Concrete Durability after Load Damage and Salt Freeze–Thaw Cycles
Abstract
:1. Introduction
2. Raw Materials and Experimental Scheme
2.1. Raw Materials
2.2. Test Methods
3. Mechanical Performance of Concrete Samples
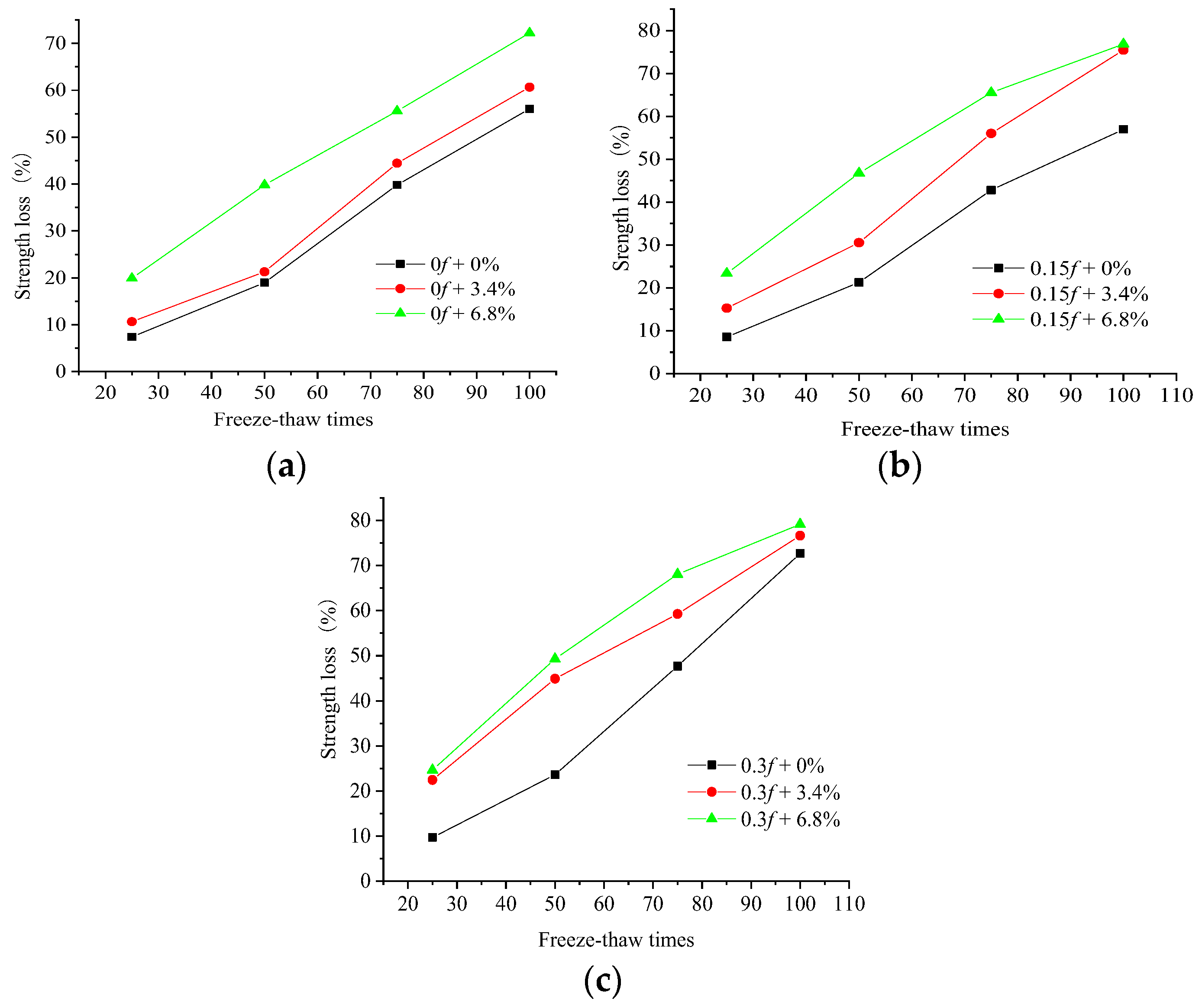
3.1. Compressive Strength Change
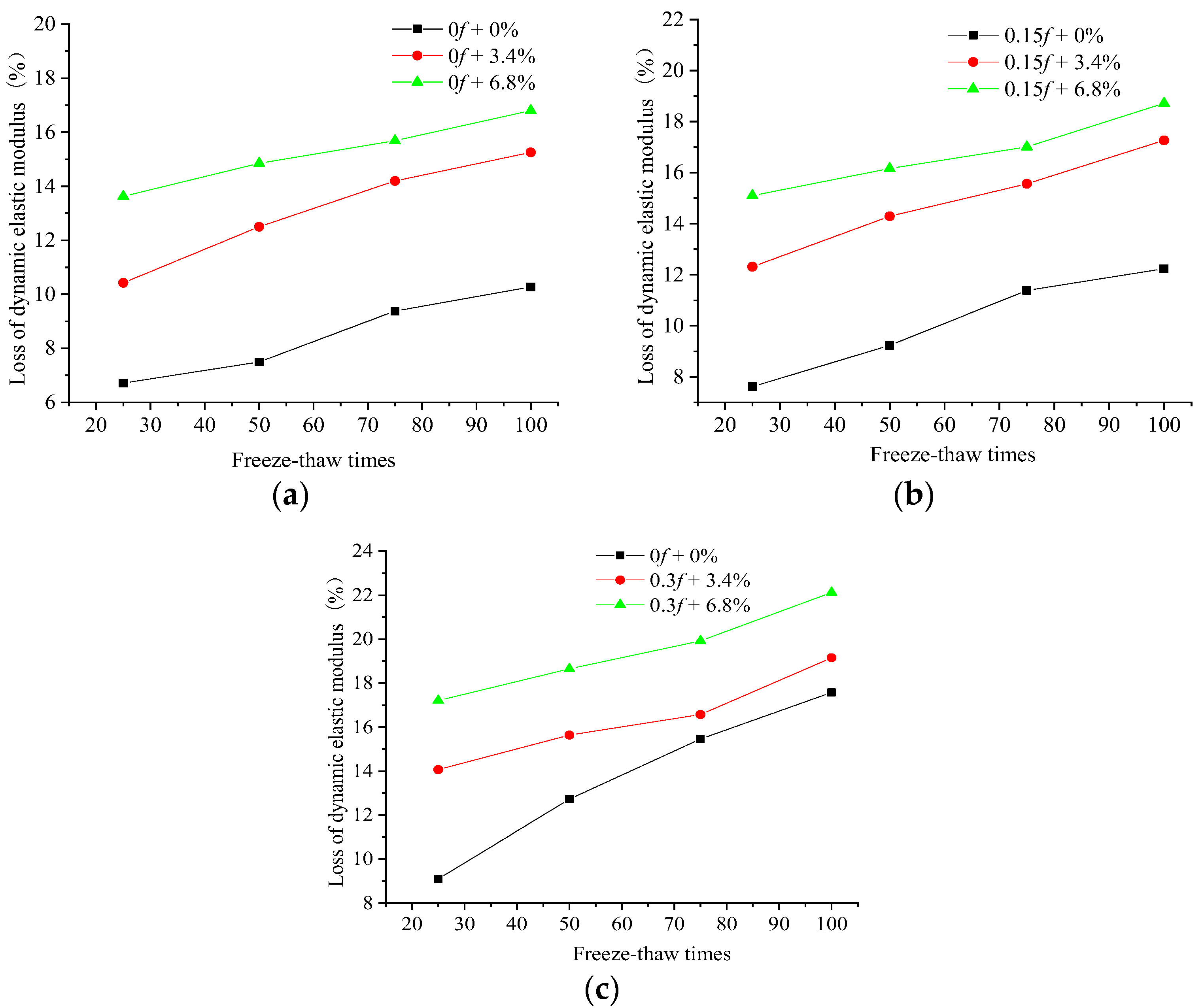
3.2. Dynamic Elastic Modulus Change
