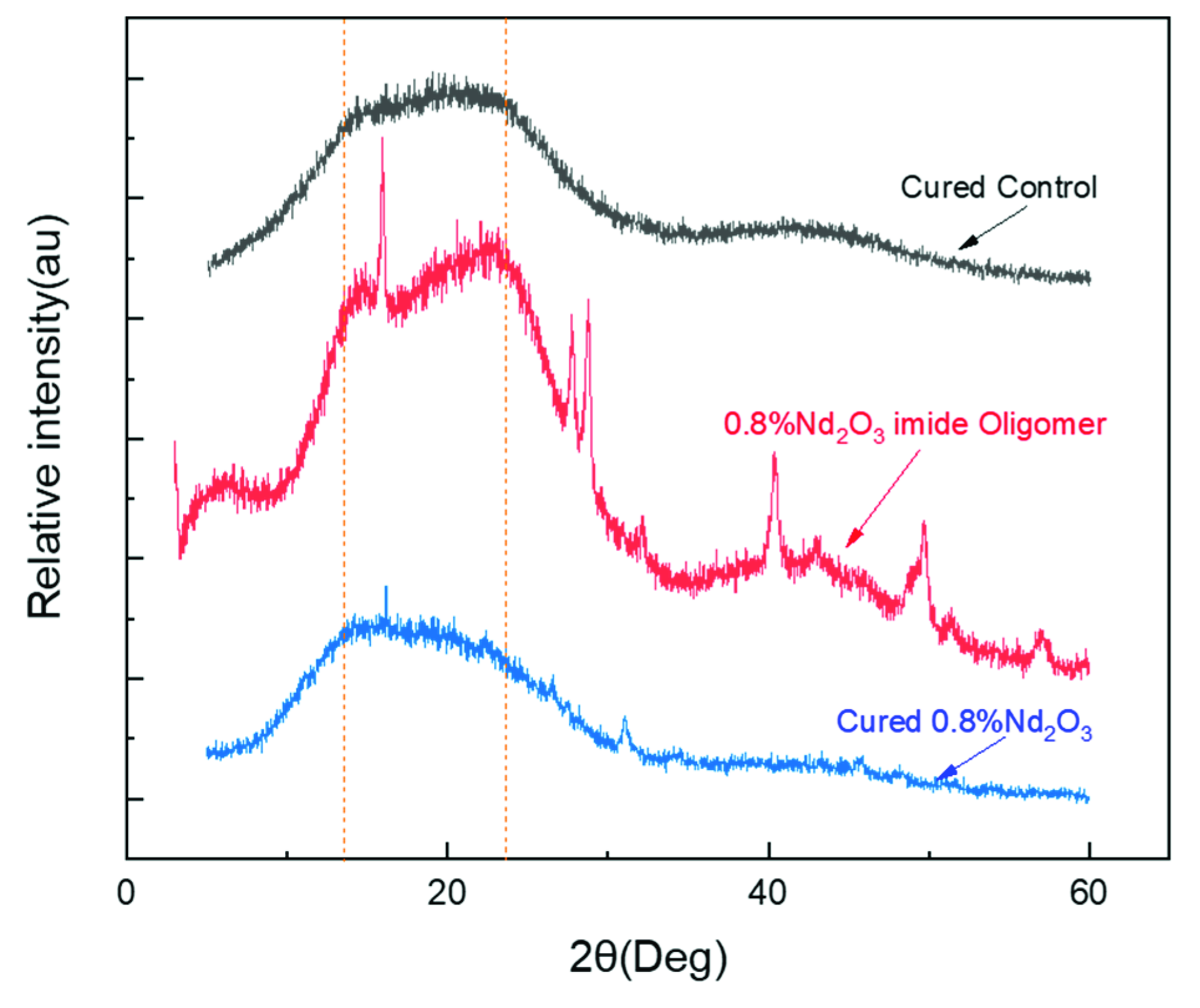
3.1. Structure Characterization of Nd2O3 Modified Imide Oligomer
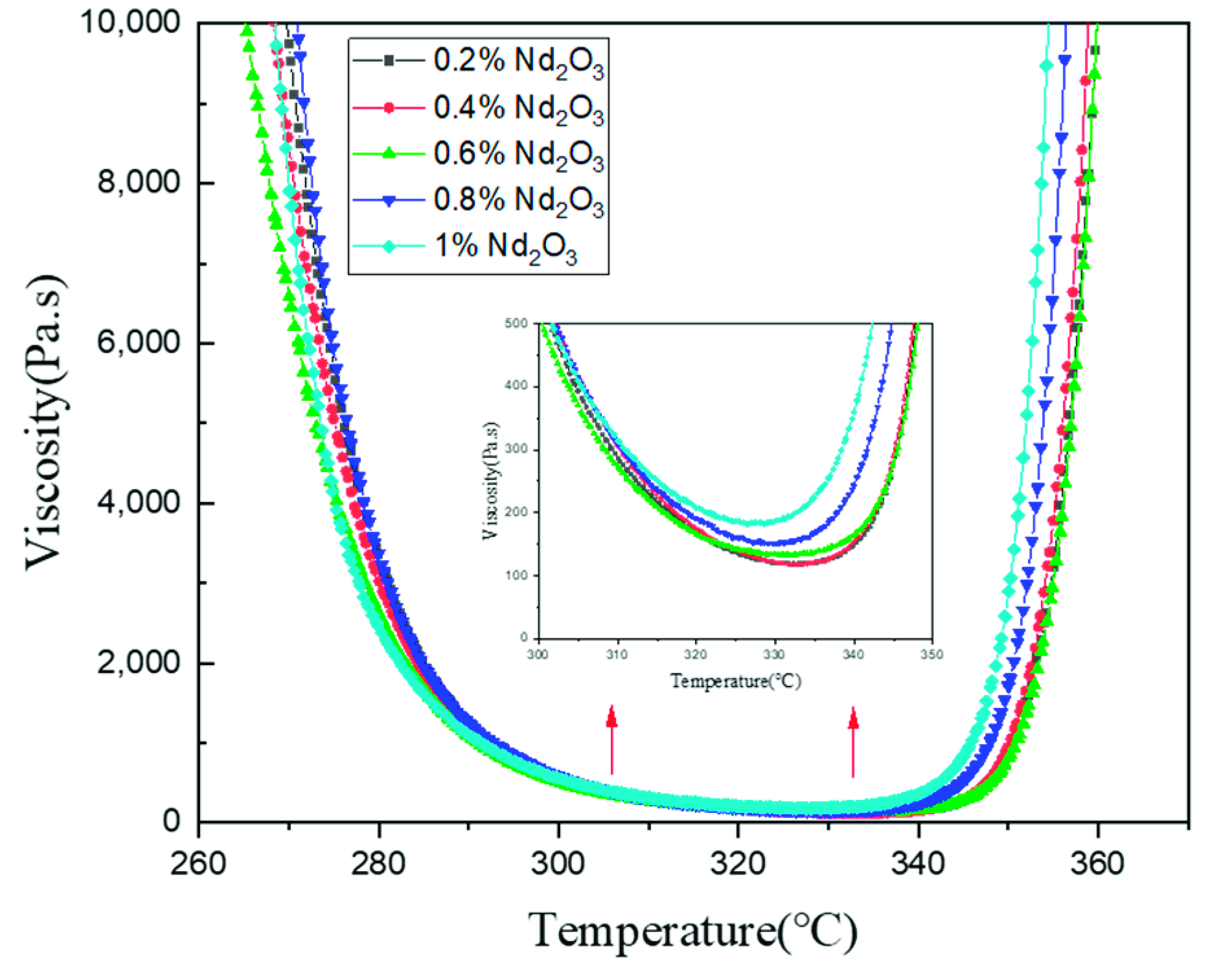
3.2. Thermomechanical Properties of Modified Polyimides
3.3. Thermal Decomposition Properties of the Modified Polyimides
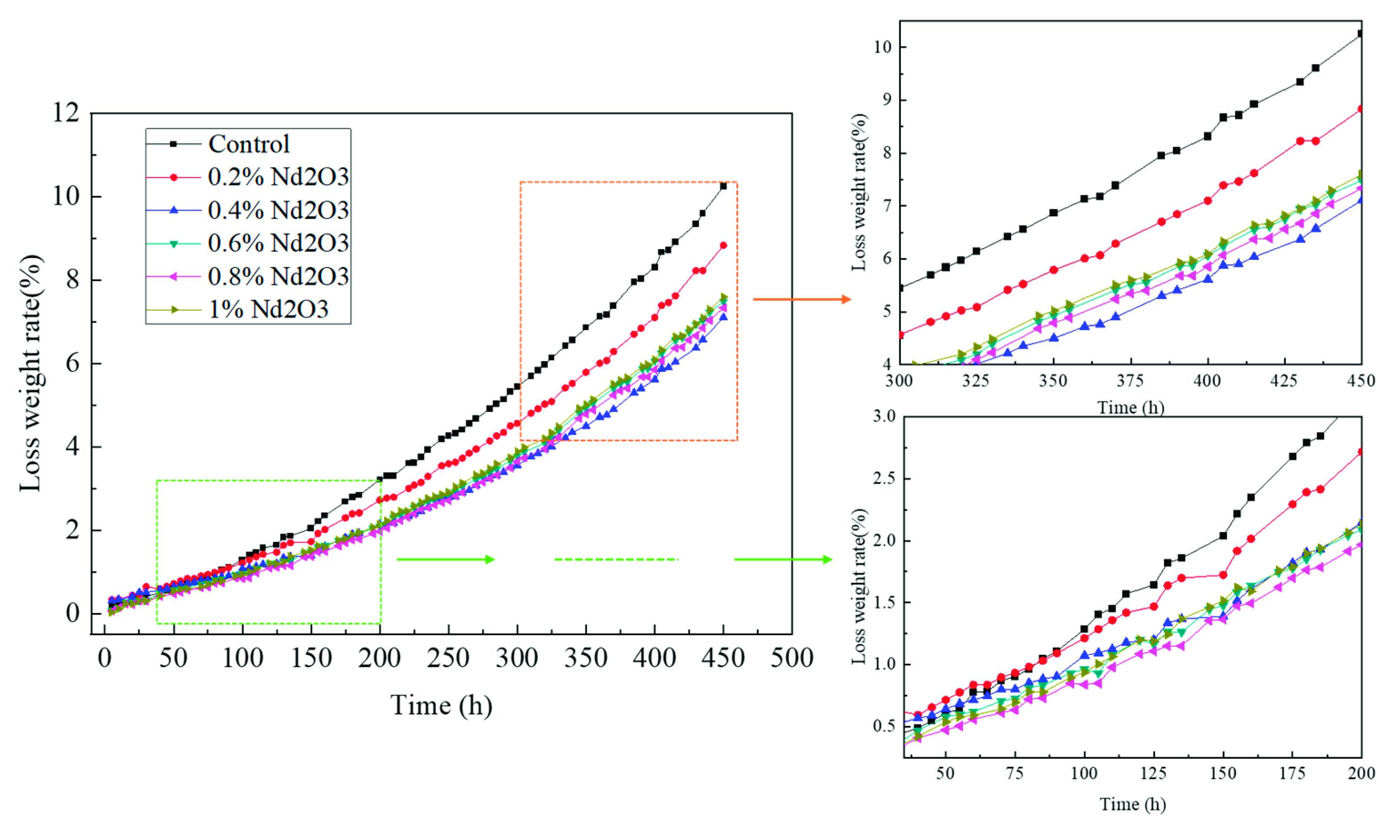
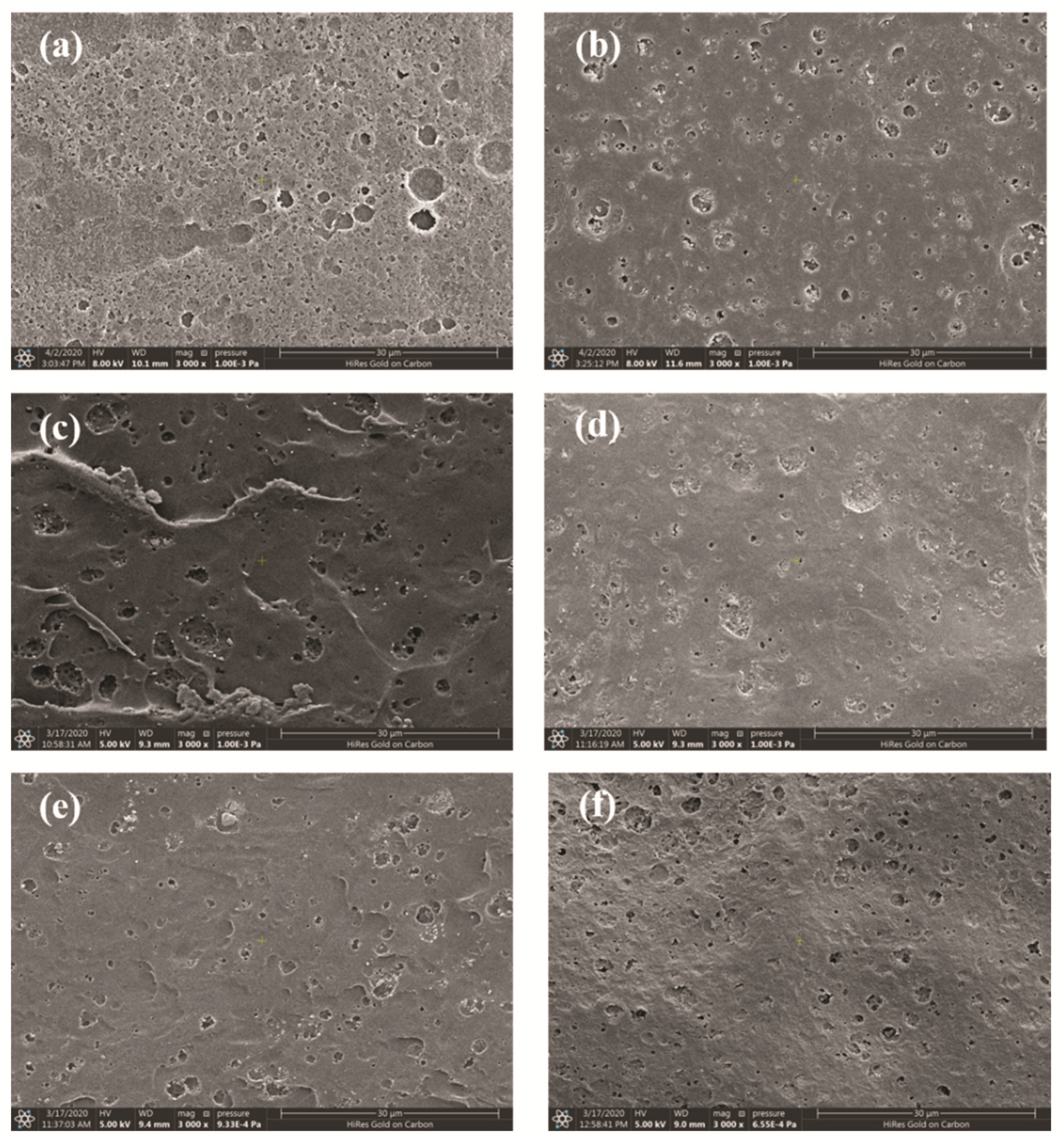
3.4. Thermal Oxidative Stability of the Modified Polyimides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Li, N.; Li, S.; Li, X.; Liu, Z.; Cheng, S.; Wang, K.; Du, S. Effect of polyetherimide sizing on surface properties of carbon fiber and interfacial strength of carbon fiber/polyetheretherketone composites. Polym. Compos. 2021, 42, 931–943. [Google Scholar] [CrossRef]
- Çakir, M.; Akin, E. Characterization of carbon fiber-reinforced thermoplastic and thermosetting polyimide matrix composites manufactured by using various synthesized PI precursor resins. Compos. Part B Eng. 2022, 231, 109559. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Zhao, Z.; Wang, G.; Zhan, S.; Ding, J.; Liu, X.; Guo, Y.; Zhou, H.; Zhao, T. Improved toughness of phthalonitrile composites through synergistic toughening methods. Compos. Commun. 2021, 26, 100779. [Google Scholar] [CrossRef]
- Alston, W.B.; Scheiman, D.A.; Sivko, G.S. A comparison study: The new extended shelf life isopropyl ester PMR technology versus the traditional methyl ester PMR approach. J. Appl. Polym. Sci. 2010, 99, 3549–3564. [Google Scholar] [CrossRef]
