Abstract
Perovskite-type catalysts were widely used in the field of automobile exhaust purification due to their inherent physicochemical properties and excellent doping characteristics. A series of La1−xMxCo1−yNyO3 (M = Ce, Ca; N = Fe, Mn) perovskite-type catalyst samples were prepared by sol-gel method for the four-way purification of PM, NOx, CO, and HC emitted by diesel exhaust. The activity of catalyst samples was tested by simulation experiments and hydrogen temperature-programmed reduction (H2-TPR). Catalyst samples were characterized by means of XRD, FT-IR, SEM, BET, and XPS analysis. The results demonstrated that the perovskite-type catalyst samples with a particle pore size of 3–5 μm can be prepared by sol-gel method. When A-site of LaCoO3 perovskite-type oxide was doped by cerium ions, the catalyst samples produced small distortion. The doping of cerium ions to A-site was more conducive to the four-way purification of diesel exhaust than calcium ions. La0.8Ce0.2CoO3 perovskite-type samples showed the best purification efficiency, and the purification efficiencies of PM, NOx, CO, and HC were 90%, 85%, 94%, and 100%, respectively. When the B-site of La0.8Ce0.2CoO3 perovskite was doped with iron ions, the purification efficiency of catalyst samples for PM and NOx can be further enhanced. When the B-site of La0.8Ce0.2CoO3 perovskite was doped with manganese ions, the purification efficiency of the catalyst samples for PM can be further enhanced. It can be seen from the simulation experiments that La0.8Ce0.2Co0.7Fe0.3O3 perovskite was the best doping amount, and the purification efficiencies of PM, NOx, CO, and HC were 95%, 92%, 94%, and 100%, respectively.
1. Introduction
The pollutants emitted by diesel exhaust mainly include particulate matter (PM), nitrogen oxides (NOx), carbon monoxide (CO), and hydrocarbons (HC) [1]. Among them, the micron PM emitted from diesel engine is an important factor causing haze weather, which can seriously damage the human respiratory system, and even lead to the increase of lung cancer incidence rate. NOx emission is the main cause of acid rain. The combination of CO and human hemoglobin will cause headache, syncope, and even endanger human life. HC causes serious damage to the human respiratory, nervous, and hematopoietic systems, which mixed with NOx under light conditions may form photochemical smog [2,3,4]. Since this century, the application research of perovskite-type catalysts in the field of engine aftertreatment technology has been rapidly developed, reasonable doping and modification of perovskite-type catalysts can effectively purify the pollutants emitted by engine [5,6]. Hong Wang et al. [7] prepared three kinds of different perovskite-type catalysts by combining sol-gel method and impregnation method. The XRD and SEM characterization results proved that the catalysts can be uniformly supported on the zeolite surface. The substitution of La3+ at the A site by Cs+ can enhance the catalytic activity of the catalysts. Xuelei Mei et al. [8] synthesized the double perovskite-type La2NiB′O6 catalyst material with a three-dimensional ordered macroporous structure successfully by crystal template method. The purification efficiency and catalytic activity of LaNiO3 on PM can be improved by doping the B site with manganese, iron, cobalt, and copper ions, respectively. When the molar ratio of cobalt ions to nickel ions is 1:1, the purification efficiency of La2NiCoO6 on PM can reach 98.2%. Shiva Abedi et al. [9] synthesized LaFeO3, LaFe0.7Mn0.3O3, and LaMn0.7Fe0.3O3 nano-scale perovskite-type catalysts with different doping amount by sol-gel method, the CO was used as a reducing agent to selectively reduce NO through simulation experiments, in addition to studying the function of synthesized catalysts and finding the optimal catalyst in the CO-SCR process by XRD, SEM characterization methods, and chemical reaction kinetic model. The results showed that the conversion efficiency of CO and NO increased with the synergistic effect of manganese ions and iron ions. When the stoichiometry of iron and manganese ions were 0.3 and 0.7, respectively, the catalyst activity can reach the highest, and the conversion efficiency of CO and NO improved with the increase of reaction temperature and decrease of space velocity. Fan Fang et al. [10] synthesized porous perovskite-type La0.6Sr0.4CoO3-δ nanotubes by sol-gel method combined with electrospinning technique following the calcination, which were treated with nitric acid to obtain a catalyst with a larger specific surface area. The catalysts were tested by temperature-programmed experiments. The results showed that the catalytic activity of La0.6Sr0.4CoO3-δ nanotubes for PM was positively correlated with its specific surface area. The research team also analyzed the specific purification mechanism. Jiang, Q et al. [11] doped the A site and B site of LaNiO3 perovskite with calcium and copper ions, respectively, and detailed the effect of different doping amount on the physicochemical structure of the catalyst materials, revealed that the doped perovskite-type catalysts can effectively promote O2− transport during the redox reaction of HC, and can be used as an effective carrier for Oads in the low-temperature oxidation reaction, while decreasing the activation temperature of the overall reaction. E. Magnone et al. [12] studied the effects of different synthesis methods of La0.6Sr0.4CoO3-δ powder on the adsorption and desorption of oxygen, particle size, specific surface area of the catalytic materials, the results showed that different synthesis methods directly affect the particle size and specific surface area of the catalysts, and indirectly affect the adsorption and desorption capacity of the catalysts for oxygen. Long Tang et al. [13] prepared LaKCoO3/γ-Al2O3/cordierite catalysts by a two-step impregnation method. The porous structure of γ-Al2O3 can evenly distribute LaKCoO3 on the surface of cordierite, and obtain a high specific surface area, thermal stability, and catalytic activity. When the reaction temperature is 390 °C, the conversion rate of LaKCoO3/γ-Al2O3/cordierite catalyst for soot particles can reach 90%, and the CO2 selectivity is 99.8%. Siran Zhang et al. [14] proposed a method of constructing highly dispersed Pt and oxygen vacancies on the catalysts. The LaCoxNi0.87−xPt0.13O3 perovskite-type catalyst was supported on SiO2 by the citric acid method and the impregnation method. Under low-temperature conditions, the LaCoxNi1−xO3 surface can form high-dispersion double active centers, namely Pt clusters and O vacancies, which showed significant activity for CO oxidation.
Although the diesel engine exhaust purification technology by perovskite-type catalysts has been developed greatly, most of them focused on the modification research of the A site or the B site. There are few studies about four-way purification of PM, NOx, CO, and HC emitted from diesel exhaust by modifying perovskite-type catalysts. A large number of studies about perovskite-type catalysts showed that the doping of the A site can help to increase the activity of the catalyst lattice oxygen and make the B site ions exist in multiple valence states, causing the increases of oxygen vacancy in the catalyst, which reduced the ignition temperature of the oxidation reaction. The A site of perovskite-type LaCoO3 is the ideal adsorption site for NOx storage. which can be doped by alkali metals, alkaline earth metals or rare earth elements. while the doping of the B site directly affects the activity of the perovskite-type catalyst, which is the redox site of the purification process, it can be doped by transition metal elements [15,16]. The doped A or B site with other cations can rationally tune the physicochemical characteristics of perovskites, including redox performance, oxygen mobility, and ionic conductivity [17]. A large number of studies showed that the catalytic activity of perovskite was comparable to that of noble metal catalysts [18,19]. Therefore, new non-precious metal-doped perovskite-type catalysts can be developed.
In this paper, the A site of the perovskite-type LaCoO3 is doped with cerium and calcium ions, and the B site is doped with iron and manganese ions. The objective of this work was to evaluate the effect of A site (cerium and calcium ions) and B site (iron and manganese ions) substitution on the structural and purification properties of perovskite-type catalyst samples. The CO, HC, and PM emitted by diesel engines are utilized as NOx reducing agents to achieve the purpose of purifying CO, HC, NOx, and PM simultaneously. Perovskite-type catalyst samples particles La1−xMxCo1−yNyO3 with different doping amount were prepared by the sol-gel method [20,21], the prepared catalyst samples were characterized by means of XRD, FT-IR, SEM, BET, and XPS analysis. Meanwhile, by related simulation experiments and H2 temperature-programmed reduction (H2-TPR) experiments, the influence factors of the four-way purification efficiency were analyzed from the perspective of purification mechanism.
2. Materials and Methods
2.1. Sample Preparation
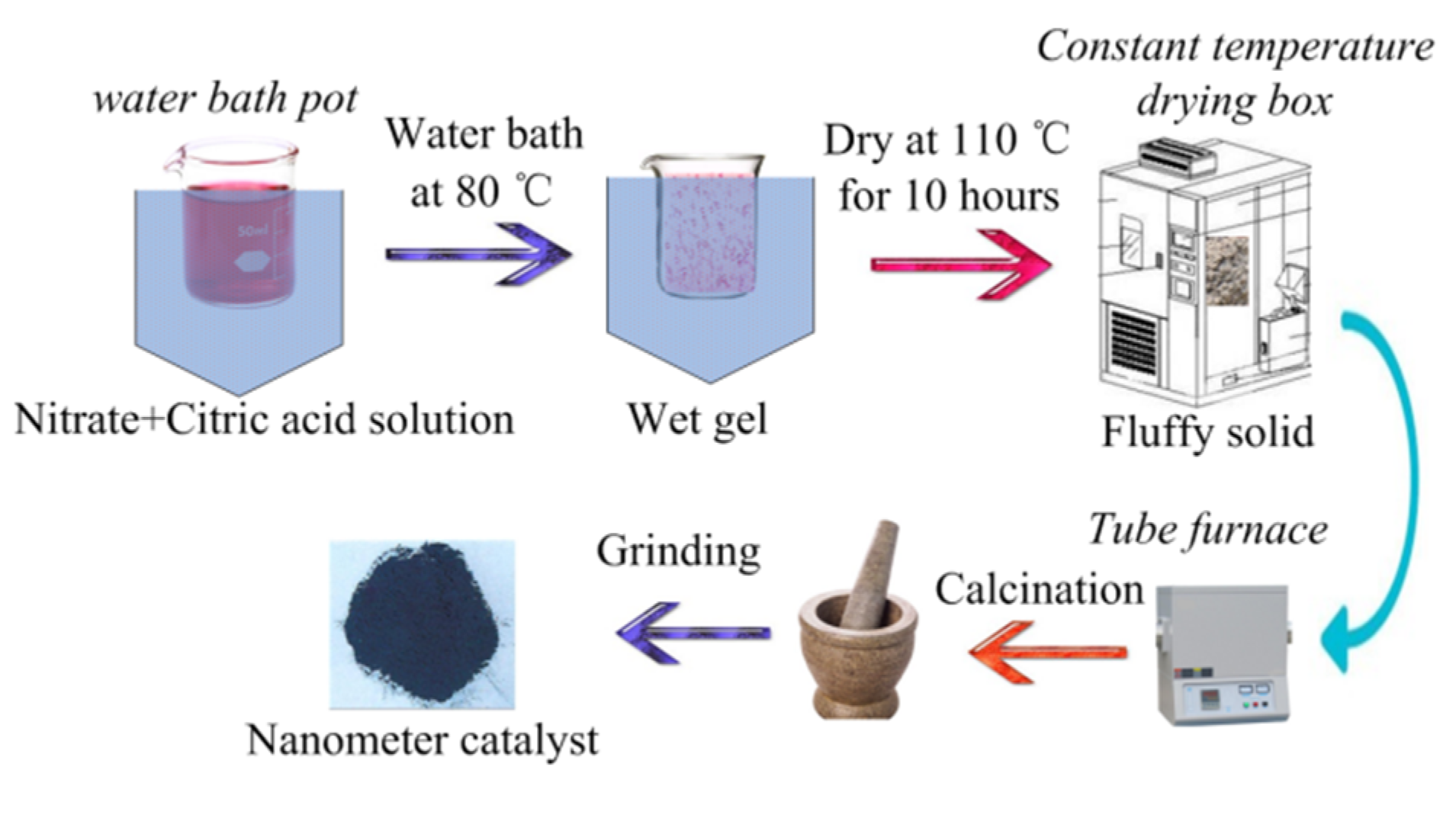
Perovskite-type catalyst samples with formula of La1−xMxCo1−yNyO3 (M = Ce, Ca; N = Fe, Mn; x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6; y = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6) were prepared by sol-gel method. La(NO3)3·6H2O, Co(NO3)2·6H2O, Ce(NO3)3·6H2O, Ca(NO3)2·6H2O, Mn(NO3)2·4H2O, Fe(NO3)3·9H2O, citrate acid, and deionized water were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. All the chemicals are analytical grade. Suitable amounts of chemicals based on stoichiometry were dissolved in deionized water to get a sol and the obtained solution was stirred vigorously using magnetic stirrer (JXX1-85-2, stirring speed 100–2000 r/min, Changzhou, China). The solution was heated on a water-bath pot (HH-1, control temperature 25–100 °C, accuracy ± 1.5 °C, Tsingtao, China); when the temperature of the solution was raised to 70 °C, a suitable amount of citric acid (the molar ratio of citric acid to metal ions is 1.2–1.5) was added and temperature was justified at 80 °C to evaporate to dryness with vigorous stirring. During the dehydration process, a poly condensation reaction carried out between nitrate ions and citric acid leading to formation of gel, the obtained wet gel was placed in a constant temperature drying box (WGL-125B, control temperature 25–300 °C, Tianjin, China) at 110 °C for 10 h to form a fluffy solid. The obtained bulk solid was placed in a Tube furnace (TL1200 Mini, Rated temperature 1100 °C, Guangzhou, China) calcined at 700 °C for 5 h and was cooled slowly to room temperature. Finally, the powders were finely ground to obtain the La1−xMxNyCo1−yO3 perovskite-type catalyst samples. The preparation process route of the catalyst samples is shown in Figure 1.
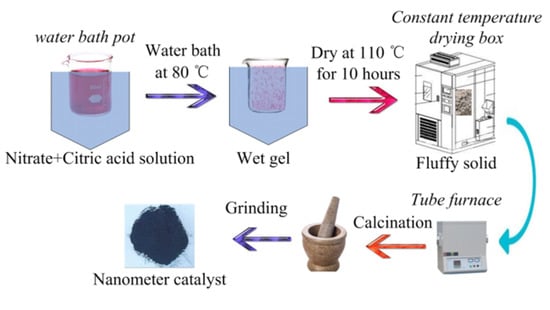
Figure 1.
Preparation process route of the catalyst samples.
2.2. Characterization
The phase structure state, and ionic valence state of the catalyst crystal have a significant impact on the catalytic performance of the catalyst samples [22,23]. The phase composition of catalyst samples was characterized by an X-ray diffractometer (XRD; XRD-6100; Shimadzu, Japan) with monochromator in the continuous mode in the range 2θ = 20~80°, using Cu Kα radiation, with a scanning rate of 8°·min−1 and time per step of 0.5 s. The fourier transform infrared spectrometer (FTIR; Spectrum 400; PerkinElmer, Waltham, MA, USA) as utilized to characterize the chemical composition of catalyst samples, using diffuse reflection method to test the samples that were prepared by mixing crushed catalyst samples and KBr with a mass ratio about 1:100. The number of scans was 80 and the resolution was set to 4 cm−1. The Scanning Electron Microscope (SEM; Quanta 200; FEI, Valley City, ND, USA) utilized to characterize the microscopic morphology of catalyst samples. The samples were mounted on the aluminum holder with conductive adhesive and then sputtered with gold. Then the microscopic morphology of the samples was observed under the acceleration voltage of 13 kV. The N2 adsorption isotherms and pore size distribution (PSD) curves of perovskite-type catalyst samples were measured by Automatic Surface Area and Porosity Analyzer (ASAP 2020). XPS analysis was performed on an Amicus spectrometer equipped with Mg Kα X-ray radiation.
