Graphene: A Path-Breaking Discovery for Energy Storage and Sustainability
Abstract
:1. Introduction
2. Graphene Synthesis
3. Supercapacitors
3.1. Performance of Supercapacitor
3.2. Electrically Conductive Polymers
3.3. Portable Electronic Devices
3.4. Hybrid Supercapacitors
4. Batteries
4.1. Li-ion Battery
4.1.1. Optimization
4.1.2. Capacity
4.1.3. Terminals
4.1.4. Advancements
4.2. Sodium and Calcium Ion Battery
4.2.1. Background
4.2.2. Performance Characteristics
5. Fuel Cells
5.1. Design and Development
5.2. Properties Based Applications
5.3. Hybrid Fuel Cells
Microbial Fuel Cell
5.4. Advancements
6. Solar Cells
6.1. Design and Development
6.2. Perovskite Solar Cells
6.3. Properties Based Applications
7. Nanolubricants
7.1. Design and Development
7.2. Hybridization
7.3. Characterization Based Studies
7.4. Advancements
8. Automotive Sector
8.1. Applications
8.2. Innovations
9. Conclusions
- (a)
- Graphene, due to its unique characteristics, has been put to multifarious uses by researchers for developing and designing energy saving, conserving and storage devices.
- (b)
- Various composite materials have been fabricated by using different derivatives of graphene with PANI, PEDOT and numerous metallic oxides. These materials have been deployed in supercapacitor applications, which resulted in improvement of specific capacitance and power density. Initial research has shown that graphene composite materials can be effectively utilized as electrode materials in Li-ion, Na-ion and Ca-ion batteries for improving the energy density, cyclability and capacity of rechargeable batteries.
- (c)
- Researchers have developed a diverse range of graphene-based fuel cell membranes, and their use resulted in the improvement of mechanical properties, ionic conductivity, chemical stability, power density and better water uptake.
- (d)
- The development of solar cells by inculcating the fascinating properties of graphene is an area which is rapidly catching the attention of researchers for improving the photovoltaic performance of solar cells.
- (e)
- Graphene, in different morphologies, has been blended with various oils/fluids, which has resulted in improvements of tribological characteristics and increased environmental friendliness.
- (f)
- Use of graphene-based lubricants has been well established in boundary layer lubrication, which has resulted in the reduction of frictional losses and enhanced product life by decreasing wear rate.
- (g)
- The excellent heat transfer characteristics of graphene in different fluids has prompted its use in the automotive sector for the rapid dissipation of heat which is generated during the functioning of engine and transmission units.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Song, J.; Yang, J. A review on structure model and energy system design of lithium-ion battery in renewable energy vehicle. Renew. Sustain. Energy Rev. 2014, 37, 627–633. [Google Scholar] [CrossRef]
- Wei, D.; Kivioja, J. Graphene for energy solutions and its industrialization. Nanoscale 2013, 5, 10108–10126. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy Environ. Sci. 2010, 4, 668–674. [Google Scholar] [CrossRef]
- Luo, B.; Liu, S.; Zhi, L. Chemical Approaches toward Graphene-Based Nanomaterials and their Applications in Energy-Related Areas. Small 2011, 8, 630–646. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.; Sutar, H.; Senapati, P.; Murmu, R.; Roy, D. Graphene, Graphene-Derivatives and Composites: Fundamentals, Synthesis Approaches to Applications. J. Compos. Sci. 2021, 5, 181. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Dubonos, S.V.; Zhang, Y.; Jiang, D. Room-temperature electric field effect and carrier-type inversion in graphene films. Nature 2004, 306. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology; World Scientific: Singapore, 2009; pp. 11–19. [Google Scholar]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Güler, Ö.; Bağcı, N. A short review on mechanical properties of graphene reinforced metal matrix composites. J. Mater. Res. Technol. 2020, 9, 6808–6833. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Gupta, T.; Chan, A. Graphene based nanofluids and nanolubricants—Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, S.K. Performance estimation of Triangular Solar air heater roughened absorber surface: An experimental and simulation modeling. Sustain. Energy Technol. Assess. 2022, 52, 102208. [Google Scholar] [CrossRef]
- Sharma, H.K.; Kumar, S.; Verma, S.K. Comparative performance analysis of flat plate solar collector having circular &trapezoidal corrugated absorber plate designs. Energy 2022, 253, 124137. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, H. Recent advances of 3D graphene-based adsorbents for sample preparation of water pollutants: A review. Chem. Eng. J. 2020, 393, 124691. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, R. Real-time monitoring of physicochemical parameters in water using big data and smart IoT sensors. Environ. Dev. Sustain. 2022, 1–48. [Google Scholar] [CrossRef]
- Paulchamy, B.; Arthi, G.; Lignesh, B.D. A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Royal Society. On the Atomic Weight of Graphite Author (s): B.c. Brodie Source: Philosophical Transactions of the Royal Society of London; Royal Society: London, UK, 1859; Volume 149, p. 249. Available online: https://www.jstor.org/stable/108699philosophicaltr (accessed on 13 May 2020).
- Staudenmaier, L. Method for the preparation of the graphite acid. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar]
- Lang, B. A LEED study of the deposition of carbon on platinum crystal surfaces. Surf. Sci. 1975, 53, 317–329. [Google Scholar] [CrossRef]
- Rokuta, E.; Hasegawa, Y.; Itoh, A.; Yamashita, K.; Tanaka, T.; Otani, S.; Oshima, C. Vibrational spectra of the monolayer films of hexagonal boron nitride and graphite on faceted Ni(755). Surf. Sci. 1999, 427–428, 97–101. [Google Scholar] [CrossRef]
- Shioyama, H. Cleavage of graphite to graphene. J. Mater. Sci. Lett. 2001, 20, 499–500. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano-Micro Lett. 2015, 8, 95–119. [Google Scholar] [CrossRef]
- Boehm, H.-P.; Stumpp, E. Citation errors concerning the first report on exfoliated graphite. Carbon 2007, 45, 1381–1383. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Davies, T.J.; Hyde, M.E.; Compton, R.G. Nanotrench Arrays Reveal Insight into Graphite Electrochemistry. Angew. Chem. Int. Ed. 2005, 44, 5121–5126. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- De Heer, W.A.; Berger, C.; Wu, X.; First, P.N.; Conrad, E.H.; Li, X.; Li, T.; Sprinkle, M.; Hass, J.; Sadowski, M.L.; et al. Epitaxial graphene. Solid State Commun. 2007, 143, 92–100. [Google Scholar] [CrossRef]
- Cambaz, Z.G.; Yushin, G.; Osswald, S.; Mochalin, V.; Gogotsi, Y. Noncatalytic synthesis of carbon nanotubes, graphene and graphite on SiC. Carbon 2008, 46, 841–849. [Google Scholar] [CrossRef]
- Rollings, E.; Gweon, G.-H.; Zhou, S.Y.; Mun, B.S.; McChesney, J.L.; Hussain, B.S.; Fedorov, A.V.; First, P.N.; de Heer, W.A.; Lanzara, A. Synthesis and characterization of atomically thin graphite films on a silicon carbide substrate. J. Phys. Chem. Solids 2006, 67, 2172–2177. [Google Scholar] [CrossRef] [Green Version]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Ruohrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E.; et al. Experimental Review of Graphene. ISRN Condens. Matter Phys. 2012, 2012, 1–56. [Google Scholar] [CrossRef]
- Ambrosi, A.; Bonanni, A.; Sofer, Z.; Cross, J.S.; Pumera, M. Electrochemistry at Chemically Modified Graphenes. Chem.—A Eur. J. 2011, 17, 10763–10770. [Google Scholar] [CrossRef]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of Graphene and Its Applications: A Review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef]
- Mattevi, C.; Kim, K.; Chhowalla, M. A review of chemical vapor deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Obraztsov, A.; Obraztsova, E.; Tyurnina, A.; Zolotukhin, A. Chemical vapor deposition of thin graphite films of nanometer thickness. Carbon 2007, 45, 2017–2021. [Google Scholar] [CrossRef]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Gilje, S.; Han, S.; Wang, M.; Wang, K.L.; Kaner, R.B. A Chemical Route to Graphene for Device Applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2012, 5, 38–51. [Google Scholar] [CrossRef]
- Wu, H.-C.; Li, Y.-Y.; Sakoda, A. Synthesis and hydrogen storage capacity of exfoliated turbostratic carbon nanofibers. Int. J. Hydrogen Energy 2010, 35, 4123–4130. [Google Scholar] [CrossRef]
- Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Chang, D.W.; Dai, L.; Baek, J.-B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Christen, T.; Carlen, M.W. Theory of Ragone plots. J. Power Sources 2000, 91, 210–216. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Rout, C.S.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, C.N.R. Graphene-based electrochemical supercapacitors. J. Chem. Sci. 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, H.; Zhu, M.; Tian, K.; Wang, J.; Kang, F.; Outlaw, R. Carbon nanosheets as the electrode material in supercapacitors. J. Power Sources 2009, 194, 1208–1212. [Google Scholar] [CrossRef]
- Shen, J.; Liu, H.; Cui, F. Effect of welding speed on microstructure and mechanical properties of friction stir welded copper. Mater. Des. 2010, 31, 3937–3942. [Google Scholar] [CrossRef]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-Based Supercapacitor with an Ultrahigh Energy Density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Mirfakhrai, T.; Chen, Z.; Casalongue, H.S.; Dai, H. Advanced asymmetrical supercapacitors based on graphene hybrid materials. Nano Res. 2011, 4, 729–736. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Choi, B.G.; Yang, M.; Hong, W.H.; Choi, J.W.; Huh, Y.S. 3D Macroporous Graphene Frameworks for Supercapacitors with High Energy and Power Densities. ACS Nano 2012, 6, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Y.; Li, H.; Pan, L.; Li, Y.; Sun, Z. Electrochemical behaviors of graphene–ZnO and graphene–SnO2 composite films for supercapacitors. Electrochim. Acta 2010, 55, 4170–4173. [Google Scholar] [CrossRef]
- Mini, P.A.; Balakrishnan, A.; Nair, S.V.; Subramanian, K.R.V. Highly super capacitive electrodes made of graphene/poly(pyrrole). Chem. Commun. 2011, 47, 5753–5755. [Google Scholar] [CrossRef] [PubMed]
- Alvi, F.; Ram, M.K.; Basnayaka, P.A.; Stefanakos, E.; Goswami, Y.; Kumar, A. Graphene–polyethylenedioxythiophene conducting polymer nanocomposite based supercapacitor. Electrochim. Acta 2011, 56, 9406–9412. [Google Scholar] [CrossRef]
- Cao, Y.; Mallouk, T.E. Morphology of Template-Grown Polyaniline Nanowires and Its Effect on the Electrochemical Capacitance of Nanowire Arrays. Chem. Mater. 2008, 20, 5260–5265. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.; Ma, Y. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Ferraris, J.P.; Eissa, M.M.; Brotherston, I.D.; Loveday, D.C. Performance Evaluation of Poly 3-(Phenylthiophene) Derivatives as Active Materials for Electrochemical Capacitor Applications. Chem. Mater. 1998, 10, 3528–3535. [Google Scholar] [CrossRef]
- Palaniappan, S.; Devi, S.L. Novel chemically synthesized polyaniline electrodes containing a fluoroboric acid dopant for supercapacitors. J. Appl. Polym. Sci. 2007, 107, 1887–1892. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Mi, H.; Zhang, X.; An, S.; Ye, X.; Yang, S. Microwave-assisted synthesis and electrochemical capacitance of polyaniline/multi-wall carbon nanotubes composite. Electrochem. Commun. 2007, 9, 2859–2862. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Graphene oxide doped polyaniline for supercapacitors. Electrochem. Commun. 2009, 11, 1158–1161. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Ramaprabhu, S. Functionalized Graphene-Based Nanocomposites for Supercapacitor Application. J. Phys. Chem. C 2011, 115, 14006–14013. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Wang, D.-W.; Ren, W.; Zhao, J.; Zhou, G.; Li, F.; Cheng, H.-M. Anchoring Hydrous RuO2 on Graphene Sheets for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2010, 20, 3595–3602. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, J.; Sim, D.H.; Tay, Y.Y.; Lu, Z.; Zhang, X.; Sharma, Y.; Srinivasan, M.; Zhang, H.; Hng, H.H.; et al. Achieving high specific charge capacitances in Fe3O4/reduced graphene oxide nanocomposites. J. Mater. Chem. 2011, 21, 3422–3427. [Google Scholar] [CrossRef]
- Qu, Q.; Yang, S.; Feng, X. 2D Sandwich-like Sheets of Iron Oxide Grown on Graphene as High Energy Anode Material for Supercapacitors. Adv. Mater. 2011, 23, 5574–5580. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, C.X.; Liu, J.; Chen, T.; Yang, H.; Li, C.M. CeO2 nanoparticles/graphene nanocomposite-based high performance supercapacitor. Dalton Trans. 2011, 40, 6388–6391. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R.; et al. Ultrathin Planar Graphene Supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, X.; Yang, S. High performance supercapacitors based on highly conductive nitrogen-doped graphene sheets. Phys. Chem. Chem. Phys. 2011, 13, 12554–12558. [Google Scholar] [CrossRef]
- Lee, K.R.; Lee, K.U.; Lee, J.W.; Ahn, B.T.; Woo, S.I. Electrochemical oxygen reduction on nitrogen doped graphene sheets in acid media. Electrochem. Commun. 2010, 12, 1052–1055. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Tian, C.; Tan, T.; Xie, Y.; Shi, K.; Li, M.; Fu, H. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012, 2, 4498–4506. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled Nitrogen-Doped Graphene Nanosheets with Ultrahigh Pore Volume for High-Performance Supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. A nanostructured graphene/polyaniline hybrid material for supercapacitors. Nanoscale 2010, 2, 2164–2170. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Shao, B.; Fan, Z.; Qian, W.; Zhang, M.; Wei, F. Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 2010, 48, 487–493. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of Graphene/Polyaniline Composite Paper via In Situ Anodic Electropolymerization for High-Performance Flexible Electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-Based Materials for Lithium-Ion Hybrid Supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef]
- Yuan, A.; Wang, X.; Wang, Y.; Hu, J. Comparison of nano-MnO2 derived from different manganese sources and influence of active material weight ratio on performance of nano-MnO2/activated carbon supercapacitor. Energy Convers. Manag. 2010, 51, 2588–2594. [Google Scholar] [CrossRef]
- Inoue, H.; Namba, Y.; Higuchi, E. Preparation and characterization of Ni-based positive electrodes for use in aqueous electrochemical capacitors. J. Power Sources 2010, 195, 6239–6244. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Chen, M.; Jiao, Y.; Wang, J. Electrochemical Performances of Nanoparticle Fe3O4/Activated Carbon Supercapacitor Using KOH Electrolyte Solution. J. Phys. Chem. C 2009, 113, 2643–2646. [Google Scholar] [CrossRef]
- Qu, Q.; Shi, Y.; Li, L.; Guo, W.; Wu, Y.; Zhang, H.; Guan, S.; Holze, R. V2O5·0.6H2O nanoribbons as cathode material for asymmetric supercapacitor in K2SO4 solution. Electrochem. Commun. 2009, 11, 1325–1328. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent Advances in Design and Fabrication of Electrochemical Supercapacitors with High Energy Densities. Adv. Energy Mater. 2013, 4, 1300816. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Liu, N.; Wang, H.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z. Enhancing the Supercapacitor Performance of Graphene/MnO2 Nanostructured Electrodes by Conductive Wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Banks, G. Graphene-Based Supercapacitor Hits New Energy Storage High. 2010. Available online: https://newatlas.com/graphene-supercapacitor-energy-density-record/17188/ (accessed on 28 April 2020).
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2018, 101, 123–145. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- Tung, V.C.; Chen, L.-M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-Temperature Solution Processing of Graphene—Carbon Nanotube Hybrid Materials for High-Performance Transparent Conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Jang, B.Z.; Liu, C.; Neff, D.; Yu, Z.; Wang, M.C.; Xiong, W.; Zhamu, A. Graphene Surface-Enabled Lithium Ion-Exchanging Cells: Next-Generation High-Power Energy Storage Devices. Nano Lett. 2011, 11, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, K.; Jones, P.; Wiseman, P.; Goodenough, J.B. LixCoO2 (0 < x ≤ 1): A new cathode material for batteries of high energy density. Solid State Ion. 1981, 3–4, 171–174. [Google Scholar] [CrossRef]
- Miroshnikov, M.; Mahankali, K.; Thangavel, N.K.; Satapathy, S.; Arava, L.M.R.; Ajayan, P.M.; John, G. Bioderived Molecular Electrodes for Next-Generation Energy-Storage Materials. ChemSusChem 2020, 13, 2186–2204. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Wang, X.; Park, J.; Dou, S.; Ahn, H.; Kim, K. Sn/graphene nanocomposite with 3D architecture for enhanced reversible lithium storage in lithium ion batteries. J. Mater. Chem. 2009, 19, 8378–8384. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Wolfenstine, J. Critical grain size for microcracking during lithium insertion. J. Power Sources 1999, 79, 111–113. [Google Scholar] [CrossRef]