- Hasegawa, M.; Nomura, R. Thermal-and solution-processable polyimides based on mellophanic dianhydride and their applications as heat-resistant adhesives for copper-clad laminates. React. Funct. Polym. 2011, 71, 109–120. [Google Scholar] [CrossRef]
- Çakir, M.; Akin, E. Mechanical properties of low-density heat-resistant polyimide-based advanced composite sandwich panels. Polym. Compos. 2022, 43, 827–847. [Google Scholar] [CrossRef]
- Wang, J.; Yi, X.S. Preparation and the properties of PMR-type polyimide composites with aluminum nitride. J. Appl. Polym. Sci. 2010, 89, 3913–3917. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Smith, J.G., Jr. Chemistry and properties of imide oligomers end-capped with phenylethynylphthalic anhydrides. Polymer 1994, 35, 4857–4864. [Google Scholar] [CrossRef]
- Bryant, R.G.; Jensen, B.J.; Hergenrother, P.M. Chemistry and properties of a phenylethynyl-terminated polyimide. J. Appl. Polym. Sci. 1996, 59, 1249–1254. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Aoki, F. Properties of Asymmetric BPDA based phenylethynyl terminated thermosetting polyimide and its composite. J. Photopolym. Sci. Technol. 2006, 19, 269–272. [Google Scholar] [CrossRef][Green Version]
- Yu, P.; Wang, Y.; Yu, J.R.; Zhu, J.; Hu, Z. Synthesis and characterization of phenylethynyl-terminated polyimide oligomers derived from 2,3,3′,4′-diphenyl ether tetracarboxylic acid dianhydride and 3,4′-oxydianiline. Chin. J. Polym. Sci. 2016, 34, 122–134. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Lan, B.; Mo, S.; Zhai, L.; He, M.; Fan, L. High Thermally Stable and Melt Processable Polyimide Resins Based on Phenylethynyl-Terminated Oligoimides Containing Siloxane Structure. Materials 2020, 13, 3742. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wang, Y.; Yu, J.R.; Zhu, J.; Hu, Z. Phenylethynyl-terminated polyimide oligomers with low viscosity and good thermal properties. Mater. Sci. Forum. 2016, 848, 167–173. [Google Scholar] [CrossRef]
- Xiao, T.J.; Gao, S.Q.; Hu, A.J.; Wang, X.C.; Yang, S.Y. Thermosetting polyimides with improved impact toughness and excellent thermal and thermo-oxidative stability. High Perform. Polym. 2001, 13, 287–300. [Google Scholar] [CrossRef]
- Shi, Z.; Hasegawa, M.; Shindo, Y.; Yokata, R.; He, F.; Yamaguchi, H.; Ozawa, H. Thermo-processable polyimides with high thermo-oxidative stability as derived from oxydiphthalic anhydride and bisphenol A type dianhydride. High Perform. Polym. 2000, 12, 377–394. [Google Scholar] [CrossRef]
- Torrecillas, R.; Baudry, A.; Dufay, J.; Mortaigne, B. Thermal degradation of high performance polymers-influence of structure on polyimide thermostability. Polym. Degrad. Stab. 1996, 54, 267–274. [Google Scholar] [CrossRef]
- Serafini, T.T.; Delvigs, P.; Lightsey, G.R. Thermally stable polyimides from solutions of monomeric reactants. J. Appl. Polym. Sci. 1972, 16, 905–915. [Google Scholar] [CrossRef]
- Hou, T.H.; Wilkinson, S.P.; Johnston, N.J.; Pater, R.H.; Schneider, T. Processing and properties of IM7/LARC™-RP46 polyimide composites. High Perform. Polym. 1996, 8, 491–505. [Google Scholar] [CrossRef]