2.3. Experimental System and Methods
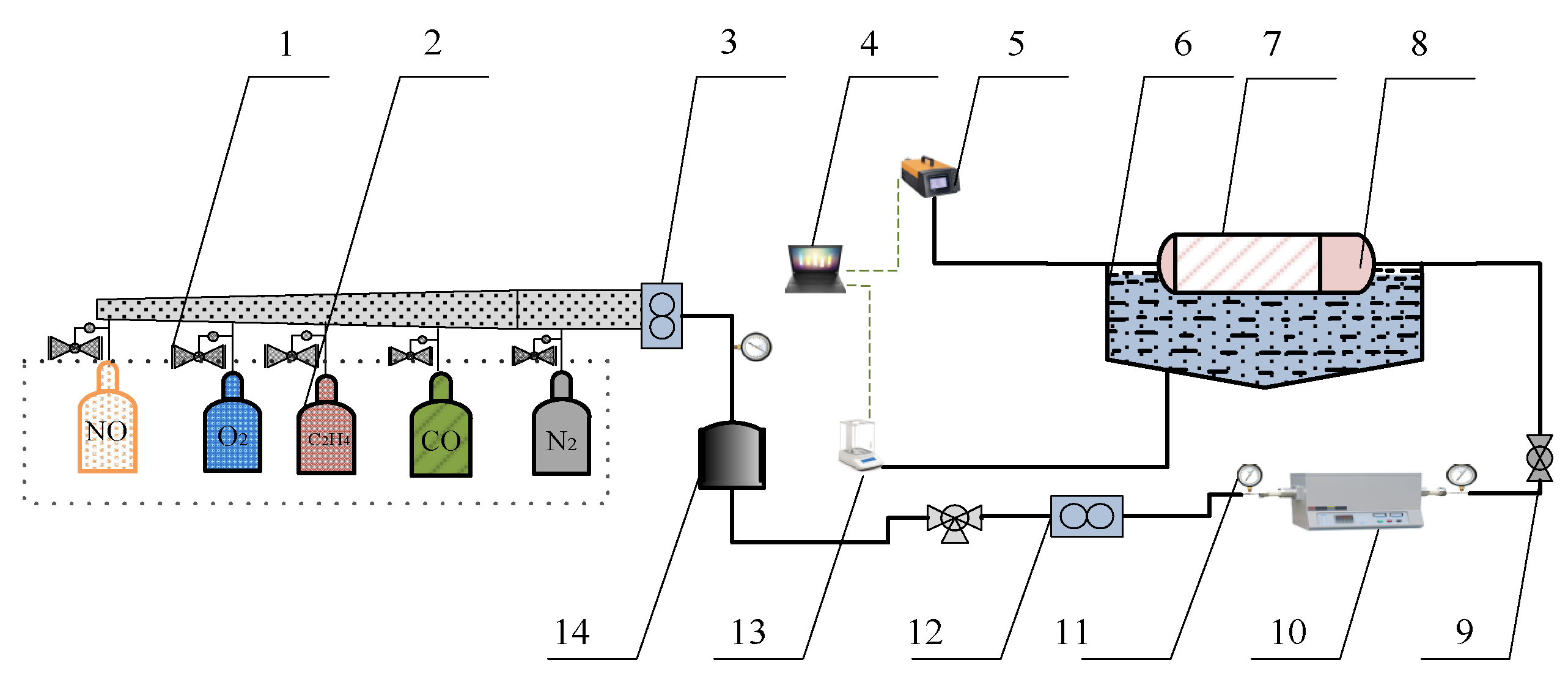
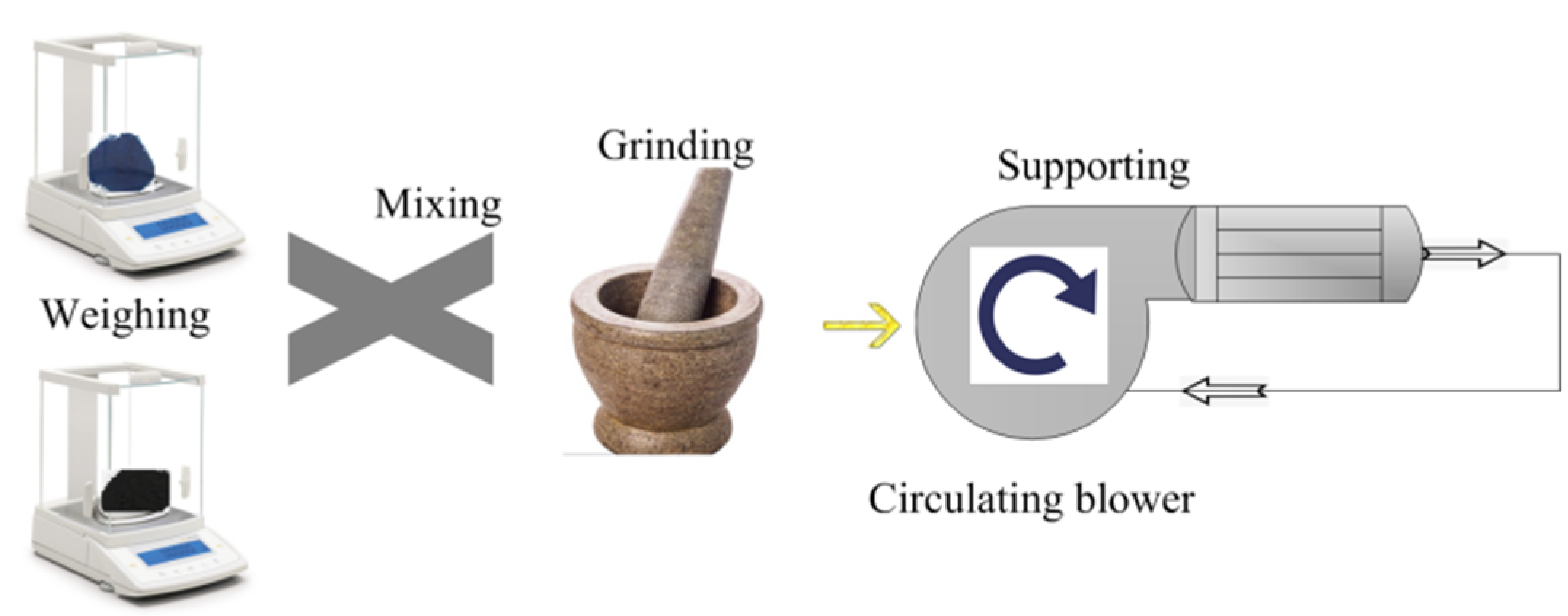
A simulation test bench for activity tests of catalyst samples is shown in Figure 2. The simulated exhaust gas can be provided by the gas cylinder group 2 (O2, N2, C2H4, CO, and NO) and mixed by the gas mixing chamber 14. The tube furnace 10 was used to control the temperature of the mixed gas and the water-bath pot 6 can maintain the exhaust temperature in the Quartz tube 7. According to reference [24], the exhaust gas temperature of diesel engine is allowed to vary from 550 °C to 650 °C. Therefore, the simulated exhaust gas temperature can be maintained at 600 °C by adjusting the tube furnace. The cordierite was used to support PM and catalyst samples, the mass of supported PM and catalyst samples was 18 mg, the mass ratio of PM to catalyst samples was 1:1, the supporting process of PM and catalyst samples is shown in Figure 3. The moderate PM and catalyst samples were set in the circulating blower, which was sealed before the supporting process. When the circulating blower started, the porous structure of cordierite can capture all of the powder PM and catalyst samples. The cordierite supported with PM and catalyst samples was placed inside the quartz tube 7, the length of quartz tube is 200 mm, which is twice that of cordierite, and their diameter is 80 mm, the gas analyzer (Horida Ltd., MEXA-4000FT, relative error 1.7%, Kyoto, Japan) was used to detect the concentration of gas generated after purification, the electronic balance (PWN224ZH, repeatability deviation 0.01 mg, Shanghai, China) was used to measure the mass of cordierite before and after purification.
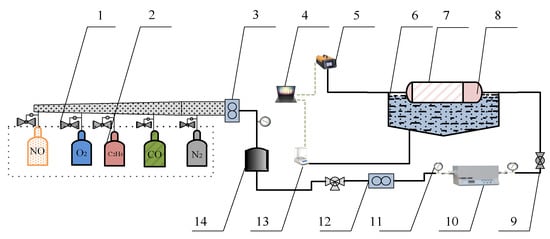
Figure 2.
The overall structure of the simulation test bench. 1. Pressure-reducing valve. 2. Gas cylinder group. 3,12. Flowmeter. 4. Computer. 5. Gas analyzer. 6. Water bath pot. 7. Quartz tube. 8. Cordierite loaded with PM and catalytic agent. 9. Ball valve. 10. Tube furnace. 11. Pressure gauge. 13. Electronic balance. 14. Gas mixing chamber.
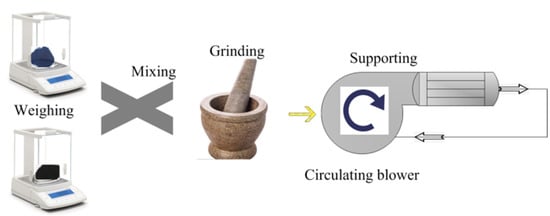
Figure 3.
The supporting process of PM and catalyst samples.
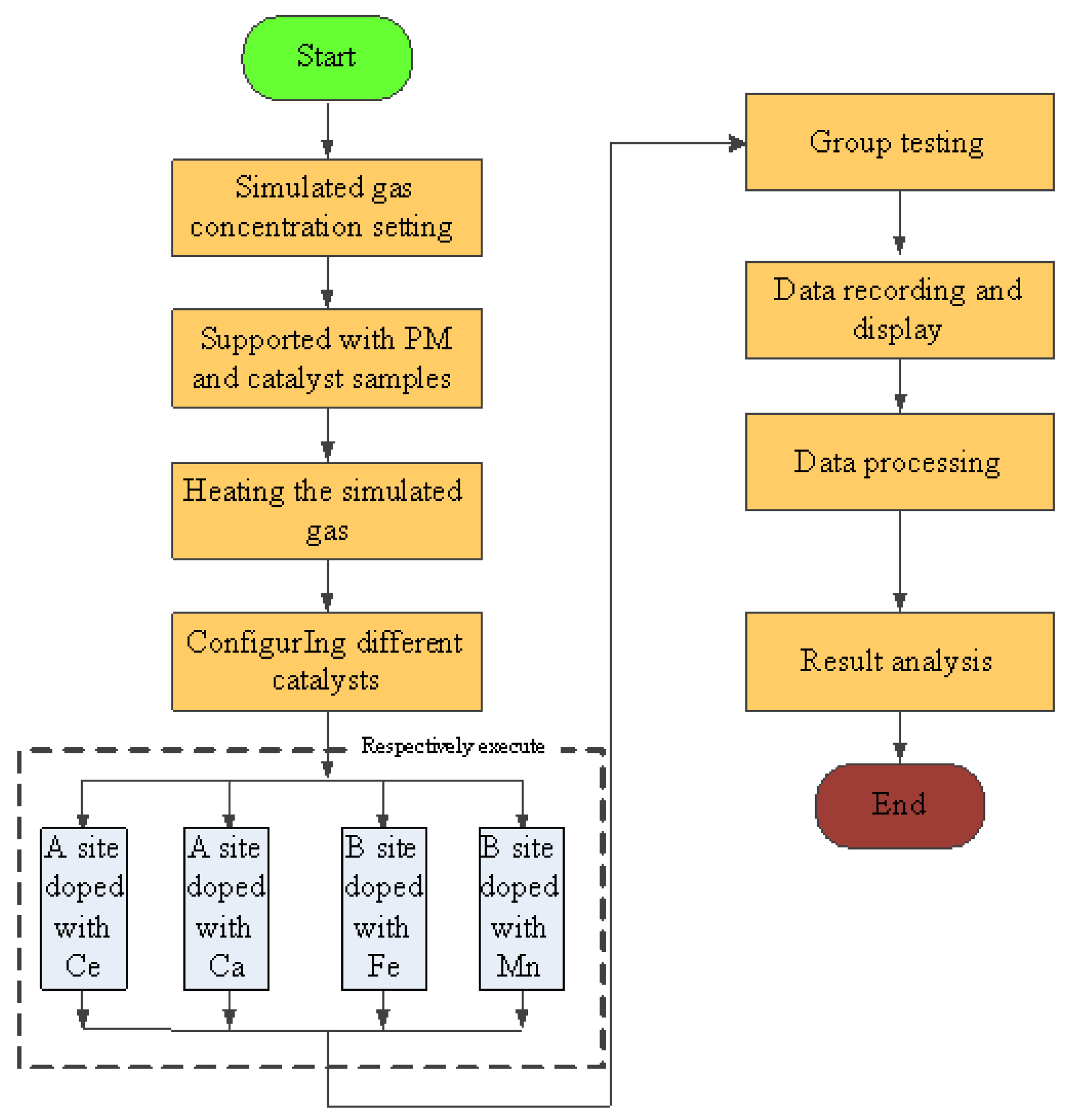
All the experiments were carried out under the condition of room temperature and shading. The simulated gas was prepared by mixing O2, N2, C2H4, CO. and NO, and the gas ratio concentration was strictly controlled by the throttle valve and the flowmeter (DMF-1-S6, accuracy ± 1%), the gas flow rate of O2, N2, C2H4, CO, and NO can be controlled to 10 mL/min, 89.81 mL/min, 0.3 mL/min, 0.8 mL/min, and 0.8 mL/min separately. And the total flow rate was set to 10 mL/min, the concentration of simulated exhaust gas was shown in Table 1 [25,26]. The space velocity was . When the experiment starts, the simulation test bench works stably for 10 min, according to the experimental requirements firstly. Then, experimental results were recorded and the test flow chart is shown in Figure 4. The conversion of CO, HC, NOx, and PM were calculated as follows:
where is the purification efficiency of CO; and are concentration of before and after the experiment, ppm; is the purification efficiency of HC; and are concentration of before and after the experiment, ppm; is the purification efficiency of NOx; and are concentration of before and after the experiment, ppm; is the purification efficiency of PM; and are the mass of the filter before and after the experiment, mg.

Table 1.
Concentration of simulated exhaust gas pollutants.
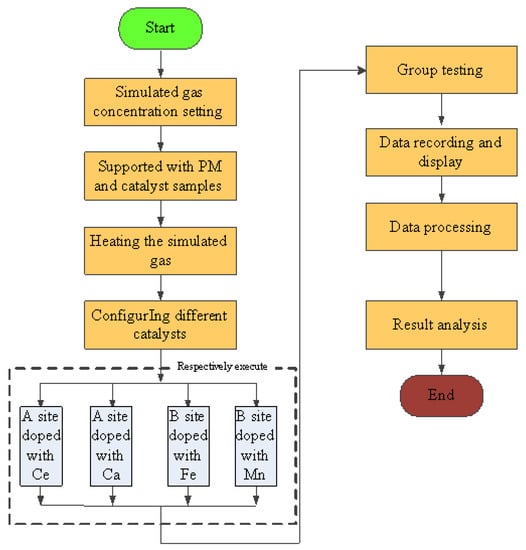
Figure 4.
Simulation test flow chart.