- Campbell, B.; Ionescu, R.; Tolchin, M.; Ahmed, K.; Favors, Z.; Bozhilov, K.N.; Ozkan, C.S.; Ozkan, M. Carbon-Coated, Diatomite-Derived Nanosilicon as a High Rate Capable Li-ion Battery Anode. Sci. Rep. 2016, 6, 33050. [Google Scholar] [CrossRef]
- Paek, S.-M.; Yoo, E.; Honma, I. Enhanced Cyclic Performance and Lithium Storage Capacity of SnO2/Graphene Nanoporous Electrodes with Three-Dimensionally Delaminated Flexible Structure. Nano Lett. 2009, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhu, X.; Lian, P.; Yang, W.; Wang, H. Superior cycle performance of Sn@C/graphene nanocomposite as an anode material for lithium-ion batteries. J. Solid State Chem. 2011, 184, 1400–1404. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Z.; Zhang, T.; Sui, D.; Ma, Y.; Chen, Y. Excellent cycling stability with high SnO2 loading on a three-dimensional graphene network for lithium ion batteries. Carbon 2016, 102, 32–38. [Google Scholar] [CrossRef]
- Kim, H.; Seo, D.-H.; Kim, S.-W.; Kim, J.; Kang, K. Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Shao, J.; Li, G.; Qu, M.; Yin, G. Co3O4@graphene Composites as Anode Materials for High-Performance Lithium Ion Batteries. Inorg. Chem. 2011, 50, 1628–1632. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.-F.; Yang, Y.; Sanchez Casalongue, H.; Robinson, J.T.; Liang, Y.; Cui, Y.; Dai, H. Mn3O4—Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, X.; Xiang, J.; Qiao, Y.; Zhang, D.; Gu, C.; Tu, J. CuO/graphene composite as anode materials for lithium-ion batteries. Electrochim. Acta 2011, 56, 2306–2311. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Xiang, H.; Li, Z.; Yang, W.; Wang, H. Enhanced cycling performance of Fe3O4–graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 56, 834–840. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, H.; Sun, Y.; Tang, L.; Zhang, X. Flexible foams of graphene entrapped SnO2–Co3O4 nanocubes with remarkably large and fast lithium storage. J. Mater. Chem. A 2016, 4, 16101–16107. [Google Scholar] [CrossRef]
- Wang, G.; Liu, T.; Luo, Y.; Zhao, Y.; Ren, Z.; Bai, J.; Wang, H. Preparation of Fe2O3/graphene composite and its electrochemical performance as an anode material for lithium ion batteries. J. Alloy Compd. 2011, 509, L216–L220. [Google Scholar] [CrossRef]
- Cai, D.; Lian, P.; Zhu, X.; Liang, S.; Yang, W.; Wang, H. High specific capacity of TiO2-graphene nanocomposite as an anode material for lithium-ion batteries in an enlarged potential window. Electrochim. Acta 2012, 74, 65–72. [Google Scholar] [CrossRef]
- Wang, D.; Choi, D.; Li, J.; Yang, Z.; Nie, Z.; Kou, R.; Hu, D.; Wang, C.; Saraf, L.V.; Zhang, J.; et al. Self-Assembled TiO2–Graphene Hybrid Nanostructures for Enhanced Li-Ion Insertion. ACS Nano 2009, 3, 907–914. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Q.; Goodman, M.D.; Zhu, H.; Kim, J.; Krueger, N.A.; Ning, H.; Huang, X.; Liu, J.; Terrones, M.; et al. Lithium-Ion Batteries: Graphene Sandwiched Mesostructured Li-Ion Battery Electrodes (Adv. Mater. 35/2016). Adv. Mater. 2016, 28, 7695. [Google Scholar] [CrossRef]
- He, J.; Li, P.; Lv, W.; Wen, K.; Chen, Y.; Zhang, W.; Li, Y.; Qin, W.; He, W. Three-dimensional hierarchically structured aerogels constructed with layered MoS 2/graphene nanosheets as free-standing anodes for high-performance lithium ion batteries. Electrochim. Acta 2016, 215, 12–18. [Google Scholar] [CrossRef]
- Ren, Y.; Lv, W.; Wen, F.; Xiang, J.; Liu, Z. Microwave synthesis of SnS2 nanoflakes anchored graphene foam for flexible lithium-ion battery anodes with long cycling life. Mater. Lett. 2016, 174, 24–27. [Google Scholar] [CrossRef]
- Chen, P.; Guo, L.; Wang, Y. Graphene wrapped SnCo nanoparticles for high-capacity lithium ion storage. J. Power Sources 2013, 222, 526–532. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Cui, K.; Li, Y. Monoclinic Li3V2(PO4)3/C nanocrystals co-modified with graphene nanosheets and carbon nanotubes as a three-dimensional-network cathode material for rechargeable lithium-ion batteries. RSC Adv. 2016, 6, 8431–8439. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, M.-S.; Kim, Y.-H.; Roh, K.C.; Kim, K.-B. High-rate Li4Ti5O12/N-doped reduced graphene oxide composite using cyanamide both as nanospacer and a nitrogen doping source. J. Power Sources 2016, 336, 376–384. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Sauvage, F.; Baudrin, E.; Morcrette, M.; Tarascon, J.-M. Pulsed Laser Deposition and Electrochemical Properties of LiFePO[sub 4] Thin Films. Electrochem. Solid-State Lett. 2004, 7, A15–A18. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.-Y.; Tang, X. Cycling degradation of an automotive LiFePO4 lithium-ion battery. J. Power Sources 2011, 196, 1513–1520. [Google Scholar] [CrossRef]
- Tu, J.; Zhao, X.; Xie, J.; Cao, G.; Zhuang, D.; Zhu, T. Enhanced low voltage cycling stability of LiMn2O4 cathode by ZnO coating for lithium ion batteries. J. Alloy Compd. 2007, 432, 313–317. [Google Scholar] [CrossRef]
- Molenda, J.; Ziemnicki, M.; Marzec, J.; Zając, W.; Molenda, M.; Bućko, M. Electrochemical and high temperature physicochemical properties of orthorhombic LiMnO2. J. Power Sources 2007, 173, 707–711. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Wang, D.; Li, X.; Geng, D.; Liang, G.; Gauthier, M.; Li, R.; Sun, X. 3D porous LiFePO4/graphene hybrid cathodes with enhanced performance for Li-ion batteries. J. Power Sources 2012, 208, 340–344. [Google Scholar] [CrossRef]
- Dhindsa, K.; Mandal, B.; Bazzi, K.; Lin, M.; Nazri, M.; Nazri, G.; Naik, V.; Garg, V.; Oliveira, A.; Vaishnava, P.; et al. Enhanced electrochemical performance of graphene modified LiFePO4 cathode material for lithium ion batteries. Solid State Ionics 2013, 253, 94–100. [Google Scholar] [CrossRef]
- Tao, S.; Huang, W.-F.; Wu, G.-X.; Zhu, X.-B.; Wang, X.-B.; Zhang, M.; Wang, S.-H.; Chu, W.-S.; Song, L.; Wu, Z.-Y. Performance enhancement of Lithium-ion battery with LiFePO4@C/RGO hybrid electrode. Electrochim. Acta 2014, 144, 406–411. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Deng, C.; Zhu, H.; Chen, G.Z.; Zhang, S.; Tian, X. Nitrogen-doped graphene guided formation of monodisperse microspheres of LiFePO4 nanoplates as the positive electrode material of lithium-ion batteries. J. Mater. Chem. A 2016, 4, 12065–12072. [Google Scholar] [CrossRef]
- Du, Y.; Tang, Y.; Huang, F.; Chang, C. Preparation of three-dimensional free-standing nano-LiFePO4/graphene composite for high performance lithium ion battery. RSC Adv. 2016, 6, 52279–52283. [Google Scholar] [CrossRef]
- Huang, H.; Faulkner, T.; Barker, J.; Saidi, M. Lithium metal phosphates, power and automotive applications. J. Power Sources 2009, 189, 748–751. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, J.; Yang, Y.; Song, G. Preparation and electrochemical performance of Li3V2(PO4)3/C cathode material by spray-drying and carbothermal method. J. Solid State Electrochem. 2009, 14, 883–888. [Google Scholar] [CrossRef]
- Ge, Y.; Yan, X.; Liu, J.; Zhang, X.; Wang, J.; He, X.; Wang, R.; Xie, H. An optimized Ni doped LiFePO4/C nanocomposite with excellent rate performance. Electrochim. Acta 2010, 55, 5886–5890. [Google Scholar] [CrossRef]
- Jiang, R.; Cui, C.; Ma, H. Using graphene nanosheets as a conductive additive to enhance the rate performance of spinel LiMn2O4 cathode material. Phys. Chem. Chem. Phys. 2013, 15, 6406–6415. [Google Scholar] [CrossRef]
- Bak, S.-M.; Nam, K.-W.; Lee, C.-W.; Kim, K.-H.; Jung, H.-C.; Yang, X.-Q. Spinel LiMn2O4/reduced graphene oxide hybrid for high rate lithium ion batteries. J. Mater. Chem. 2011, 21, 17309–17315. [Google Scholar] [CrossRef]
- Pyun, M.H.; Park, Y.J. Graphene/LiMn2O4 nanocomposites for enhanced lithium ion batteries with high rate capability. J. Alloy Compd. 2015, 643, S90–S94. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Cui, L.-F.; Casalongue, H.S.; Li, Y.; Hong, G.; Cui, Y.; Dai, H. LiMn1−xFexPO4 Nanorods Grown on Graphene Sheets for Ultrahigh-Rate-Performance Lithium Ion Batteries. Angew. Chem. Int. Ed. 2011, 50, 7364–7368. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.-U.; Seo, D.-H.; Kim, J.; Kim, S.-W.; Kang, K. Mg and Fe Co-doped Mn Based Olivine Cathode Material for High Power Capability. J. Electrochem. Soc. 2011, 158, A250–A254. [Google Scholar] [CrossRef]