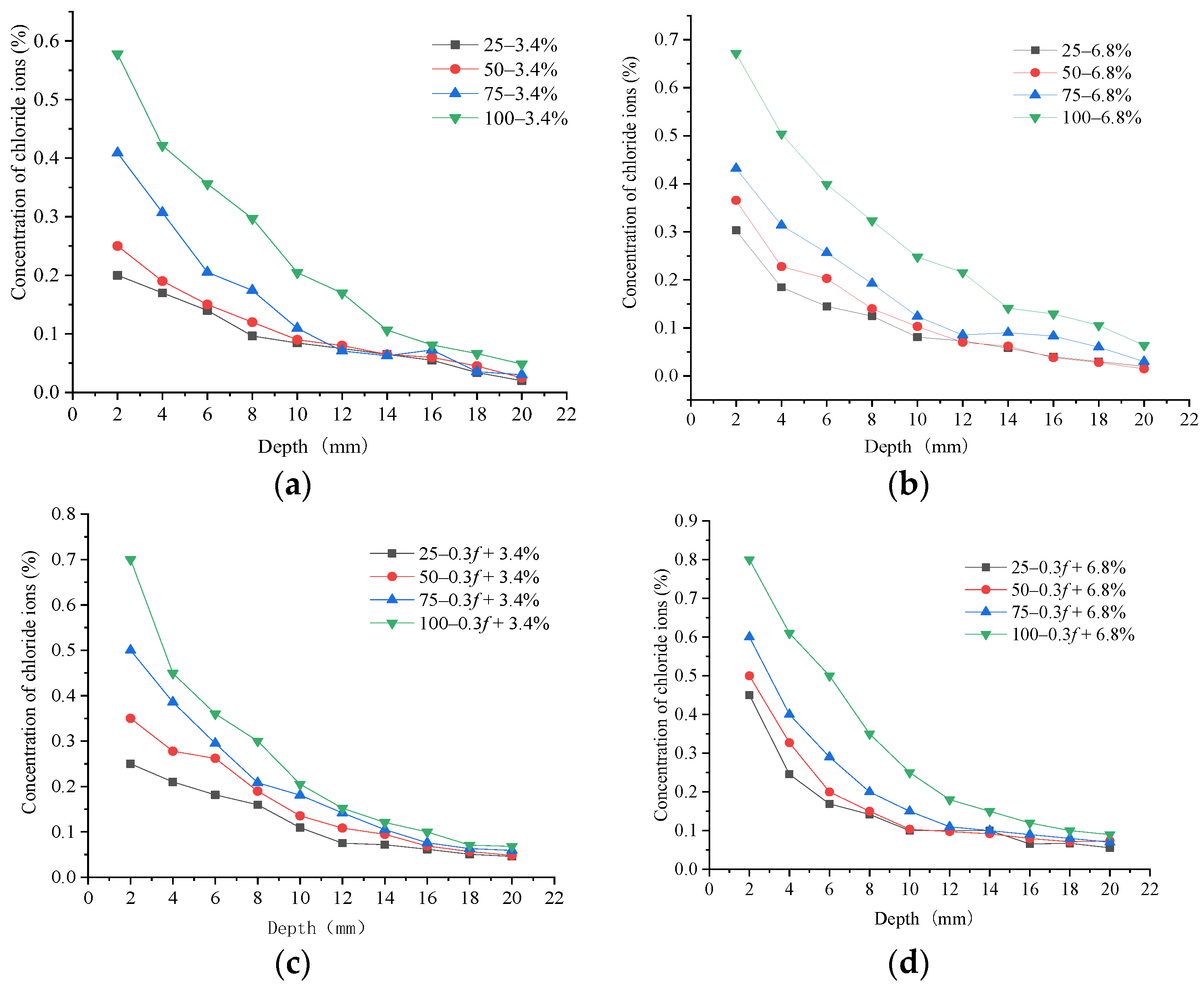
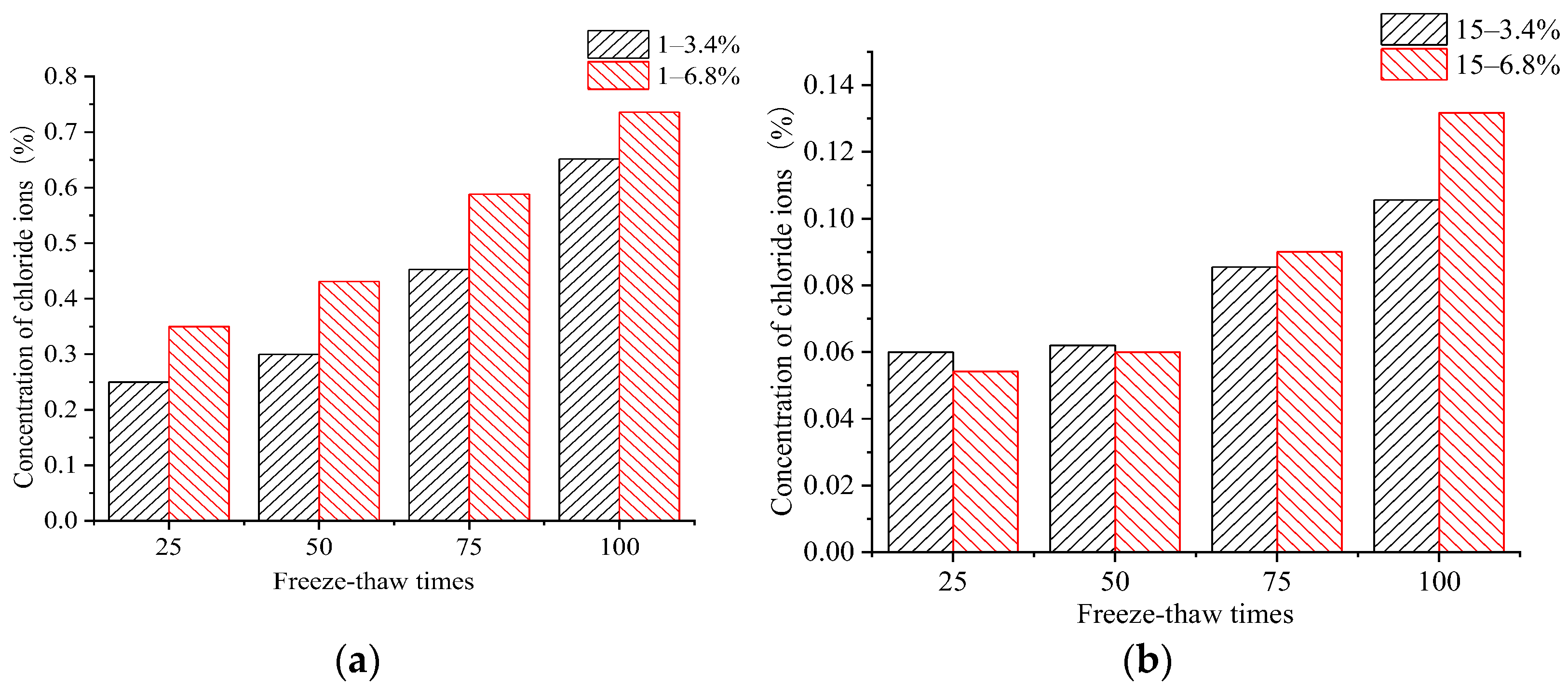
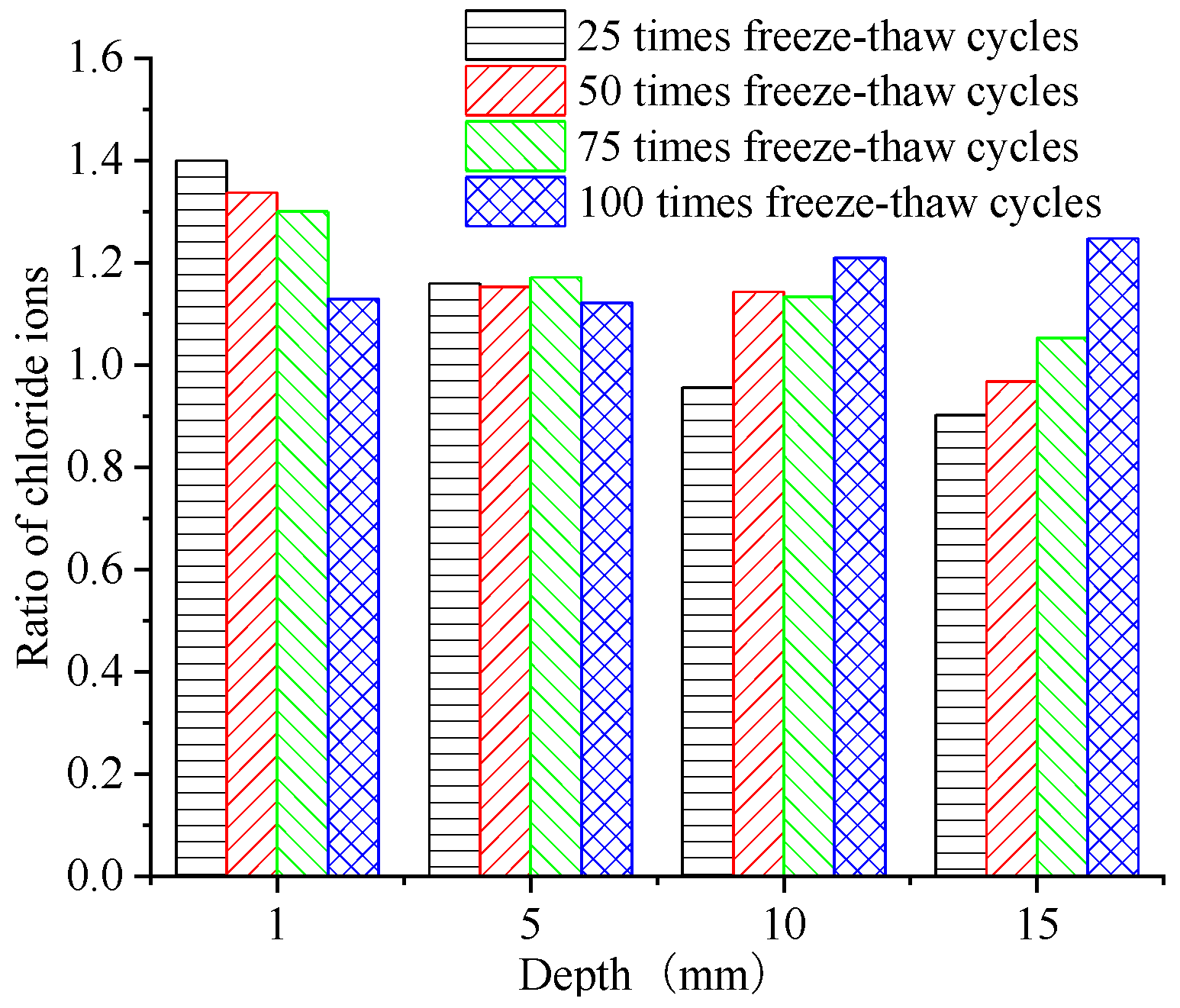
4. Performance of Chloride Ion Erosion in Concrete Samples
5. Pore Structure Characteristics of Concrete Samples
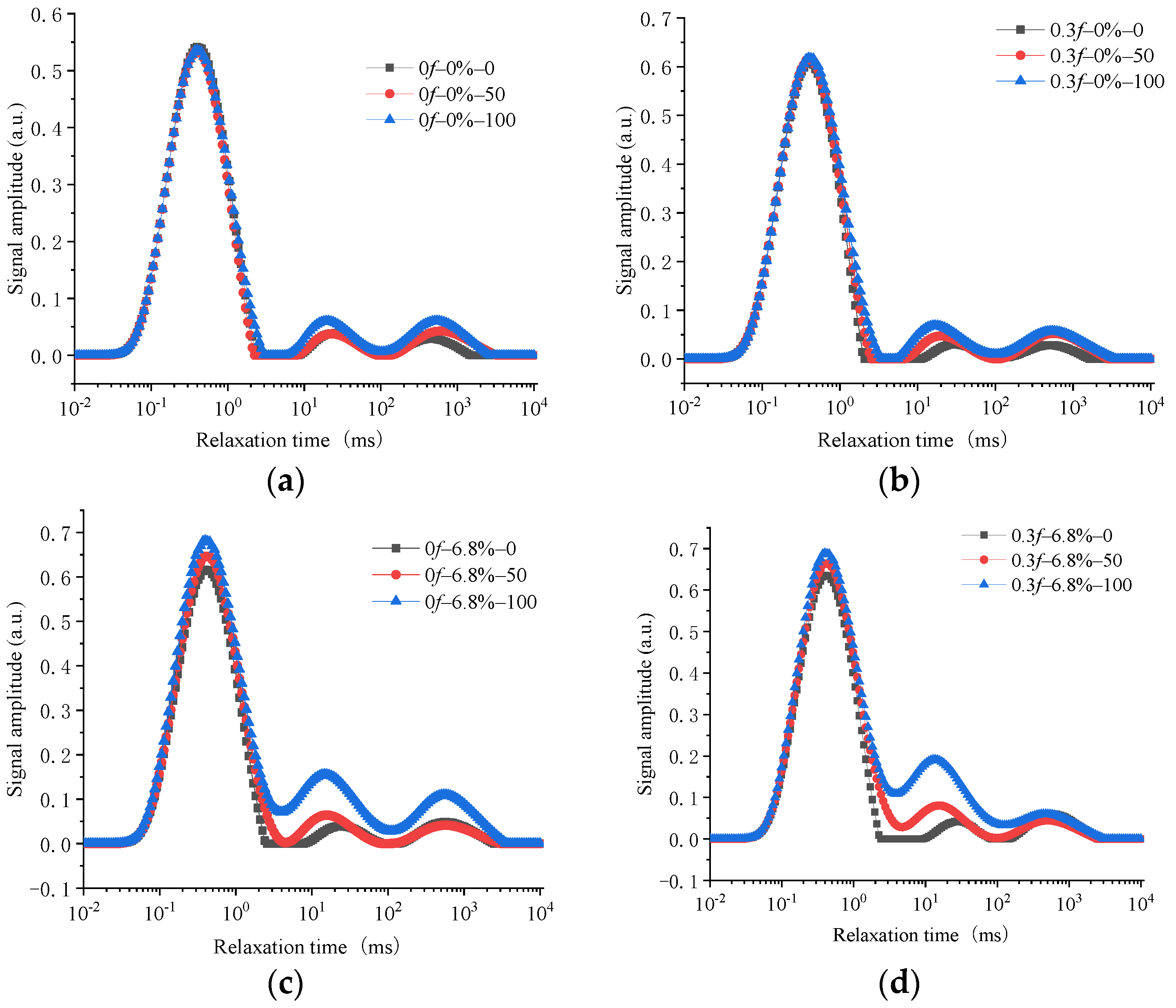
5.1. T2 Spectrum Characteristics
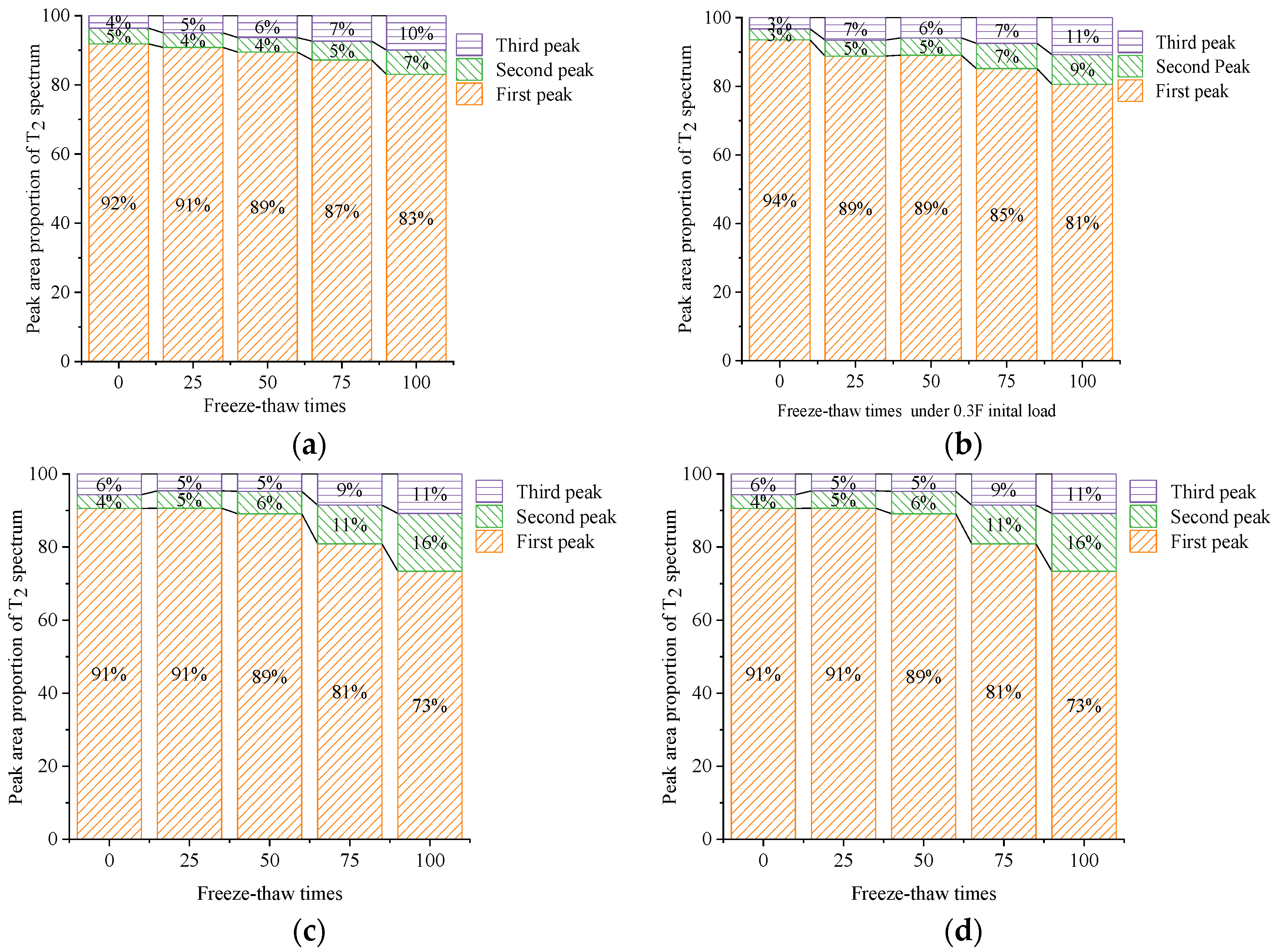