- Chuang, K.C.; Bowles, K.J.; Papadopoulos, D.S.; DeNise, H.G.; Linda, M.G. A high Tg PMR polyimide composites (DMBZ-15). J. Adv. Mater. 2001, 33, 33–38. [Google Scholar]
- Connell, J.W.; Smith, J.J.; Hergenrother, P.M.; Criss, J.M. High Temperature Transfer Molding Resins: Laminate Properties of PETI-298 and PETI-330. High Perform. Polym. 2003, 15, 375–394. [Google Scholar] [CrossRef]
- Connell, J.W.; Smith, J.J.; Hergenrother, P.M. Criss High Temperature Transfer Molding Resin: Preliminary Composite Properties of PETI-375. Int. SAMPE Symp. Exhi. 2004, 5, 16–20. [Google Scholar]
- Whitley, K.S.; Collins, T.J. Mechanical Properties of T650-35/AFR-PE-4 at Elevated Temperatures for Lightweight Aeroshell Designs. In Proceedings of the 47th AIAA/ASME/ASCE/AHS/ASC SDM Conference, Newport, RI, USA, 1–4 May 2006; pp. 2006–2202. [Google Scholar]
- Ogasawara, T.; Takashi, I.; Yokota, R.; Ozawa, H.; Taguchi, M.; Sato, R.; Shigenari, Y.; Miyagawa, K. Processing and properties of carbon fiber reinforced triple-A Polyimide (Tri-A PI) matrix composites. Adv. Compos. Mater. 2003, 11, 277–286. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Influence of different ratios of a-ODPA/a-BPDA on the properties of phenylethynyl terminated polyimide. J. Polym. Res. 2018, 25, 110. [Google Scholar] [CrossRef]
- Ogasawara, T.; Ishida, Y.; Ishikawa, T.; Yokota, R. Characterization of multi-walled carbon nanotube/phenylethynyl terminated polyimide composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 67–74. [Google Scholar] [CrossRef]