- Damen, L.; De Giorgio, F.; Monaco, S.; Veronesi, F.; Mastragostino, M. Synthesis and characterization of carbon-coated LiMnPO4 and LiMn1−xFexPO4 (x = 0.2, 0.3) materials for lithium-ion batteries. J. Power Sources 2012, 218, 250–253. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, X.; Zan, L.; Zhang, Y. Three-Dimensional Macroporous Graphene–Li2FeSiO4 Composite as Cathode Material for Lithium-Ion Batteries with Superior Electrochemical Performances. ACS Appl. Mater. Interfaces 2014, 6, 11724–11733. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Hou, Z.F.; Wu, L.-M. First-Principles Study of Lithium Adsorption and Diffusion on Graphene with Point Defects. J. Phys. Chem. C 2012, 116, 21780–21787. [Google Scholar] [CrossRef]
- Datta, D.; Li, J.; Shenoy, V.B. Defective graphene as promising anode material for na-ion battery and ca-ion bat-tery. ACS Appl. Mater. Interfaces 2014, 6, 1788–1795. [Google Scholar] [CrossRef]
- Xu, J.; LaVan, D.A. Designing artificial cells to harness the biological ion concentration gradient. Nat. Nanotechnol. 2008, 3, 666–670. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Zhang, P.; Li, H.; Sheng, W.; Zhang, T.; Jordan, R.; Wu, Y.; Zhuang, X.; Feng, X. Dual-Graphene Rechargeable Sodium Battery. Small 2017, 13, 1702449. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Prikhodchenko, P.V.; Mason, C.W.; Batabyal, S.K.; Gun, J.; Sladkevich, S.; Medvedev, A.G.; Lev, O. High-capacity antimony sulphide nanoparticle-decorated graphene composite as anode for sodium-ion batteries. Nat. Commun. 2013, 4, 2922. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Liang, Q.; Liu, X.; Weng, Q.; Liu, J.; Yang, Y.; Dai, Z.; Ding, K.; Bando, Y.; et al. Amorphous Phosphorus/Nitrogen-Doped Graphene Paper for Ultrastable Sodium-Ion Batteries. Nano Lett. 2016, 16, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Dobrota, A.S.; Pašti, I.A.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Functionalized graphene for sodium battery applications: The DFT insights. Electrochim. Acta 2017, 250, 185–195. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, Y.-U.; Chang, S.-J.; Kim, B.H.; Choi, J.; Wang, J.; Zhang, D.; Braun, P.V.; Jin, H.-J.; Kang, K. Crumpled graphene paper for high power sodium battery anode. Carbon 2015, 99, 658–664. [Google Scholar] [CrossRef]
- Sun, J.; Lee, H.-W.; Pasta, M.; Yuan, H.; Zheng, G.; Sun, Y.; Li, Y.; Cui, Y. A phosphorene–graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat. Nanotechnol. 2015, 10, 980–985. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.; Dai, L. High-Performance Sodium Ion Batteries Based on a 3D Anode from Nitrogen-Doped Graphene Foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef]
- Xie, X.; Su, D.; Zhang, J.; Chen, S.; Mondal, A.K.; Wang, G. A comparative investigation on the effects of nitrogen-doping into graphene on enhancing the electrochemical performance of SnO2/graphene for sodium-ion batteries. Nanoscale 2015, 7, 3164–3172. [Google Scholar] [CrossRef]
- Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y.S.; Wang, T.; Lee, J.Y. Layered SnS2-Reduced Graphene Oxide Composite—A High-Capacity, High-Rate, and Long-Cycle Life Sodium-Ion Battery Anode Material. Adv. Mater. 2014, 26, 3854–3859. [Google Scholar] [CrossRef]
- Medeiros, P.V.C.; Mascarenhas, A.J.S.; Mota, F.D.B.; De Castilho, C.M.C. A DFT study of halogen atoms adsorbed on graphene layers. Nanotechnology 2010, 21, 485701. [Google Scholar] [CrossRef]
- Stournara, M.E.; Shenoy, V.B. Enhanced Li capacity at high lithiation potentials in graphene oxide. J. Power Sources 2011, 196, 5697–5703. [Google Scholar] [CrossRef]
- Liu, Y.; Merinov, B.V.; Goddard, W.A. Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals. Proc. Natl. Acad. Sci. USA 2016, 113, 3735–3739. [Google Scholar] [CrossRef]
- Niaei, A.H.F.; Hussain, T.; Hankel, M.; Searles, D.J. Hydrogenated defective graphene as an anode material for sodium and calcium ion batteries: A density functional theory study. Carbon 2018, 136, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Niaei, A.H.F.; Roman, T.; Hussain, T.; Searles, D.J. Computational Study on the Adsorption of Sodium and Calcium on Edge-Functionalized Graphene Nanoribbons. J. Phys. Chem. C 2019, 123, 14895–14908. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Singh, G. MoS2/Graphene Composite Paper for Sodium-Ion Battery Electrodes. ACS Nano 2014, 8, 1759–1770. [Google Scholar] [CrossRef]
- Farooqui, U.; Ahmad, A.; Hamid, N. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Zhu, H. Recent Developments in Graphene-Based Membranes: Structure, Mass-Transport Mechanism and Potential Applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Cao, Y.-C.; Xu, C.; Wu, X.; Wang, X.; Xing, L.; Scott, K. A poly (ethylene oxide)/graphene oxide electrolyte membrane for low temperature polymer fuel cells. J. Power Sources 2011, 196, 8377–8382. [Google Scholar] [CrossRef]
- Lee, D.; Yang, H.; Park, S.; Kim, W. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Lee, D.; Yang, H.; Park, S.; Park, K.; Kim, W. Self-humidifying Pt–graphene/SiO2 composite membrane for polymer electrolyte membrane fuel cell. J. Membr. Sci. 2015, 474, 254–262. [Google Scholar] [CrossRef]
- Yang, H.; Lee, W.; Choi, B.; Kim, W. Preparation of Nafion/Pt-containing TiO2/graphene oxide composite membranes for self-humidifying proton exchange membrane fuel cell. J. Membr. Sci. 2016, 504, 20–28. [Google Scholar] [CrossRef]
- Lee, S.-H.; Choi, S.-H.; Gopalan, S.-A.; Lee, K.-P.; Anantha-Iyengar, G. Preparation of new self-humidifying composite membrane by incorporating graphene and phosphotungstic acid into sulfonated poly(ether ether ketone) film. Int. J. Hydrogen Energy 2014, 39, 17162–17177. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Lee, C.; Jung, Y.; Cheong, S.; Ku, B.; Jang, S. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- He, Y.; Tong, C.; Geng, L.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Ansari, S.; Kelarakis, A.; Estevez, L.; Giannelis, E.P. Oriented Arrays of Graphene in a Polymer Matrix by in situ Reduction of Graphite Oxide Nanosheets. Small 2010, 6, 205–209. [Google Scholar] [CrossRef]
- Wang, L.; Kang, J.; Nam, J.-D.; Suhr, J.; Prasad, A.K.; Advani, S. Composite Membrane Based on Graphene Oxide Sheets and Nafion for Polymer Electrolyte Membrane Fuel Cells. ECS Electrochem. Lett. 2014, 4, F1–F4. [Google Scholar] [CrossRef]
- Peng, K.-J.; Lai, J.-Y.; Liu, Y.-L. Nanohybrids of graphene oxide chemically-bonded with Nafion: Preparation and application for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 514, 86–94. [Google Scholar] [CrossRef]
- Kumar, R.; Xu, C.; Scott, K. Graphite oxide/Nafion composite membranes for polymer electrolyte fuel cells. RSC Adv. 2012, 2, 8777–8782. [Google Scholar] [CrossRef]
- Kim, Y.; Ketpang, K.; Jaritphun, S.; Park, J.S.; Shanmugam, S. A polyoxometalate coupled graphene oxide–Nafion composite membrane for fuel cells operating at low relative humidity. J. Mater. Chem. A 2015, 3, 8148–8155. [Google Scholar] [CrossRef]
- Choi, B.G.; Hong, J.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Hammond, P.T.; Park, H. Innovative Polymer Nanocomposite Electrolytes: Nanoscale Manipulation of Ion Channels by Functionalized Graphenes. ACS Nano 2011, 5, 5167–5174. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized Graphene Oxide Nanocomposite Membrane for Low Humidity and High Temperature Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kim, N.H.; Jung, D.; Lee, J.H. Enhanced mechanical properties and proton conductivity of Nafion–SPEEK–GO composite membranes for fuel cell applications. J. Membr. Sci. 2014, 458, 128–135. [Google Scholar] [CrossRef]
- Özdemir, Y.; Üregen, N.; Devrim, Y. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2648–2657. [Google Scholar] [CrossRef]
- Feng, K.; Tang, B.; Wu, P. “Evaporating” Graphene Oxide Sheets (GOSs) for Rolled up GOSs and Its Applications in Proton Exchange Membrane Fuel Cell. ACS Appl. Mater. Interfaces 2013, 5, 1481–1488. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, S.; Jang, H.; Hossain, A.; Gwak, G.; Ju, H.; Kim, D.; Kim, W. Sulfonated poly(ether sulfone) electrolytes structured with mesonaphthobifluorene graphene moiety for PEMFC. Int. J. Hydrogen Energy 2014, 39, 1532–1538. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Gao, L.; Wang, J.; Xu, Y.; He, R. Novel composite membranes of triazole modified graphene oxide and polybenzimidazole for high temperature polymer electrolyte membrane fuel cell applications. RSC Adv. 2015, 5, 101049–101054. [Google Scholar] [CrossRef]
- Sharma, P.P.; Kulshrestha, V. Synthesis of highly stable and high water retentive functionalized biopolymer-graphene oxide modified cation exchange membranes. RSC Adv. 2015, 5, 56498–56506. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Tseng, C.-Y.; Shen, W.-C.; Wang, J.-S.; Chen, K.-J.; Cheng, M.-Y.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. A new graphene-modified protic ionic liquid-based composite membrane for solid polymer electrolytes. J. Mater. Chem. 2011, 21, 10448–10453. [Google Scholar] [CrossRef]