3. Results and Discussion
3.1. Results of Characterization on Catalyst Samples Doping at A Site
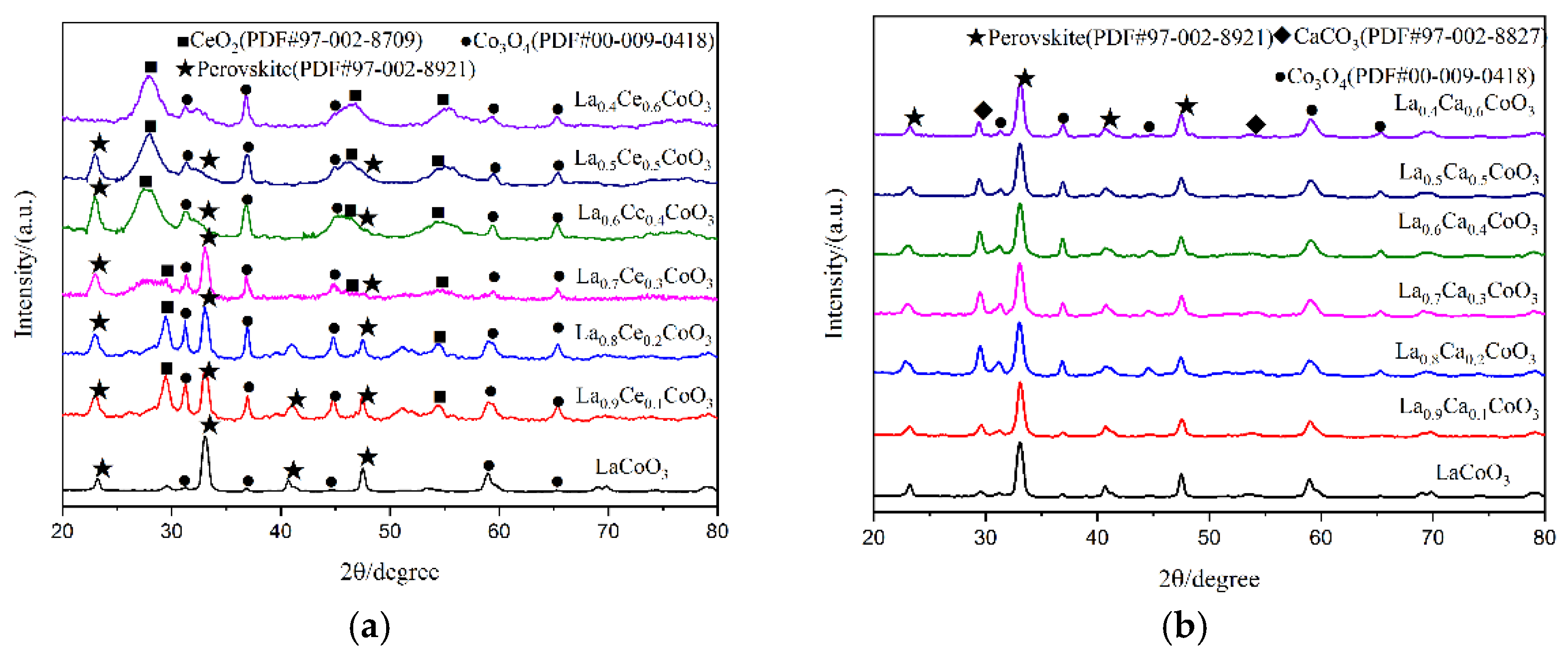
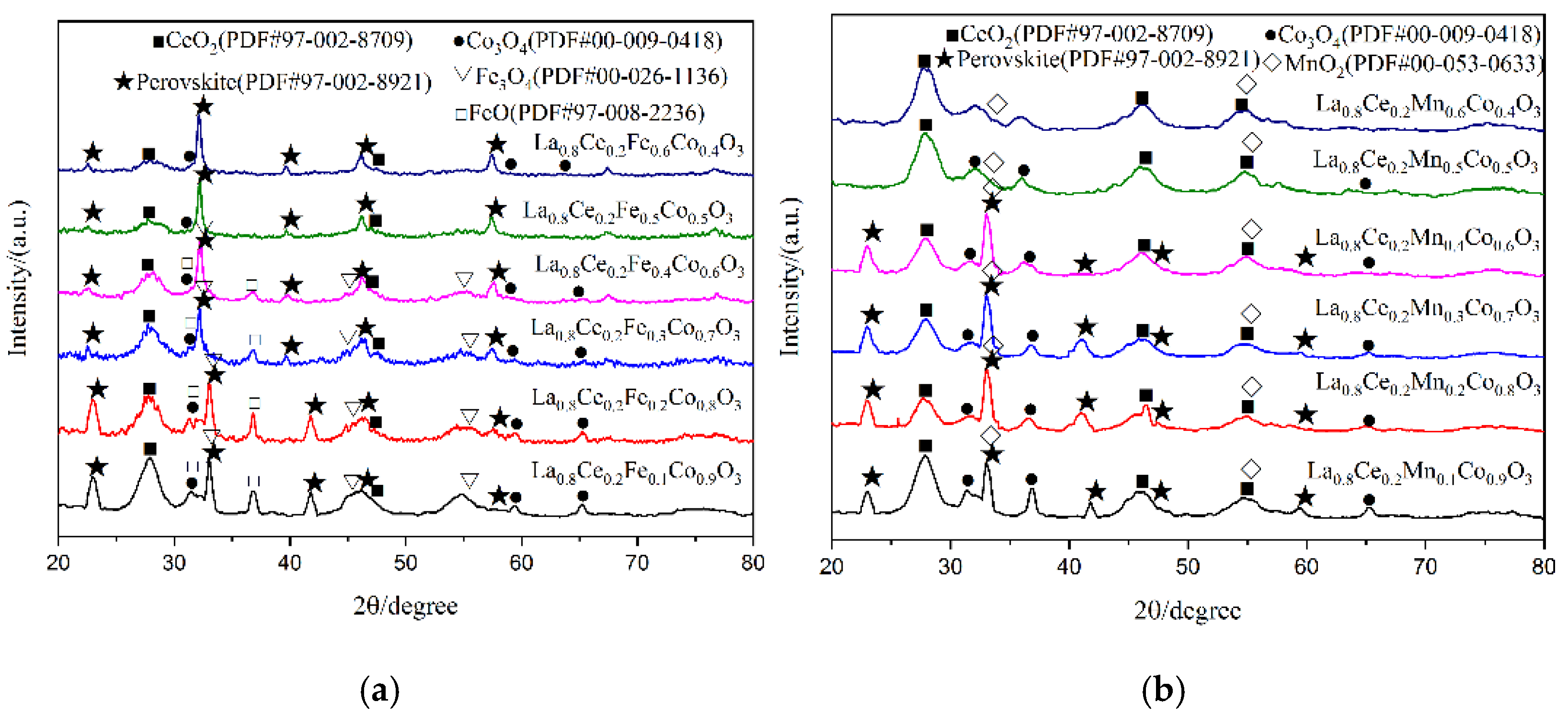
X-ray diffraction patterns of catalyst samples La1−xMxCoO3 (M = Ce, Ca; x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6) are shown in Figure 5. As can be observed from Figure 5a, perovskite diffraction patterns were observed for LaCoO3, La0.9Ce0.1CoO3, La0.8Ce0.2CoO3, and La0.7Ce0.3CoO3. These samples have characteristic diffraction peaks of LaCoO3 near 2θ = 23.3°, 33.0°, 42.4°, and 47.0°, and they all correspond to the perovskite-type cubic crystallized (PDF# 97-002-8921). There are CeO2 (PDF# 97-002-8709), Co3O4 (PDF# 00-009-0418) impurity phases in the samples. Therefore, when Ce-content > 0.3, the XRD characteristic diffraction peaks of LaCoO3 near 2θ = 33.0° was extraordinarily inconspicuous. According to reference [27], the effective ionic radius of lanthanum ions and cerium ions are 1.10 Å and 0.87 Å, respectively, therefore, excessive cerium ions and cobalt ions cannot be completely dissolved in the LaCoO3 lattice. As can be observed from Figure 5b, perovskite diffraction patterns were observed for La0.9Ca0.1CoO3, La0.8Ca0.2CoO3, La0.7Ca0.3CoO3, La0.6Ca0.4CoO3, La0.5Ca0.5CoO3, and La0.4Ca0.6CoO3. All samples have characteristic diffraction peaks of LaCoO3, and they all correspond to the perovskite-type cubic crystallized (PDF# 97-002-8921). The effective ionic radius of calcium ions is 1.06 Å, which is close to the ion radius of lanthanum ions, therefore, there are only small amounts of CaCO3 (PDF# 97-002-8827) and Co3O4 (PDF# 00-009-0418) in the impurity phase in the crystal samples. Through the above analysis, it can be concluded that the structure of La0.8Ce0.2CoO3 and La0.8Ca0.2CoO3 perovskite-type catalyst samples reaches the best catalyst conditions. As can be observed from Figure 5a,b, the characteristic diffraction peaks of LaCoO3 doping with cerium ions and calcium ions near 2θ = 23.3° moved left slightly. This information would confirm the accommodation of these compounds within the perovskite lattice.
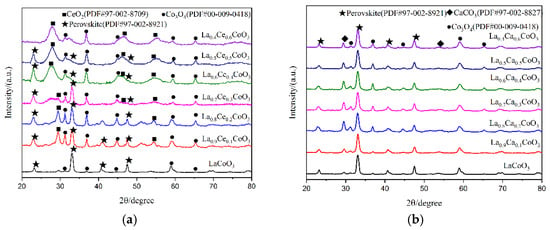
Figure 5.
XRD patterns of catalyst samples: (a) La1−xCexCoO3 catalyst samples; and (b) La1−xCaxCoO3 catalyst samples.
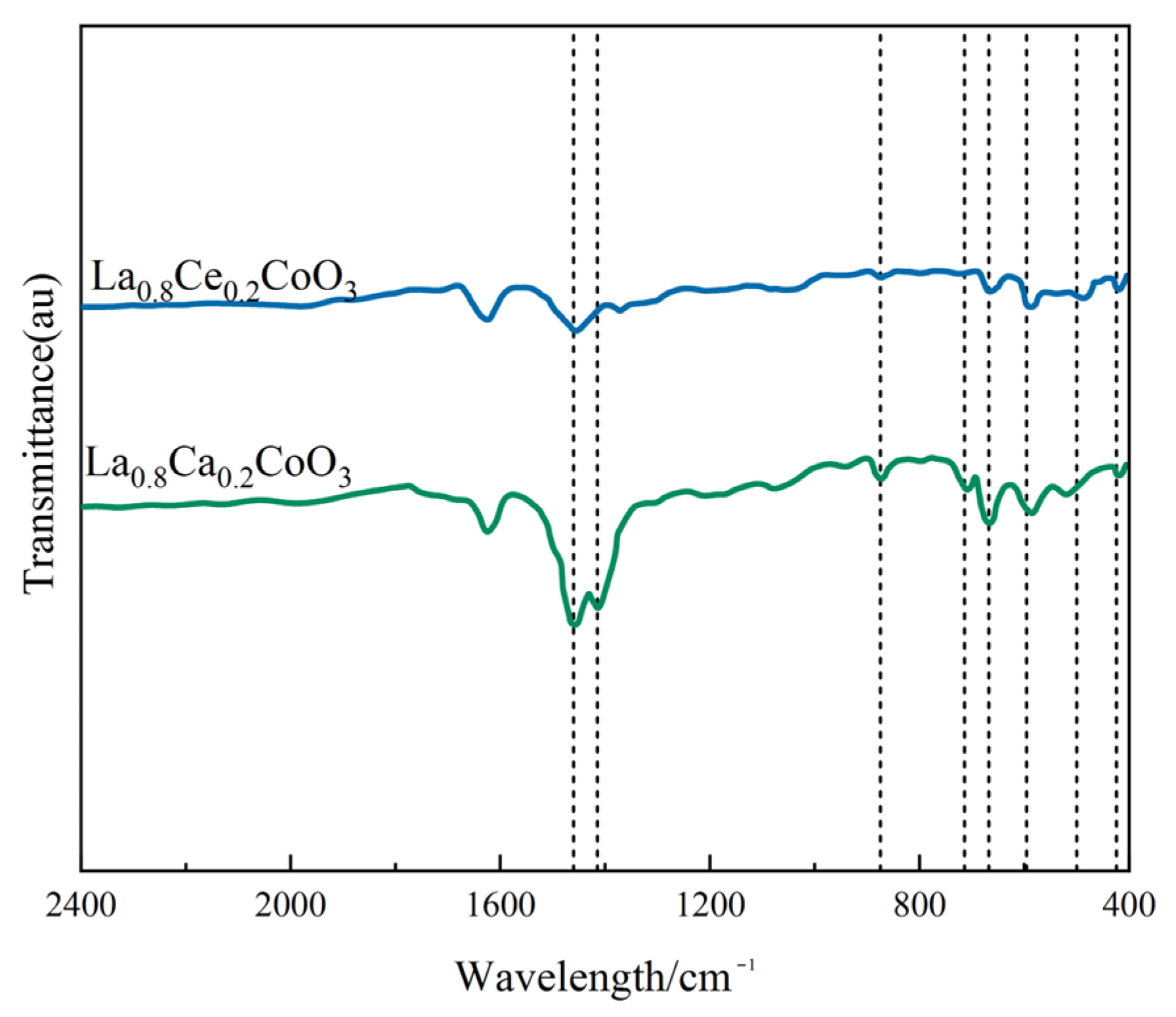
Figure 6 shows the FT-IR spectra of La0.8Ce0.2CoO3 and La0.8Ca0.2CoO3 catalyst samples. As can be observed from Figure 6, there are several vibration bands at 424, 500, 596, 668, 712, 875, 1415, 1460, and 1628 cm−1. FT-IR peaks located below 1000 cm−1 has been reported to represent metal oxides [28]. The vibration band at 424 cm−1 and 596 cm−1 belongs to the bending vibration of Co–O bonding in the BO6 octahedron, and the vibration band at 500 and 669 cm−1 belongs to the bending vibration of Co–O and Ce–O bonding, which are attributed to Co3O4 and CeO2 [29,30]. The absorption peaks of 875 and 712 cm−1 belong to the calcite crystals, which are related to the bending vibration of C–O bond. The absorption peaks appeared at 1415, 1460, and 1628 cm−1 of catalyst samples, representing the bending mode of C–H. Compared to FTIR spectra of La0.8Ca0.2CoO3 and La0.8Ce0.2CoO3 catalyst samples, in the FTIR spectra of La0.8Ce0.2CoO3, the band at 596 cm−1 becomes broad and up-shifting, suggesting that some amounts of Co3+ changed to Co2+ when some La3+ was replaced by Ce3+ and Ce4+. However, there is no change in the valence state of Ca2+, so there is no other absorption spectrum in the FTIR spectra of La0.8Ca0.2CoO3. After the FTIR analysis, it can be confirmed that some impurities like carbonate group and hydroxyl group presented in samples.
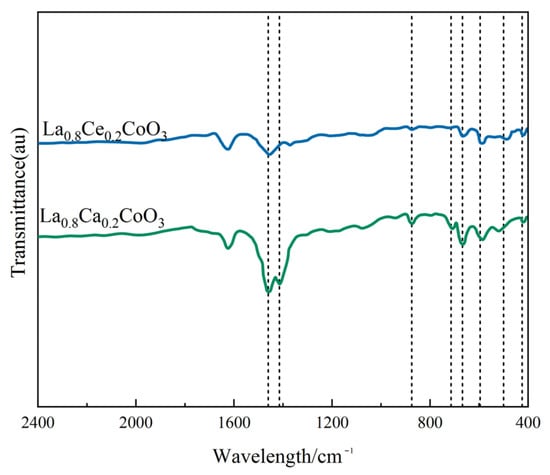
Figure 6.