- Lebrón-Colón, M.; Meador, M.A.; Gaier, J.R.; Solá, F.; McCorkle, L.S. Reinforced thermoplastic polyimide with dispersed functionalized single wall carbon nanotubes. ACS Appl. Mater. Interfaces 2010, 2, 669–676. [Google Scholar] [CrossRef]
- Chou, W.J.; Wang, C.C.; Chen, C.Y. Thermal behaviors of polyimide with plasma-modified carbon nanotubes. Polym. Degrad. Stab. 2008, 93, 745–752. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.; Li, H.; Zhang, S.; Tan, Z. Thermal stability of several polyaniline/rare earth oxide composites. J. Therm. Anal. Calorim. 2014, 115, 259. [Google Scholar] [CrossRef]
- Babanzadeh, S.; Mehdipour-Ataei, S.; Mahjoub, A.R. Effect of nanosilica on the dielectric properties and thermal stability of polyimide/SiO2 nanohybrid. Des. Monomers Polym. 2013, 16, 417–424. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Gulii, N.S.; Teplonogova, M.A.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Interplay of polymer matrix and nanosized Redox dopant with regard to thermo-oxidative and pyrolytic stability: CeO2 nanoparticles in a milieu of aromatic polyimides. Mater. Today Commun. 2020, 22, 100803. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, J.; Feng, J.; Li, M.; Hu, Y.; Hao, W.; Feng, F. Study on the use of rare earth stabilizer as poly (vinyl chloride) stabilizer. J. Vinyl Addit. Technol. 2020, 26, 536–547. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, W.; Wu, Z.; Tang, Y.; Huang, Y.; Ding, X.T.; Chen, D. Poly (styrene-alt-maleic anhydride) ionic salt functionalized reduced graphene oxide doped with rare earth ions for anti-corrosion performance enhancement. J. Appl. Polym. Sci. 2021, 138, 50666. [Google Scholar] [CrossRef]