- Pandey, R.P.; Thakur, A.K.; Shahi, V.K. Sulfonated Polyimide/Acid-Functionalized Graphene Oxide Composite Polymer Electrolyte Membranes with Improved Proton Conductivity and Water-Retention Properties. ACS Appl. Mater. Interfaces 2014, 6, 16993–17002. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Shen, J.; Hu, Z. From structures, packaging to application: A system-level review for micro direct methanol fuel cell. Renew. Sustain. Energy Rev. 2017, 80, 669–678. [Google Scholar] [CrossRef]
- Yan, X.; Wu, R.; Xu, J.; Luo, Z.; Zhao, T. A monolayer graphene—Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, H.; Cao, L.; Li, Z.; Li, Z.; Gang, M.; Wang, C.; Wu, H.; Jiang, Z.; Zhang, P. Sulfonated poly(ether ether ketone)-based hybrid membranes containing graphene oxide with acid-base pairs for direct methanol fuel cells. Electrochim. Acta 2016, 203, 178–188. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Fu, Y.; Manthiram, A. Composite membranes based on sulfonated poly(ether ether ketone) and SDBS-adsorbed graphene oxide for direct methanol fuel cells. J. Mater. Chem. 2012, 22, 24862–24869. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Zhang, H.; Zhang, T.; Zhang, B.; Cao, S.; Liu, J. Polydopamine-modified graphene oxide nanocomposite membrane for proton exchange membrane fuel cell under anhydrous conditions. J. Mater. Chem. A 2014, 2, 9548–9558. [Google Scholar] [CrossRef]
- Chien, H.-C.; Tsai, L.-D.; Huang, C.-P.; Kang, C.-Y.; Lin, J.-N.; Chang, F.-C. Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 13792–13801. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on Sulfonated Poly (Ether Ether Ketone) membrane for direct methanol fuel cells. J. Membr. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Bagheri, A.; Salarizadeh, P.; Zahmatkesh, H.G.; Kashefi, S.; Kowsari, E. Novel nanocomposite membranes based on blended sulfonated poly(ether ether ketone)/poly(vinyl alcohol) containing sulfonated graphene oxide/Fe3O4 nanosheets for DMFC applications. RSC Adv. 2015, 5, 74054–74064. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. A Graphite Oxide Paper Polymer Electrolyte for Direct Methanol Fuel Cells. Int. J. Electrochem. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Yuan, T.; Pu, L.; Huang, Q.; Zhang, H.; Li, X.; Yang, H. An effective methanol-blocking membrane modified with graphene oxide nanosheets for passive direct methanol fuel cells. Electrochim. Acta 2014, 117, 393–397. [Google Scholar] [CrossRef]
- Choi, B.G.; Huh, Y.S.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Park, H. Enhanced transport properties in polymer electrolyte composite membranes with graphene oxide sheets. Carbon 2012, 50, 5395–5402. [Google Scholar] [CrossRef]
- Lin, C.; Lu, Y. Highly ordered graphene oxide paper laminated with a Nafion membrane for direct methanol fuel cells. J. Power Sources 2013, 237, 187–194. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing Biomass-Based Graphene Oxide–Polyaniline–Ag Electrodes in Microbial Fuel Cells to Boost Energy Generation and Heavy Metal Removal. Polymers 2022, 14, 845. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Ghangrekar, M.M.; Pradhan, D.; Das, D. Graphene Oxide-Impregnated PVA–STA Composite Polymer Electrolyte Membrane Separator for Power Generation in a Single-Chambered Microbial Fuel Cell. Ind. Eng. Chem. Res. 2013, 52, 11597–11606. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Cheng, M.-Y.; Xie, X.-L.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J. Power Sources 2013, 239, 424–432. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, G.; Li, X.; Zhang, W.; Zhang, J.; Ye, J.; Huang, X.; Yu, C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources 2011, 196, 5402–5407. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Liu, X.-W.; Xie, J.-F.; Sheng, G.-P.; Wang, G.-Y.; Zhang, Y.-Y.; Xu, A.-W.; Yu, H.-Q. Graphene oxide nanoribbons greatly enhance extracellular electron transfer in bio-electrochemical systems. Chem. Commun. 2011, 47, 5795–5797. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Damien, J.; Luo, J.; Jang, H.D.; Huang, J.; He, Z. Crumpled graphene particles for microbial fuel cell electrodes. J. Power Sources 2012, 208, 187–192. [Google Scholar] [CrossRef]
- Ren, Y.; Pan, D.; Li, X.; Fu, F.; Zhao, Y.; Wang, X. Effect of polyaniline-graphene nanosheets modified cathode on the performance of sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2013, 88, 1946–1950. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C. A Novel Aluminum-Graphite Dual-Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Ahmad, A.; Ismail, M.; Ben Liew, K. Composite membrane containing graphene oxide in sulfonated polyether ether ketone in microbial fuel cell applications. Int. J. Hydrogen Energy 2015, 40, 11604–11614. [Google Scholar] [CrossRef]
- Wang, C.; Lin, B.; Qiao, G.; Wang, L.; Zhu, L.; Chu, F.; Feng, T.; Yuan, N.; Ding, J. Polybenzimidazole/ionic liquid functionalized graphene oxide nanocomposite membrane for alkaline anion exchange membrane fuel cells. Mater. Lett. 2016, 173, 219–222. [Google Scholar] [CrossRef]
- Liu, L.; Tong, C.; He, Y.; Zhao, Y.; Lü, C. Enhanced properties of quaternized graphenes reinforced polysulfone based composite anion exchange membranes for alkaline fuel cell. J. Membr. Sci. 2015, 487, 99–108. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Daio, T.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Alkaline anion exchange membranes based on KOH-treated multilayer graphene oxide. J. Membr. Sci. 2016, 508, 51–61. [Google Scholar] [CrossRef]
- Low, F.W.; Lai, C.W. Recent developments of graphene-TiO2 composite nanomaterials as efficient photoelectrodes in dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 103–125. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Miao, Z.; Meng, X.; Liu, L. Analyzing and optimizing the power generation performance of thermoelectric generators based on an industrial environment. J. Power Sources 2022, 541, 231699. [Google Scholar] [CrossRef]
- Miao, X.; Tongay, S.; Petterson, M.K.; Berke, K.; Rinzler, A.G.; Appleton, B.R.; Hebard, A.F. High Efficiency Graphene Solar Cells by Chemical Doping. Nano Lett. 2012, 12, 2745–2750. [Google Scholar] [CrossRef]
- Jiao, K.; Wang, X.; Wang, Y.; Chen, Y. Graphene oxide as an effective interfacial layer for enhanced graphene/silicon solar cell performance. J. Mater. Chem. C 2014, 2, 7715–7721. [Google Scholar] [CrossRef]
- Li, X.; Xie, D.; Park, H.; Zhu, M.; Zeng, T.H.; Wang, K.; Wei, J.; Wu, D.; Kong, J.; Zhu, H. Ion doping of graphene for high-efficiency heterojunction solar cells. Nanoscale 2013, 5, 1945–1948. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, L.; Li, Z.; Shi, E.; Li, X.; Li, H.; Ji, C.; Jia, Y.; Wei, J.; Wang, K.; et al. Graphene-CdSe nanobelt solar cells with tunable configurations. Nano Res. 2011, 4, 891–900. [Google Scholar] [CrossRef]
- Ye, Y.; Dai, Y.; Dai, L.; Shi, Z.; Liu, N.; Wang, F.; Fu, L.; Peng, R.; Wen, X.; Chen, Z.; et al. High-Performance Single CdS Nanowire (Nanobelt) Schottky Junction Solar Cells with Au/Graphene Schottky Electrodes. ACS Appl. Mater. Interfaces 2010, 2, 3406–3410. [Google Scholar] [CrossRef]
- Bi, H.; Huang, F.; Liang, J.; Xie, X.; Jiang, M. Transparent Conductive Graphene Films Synthesized by Ambient Pressure Chemical Vapor Deposition Used as the Front Electrode of CdTe Solar Cells. Adv. Mater. 2011, 23, 3202–3206. [Google Scholar] [CrossRef]
- Geng, H.-Z.; Kim, K.K.; Song, C.; Xuyen, N.T.; Kim, S.M.; Park, K.A.; Lee, D.S.; An, K.H.; Lee, Y.S.; Chang, Y.; et al. Doping and de-doping of carbon nanotube transparent conducting films by dispersant and chemical treatment. J. Mater. Chem. 2008, 18, 1261–1266. [Google Scholar] [CrossRef]
- Shi, E.; Li, H.; Yang, L.; Zhang, L.; Li, Z.; Li, P.; Shang, Y.; Wu, S.; Li, X.; Wei, J.; et al. Colloidal Antireflection Coating Improves Graphene–Silicon Solar Cells. Nano Lett. 2013, 13, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Duan, C.; Wu, X.; Chen, J.; Wang, Y.; Chen, Y. The role of MoS2 as an interfacial layer in graphene/silicon solar cells. Phys. Chem. Chem. Phys. 2015, 17, 8182–8186. [Google Scholar] [CrossRef]