FTIR spectra of La0.8Ce0.2CoO3 and La0.8Ca0.2CoO3 catalyst samples.
3.2. Results of Simulation Experiments on Catalyst Samples Doping at A Site
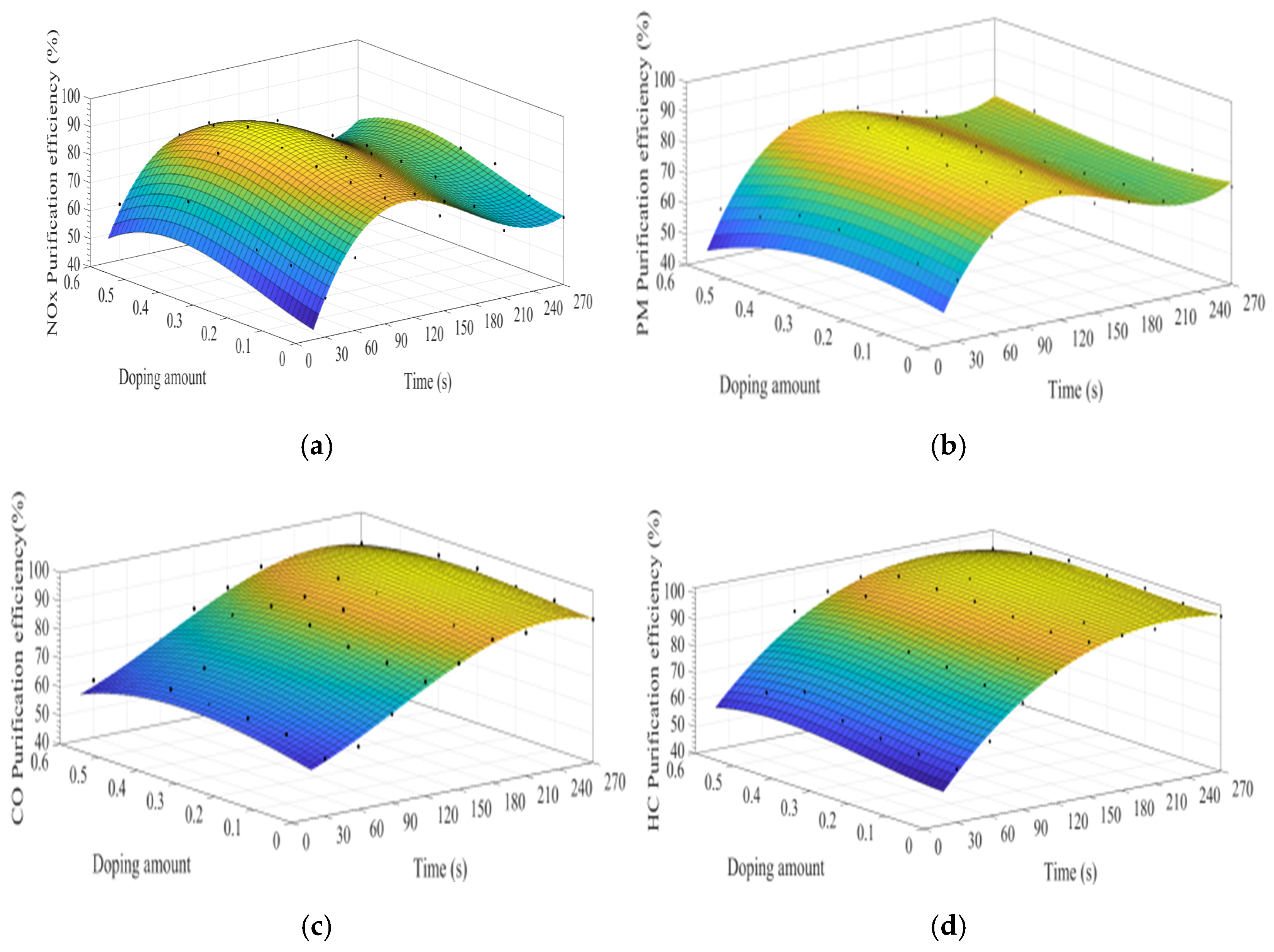
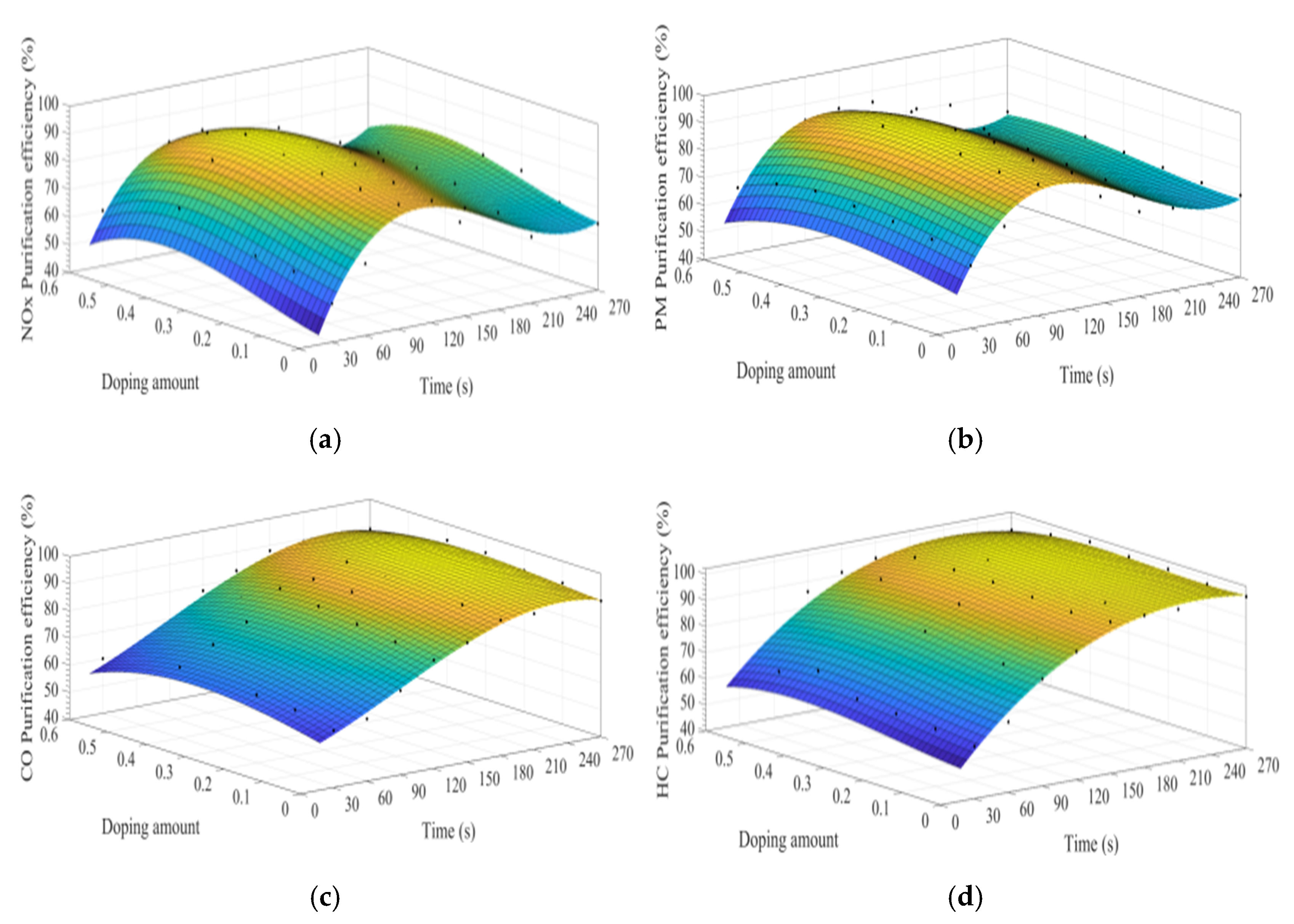
MATLAB software was used to fit the experimental data. Figure 7a–d show the time curve of the purification efficiency of NOx, PM, HC, and CO when the A site was doped with different concentrations of cerium ions. As can be observed from Figure 7, when the purification time does not exceed 90 s, the purification efficiency increases with time. At this time, the catalyst samples are in the heating stage and do not exert the best catalytic performance. When the time exceeds 90 s, the purification efficiency reaches the highest. When the purification time exceeds 120 s, the purification efficiencies of NOx and PM show an obvious downward trend, which can be attributed to the decrease of PM load with purification time increases, decreasing the NOx and PM redox reaction rate. When the doping amount does not exceed 0.2, the purification efficiencies of NOx, PM, HC, and CO improve with the increase of the doping amount. When x = 0.2, the purification efficiency can reach the highest, which can reach 85%, 90%, 94%, and 100%, respectively. When x > 0.3, excessive cerium ions were not doped in perovskite-type oxide, too much impurity phase was introduced into the catalyst samples. These impurities have poor purification efficiency on NOx, PM, HC, and CO, which are consistent with the results of XRD.
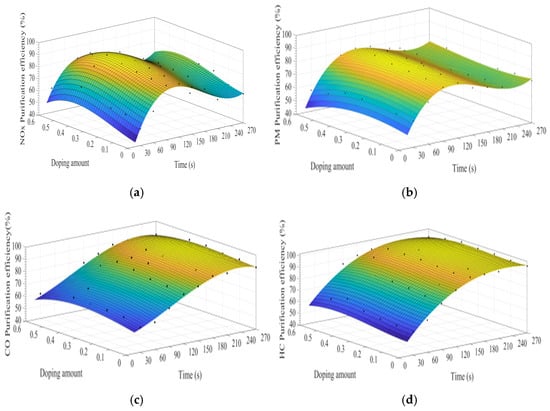
Figure 7.
Purification efficiency curve of catalyst when A site was doped with ceriumions: (a) NOx; (b) PM; (c) CO; and (d) HC.
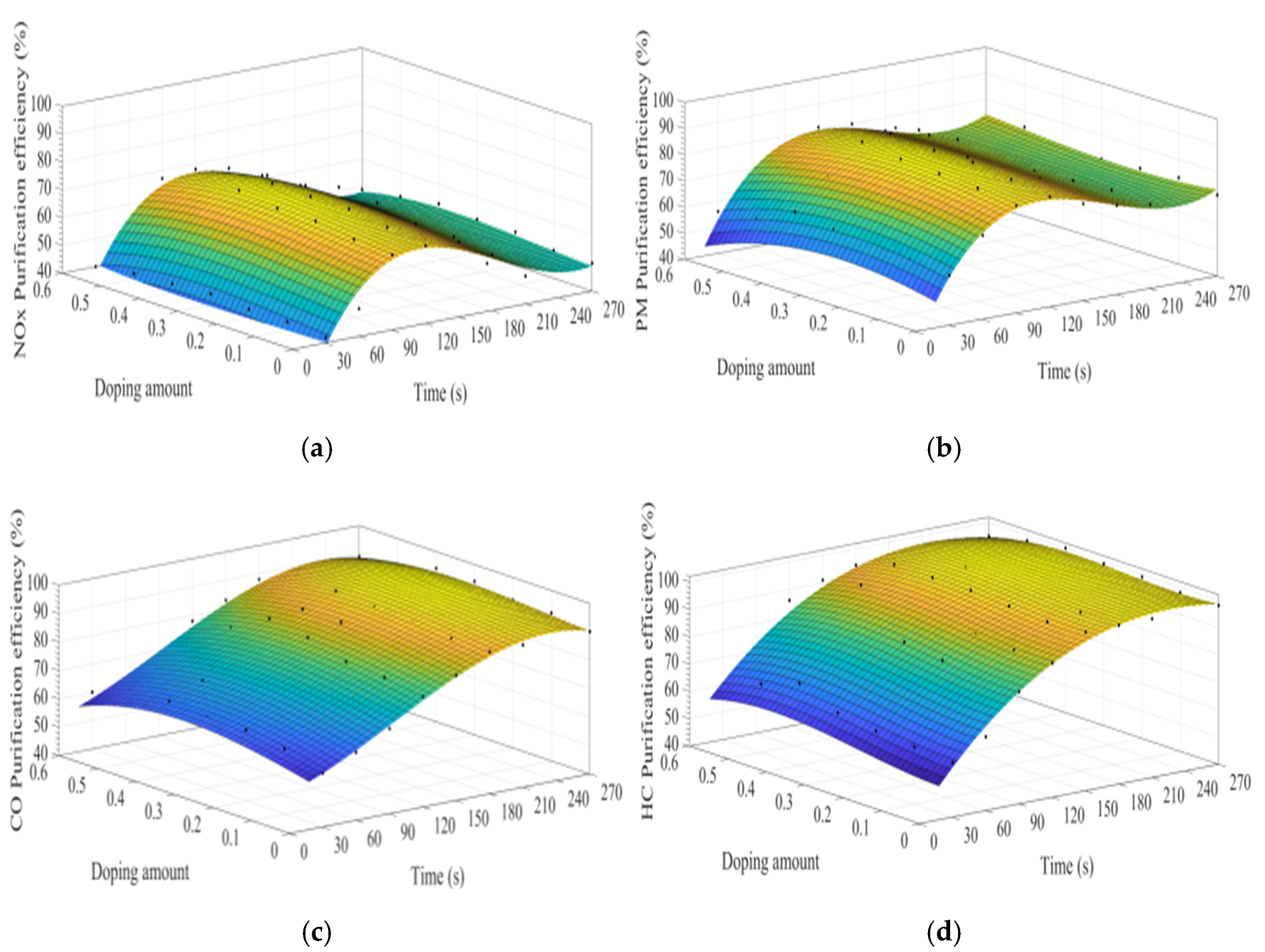
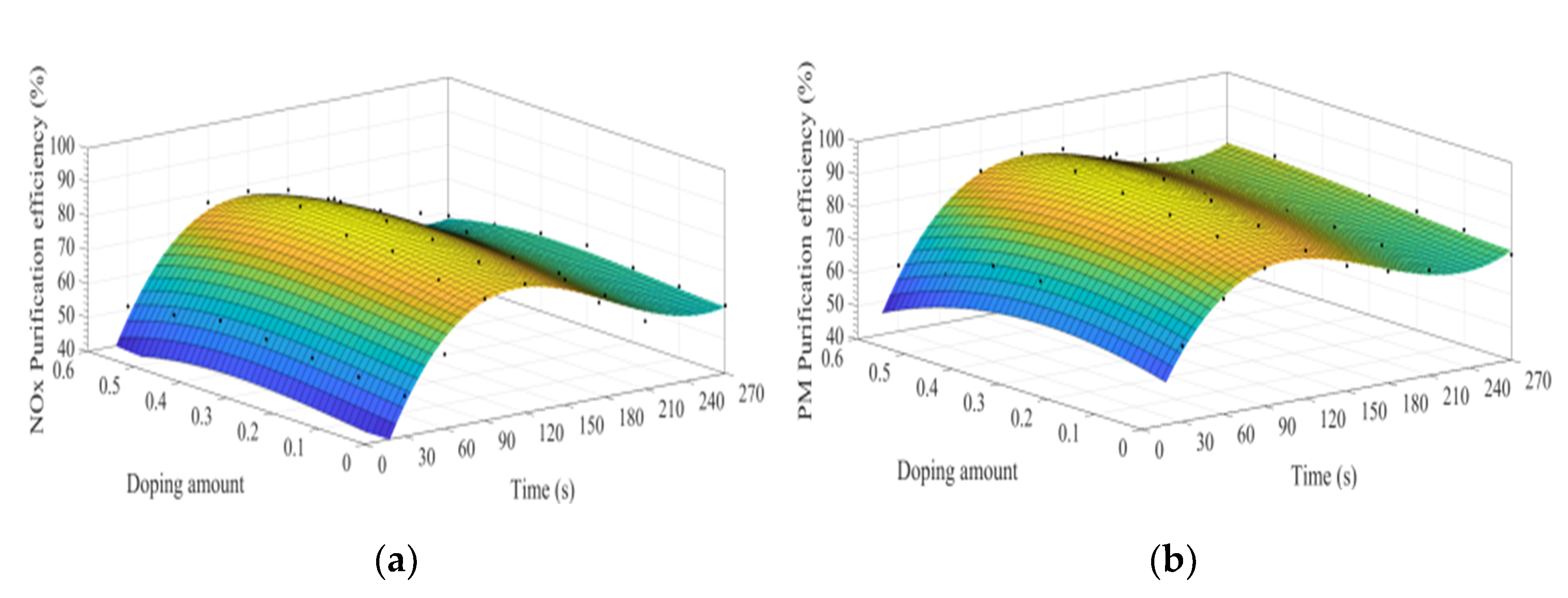
Figure 8a–d show the time curve of the purification efficiency of NOx, PM, HC, and CO when the A site was doped with different concentrations of calcium ions. Upon comparison of perovskite-type catalyst samples doped with calcium ions and cerium ions, the purification trend and efficiency of PM, HC, and CO are basically the same. However, the doping of calcium ions shows a lower efficiency in purifying NOx, and the highest purification efficiency is 72%, which can be attributed to the factor that the valence state of the doped calcium ions has not changed, and the Co3+ in the catalyst samples change to Co4+, but not to Co2+. The existence of Co2+ is more conducive to the formation of O vacancies and adsorbed NO, which is beneficial to the reduction of NO, and the change of ions valence state promotes the adsorption and desorption of O. In addition, When A-site of LaCoO3 perovskite was doped with cerium ions, the catalyst crystal produces small distortion and enhances the purification efficiency of PM, NOx, CO, and HC, which is consistent with the research content reported in the literature [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. According to the literature [32,33,34,35,36] on the mechanism analysis of purifying the exhaust pollutants by perovskite-type catalysts, combined with the experiment results of this study, the overall purification and purification process is inferred as Formulas (5)–(19).