5.2. Ratio of the Peak Area of the T2 Spectrum
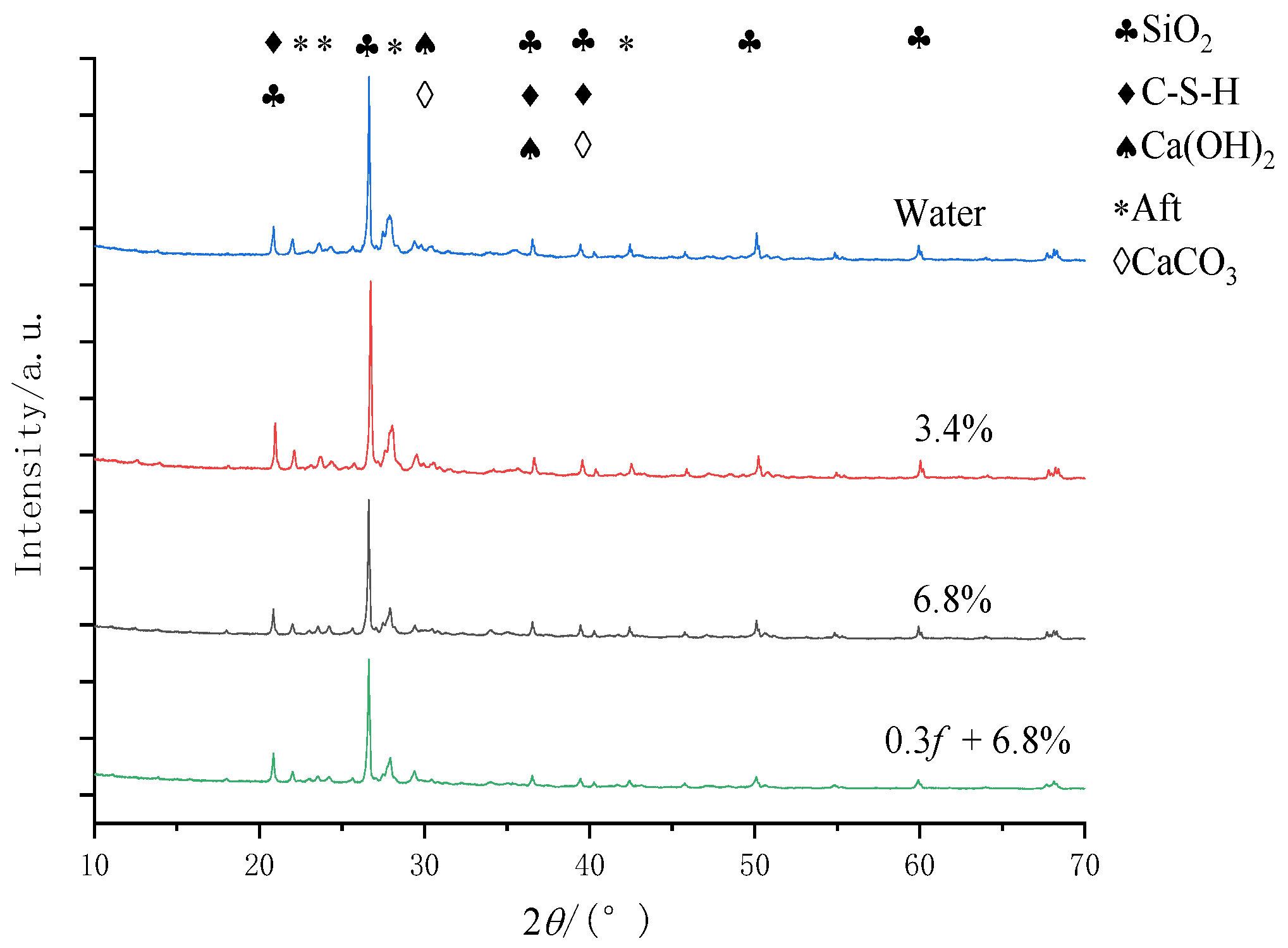
6. Ingredient Characteristics of Concrete Samples
6.1. Principal Component Analysis
6.2. Component Characteristics
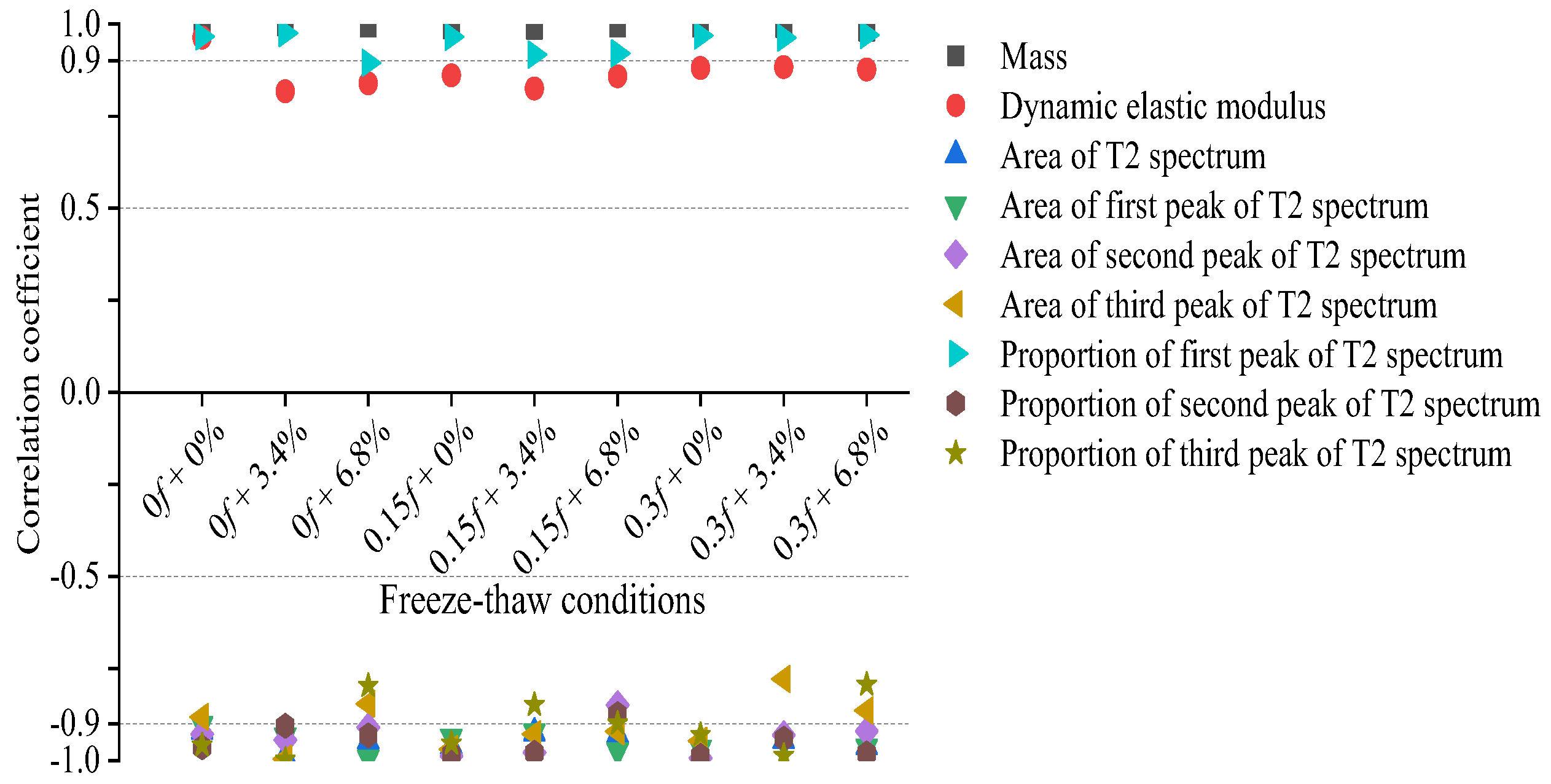
7. Correlation Analysis
8. Conclusions
- (1)
- When the initially loaded concrete samples underwent FTCs in freshwater or salt solutions, internal damage occurred, and more internal cracks were generated. The combined action of the initial load and the salt freeze–thaws led to more severe damage to the concrete.
- (2)
- When concrete samples underwent FTCs in the low-concentration salt solution, the negative effect of the salt produced considerable freezing pressures inside the concrete, which led to more severe damage. The increase in the number of FTCs intensified the concentration of chloride ions, which eroded the concrete samples.
- (3)
- It can be seen from the change in the microscopic structure of the concrete materials under FTCs in freshwater and salt solutions that the overall porosity of the concrete increased with an increasing number of FTCs, and the proportion of mid-sized pores and large pores and cracks gradually increased inside the concrete. During the FTCs, the compressive strength and DEM of the concrete gradually decreased, which had a close correlation with the proportion of the increase in the mid-sized pores and large pores and cracks.