- Singh, E.; Nalwa, H.S. Stability of graphene-based heterojunction solar cells. RSC Adv. 2015, 5, 73575–73600. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, X.; Wu, Y.; Zhang, X.; Zhang, X.; Wang, Y.; Zhang, W.; Gao, P.; Han, Y.; Jie, J. Surface passivation and band engineering: A way toward high efficiency graphene–planar Si solar cells. J. Mater. Chem. A 2013, 1, 8567–8574. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhang, S.; Wu, Z.; Wang, P.; Xu, Z.; Chen, H.; Yin, W.; Zhong, H.; Lin, S. 18.5% efficient graphene/GaAs van der Waals heterostructure solar cell. Nano Energy 2015, 16, 310–319. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Chi, H.; Liu, Y.; Hou, C.L.; Fang, D. Recent development of graphene materials applied in polymer solar cell. Renew. Sustain. Energy Rev. 2014, 43, 973–980. [Google Scholar] [CrossRef]
- Stotter, J.; Show, Y.; Wang, S.; Swain, G. Comparison of the Electrical, Optical, and Electrochemical Properties of Diamond and Indium Tin Oxide Thin-Film Electrodes. Chem. Mater. 2005, 17, 4880–4888. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huang, X.; He, Q.; Lam, Y.M.; Zhang, H. Organic Photovoltaic Devices Using Highly Flexible Reduced Graphene Oxide Films as Transparent Electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef]
- Yusoff, A.R.B.M.; Kim, D.; Schneider, F.K.; da Silva, W.J.; Jang, J. Au-doped single layer graphene nanoribbons for a record-high efficiency ITO-free tandem polymer solar cell. Energy Environ. Sci. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, F.; Lin, P.; Choy, W.C.H. Al-TiO2 Composite-Modified Single-Layer Graphene as an Efficient Transparent Cathode for Organic Solar Cells. ACS Nano 2013, 7, 1740–1747. [Google Scholar] [CrossRef]
- Agresti, A.; Pescetelli, S.; Taheri, B.; Del Rio Castillo, A.E.; Cinà, L.; Bonaccorso, F.; Di Carlo, A. Graphene-Perovskite Solar Cells Exceed 18% Efficiency: A Stability Study. ChemSusChem 2016, 9, 2609–2619. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Hu, J.; Cui, N.; Xue, Y.; Li, L.; Long, G.; Lin, S. The roles of graphene and its derivatives in perovskite solar cells: A review. Mater. Des. 2021, 211, 110170. [Google Scholar] [CrossRef]
- Smith, I.C.; Hoke, E.T.; Solis-Ibarra, D.; McGehee, M.D.; Karunadasa, H.I. A Layered Hybrid Perovskite Solar-Cell Absorber with Enhanced Moisture Stability. Angew. Chem. 2014, 126, 11414–11417. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Girtan, M.; Rusu, M. Role of ITO and PEDOT:PSS in stability/degradation of polymer:fullerene bulk heterojunctions solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 446–450. [Google Scholar] [CrossRef]
- Cao, H.; He, W.; Mao, Y.; Lin, X.; Ishikawa, K.; Dickerson, J.H.; Hess, W.P. Recent progress in degradation and stabilization of organic solar cells. J. Power Sources 2014, 264, 168–183. [Google Scholar] [CrossRef]
- Roesch, R.; Eberhardt, K.-R.; Engmann, S.; Gobsch, G.; Hoppe, H. Polymer solar cells with enhanced lifetime by improved electrode stability and sealing. Sol. Energy Mater. Sol. Cells 2013, 117, 59–66. [Google Scholar] [CrossRef]
- Wang, J.T.-W.; Ball, J.M.; Barea, E.M.; Abate, A.; Alexander-Webber, J.A.; Huang, J.; Saliba, M.; Mora-Sero, I.; Bisquert, J.; Snaith, H.J.; et al. Low-Temperature Processed Electron Collection Layers of Graphene/TiO2 Nanocomposites in Thin Film Perovskite Solar Cells. Nano Lett. 2013, 14, 724–730. [Google Scholar] [CrossRef]
- Feng, T.; Xie, D.; Lin, Y.; Zhao, H.; Chen, Y.; Tian, H.; Ren, T.; Li, X.; Li, Z.; Wang, K.; et al. Efficiency enhancement of graphene/silicon-pillar-array solar cells by HNO3 and PEDOT-PSS. Nanoscale 2012, 4, 2130–2133. [Google Scholar] [CrossRef]
- Park, H.; Brown, P.R.; Bulović, V.; Kong, J. Graphene As Transparent Conducting Electrodes in Organic Photovoltaics: Studies in Graphene Morphology, Hole Transporting Layers, and Counter Electrodes. Nano Lett. 2011, 12, 133–140. [Google Scholar] [CrossRef]
- Tong, S.W.; Wang, Y.; Zheng, Y.; Ng, M.-F.; Loh, K.P. Graphene Intermediate Layer in Tandem Organic Photovoltaic Cells. Adv. Funct. Mater. 2011, 21, 4430–4435. [Google Scholar] [CrossRef]
- Tung, V.C.; Kim, J.; Cote, L.J.; Huang, J. Sticky Interconnect for Solution-Processed Tandem Solar Cells. J. Am. Chem. Soc. 2011, 133, 9262–9265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, S.W.; Xu, X.F.; Özyilmaz, B.; Loh, K.P. Interface Engineering of Layer-by-Layer Stacked Graphene Anodes for High-Performance Organic Solar Cells. Adv. Mater. 2011, 23, 1514–1518. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Tu, K.-H.; Lin, C.-C.; Chen, C.-W.; Chhowalla, M. Solution-Processable Graphene Oxide as an Efficient Hole Transport Layer in Polymer Solar Cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef]
- Liu, X.; Kim, H.; Guo, L.J. Optimization of thermally reduced graphene oxide for an efficient hole transport layer in polymer solar cells. Org. Electron. 2012, 14, 591–598. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, S.; Zhou, X.; Huang, X.; Zhang, Q.; Boey, F.; Zhang, H. Electrochemical Deposition of ZnO Nanorods on Transparent Reduced Graphene Oxide Electrodes for Hybrid Solar Cells. Small 2010, 6, 307–312. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Tsao, H.N.; Tomović; Li, J.; Müllen, K. Transparent Carbon Films as Electrodes in Organic Solar Cells. Angew. Chem. Int. Ed. 2008, 47, 2990–2992. [Google Scholar] [CrossRef]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent graphene/PEDOT–PSS composite films as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Yong, V.; Tour, J.M. Theoretical Efficiency of Nanostructured Graphene-Based Photovoltaics. Small 2010, 6, 313–318. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, B.; Li, L.-S. Large, Solution-Processable Graphene Quantum Dots as Light Absorbers for Photovoltaics. Nano Lett. 2010, 10, 1869–1873. [Google Scholar] [CrossRef]
- Guo, C.X.; Bin Yang, H.; Sheng, Z.M.; Lu, Z.S.; Song, Q.L.; Li, C.M. Layered Graphene/Quantum Dots for Photovoltaic Devices. Angew. Chem. 2010, 122, 3078–3081. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells. Nano Lett. 2007, 8, 323–327. [Google Scholar] [CrossRef]
- Li, S.; Luo, Y.; Lv, W.; Yu, W.; Wu, S.; Hou, P.; Yang, Q.; Meng, Q.; Liu, C.; Cheng, H.-M. Vertically Aligned Carbon Nanotubes Grown on Graphene Paper as Electrodes in Lithium-Ion Batteries and Dye-Sensitized Solar Cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Yang, N.; Zhai, J.; Wang, D.; Chen, Y.; Jiang, L. Two-Dimensional Graphene Bridges Enhanced Photoinduced Charge Transport in Dye-Sensitized Solar Cells. ACS Nano 2010, 4, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Roy-Mayhew, J.D.; Bozym, D.J.; Punckt, C.; Aksay, I.A. Functionalized Graphene as a Catalytic Counter Electrode in Dye-Sensitized Solar Cells. ACS Nano 2010, 4, 6203–6211. [Google Scholar] [CrossRef]
- Kavan, L.; Yum, J.-H.; Nazeeruddin, M.K.; Grätzel, M. Graphene Nanoplatelet Cathode for Co(III)/(II) Mediated Dye-Sensitized Solar Cells. ACS Nano 2011, 5, 9171–9178. [Google Scholar] [CrossRef]
- Song, J.; Yin, Z.; Yang, Z.; Amaladass, P.; Wu, S.; Ye, J.; Zhao, Y.; Deng, W.-Q.; Zhang, H.; Liu, X.-W. Enhancement of Photogenerated Electron Transport in Dye-Sensitized Solar Cells with Introduction of a Reduced Graphene Oxide-TiO2 Junction. Chem.—A Eur. J. 2011, 17, 10832–10837. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Kucherova, A.; Memetov, N.; Pasko, T.; Ovchinnikov, K.; Pershin, V.; Kuznetsov, D.; Galunin, E.; Grachev, V.; et al. Advances in carbon nanomaterials as lubricants modifiers. J. Mol. Liq. 2019, 279, 251–266. [Google Scholar] [CrossRef]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf. Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Stachowiak, G.; Batchelor, A.W. Experimental Methods in Tribology; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Wählisch, F.; Hoth, J.; Held, C.; Seyller, T.; Bennewitz, R. Friction and atomic-layer-scale wear of graphitic lubricants on SiC(0001) in dry sliding. Wear 2013, 300, 78–81. [Google Scholar] [CrossRef]
- Penkov, O.; Kim, H.-J.; Kim, H.-J.; Kim, D.-E. Tribology of graphene: A review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Filleter, T.; McChesney, J.; Bostwick, A.; Rotenberg, E.; Emtsev, K.V.; Seyller, T.; Horn, K.; Bennewitz, R. Friction and Dissipation in Epitaxial Graphene Films. Phys. Rev. Lett. 2009, 102, 086102. [Google Scholar] [CrossRef]
- Huang, H.; Tu, J.; Gan, L.; Li, C. An investigation on tribological properties of graphite nanosheets as oil additive. Wear 2006, 261, 140–144. [Google Scholar] [CrossRef]
- Guo, Y.-B.; Zhang, S.-W. The Tribological Properties of Multi-Layered Graphene as Additives of PAO2 Oil in Steel–Steel Contacts. Lubricants 2016, 4, 30. [Google Scholar] [CrossRef]