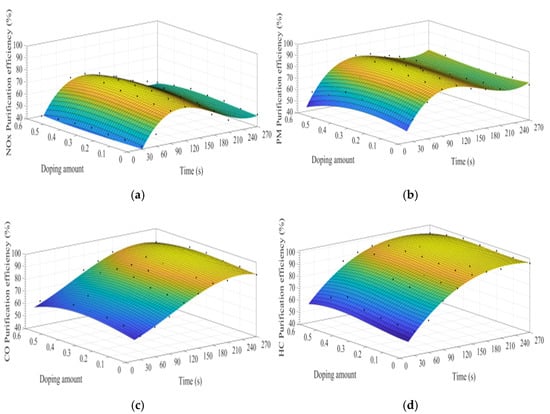
Figure 8.
Purification efficiency curve of catalyst when A site was doped with calcium ions: (a) NOx; (b) PM; (c) CO; and (d) HC.
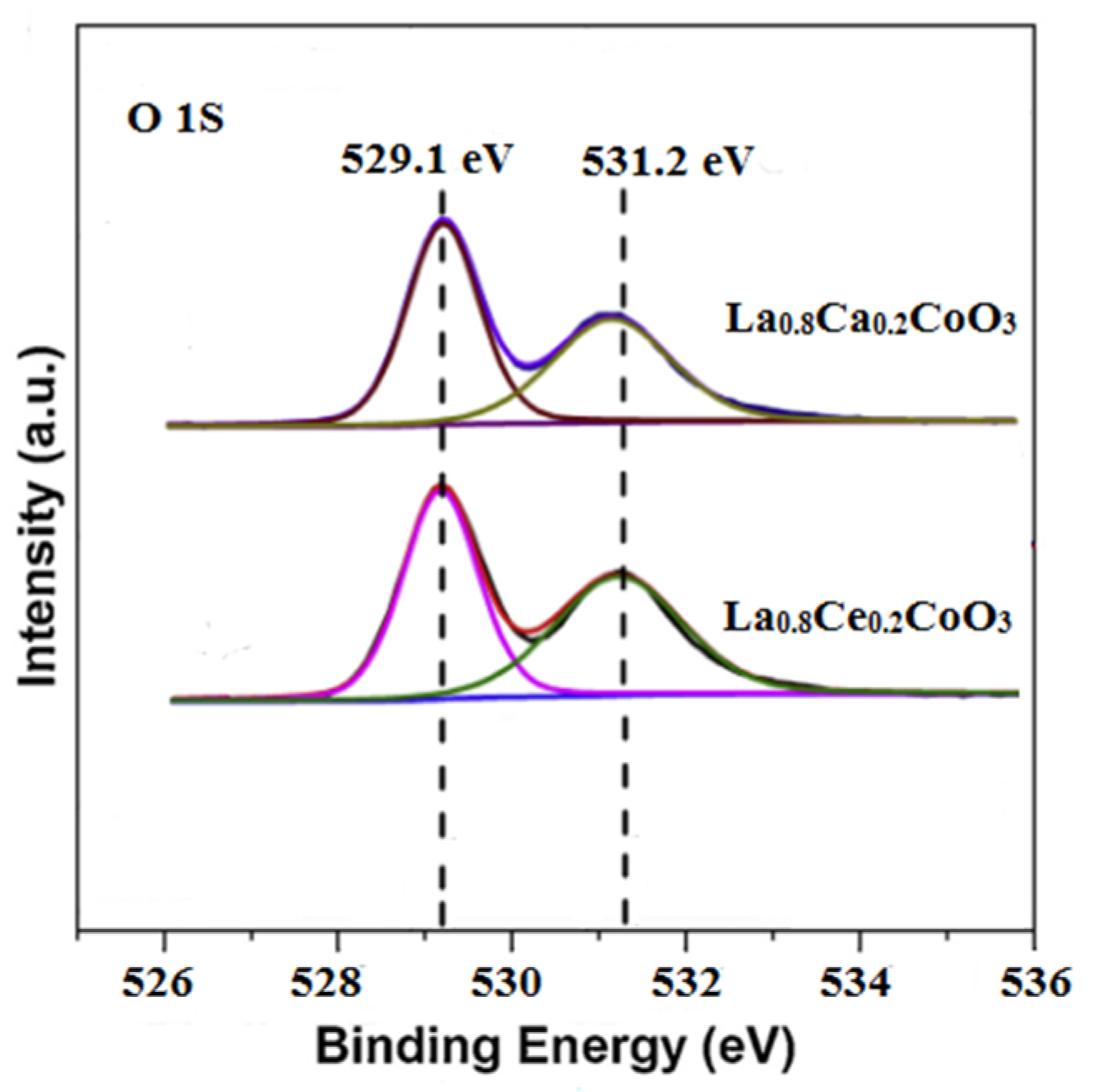
The deconvolution of O 1 s signals was conducted in order to estimate the species in detail. As shown in Figure 9, The O 1 s spectrum for La0.8Ca0.2CoO3 and La0.8Ce0.2CoO3 could be decomposed into two components. The peak at about 529.1 eV was ascribed to the lattice oxygen species (Olatt). Another component at 531.2 eV was assigned to adsorbed oxygen species (Oads) [37], which has been reported as active species in oxidation reactions due to the electrophilic nature [38]. Since the surface Oads/Olatt molar ratio of the La0.8Ce0.2CoO3 sample (0.78) was higher than that of the La0.8Ca0.2CoO3 sample (0.62). Therefore, the La0.8Ce0.2CoO3 sample showed the better purification efficiency in NO, which are consistent with the reaction pathway reported in Equations (5)–(19).
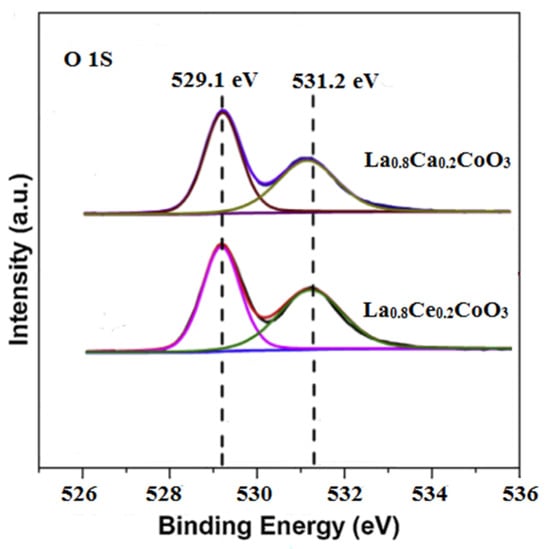
Figure 9.
X-ray photoelectron of the O 1 s for La0.8Ca0.2CoO3 and La0.8Ce0.2CoO3.
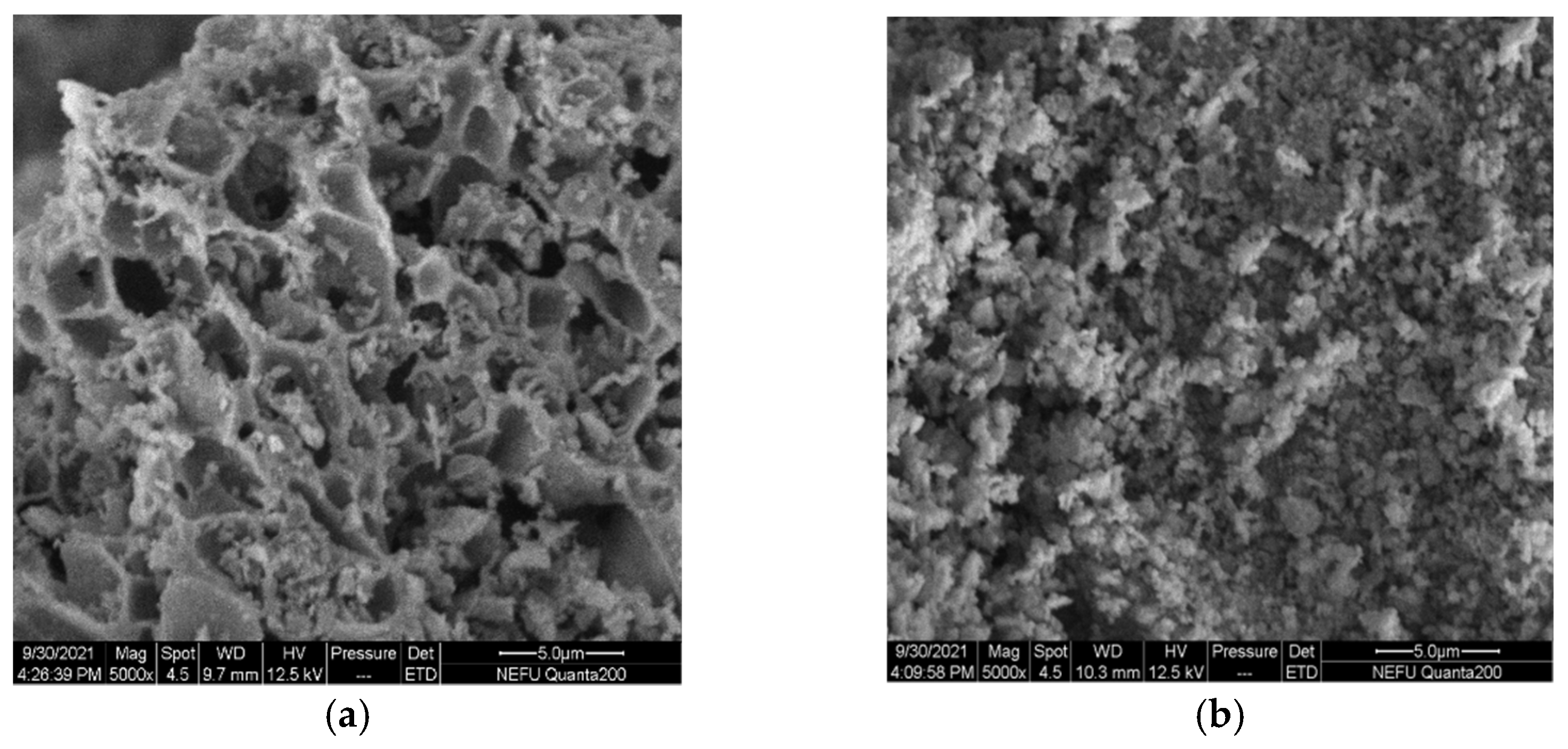
The SEM micrographs clearly show large differences in the micro-structure and morphology of La0.8Ce0.2CoO3 and La0.8Ca0.2CoO3 perovskite-type catalyst samples from Figure 10a,b, La0.8Ce0.2CoO3 perovskite-type catalyst samples represent porous morphologies, La0.8Ca0.2CoO3 perovskite-type catalyst samples represent spongy morphologies. Both samples are uniformly dispersed without sintering. The porous structure of La0.8Ce0.2CoO3 perovskite-type catalyst samples are clearer than La0.8Ca0.2CoO3, the pore size of which ranging from 3 µm to 5 µm.
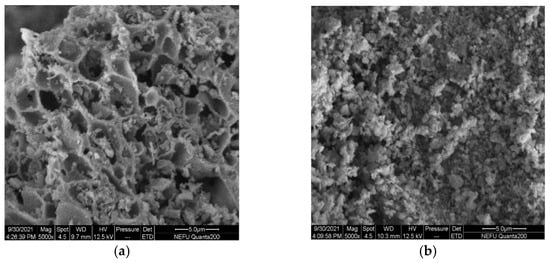
Figure 10.
SEM of catalyst samples: (a) La0.8Ce0.2CoO3; and (b) La0.8Ca0.2CoO3.
3.3. Results of Characterization on Catalyst Samples Doping at B Site
On the basis of the above research results, the A-site doping amount of cerium ions was selected, and the doping amount was 0.2. The B-site doping of manganese and iron ions on the catalytic performance of the La-based perovskite-type catalyst should be further studied. According to reference [27], the effective ionic radius of iron ions, cobalt ions and manganese ions are 0.645 Å, 0.61 Å, and 0.645 Å, respectively, which meet the 15% rule. Therefore, La0.8Ce0.2Co1−yNyO3 (N = Fe, Mn; y = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6) can form continuous series of solid solutions.