- Xiao, W.; Li, X.; Guo, H.; Wang, Z.; Yang, B.; Wu, X. Preparation and properties of composite polymer electrolyte modified with nano-size rare earth oxide. J. Cent. South Univ. 2012, 19, 3378–3384. [Google Scholar] [CrossRef]
- Phani, K.K.; Niyogi, S.K. Elastic modulus-porosity relation in polycrystalline rare-earth oxides. J. Am. Ceram. Soc. 1987, 70, 362–366. [Google Scholar] [CrossRef]
- Dong, B.; Cao, B.; He, Y.; Liu, Z.; Li, Z.; Feng, Z. Temperature sensing and in vivo imaging by molybdenum sensitized visible upconversion luminescence of rare-earth oxides. Adv. Mater. 2012, 24, 1987–1993. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Z.; Wang, J.; Li, Y.; Tan, Z. Thermal stability of several polyaniline/rare earth oxide composites (I): Polyaniline/CeO2 composites. J. Therm. Anal. Calorim. 2012, 107, 1199–1203. [Google Scholar] [CrossRef]
- Feng, C.; Liang, M.; Zhang, Y.; Jiang, J.; Huang, J.; Liu, H. Synergism effect of CeO2 on the flame retardant performance of intumescent flame retardant polypropylene composites and its mechanism. J. Anal. Appl. Pyrolysis 2016, 122, 405–414. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, Y.; Liu, S.; Chi, Z.; Xu, J. Synergistic effect of La2O3 on the flame retardant properties and the degradation mechanism of a novel PP/IFR system. Polym. Degrad. Stab. 2012, 97, 707–714. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Liu, W. Tribological behavior of LaF3 nanoparticles as additives in poly-alpha-olefin. Ind. Lubr. Tribol. 2013, 65, 226–235. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Shi, Y.; Lu, X. A study on the tribological properties of self-assembled method treated nano-La2O3 filled PTFE/PEEK composites. J. Reinf. Plast. Compos. 2009, 28, 645–655. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.X.; Huang, Z.; Zhang, S.; Li, Y.; Han, L.; Tan, Z. Effect Nd2O3 content on electrochemical performance of polyaniline/Nd2O3 composites. Polym. Adv. Technol. 2014, 25, 1163–1168. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Gotoh, H. Basic properties of rare earth oxides. Appl. Catal. A 2009, 356, 57–63. [Google Scholar] [CrossRef]
- Bian, L.J.; Qian, X.F.; Yin, J.; Lu, Q.H.; Liu, L.; Zhu, Z.K. Preparation and properties of rare earth oxide/polyimide hybrids. Polym. Test. 2002, 21, 841–845. [Google Scholar] [CrossRef]
- Falcao, E.A.; Aguiar, L.W.; Guo, R.; Bhalla, A.S. Optical absorption of Nd2O3-Doped polyvinylidene fluoride films. Mater. Chem. Phys. 2021, 258, 123904. [Google Scholar] [CrossRef]
- Nagao, M.; Hamano, H.; Hirata, K.; Kumashiro, R.; Kuroda, Y. Hydration process of rare-earth sesquioxides having different crystal structures. Langmuir 2003, 19, 9201. [Google Scholar] [CrossRef]
- Zhang, P.; Bao, J.; Jiang, Z.; Dong, B.; Chen, X. Enhancing thermo-oxidative stability of thermoset polyimide composites using nano neodymium oxide particles. J. Mater. Res. Technol. 2021, 14, 2638–2649. [Google Scholar] [CrossRef]