- (4)
- This paper discusses the mechanical, physical, and microscopic pore change properties of ordinary cement specimens under FTCs in a low-concentration salt solution and concrete specimens subjected to low-stress initial loads. There is still a need for further studies on the mechanical, physical, and internal pore morphology changes of high-strength cement concrete or concrete under FTCs subjected to high-stress initial loads.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, B.; Li, W.; Tang, Z.; Tam, V.W.; Sun, Z. Investigation on properties of recycled aggregate concrete under coupling loading and freeze-thaw cycles in salt-solution. Constr. Build. Mater. 2018, 163, 840–849. [Google Scholar] [CrossRef]
- Chalangaran, N.; Jabbari, M.M. Experimental Investigation into Sound Transmission Loss through Concrete Containing Recycled Rubber. Amirkabir J. Mech. Eng. 2019, 51, 131–140. [Google Scholar]
- Chalangaran, N.; Farzampour, A.; Paslar, N. Nano Silica and Metakaolin Effects on the Behavior of Concrete Containing Rubber Crumbs. CivilEng 2020, 1, 264–274. [Google Scholar] [CrossRef]
- Wu, P.; Yang, Q.; Xu, J. Effects of a low-harm deicing salt on the salt-frost scaling of concrete and its mechanism. J. Build. Mater. 2020, 23, 317–321+327. [Google Scholar]
- Wu, J.; Zhang, J.; Diao, B.; Cheng, S.; Ye, Y. Hysteretic behavior of eccentrically load reinforced air-entrained concrete columns under combined effects of freeze-thaw cycles and seawater corrosion. Adv. Civ. Eng. 2018, 2018, 3931791. [Google Scholar]
- Wang, R.; Zhang, Q.; Li, Y. Deterioration of concrete under the coupling effects of freeze–thaw cycles and other actions: A review. Constr. Build. Mater. 2022, 319, 126045. [Google Scholar] [CrossRef]
- Chen, D.; Deng, Y.; Shen, J.; Sun, G.; Shi, J. Study on damage rules on concrete under corrosion of freeze-thaw and saline solution. Constr. Build. Mater. 2021, 304, 124617. [Google Scholar] [CrossRef]
- Li, Y. Study on the Physical and Mechanical Properties and Damage Mechanism of Concrete Mixed with Mineral Admixture in Marine Corrosion and Freeze-Thaw Environment; China University Mining Technology: Xuzhou, China, 2015. [Google Scholar]
- Xu, G.; Gong, C.; Liu, J.; Gao, D.J.; Zeng, Z. Correlation between water freeze–thaw resistance and salt freeze–thaw resistance of concrete. J. Build. Mater. 2020, 23, 552–556+562. [Google Scholar]
- Yang, Q.B. One of mechanisoms on the deicer-frost scaling of concrete (II): Degree of saturation and ice-formation pressure during freeze–thaw cycles. J. Build. Mater. 2012, 15, 741–746. [Google Scholar]
- Wang, F.; Zhang, Z.; Li, M.; Huang, D.; Zhu, Y. Influence of chloride solution concentration difference on salt frost scaling of concrete. J. Build. Mater. 2014, 17, 138–142. [Google Scholar]
- Xiaoping, S.U. Research on the Concrete Durability Due to Salinized Soil in the Western Region of Jilin Province; Jilin University-China: Changchun, China, 2013. [Google Scholar]
- Jiang, W.Q.; Liu, Q.F. Chloride transport in concrete subjected to freeze–thaw cycles- a short review. J. Chin. Ceram. Soc. 2020, 48, 258–272. [Google Scholar]
- Zhao, J.; Cai, G.; Gao, D.; Zhao, S. Influences of freeze–thaw cycle and curing time on chloride ion penetration resistance of Sulphoaluminate cement concrete. Constr. Build. Mater. 2014, 53, 305–311. [Google Scholar] [CrossRef]
- Wang, X. Research on the Durability of Natural Pumice Concrete in Saline Solution Environment of Cold Regions; Inner Mongolia University: Huhehaote, China, 2015. [Google Scholar]
- Zhao, Y.; Liu, F.; Wang, L.; Guo, Z. Modeling of compressive strength of concrete based on pore structure under single-side freeze–thaw condition. J. Build. Mater. 2020, 23, 1328–1336+1344. [Google Scholar]
- Han, J.W.; Yan, C.C.; Hu, W.L.; Zhu, S.R.; Yan, S.L. Pores evolution and characterization of hardened cement mortar with different water/cement ratios under capillary suction of deicing solution freeze-thaw action. J. Chin. Ceram. Soc. 2020, 48, 1842–1851. [Google Scholar]
- Wang, Y.; An, M.Z.; Yu, Z.R. Durability of C50 high performance concrete under the coupled action of chloride salt erosion and freeze-thaw cycle. China Railw. Sci. 2014, 35, 41–46. [Google Scholar]
- Mehta, P.K.; Monteiro, P. Concrete: Structure, Properties and Materials; Prentice-Hall: Upper Saddle River, NJ, USA, 2013; p. 499. [Google Scholar]
- Samaha, H.R.; Hover, K.C. In¯uence of microcracking on the mass transport properties of concrete. ACI Mater. J. 1992, 89, 416–424. [Google Scholar]
- Lim, C.C.; Gowripalan, N.; Sirivivatnanon, V. Microcracking and chloride permeability of concrete under uniaxial compression. Cem. Concr. Compos. 2000, 22, 353–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Transport properties in unsaturated cement-based materials—A review. Constr. Build. Mater. 2014, 72, 367–379. [Google Scholar] [CrossRef]
- Luo, D.; Di-tao, N.D.; Li, S. Research progress on durability of stressed concrete under environmental actions. Eng. Mech. 2019, 36, 1–14. [Google Scholar]
- Du, X.; Jin, L.; Zhang, R. Review on effect of external mechanical loadings on chloride penetration and diffusion into concrete. J. Build. Struct. 2016, 37, 107–125. [Google Scholar]
- GB/T50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Architecture & Building Press: Beijing, China, 2019.