X-ray diffraction patterns of catalyst samples La0.8Ce0.2Co1−yNyO3 (N = Fe, Mn; y = 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6) are shown in Figure 11. As can be observed from Figure 11a, perovskite diffraction patterns can be observed for La0.8Ce0.2Co0.9Fe0.1O3, La0.8Ce0.2Co0.8Fe0.2O3, La0.8Ce0.2Co0.7Fe0.3O3, La0.8Ce0.2Co0.6Fe0.4O3, La0.8Ce0.2Co0.5Fe0.5O3, and La0.8Ce0.2Co0.4Fe0.6O3. All samples have characteristic diffraction peaks of LaCoO3 near 2θ = 23.3°, 33.0°, 42.4°, and 47.0°, they all correspond to the perovskite-type cubic crystallized (PDF# 97-002-8921). There are CeO2 (PDF# 97-002-8709), FeO (PDF# 97-008-2236), and Fe3O4 (PDF# 00-026-1136), as well as a small amount of Co3O4 (PDF# 00-009-0418) impurity phases in the crystal samples. However, the doping of iron ions enhances the solubility of cerium ions in the crystal lattice. As can be observed from Figure 11b, perovskite diffraction patterns can be observed for La0.8Ce0.2Co0.9Mn0.1O3, La0.8Ce0.2Co0.8Mn0.2O3, La0.8Ce0.2Co0.7Mn0.3O3, and La0.8Ce0.2Co0.6Mn0.4O3, they all correspond to the perovskite-type cubic crystallized. There are amount of MnO2 (PDF# 00-053-0633), CeO2 (PDF# 97-002-8709), and Co3O4 (PDF# 00-009-0418) impurity phases in the crystal samples. LaCoO3 perovskite seems to accept a higher cobalt ions substitution degree by iron ions than manganese ions, without destabilizing the perovskite structure. In fact, the continuous series of solid solutions of atoms in perovskite crystals has not been realized. This may be due to the fact that cerium atoms cannot achieve continuous series of solid solutions in perovskite crystals. Through the above analysis, it can be concluded that the structure of La0.8Ce0.2Co0.7Fe0.3O3 and La0.8Ce0.2Co0.7Mn0.3O3 perovskite-type catalyst samples reaches the best catalyst conditions. As can be observed from Figure 11a,b, the characteristic diffraction peaks of La0.8Ce0.2CoO3 doping with cerium ions and calcium ions near 2θ = 33° moved right slightly. This information would confirm the accommodation of these compounds within the perovskite lattice.
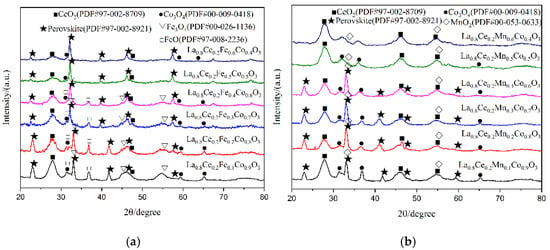
Figure 11.
XRD patterns of catalyst samples: (a) La0.8Ce0.2Co1−yFeyO3 catalyst samples; and (b) La0.8Ce0.2Co1−yMnyO3 catalyst samples.
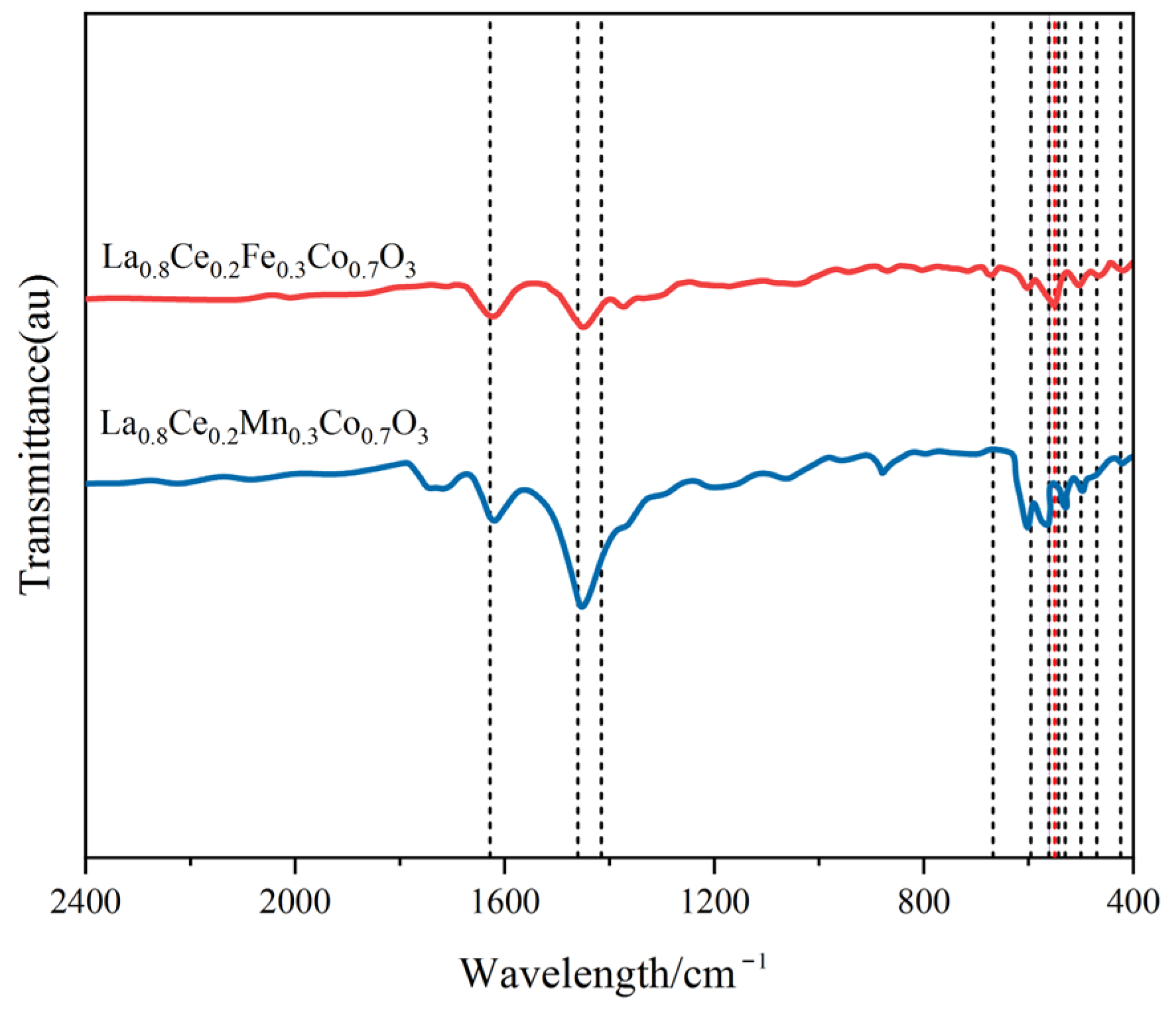
Figure 12 shows the FT-IR spectra of La0.8Ce0.2Co0.7Fe0.3O3 and La0.8Ce0.2Co0.7Mn0.3O3 catalyst samples. As can be observed from Figure 12, there are several vibration bands at 424, 470, 500, 530, 543, 550, 561, 562, 596, 668, 1460, and 1628 cm−1 for La0.8Ce0.2Co0.7Fe0.3O3 and La0.8Ce0.2Co0.7Mn0.3O3 catalyst samples. The vibration band at 470 and 550 cm−1 belongs to the bending vibration of Fe–O bonding in the BO6 octahedron, and the peak at 561 cm−1 is attributed to asymmetrical modes of the Mn–O bond of MnO6 octahedrons groups of LaMnO3 perovskite. The vibration band at 543 and 560 cm–1 belongs to the bending vibration of Fe–O bonding in the FeO and Fe3O4 crystals, respectively. The vibration band at 530 cm–1 belongs to the bending vibration of Mn–O bonding in the MnO2 crystals [39,40]. Several vibration band of some impurities like carbonate group and hydroxyl group also can be observed from Figure 12, which would not be analysed in this paper.
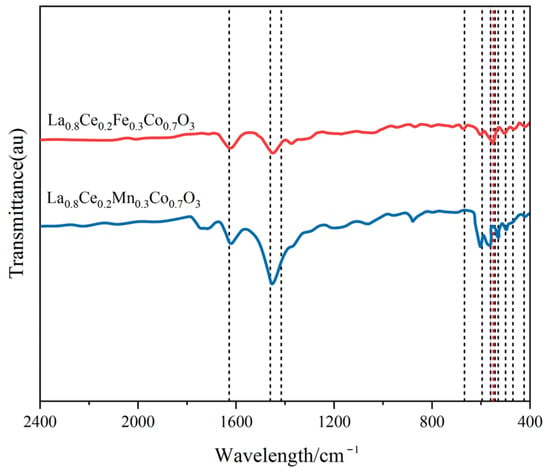
Figure 12.
FTIR spectra of La0.8Ce0.2Fe0.3Co0.7O3 and La0.8Ce0.2Mn0.3Co0.7O3 catalyst samples.
3.4. Results of Simulation Experiments on Catalyst Samples Doping at B Site
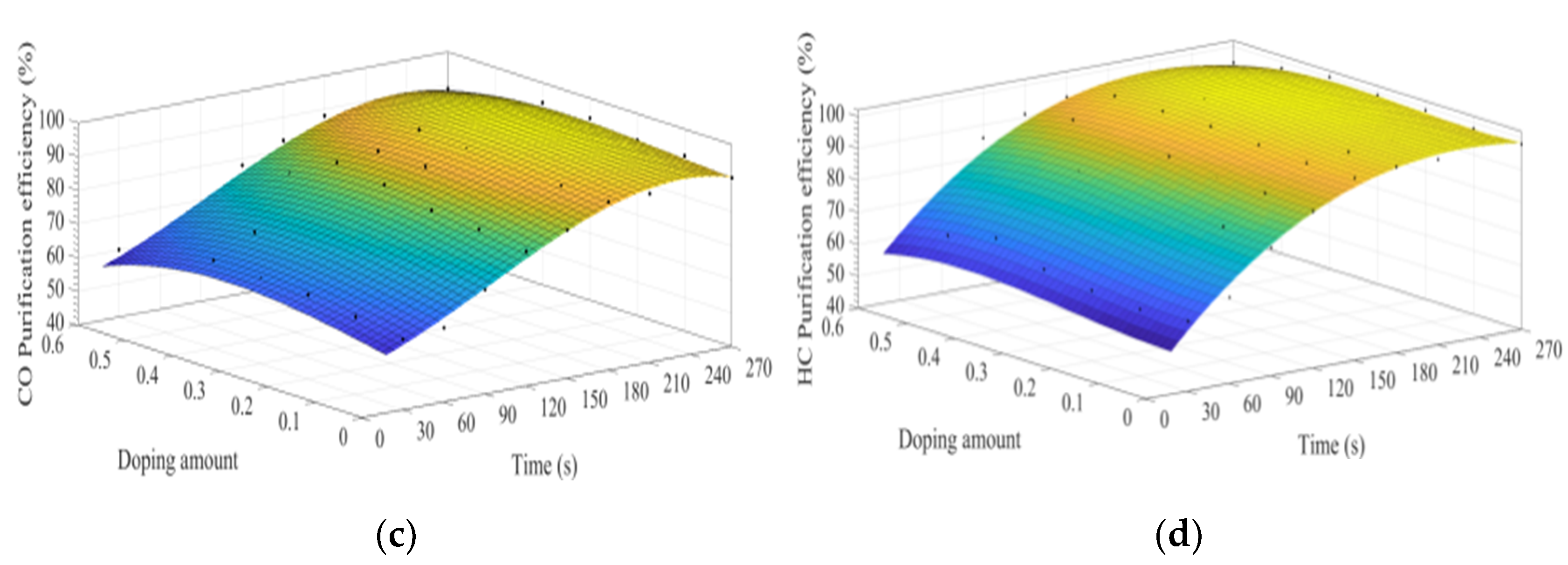
Figure 13a–d shows the fitting curves of the purification efficiencies of NOx, PM, HC, and CO over time when the B site was doped with different concentrations of iron ions. As can be observed from Figure 13, when the purification time does not exceed 90 s, the purification efficiency increases with time. When the time exceeds 90 s, the purification efficiency reaches the highest. When the purification time exceeds 180 s, the purification efficiency of NOx and PM shows an obvious downward trend. When y = 0.3, the purification efficiency can reach the highest. Compared with La0.8Ce0.2CoO3, the highest purification efficiency for PM and NOx is increased by 5% and 7%, respectively, while the purification efficiency for HC and CO is hardly improved.
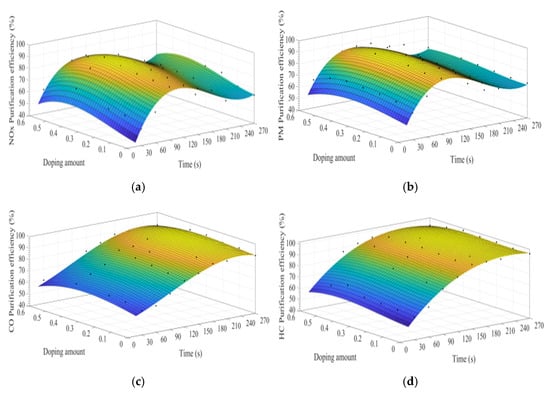
Figure 13.
Purification efficiency curve of catalyst when B site was doped with iron ions: (a) NOx; (b) PM; (c) CO; and (d) HC.
Figure 14a–d shows the time curve of the purification efficiency of NOx, PM, HC, and CO when the B site is doped with different concentrations of manganese ions. Comparison of perovskite-type catalysts doped with iron ions and manganese ions, the purification trend of NOx, PM, HC, and CO are basically the same. Compared with La0.8Ce0.2CoO3, when y = 0.3, the highest purification efficiency for PM and NOx is increased by 6% and 2%, which can be speculated that the doping of manganese ions at the B site is more conducive to the oxidation reaction, and the doping of iron ions at the B site is more conducive to the reduction reaction.
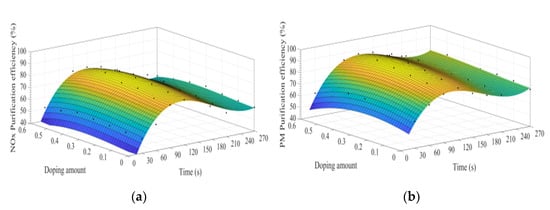
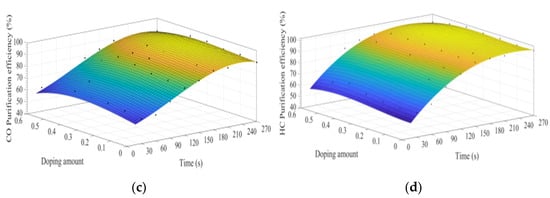
Figure 14.
Purification efficiency curve of catalyst when B site was doped with manganese ions: (a) NOx; (b) PM; (c) CO; and (d) HC.