- Hamano, H.; Kuroda, Y.; Yoshikawa, Y.; Nagao, M. Adsorption of water on Nd2O3: Protecting a Nd2O3 sample from hydration through surface fluoridation. Langmuir 2000, 16, 6961. [Google Scholar] [CrossRef]


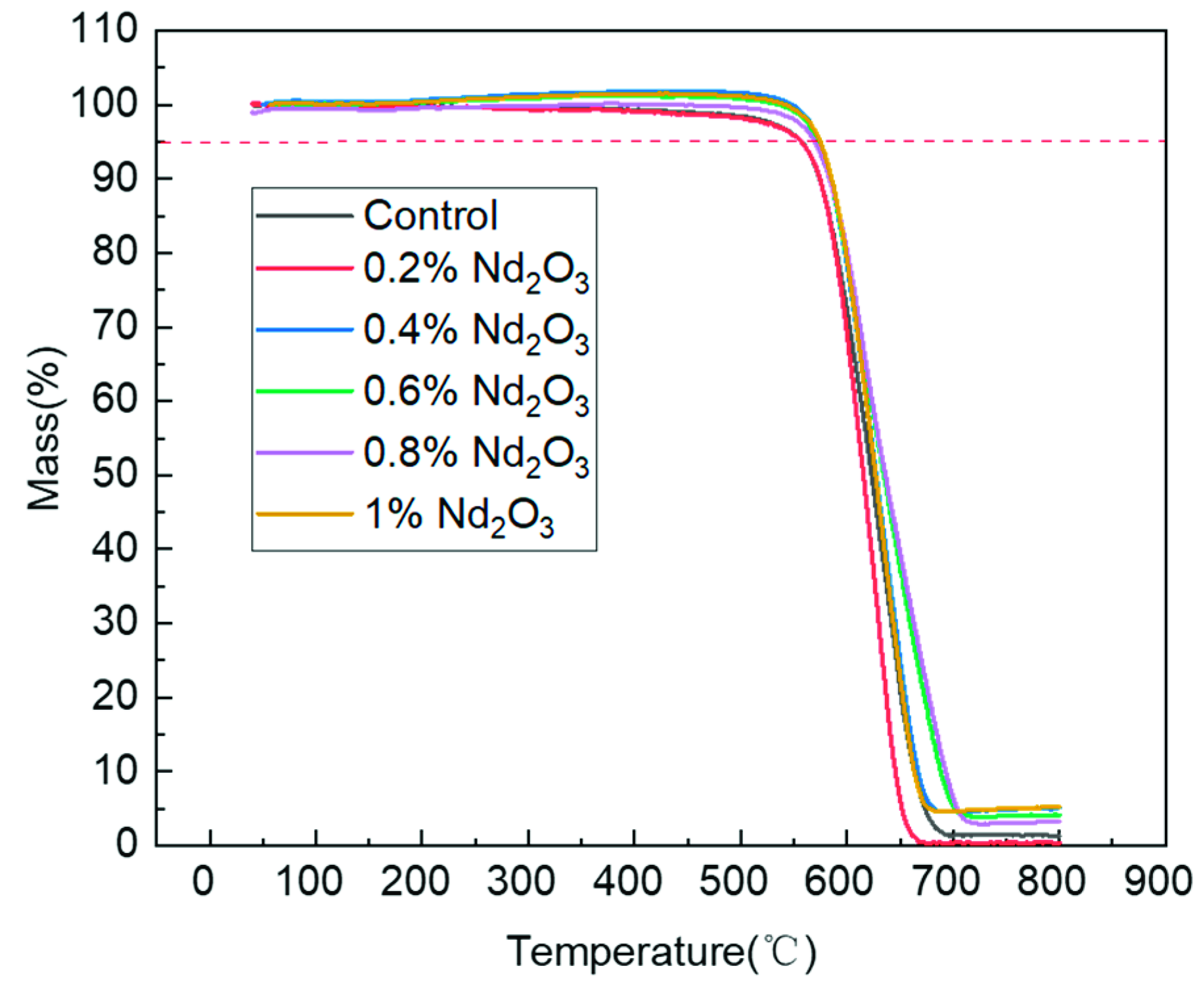

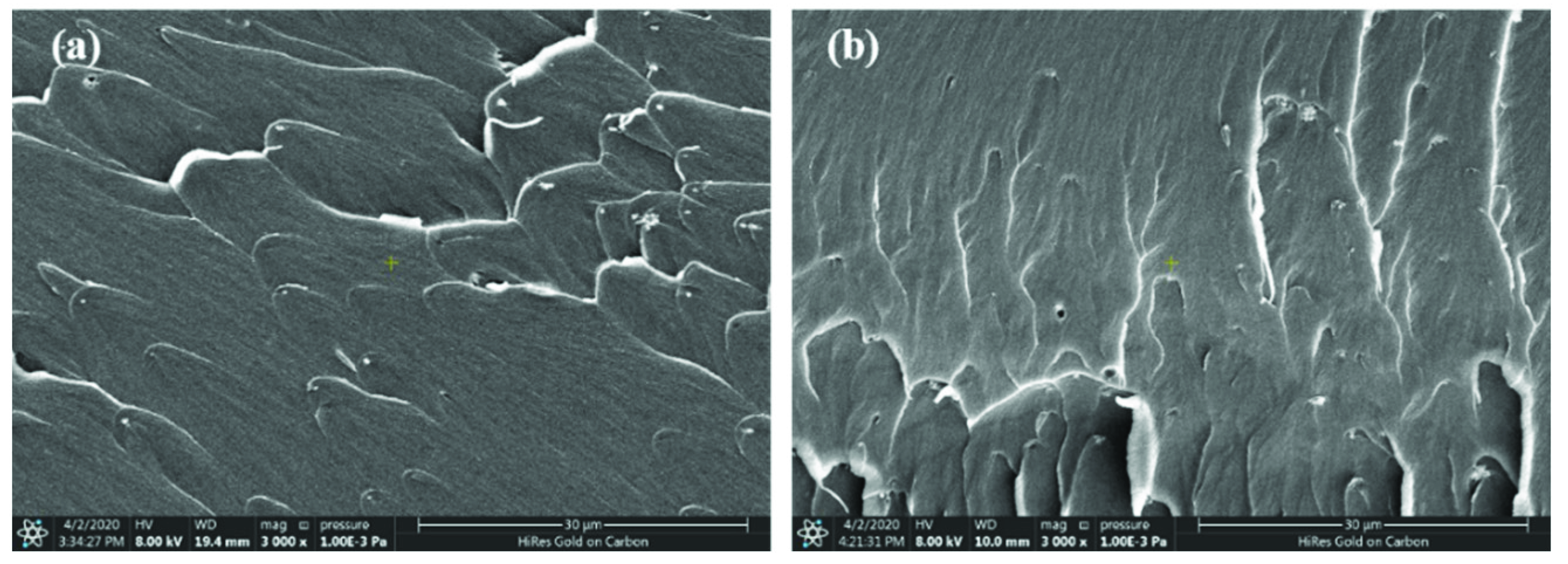
| Content of Nd2O3/% | Td5% (Air)/°C | Td10% (Air)/°C |
|---|---|---|
| 0 | 557 | 574 |
| 0.2 | 557 | 574 |
| 0.4 | 574 | 584 |
| 0.6 | 573 | 584 |
| 0.8 | 570 | 584 |
| 1.0 | 575 | 585 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, H.; Yao, Y.; Yang, T.; Sun, J.; Zhong, X.; Bao, J.; Zhao, Y.; Chen, X. Preparation and Properties of Modified Phenylethynyl Terminated Polyimide with Neodymium Oxide. Materials 2022, 15, 4148. https://doi.org/10.3390/ma15124148
Zhang P, Liu H, Yao Y, Yang T, Sun J, Zhong X, Bao J, Zhao Y, Chen X. Preparation and Properties of Modified Phenylethynyl Terminated Polyimide with Neodymium Oxide. Materials. 2022; 15(12):4148. https://doi.org/10.3390/ma15124148
Chicago/Turabian StyleZhang, Peng, Hansong Liu, Yilun Yao, Tao Yang, Jinsong Sun, Xiangyu Zhong, Jianwen Bao, Yan Zhao, and Xiangbao Chen. 2022. "Preparation and Properties of Modified Phenylethynyl Terminated Polyimide with Neodymium Oxide" Materials 15, no. 12: 4148. https://doi.org/10.3390/ma15124148
APA StyleZhang, P., Liu, H., Yao, Y., Yang, T., Sun, J., Zhong, X., Bao, J., Zhao, Y., & Chen, X. (2022). Preparation and Properties of Modified Phenylethynyl Terminated Polyimide with Neodymium Oxide. Materials, 15(12), 4148. https://doi.org/10.3390/ma15124148