- Senatore, A.; D’Agostino, V.; Petrone, V.; Ciambelli, P.; Sarno, M. Graphene Oxide Nanosheets as Effective Friction Modifier for Oil Lubricant: Materials, Methods, and Tribological Results. ISRN Tribol. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Kamel, B.M.; Mohamed, A.; El Sherbiny, M.; Abed, K.A.; Abd-Rabou, M. Tribological properties of graphene nanosheets as an additive in calcium grease. J. Dispers. Sci. Technol. 2016, 38, 1495–1500. [Google Scholar] [CrossRef]
- Elomaa, O.; Singh, V.K.; Iyer, A.; Hakala, T.J.; Koskinen, J. Graphene oxide in water lubrication on diamond-like carbon vs. stainless steel high-load contacts. Diam. Relat. Mater. 2015, 52, 43–48. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2010, 41, 209–215. [Google Scholar] [CrossRef]
- Cui, M.; Wu, X.; Mao, J.; Wang, X.; Nie, M. T2DM Self-Management via Smartphone Applications: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166718. [Google Scholar] [CrossRef]
- Marchetto, D.; Restuccia, P.; Ballestrazzi, A.; Righi, M.; Rota, A.; Valeri, S. Surface passivation by graphene in the lubrication of iron: A comparison with bronze. Carbon 2017, 116, 375–380. [Google Scholar] [CrossRef]
- Dang, R.K.; Goyal, D.; Chauhan, A.; Dhami, S.S. Numerical and Experimental Studies on Performance Enhancement of Journal Bearings Using Nanoparticles Based Lubricants. Arch. Comput. Methods Eng. 2021, 28, 3887–3915. [Google Scholar] [CrossRef]
- Mungse, H.P.; Khatri, O.P. Chemically Functionalized Reduced Graphene Oxide as a Novel Material for Reduction of Friction and Wear. J. Phys. Chem. C 2014, 118, 14394–14402. [Google Scholar] [CrossRef]
- Kiong, K.S.S.; Yusup, S.; Soon, C.V.; Arpin, T.; Samion, S.; Kamil, R.N.M. Tribological Investigation of Graphene as Lubricant Additive in Vegetable Oil. J. Phys. Sci. 2017, 28, 257–267. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-Based Engine Oil Nanofluids for Tribological Applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, X.; Ren, T.; de Vries, E.; van der Heide, E. The emulsifying and tribological properties of modified graphene oxide in oil-in-water emulsion. Tribol. Int. 2017, 105, 304–316. [Google Scholar] [CrossRef]
- Cho, D.-H.; Wang, L.; Kim, J.-S.; Lee, G.-H.; Kim, E.S.; Lee, S.; Lee, S.Y.; Hone, J.; Lee, C. Effect of surface morphology on friction of graphene on various substrates. Nanoscale 2013, 5, 3063–3069. [Google Scholar] [CrossRef]
- Dou, X.; Koltonow, A.R.; He, X.; Jang, H.D.; Wang, Q.; Chung, Y.-W.; Huang, J. Self-dispersed crumpled graphene balls in oil for friction and wear reduction. Proc. Natl. Acad. Sci. USA 2016, 113, 1528–1533. [Google Scholar] [CrossRef]
- MCai, H.; Zhao, Z.B.; Zhang, L.; Yue, X.; Zhu, W. Combined effect of textured patterns and graphene flake ad-ditives on tribological behavior under boundary lubrication. PLoS ONE 2016, 11, e0152143. [Google Scholar]
- Azman, S.S.N.; Zulkifli, N.W.M.; Masjuki, H.; Gulzar, M.; Zahid, R. Study of tribological properties of lubricating oil blend added with graphene nanoplatelets. J. Mater. Res. 2016, 31, 1932–1938. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Qin, X.-X. Study on friction performance of graphene-based semi-solid grease. Chin. Chem. Lett. 2014, 25, 1305–1307. [Google Scholar] [CrossRef]
- Kumar, P.; Wani, M.F. Tribological Characterisation of Graphene Oxide as Lubricant Additive on Hypereutectic Al-25Si/Steel Tribopair. Tribol. Trans. 2017, 61, 335–346. [Google Scholar] [CrossRef]
- Vidal, F.A.C.; Avila, A.F. Tribological Investigation of Nanographite Platelets as Additive in Anti-Wear Lubricant: A Top-Down Approach. J. Tribol. 2014, 136, 031603. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Ma, S. In-situ exfoliated graphene for high-performance water-based lubricants. Carbon 2016, 96, 1181–1190. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P.; et al. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Synthesis of nano-Cu/graphene oxide composites by supercritical CO2-assisted deposition as a novel material for reducing friction and wear. Chem. Eng. J. 2015, 281, 11–19. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 31246. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Melvin, A.A.; Joshi, S.; Dash, S.; Tyagi, A.K. Effective Noncovalent Functionalization of Poly(ethylene glycol) to Reduced Graphene Oxide Nanosheets through γ-Radiolysis for Enhanced Lubrication. J. Phys. Chem. C 2016, 120, 2139–2148. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Luo, J. Tribological properties of few-layer graphene oxide sheets as oil-based lubricant additives. Chin. J. Mech. Eng. 2015, 29, 439–444. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nishina, Y.; Alias, A.A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2013, 66, 720–723. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, J.; Wang, J.; Yang, Z.; Liu, S.; Wang, Z.; Yang, S. Preparation of a reduced graphene oxide/zirconia nanocomposite and its application as a novel lubricant oil additive. RSC Adv. 2015, 5, 91802–91812. [Google Scholar] [CrossRef]
- Ou, J.; Wang, J.; Liu, S.; Mu, B.; Ren, J.; Wang, H.; Yang, S. Tribology Study of Reduced Graphene Oxide Sheets on Silicon Substrate Synthesized via Covalent Assembly. Langmuir 2010, 26, 15830–15836. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L. High-performance lubricant additives based on modified graphene oxide by ionic liquids. J. Colloid Interface Sci. 2015, 452, 98–108. [Google Scholar] [CrossRef]
- Ismail, N.A.; Bagheri, S. Highly oil-dispersed functionalized reduced graphene oxide nanosheets as lube oil friction modifier. Mater. Sci. Eng. B 2017, 222, 34–42. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, G.; Zhao, Q.; Gong, K.; Wang, X.; Liu, W.; Liu, W. Investigating the tribological performance of nanosized MoS2 on graphene dispersion in perfluoropolyether under high vacuum. RSC Adv. 2016, 6, 98606–98610. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Wang, Y.; Ren, T. Tribological studies on a novel borate ester containing benzothiazol-2-yl and disulfide groups as multifunctional additive. Tribol. Int. 2010, 43, 1048–1053. [Google Scholar] [CrossRef]
- Kumar, A.; Behera, B.; Ray, S.S. Microwave-assisted surface-initiated redox polymerization of acrylamide with functionalized graphene oxide for aqueous lubricant additive. RSC Adv. 2015, 5, 39474–39481. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, W.; Liu, Z. Preparation, characterization, and tribological properties of oleic diethanolamide-capped zinc borate-coated graphene oxide composites. J. Alloy Compd. 2017, 705, 384–391. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Miura, K.; Ishikawa, M. C60 Intercalated Graphite as Nanolubricants. Materials 2010, 3, 4510–4517. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Mao, J.; He, Y.; Luo, J. Synthesis of thermally reduced graphite oxide in sulfuric acid and its application as an efficient lubrication additive. Tribol. Int. 2017, 116, 303–309. [Google Scholar] [CrossRef]
- Song, H.; Wang, Z.; Yang, J.; Jia, X.; Zhang, Z. Facile synthesis of copper/polydopamine functionalized graphene oxide nanocomposites with enhanced tribological performance. Chem. Eng. J. 2017, 324, 51–62. [Google Scholar] [CrossRef]
- Singh, V.K.; Elomaa, O.; Johansson, L.-S.; Hannula, S.-P.; Koskinen, J. Lubricating properties of silica/graphene oxide composite powders. Carbon 2014, 79, 227–235. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Ju, M.J.; Xu, J.; Choi, H.-J.; Seo, J.-M.; Kim, M.-J.; Choi, I.T.; Kim, H.M.; Kim, J.C.; Lee, J.-J.; et al. Edge-Fluorinated Graphene Nanoplatelets as High Performance Electrodes for Dye-Sensitized Solar Cells and Lithium Ion Batteries. Adv. Funct. Mater. 2015, 25, 1170–1179. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent Functionalization of Fluorinated Graphene and Subsequent Application as Water-based Lubricant Additive. ACS Appl. Mater. Interfaces 2016, 8, 7483–7488. [Google Scholar] [CrossRef]
- Paul, G.; Hirani, H.; Kuila, T.; Murmu, N.C. Nanolubricants dispersed with graphene and its derivatives: An assessment and review of the tribological performance. Nanoscale 2019, 11, 3458–3483. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, B.; Dai, J.; Peng, C.; Li, C.; Li, Q.; Pan, F. Tribological Behaviors of Graphene and Graphene Oxide as Water-Based Lubricant Additives for Magnesium Alloy/Steel Contacts. Materials 2018, 11, 206. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, S.K. Design and development of e-smart robotics-based underground solid waste storage and transportation system. J. Clean. Prod. 2022, 343, 130987. [Google Scholar] [CrossRef]