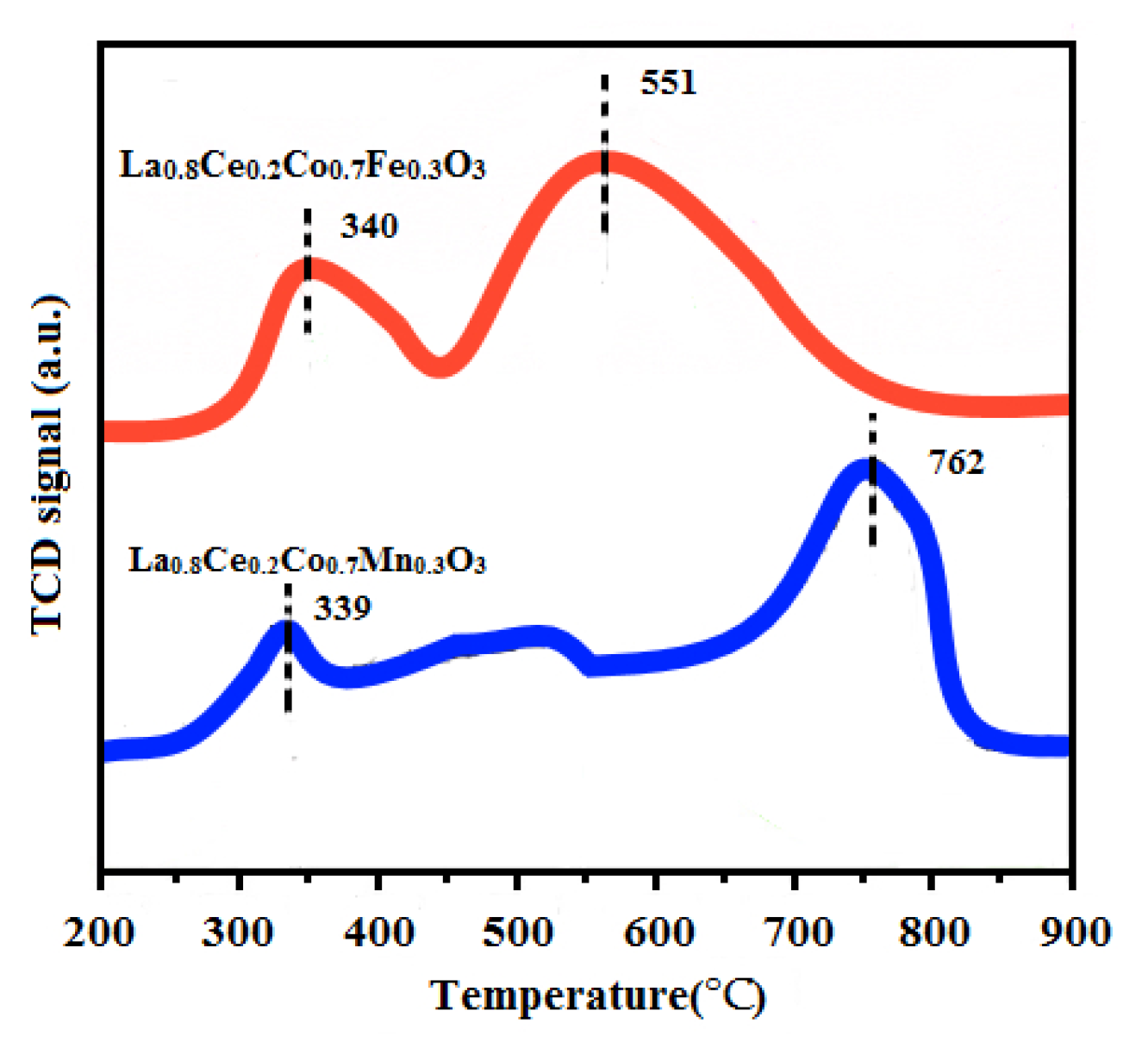
The H2-TPR experiments were conducted on a TPDRO instrument (TP-5080, Xianquan, Tianjin, China) using a thermal conductivity detector (TCD). H2-TPR techniques were used to measure the reducibility of La0.8Ce0.2Co0.7Fe0.3O3 and La0.8Ce0.2Co0.7Mn0.3O3 catalyst samples. The corresponding H2-TPR profiles are presented in Figure 15. Both of the samples exhibit two major reduction peaks, the first reduction peak of La0.8Ce0.2Co0.7Fe0.3O3 at 340 °C can be attributed to the reduction of Fe4+ to Fe3+ and consumption of adsorbed oxygen [41]. The first reduction peak of La0.8Ce0.2Co0.7Mn0.3O3 at 339 °C can be attributed to the reduction of Mn4+ to Mn3+ consumption of adsorbed oxygen [42]. The second reduction peak of La0.8Ce0.2Co0.7Fe0.3O3 appears at 551 °C, which can be ascribed to the reduction of Fe3+ to Fe2+ and consumption of lattice oxygen [43]. The second reduction peak of La0.8Ce0.2Co0.7Mn0.3O3 appears at 762 °C, which can be ascribed to the reduction of Mn3+ to Mn2+ and consumption of lattice oxygen [44]. Through the analysis above, it can be concluded that the redox properties and the oxygen mobility of the catalysts are important influencing factors in purification efficiency of NOx, PM, HC, and CO.
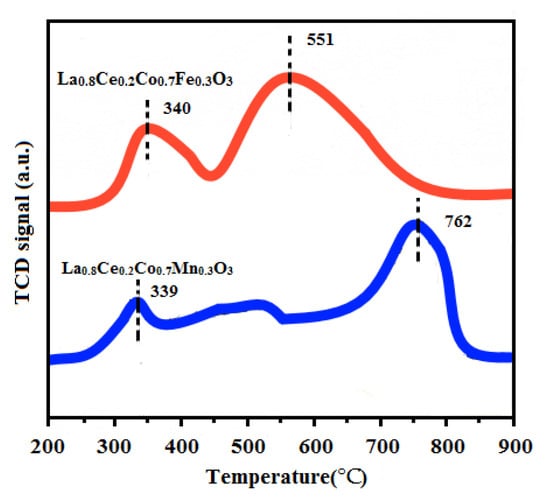
Figure 15.
H2-TPR profiles of La0.8Ce0.2Co0.7Fe0.3O3 and La0.8Ce0.2Co0.7Mn0.3O3.
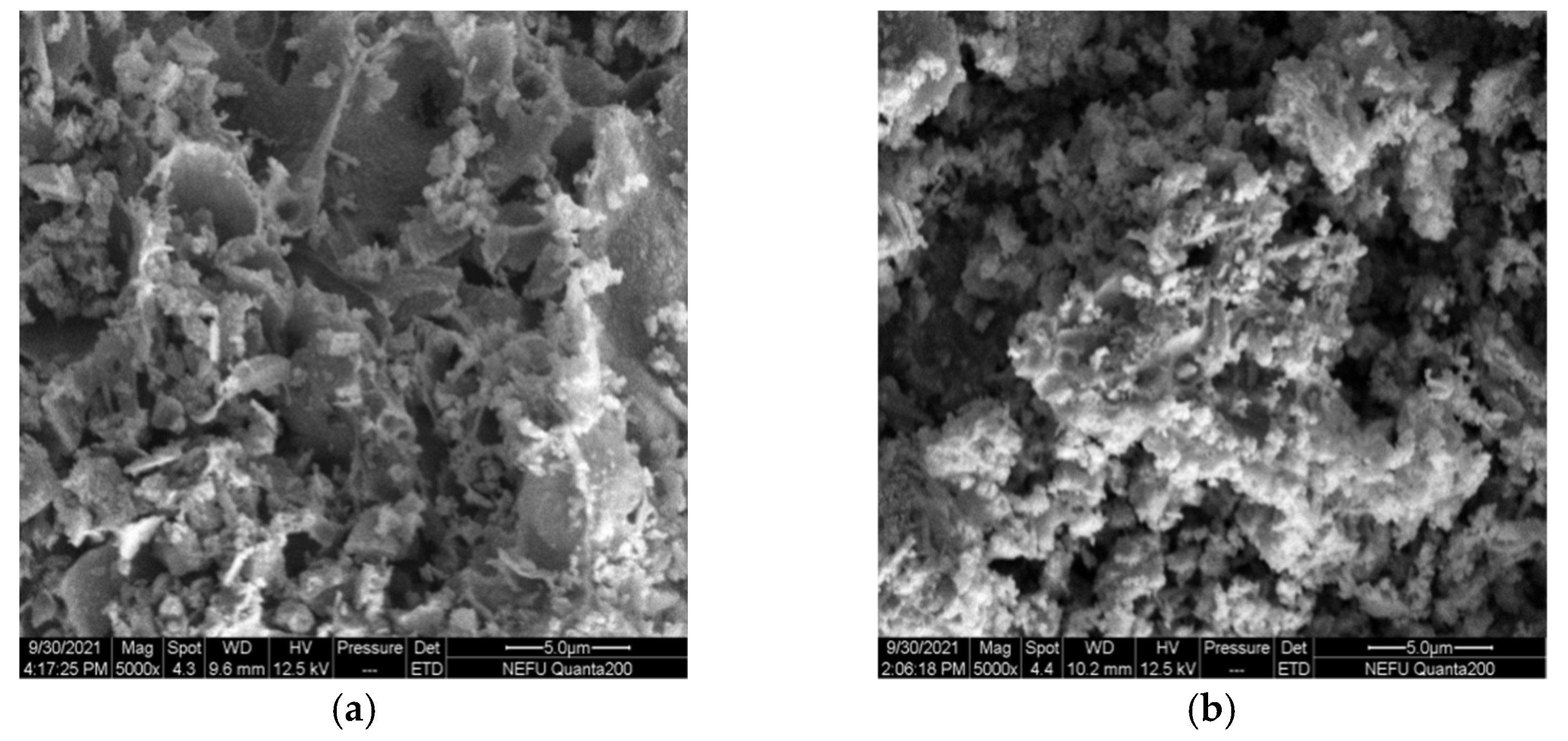
The SEM micrographs of La0.8Ce0.2Co0.7Fe0.3O3 and La0.8Ce0.2Co0.7Mn0.3O3 perovskite-type catalyst crystal samples are shown in Figure 16a,b, La0.8Ce0.2Co0.7Fe0.3O3 perovskite-type catalyst samples are more uniformly dispersed, and La0.8Ce0.2Co0.7Mn0.3O3 perovskite-type catalyst samples are partially reunited. Both of the perovskite-type catalyst samples exist porous structure. The macropore with diameter ranging from 3 µm to 5 µm in perovskite-type catalyst samples. Perovskite-type catalyst crystal samples can contact the PM and hazardous gas sufficiently emitted from engine owing to their high specific surface area and porosity [45]. La0.8Ce0.2Co0.7Fe0.3O3 perovskite-type catalyst samples were more conducive to the application in the field of automobile exhaust purification.
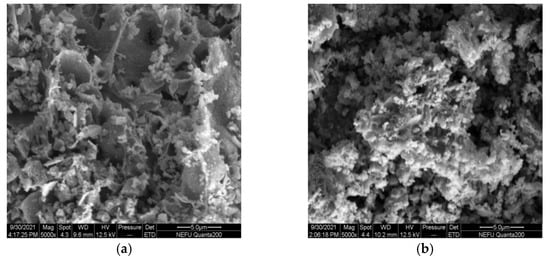
Figure 16.
SEM of catalyst samples: (a) La0.8Ce0.2Co0.7Fe0.3O3; and (b) La0.8Ce0.2Co0.7Mn0.3O3.
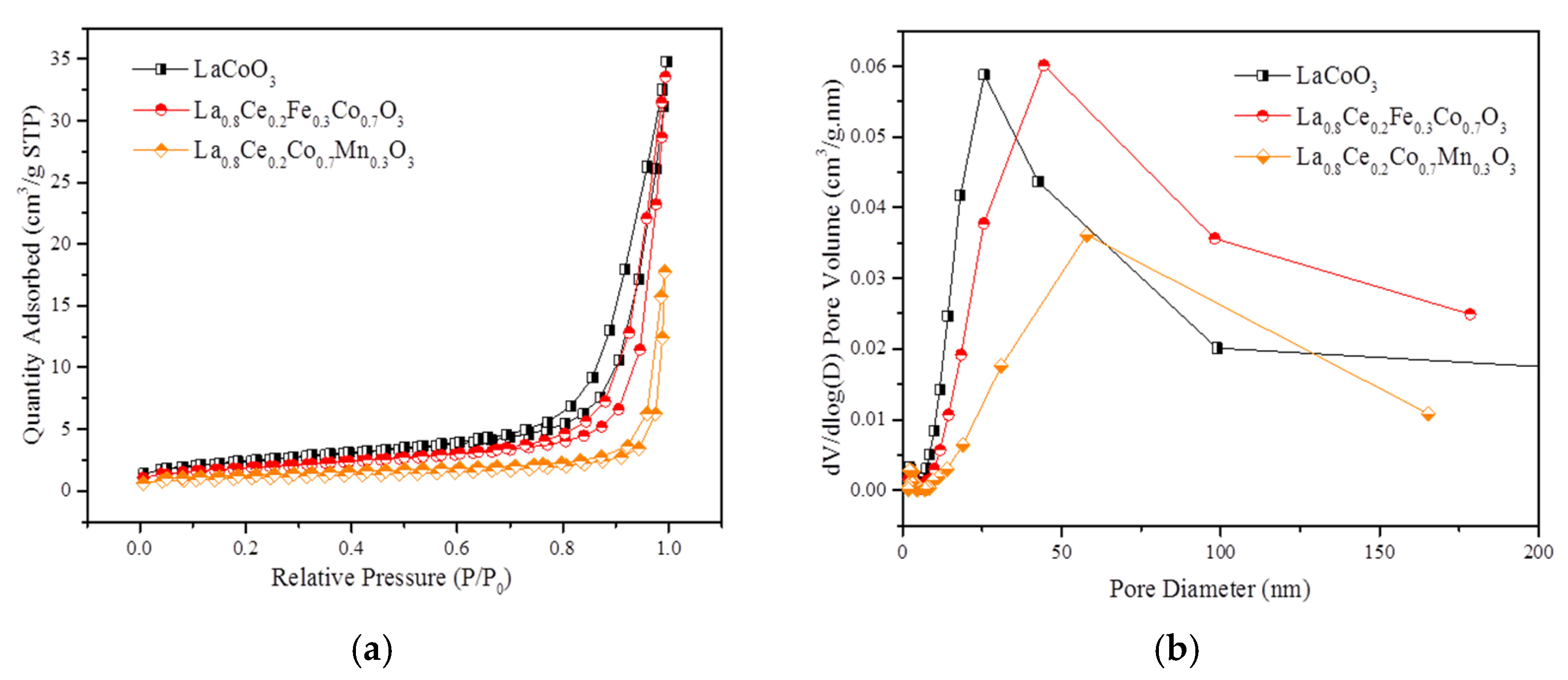
Figure 17a,b show N2 adsorption isotherms and pore size distribution (PSD) curves of LaCoO3, La0.8Ce0.2Fe0.3Co0.7O3, and La0.8Ce0.2Co0.7Mn0.3O3 perovskite-type catalyst samples separately. Based on the International Union of Pure and Applied Chemistry classification (IUPAC), the isotherms curves in Figure 17a reveals an H3 hysteresis loop related to mesoporous or macroporous materials. It can be seen that the order of specific surface area is LaCoO3, La0.8Ce0.2Fe0.3Co0.7O3, and La0.8Ce0.2Co0.7Mn0.3O3. Significant increase in nitrogen adsorption at high relative pressure (0.9 < P/P0 < 1) and the PSD curves illustrate the extensive existence of the mesopores and macropore, which are well consistent with the analysis of SEM. Figure 17b reveals that La0.8Ce0.2Fe0.3Co0.7O3 perovskite -type catalyst sample has the largest pore volume of mesopores (mainly concentrated in 50 nm). The detailed textural parameters of catalyst samples are listed in Table 2. It is widely accepted that the impurities formation as a consequence of the limited accommodation of A and B cations leads to a progressive decrease of specific surface area [46]. Based on the above analysis, it can be further inferred that the impurity content of La0.8Ce0.2Co0.7Fe0.3O3 perovskite-type catalyst crystal samples is less than that of La0.8Ce0.2Co0.7Mn0.3O3 perovskite-type catalyst crystal samples, which are consistent with the results of XRD and FT-IR.