- Lei, B.; Lu, Y.; Li, X.; Xiong, J.; Ding, C. Laboratory simulation method of concrete durability under multi-factor coupling actions. Exp. Technol. Manag. 2020, 37, 58–63. [Google Scholar]
- GB/T 50082–2009; Standard of Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2019.
- Xue, W.; Liu, X.; Yao, Z.; Hua, C.; Li, H. Effects of different damage sources on pore structure change characteristics of basalt fiber reinforced concrete. Acta Mater. Compos. Sin. 2020, 37, 2285–2293. [Google Scholar]
- Xing, B.Y.; Cheng, P.Y.; Tang, J.P.; Xie, E.H.; Zeng, Q.; Zhou, C.S. Pore structure evolution of water-saturated mortar under freeze-thaw cycles. J. Chin. Ceram. Soc. 2021, 49, 331–339. [Google Scholar]
- Zhou, Y.X.; Shen, X.D.; Li, G.F. Micropore of aeolian sand concrete under MgSO4-freeze-thaw cycles. J. Build. Mater. 2018, 21, 817–824. [Google Scholar]
- Dong, R.; Shen, X.; Xue, H.; Liu, Q. Sulfate resistance mechanism of aeolian sand concrete under dry-wet cycles. Mater. Rep. 2020, 34, 24040–24044. [Google Scholar]
- Zhou, K.P.; Hu, Z.X.; Gao, F.; Wang, M.Q.; Yang, Z. Study of marble damage laws under triaxial compression condition based on nuclear magnetic resonance technique. Rock Soil Mech. 2014, 35, 3117–3122. [Google Scholar]
- Lu, Y.; Wang, L.; Ge, Z.; Zhou, Z.; Deng, K.; Zuo, S. Fracture and pore structure dynamic evolution of coals during hydraulic fracturing. Fuel 2019, 10, 116272. [Google Scholar] [CrossRef]


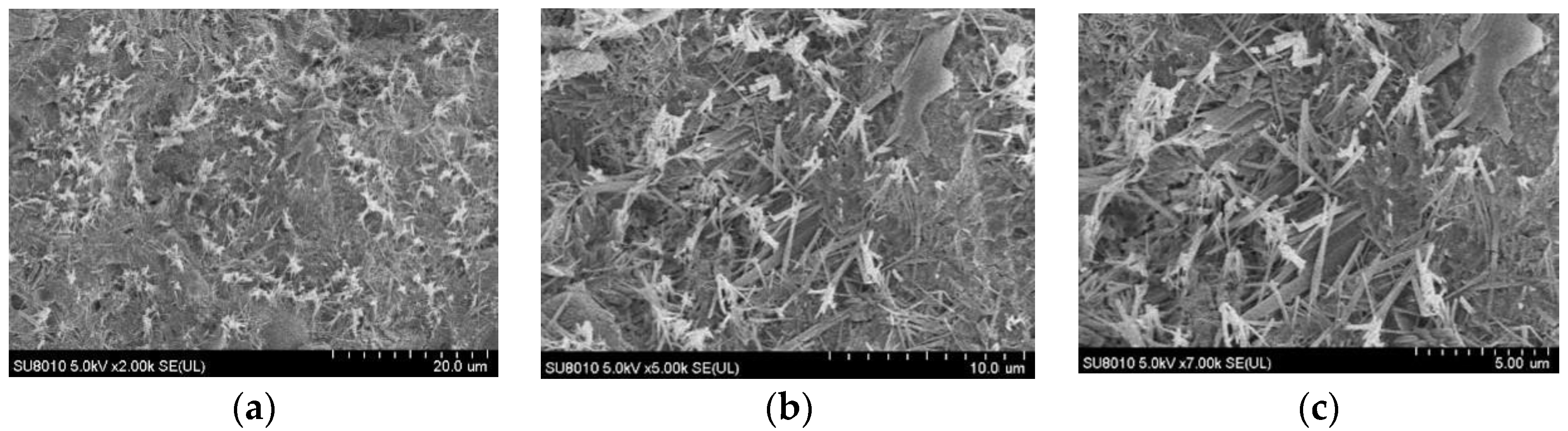

| Cement | Water | Fly Ash | Fine Aggregate | Coarse Aggregate | 7 d Compressive Strength | 28 d Compressive Strength |
|---|---|---|---|---|---|---|
| 500 | 250 | 55 | 600 | 1105 | 32.8 | 42.5 |
| Stress Levels | Salt Solution Ratio of Rapid Freeze–Thaw (g/L) | Solution Concentration | Freeze–Thaw Cycles | ||
|---|---|---|---|---|---|
| Na2SO4 | NaCl | NaHCO3 | |||
| 0, 15%, 30% | 0 | 0 | 0 | 0 | 0, 25, 50, 100 |
| 0, 15%, 30% | 13.36 | 7.46 | 14.38 | 3.4% | 0, 25, 50, 100 |
| 0, 15%, 30% | 26.72 | 14.92 | 28.76 | 6.8% | 0, 25, 50, 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, G.; Liu, P.; Guo, X.; Xu, J. Concrete Durability after Load Damage and Salt Freeze–Thaw Cycles. Materials 2022, 15, 4380. https://doi.org/10.3390/ma15134380
Zhou J, Wang G, Liu P, Guo X, Xu J. Concrete Durability after Load Damage and Salt Freeze–Thaw Cycles. Materials. 2022; 15(13):4380. https://doi.org/10.3390/ma15134380
Chicago/Turabian StyleZhou, Jiguo, Guihua Wang, Peng Liu, Xuefeng Guo, and Jun Xu. 2022. "Concrete Durability after Load Damage and Salt Freeze–Thaw Cycles" Materials 15, no. 13: 4380. https://doi.org/10.3390/ma15134380