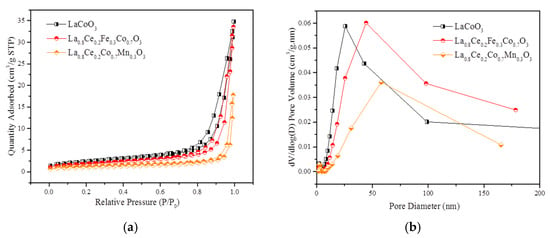
Figure 17.
N2 adsorption isotherms and PSD curves of catalyst samples: (a) N2 adsorption isotherms; and (b) PSD curves.

Table 2.
The Textural parameters of catalyst samples.
Based on the above analysis, the purification data for the best samples (x = 0.2; y = 0; 0.3) are shown in Table 3. It can be seen that appropriate doping of A and B sites is effective for the four-way purification efficiency. Combined with the analysis results of t SEM and BET, La0.8Ce0.2Co0.7Fe0.3O3 perovskite-type catalyst samples can be further proved to be the optimal doping concentration.

Table 3.
The purification data for the best samples (x = 0.2; y = 0; 0.3).
4. Conclusions
Several perovskite-type catalyst samples were developed for the four-way purification of diesel engine exhaust by doping A and B Sites in perovskite LaCoO3. The prepared perovskite-type catalyst samples were characterized by XRD, SEM, FT-IR, and BET analysis, and their activity were tested by simulation experiments. The following conclusions were reached:
- The porous perovskite structures can be observed from prepared perovskite-type catalyst samples. When the A-site and B-site doping amount of LaCoO3 exceeds a certain value, more impurity phases will be produced;
- Macropores of 3 µm to 5 µm are presented in La0.8Ce0.2Fe0.3Co0.7O3 and La0.8Ce0.2Co0.7Mn0.3O3 perovskite-type catalyst samples, and La0.8Ce0.2Fe0.3Co0.7O3 has the largest pore volume of mesopores (mainly concentrated in 50 nm);
- Doping the A site of LaCoO3 perovskite-type oxide can change the valence state of the B site ions to a certain extent, which is conducive to the occurrence of redox reactions;
- On the basis of cerium ions doping the A site of LaCoO3 perovskite-type oxide, the doping of manganese ions at the B site can further improve the purification ability on PM. However, the doping of iron ions at the B site can enhance the purification ability on PM and NOx; and
- La0.8Ce0.2Co0.7Fe0.3O3 shows the best purification ability and the least impurity phase in the samples, the purification efficiency of PM, NOx, HC, and CO are 95%, 92%, 94%, and 100%, respectively.
Author Contributions
X.G. and Y.W., data curation; Y.W. and S.Y., formal analysis; D.D. and S.Y., resources; Y.W. and D.D. methodology, Y.W. writing—original draft; X.G. and Y.W., writing—review and editing; X.G., funding acquisition and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (grant number 51972050).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be made available upon request.
Acknowledgments
The authors gratefully acknowledge the academic support of the Lab of Automobile Emission Testing and Control Laboratory, College of Mechanical and Electric Engineering at Northeast Forestry University, Harbin, China.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced, or appeared to have influenced, the work reported in this paper.
References
- Fujita, E.M.; Zielinska, B.; Campbell, D.E.; Arnott, W.P.; Sagebiel, J.C.; Mazzoleni, L.; Chow, J.C.; Gabele, P.A.; Crews, W.; Snow, R.; et al. Variations in Speciated Emissions from Spark-Ignition and Compression-Ignition Motor Vehicles in California’s South Coast Air Basin. J. Air Waste Manag. Assoc. 2007, 57, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Z.; Ge, D.-D.; Zhou, L.-F.; Hou, L.-Y.; Zhou, Y.; Li, Q.-Y. Effects of particulate matter on allergic respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, J.; Zhao, Z.; Song, W.; Wei, Y. A new 3DOM Ce-Fe-Ti material for simultaneously catalytic removal of PM and NOx from diesel engines. J. Hazard. Mater. 2018, 342, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Pacultová, K.; Draštíková, V.; Chromčcáková, Ž.; Bílková, K.T.; Kutláková, M.; Kotarba, A.; Obalová, L. On the stability of alkali metal promoters in Co mixed oxides during direct NO catalytic decomposition. Mol. Catal. 2017, 428, 33–40. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Dhal, G.C.; Mohan, D.; Prasad, R. Preparation and application of effective different catalysts for simultaneous control of diesel soot and NOX emissions: An overview. Catal. Sci. Technol. 2017, 7, 1803–1825. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Hu, Y.; Chi, Y.; Song, Y.; Ren, X. Research into Eliminating Particulate from Diesel Engine Exhaust over Zeolite Covered with Catalysts of Perovskite-Type Oxides. In Proceedings of the International Conference on Energy and Environment Technology, Guilin, China, 16–18 October 2009. [Google Scholar]
- Mei, X.; Xiong, J.; Wei, Y.; Zhang, Y.; Zhang, P.; Yu, Q.; Zhao, Z.; Liu, J. High-efficient non-noble metal catalysts of 3D ordered macroporous perovskite-type La2NiB’O6 for soot combustion: Insight into the synergistic effect of binary Ni and B’ sites. Appl. Catal. B Environ. 2020, 275, 119108. [Google Scholar] [CrossRef]
- Abedi, S.; Niaei, A.; Namjou, N.; Salari, D.; Tarjomannejad, A.; Izadkhah, B. Experimental and Modeling Study of CO-Selective Catalytic Reduction of NO Over Perovskite-Type Nanocatalysts. Period. Polytech. Chem. Eng. 2020, 64, 46–53. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, P.; Feng, N.; Wan, H.; Guan, G. Surface engineering on porous perovskite-type La0.6Sr0.4CoO3-δ nanotubes for an enhanced performance in diesel soot elimination. J. Hazard. Mater. 2020, 399, 123014. [Google Scholar] [CrossRef]
- Jiang, Q.; Cao, Y.; Liu, X.; Zhang, H.; Hong, H.; Jin, H. Chemical Looping Combustion over a Lanthanum Nickel Perovskite-Type Oxygen Carrier with Facilitated O2-Transport. Energy Fuels 2020, 34, 8732–8739. [Google Scholar] [CrossRef]
- Magnone, E.; Kim, J.R.; Park, J.H. Effect of synthesis method on oxygen adsorption/desorption properties of La-Sr-Co-FeO perovskite-typeoxide. J. Anal. Calorim. 2014, 117, 1221–1229. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, Z.; Li, K.; Yu, X.; Wei, Y.; Liu, J.; Peng, Y.; Li, Y.Z.; Chen, Y.S. Highly active monolith catalysts of LaKCoO3 perovskite-type complex oxide on alumina-wash coated diesel particulate filter and the catalytic performances for the combustion of soot. Catal. Today 2020, 339, 159–173. [Google Scholar] [CrossRef]
- Zhang, S.; An, K.; Li, S.; Zhang, Z.; Sun, R.; Liu, Y. Bi-active sites of stable and highly dispersed platinum and oxygen vacancy constructed by reducing a loaded perovskite-type oxide for CO oxidation. Appl. Surf. Sci. 2020, 532, 147455. [Google Scholar] [CrossRef]
- Peng, X.; Lin, H.; Shangguan, W.; Huang, Z. A highly efficient and porous catalyst for simultaneous removal of NOx and diesel soot. Catalysis Communications 2007, 8, 157–161. [Google Scholar] [CrossRef]
- De Lim, S.M.; Assaf, J.M. Synthesis and characterization of LaNiO3, LaNi(1−x)FexO3 and LaNi(1−x)CoxO3 perovskite oxides for catalysis application. Mater. Res. Bull. 2002, 5, 329–335. [Google Scholar]
- Yan, X.; Liu, J.; Yang, Y.; Wang, Z.; Zheng, Y. A catalytic reaction scheme for NO reduction by CO over Mn-terminated LaMnO3 perovskite: A DFT study. Fuel Processing Technol. 2021, 216, 106798. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Long, G.; Buckley, C.E.; Hu, X.; Dong, D. Electrospun La0.8Ce0.2Fe1−xNixO3 perovskite nanofibrous catalysts for CO oxidation. Appl. Surf. Sci. Adv. 2020, 2, 100030. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Strontium doping and impregnation onto alumina improve the NOx storage and reduction capacity of LaCoO3 perovskites. Catal. Today 2019, 333, 208–218. [Google Scholar] [CrossRef]
- Natile, M.M.; Ugel, E.; Maccato, C.; Glisenti, A. LaCoO3: Effect of synthesis conditions on properties and reactivity. Appl. Catal. B Environ. 2007, 72, 351–362. [Google Scholar] [CrossRef]
- Zhao, P.; Fang, F.; Feng, N.; Chen, C.; Liu, G.; Chen, L.; Zhu, Z.; Meng, J.; Wan, H.; Guan, G. Self-templating construction of mesopores on three-dimensionally ordered macroporous La0.5Sr0.5MnO3 perovskite with enhanced performance for soot combustion. Catal. Sci. Technol. 2019, 9, 1835–1846. [Google Scholar] [CrossRef]
- Xing, Z.; Kongzhai, L.; Neal, L.M.; Fanxing, L. Perovskites as geo-inspired oxygen storage materials for chemical looping and three-way catalysis—A perspective. ACS Catal. 2018, 8, 8213–8236. [Google Scholar]
- Yu, L.; Zhong, Q.; Deng, Z.; Zhang, S. Enhanced NOx removal performance of amorphous Ce-Ti catalyst by hydrogen pretreatment. J. Mol. Catal. A Chem. 2016, 423, 371–378. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.Y.; Kim, H.G. NO2-Assisted Soot Regeneration Behavior in a Diesel Particulate Filter with Heavy-Duty Diesel Exhaust Gases. Numer. Heat Transf. Part A Appl. 2010, 58, 725–739. [Google Scholar] [CrossRef]
- Danfeng, D.; Yinghui, W.; Xiurong, G. Experiment on the performance of the NTP synergisticwood fiber diesel exhaust purifier. J. Harbin. Eng. Univ. 2019, 40, 419–425. [Google Scholar]
- Qin, X.; Jie, X. Modern Catalytic Chemistry; Science Press: Beijing, China, 2016; pp. 310–312. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Jafari, M.F.; Khorram, S. Non-thermal plasma assisted synthesis and physicochemical characterizations of Co and Cu doped Ni/Al2O3 nanocatalysts used for dry reforming of methane. Int. J. Hydrog. Energy 2013, 38, 16048–16061. [Google Scholar] [CrossRef]
- Dhal, G.C.; Dey, S.; Mohan, D.; Prasad, R. Study of Fe, Co, and Mn-based perovskite-type catalysts for the simultaneous control of soot and NOx from diesel engine exhaust. Mater. Discov. 2017, 10, 37–42. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Zha, Y.; Dai, F.; Hu, T.; Xie, Y.; Zhang, J. Highly efficient multifunctional dually-substituted perovskite catalysts La1−xKxCo1−yCuyO3-δ used for soot combustion, NOx storage and simultaneous NOx-soot removal. Appl. Catal. B Environ. 2012, 121, 65–74. [Google Scholar] [CrossRef]
- Dacquin, J.P.; Dujardin, C.; Granger, P. Surface reconstruction of supported Pd on LaCoO3: Consequences on the catalytic properties in the decomposition of N2O. J. Catal. 2008, 253, 37–49. [Google Scholar] [CrossRef]
- Wu, S.; Song, C.; Bin, F.; Lv, G.; Song, J.; Gong, C. La1−xCexMn1−yCoyO3 perovskite oxides: Preparation, physico-chemical properties and catalytic activity for the reduction of diesel soot. Mater. Chem. Phys. 2014, 148, 181–189. [Google Scholar] [CrossRef]
- Zhu, J.; Thomas, A. Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B Environ. 2009, 92, 225–233. [Google Scholar] [CrossRef]
- Leontiou, A.A.; Ladavos, A.K.; Armatas, G.S.; Trikalitis, P.N.; Pomonis, P.J. Kinetics investigation of NO + CO reaction on La–Sr–Mn–O perovskite-type mixed oxides. Appl. Catal. A Gen. 2004, 263, 227–239. [Google Scholar] [CrossRef]
- Ladavos, A.K.; Pomonis, P.J. Mechanistic aspects of NO + CO reaction on La2−xSrxNiO4-δ (x = 0.00–1.50) perovskite-type oxides. Appl. Catal. A Gen. 1997, 165, 73–85. [Google Scholar] [CrossRef]
- Yao, W.; Wang, R.; Yang, X. LaCo1−xPdxO3 Perovskite-Type Oxides: Synthesis, Characterization and Simultaneous Removal of NOx and Diesel Soot. Catal. Lett. 2009, 130, 613–621. [Google Scholar] [CrossRef]
- Wu, H.; Hu, R.; Zhou, T.; Li, C.; Meng, W.; Yang, J. A novel efficient boron-doped LaFeO3 photocatalyst with large specific surface area for phenol degradation under simulated sunlight. Cryst. Eng. Comm. 2015, 17, 3859–3865. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Zhao, Z.; Xu, C.; Zheng, J.; Duan, A.; Jiang, G. Easy synthesis of three dimensionally ordered macroporous La1−xKxCoO3 catalysts and their high activities for the catalytic combustion of soot. J. Catal. 2011, 282, 1–12. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, Y.; Li, X. Hierarchically porous LaFeO3 perovskite prepared from the pomelo peel bio-template for catalytic oxidation of NO. J. Phys. Chem. Solids 2018, 116, 43–49. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Pantaleo, G.; La Parola, V.; Valverde, J.L.; Gil, S.; Liotta, L.F.; Giroir-Fendler, A. The effect of citric acid concentration on the properties of LaMnO3 as a catalyst for hydrocarbon oxidation. Catalysts 2019, 9, 226. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, X.; Hu, W.; Zheng, C.; Yang, Y.; Chen, M.; Gao, X. Plasma-catalytic decomposition of ethyl acetate over LaMO3 (M = Mn, Fe, and Co) perovskite catalysts. J. Ind. Eng. Chem. 2019, 70, 447–452. [Google Scholar] [CrossRef]
- Royer, S.; Alamdari, H.; Duprez, D.; Kaliaguine, S. Oxygen storage capacity of La1−xA’xBO3 perovskites (with A’ = Sr, Ce; B = Co, Mn)-relation with catalytic activity in the CH4 oxidation reaction. Appl. Catal. B Environ. 2005, 58, 273–288. [Google Scholar] [CrossRef]
- Tarjomannejad, A.; Zonouz, P.R.; Masoumi, M.E.; Niaei, A.; Farzi, A. LaFeO3 perovskites obtained from different methods for NO + CO reaction, modeling and optimization of synthesis process by response surface methodology. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2012–2022. [Google Scholar] [CrossRef]
- Ponce, S.; Peña, M.; Fierro, J.L. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Appl. Catal. B Environ. 2000, 24, 193–205. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Wang, J.; Su, Y.; Zhao, Z. Catalytic performance of NO oxidation over LaMeO3 (Me = Mn, Fe, Co) perovskite prepared by the sol-gel method. Catal. Commun. 2013, 37, 105–108. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; Cabrejas, I.; De-La-Torre, U.; González-Velasco, J.R. Ba-doped vs. Sr-doped LaCoO3 perovskites as base catalyst in diesel exhaust purification. Mol. Catal. 2020, 488, 110913. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).