- Skszek, T.; Conklin, J.; Zaluzec, M.; Wagner, D. Multi-Material Lightweight Vehicles: Mach-II Design. 2014. Available online: http://energy.gov/sites/prod/files/2014/07/f17/lm088_skszek_2014_o.pdf (accessed on 8 August 2022).
- Rasheed, A.; Khalid, M.; Javeed, A.; Rashmi, W.; Gupta, T.; Chan, A. Heat transfer and tribological performance of graphene nanolubricant in an internal combustion engine. Tribol. Int. 2016, 103, 504–515. [Google Scholar] [CrossRef]
- Mang, T.; Dresel, W. Lubricants and Lubrication, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Sarafraz, M.; Hormozi, F. Heat transfer, pressure drop and fouling studies of multi-walled carbon nanotube nano-fluids inside a plate heat exchanger. Exp. Therm. Fluid Sci. 2016, 72, 1–11. [Google Scholar] [CrossRef]
- Srinivas, V.; Moorthy, C.V.K.N.S.N.; Dedeepya, V.; Manikanta, P.V.; Satish, V. Nanofluids with CNTs for automotive applications. Heat Mass Transf. 2015, 52, 701–712. [Google Scholar] [CrossRef]
- M’Hamed, B.; Sidik, N.A.C.; Akhbar, M.F.A.; Mamat, R.; Najafi, G. Experimental study on thermal performance of MWCNT nanocoolant in Perodua Kelisa 1000cc radiator system. Int. Commun. Heat Mass Transf. 2016, 76, 156–161. [Google Scholar] [CrossRef]
- Ramón-Raygoza, E.; Rivera-Solorio, C.; Giménez-Torres, E.; Cortés, D.M.; Cardenas-Alemán, E.; Sampedro, R.C. Development of nanolubricant based on impregnated multilayer graphene for automotive applications: Analysis of tribological properties. Powder Technol. 2016, 302, 363–371. [Google Scholar] [CrossRef]
- Selvam, C.; Raja, R.S.; Lal, D.M.; Harish, S. Overall heat transfer coefficient improvement of an automobile radiator with graphene based suspensions. Int. J. Heat Mass Transf. 2017, 115, 580–588. [Google Scholar] [CrossRef]
- Contreras, E.M.C.; Oliveira, G.A.; Filho, E.P.B. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Esquivel-Gaon, M.; Nguyen, N.H.A.; Sgroi, M.F.; Pullini, D.; Gili, F.; Mangherini, D.; Pruna, A.I.; Rosicka, P.; Sevcu, A.; Castagnola, V. In vitro and environmental toxicity of reduced graphene oxide as an additive in automotive lubricants. Nanoscale 2018, 10, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Izzaty, N.; Sastra, H. Ilyas The Implementation of Graphene Composites for Automotive: An Industrial Perspective. IOP Conf. Ser. Mater. Sci. Eng. 2019, 536, 012133. [Google Scholar] [CrossRef]
- Kojima, E.; Kano, K.; Wado, H.; Iwamori, N.; Corp, D. Magnetic Field Sensor of Graphene for Automotive Applications Principle of a Hall Sensor for Detecting Magnetic Manufacturing a Graphene Hall Sensor the Manufacturing Process for the Graphene Hall Sensor is Shown in; SAE: Warrendale, PA, USA, 2017. [Google Scholar] [CrossRef]
- Toh, L.K.L.; Ting, T.W. Thermal performance of automotive radiator with graphene nanoplatelets suspension. AIP Conf. Proc. 2019, 2059, 020012. [Google Scholar] [CrossRef]
- Sumanth, S.; Rao, P.B.; Krishna, V.; Seetharam, T.; Seetharamu, K. Effect of carboxyl graphene nanofluid on automobile radiator performance. Heat Transfer—Asian Res. 2018, 47, 669–683. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Abdelkareem, M.A.; Gulzar, M.; Elsheikh, A. Novel approach of the graphene nanolubricant for energy saving via anti-friction/wear in automobile engines. Tribol. Int. 2018, 124, 209–229. [Google Scholar] [CrossRef]
- Selvam, C.; Lal, D.M.; Harish, S. Enhanced heat transfer performance of an automobile radiator with graphene based suspensions. Appl. Therm. Eng. 2017, 123, 50–60. [Google Scholar] [CrossRef]
- Amiri, A.; Sadri, R.; Shanbedi, M.; Ahmadi, G.; Kazi, S.; Chew, B.; Zubir, M.N.M. Synthesis of ethylene glycol-treated Graphene Nanoplatelets with one-pot, microwave-assisted functionalization for use as a high performance engine coolant. Energy Convers. Manag. 2015, 101, 767–777. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Approaches for Achieving Superlubricity in Two-Dimensional Materials. ACS Nano 2018, 12, 2122–2137. [Google Scholar] [CrossRef]








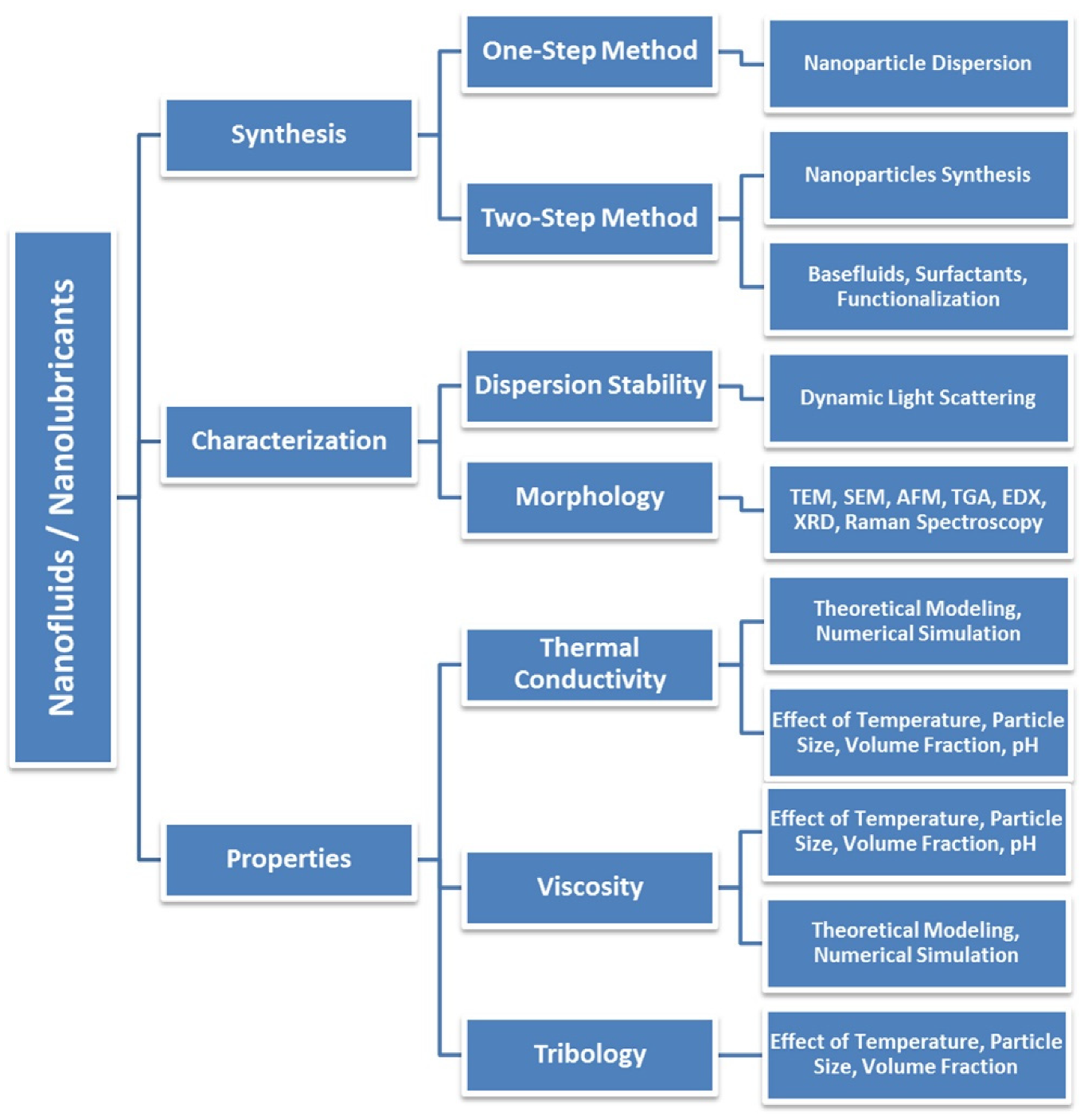


| Property | Value | Property | Value |
|---|---|---|---|
| Bond type | sp2 | Crystal structure | Hexagonal |
| Dimension | 2D | Surface area | 2630 m2/g |
| Melting point | ~3852 °C | C-C bond length | 0.142 nm |
| Mobility (typical) | ~200,000 cm2V−1s−1 | Mobility (intrinsic) | 108 A cm−2 |
| Real density | 2.25 g/cm3 | Mass (bulk) density | ~0.3 g/cm3 |
| Relaxation length | ~15,000 cm2V−1s−1 | Thickness | ~1–2 nm |
| Thermal conductivity | ~5000 W/m-K | Electrical conductivity | ~20,000 S/cm |
| Elasticity modulus | ~1 TPa | Intrinsic strength | ~130 GPa |
| Fracture toughness | ~4 MPa-m0.5 | Breaking strength | 42 Nm−1 |
| Electron mobility | ~2.5 × 105 cm2/(V·s) | Electron density | 2 × 1011 cm−2 |
| High temp. resistivity | −75 + 200 °C between not changing | Optical transmittance | 97.7% |
| Interplanar spacing b/w Gr sheets | 0.335 nm | Spin R | 1.5–2 µm |
| Fermi velocity | 300–500 nm | Phase coherence length | 3–5 µm |
| Current density | c/300 = 1,000,000 ms−1 | Sheet resistance | 1.3 × 10−4–5.1 × 10−4 Ω/sq |
| Materials | Process/Electrolyte | Power Density (kW kg−1) | Energy Density (W h kg−1) | Specific Capacitance (Fg−1) | Ref. |
|---|---|---|---|---|---|
| r-GRO | Reduced GR and convection dry | 9.8 | 85.6 | 250 | [53] |
| PPy-GR | Electric deposition on GRO | 3 | 5.7 | 1510 | [58] |
| PANI-GR | In situ polymerization | 0.14 | 37.9 | 1126 | [80] |
| PEDOT-GR | Oxidative polymerization | 0.038 | 12 | 304 | [59] |
| MnO2-exfolitated graphite | Dip and dry deposition | 110 | 12.5 | 315 | [89] |
| RuO2-GR | Sol-gel treatment with RuO2 and GRO | 0.05 | 20.1 | 570 | [70] |
| Fe3O4-GR | Crystallization of metal oxide with r-GRO | 2.4 | 85 | 326 | [72] |
| N-doped GR | N2 plasma | 800 | 48 | 282 | [75] |
| N-doped GR | Et4NBF4/PC (2 at% N) | 1 | 76.7 | 138.1 | [77] |
| N-doped GR | KOH (10.1 at% N) | 7.98 | 25 | 326 | [78] |
| N-doped GR | Bu4NBF4 (10 wt% N) | - | - | 248.4 | [79] |
| PANI-GR | in situ polymerization/reduction–dedoping/redoping | 136 | 34.8 | 1126 | [80] |
| Material | Type | Capacity (mAh/g) | Cycling Condition | Ref. |
|---|---|---|---|---|
| 3D GR integrated LiFePO4 | Cathode | 146 | 17 mA/g | [128] |
| LiFePO4/3D GR Composite | Cathode | 160 | C/3 | [129] |
| LiFePO4@C/r-GRO | Cathode | 119 | 20 C | [130] |
| Free-standing 3D GR/LiFePO4 | Cathode | 115 | 10 C | [132] |
| N-doped GR/LiFePO4 | Cathode | 78 | 100 C | [131] |
| GR/LiMn2O4 | Cathode | 113 | 0.5 C | [136] |
| GR/LiMn2O4 Nanoparticles | Cathode | 140 | 70 mA/g | [138] |
| 3D macroporous GR-based Li2FeSiO4 | Cathode | 313 | 0.1 C | [142] |
| GR and carbon nanotube co-modified Li3V2(PO4)3/C | Cathode | 147.5 | 20 C | [121] |
| GR@Si@GR 3D sandwich structure | Anode | 2515 | 0.4 C | [116] |
| 3D GR/SnO2 | Anode | 1096 | 1 A/g | [106] |
| Li4Ti5O12/N-reduced graphene oxide | Anode | 117.8 | 30 C | [122] |
| Graphene/SnO2-Co3O4 Nanocubes | Anode | 1665 | 100 mA/g | [112] |
| MoS2/graphene | Anode | 870 | 1 A/g | [117] |
| 3D graphene/SnS2 | Anode | 1386.7 | 100 mA/g | [118] |
| 3D graphene/SnCo Nanoparticles | Anode | 1117 | 72 mA/g | [119] |
| Ion | Divacancy | Pristine | Stone-Wales |
|---|---|---|---|
| Na+ | 0.8848 e | 0.6617 e | 0.8073 e |
| Ca2+ | 1.3727 e | 0.8208 e | 1.1189 e |
| Polymers | GR (Content) | Solvent | Contribution | Ref. |
|---|---|---|---|---|
| Graphene based membranes in PEMFCs | ||||
| Poly (ethylene oxide) (PEO) | GRO (0.5 wt%) | Distilled H2O | Improvements to the Young’s modulus, electronic resistance tensile strength and ionic conductivity | [172] |
| Polybenzimidazole (PBI) | Graphite oxide & Sulfonated GRO (2 wt%) | N,N-dimethylacetamide (DMAc) | Enhancement of ionic and proton conductivities | [183] |
| Sulfonated polyimide | Ionic liquid polymer adapted GR sheets (10 wt%) | Dimethyl sulfoxide (DMSO) | Increase in tensile strength, ionic conductivity and other mechanical properties | [197] |
| Nafion | Rolled up graphene oxide sheets | N,N-dimethylformamide (DMF) | Enrichment of water retention ability, better proton transport and conductivity, reduced activation energy | [193] |
| Nafion | f-GRO (5 and 10 wt%) | Ethanol | Improvement to chemical and mechanical stability, better water intake and IEC with higher proton conductivity | [190] |
| Sulfonated poly(ether sulfone) | Mesonaphth o-bifluorenegraphene moiety | Dimethyl sulfoxide (DMSO) | Enhancement of thermal stability, better water intake and IEC with increased proton conductivity | [194] |
| Polybenzimidazole (PBI) | 3-amino propyl-triethoxysilane ionic liquid f-graphite oxide (5 wt%) | N,N-dimethylacetamide (DMAc) | Better ionic and proton conductivities | [181] |
| Nafion–SPEEK | GRO (0.75 wt%) | Ethanol/Water(75:25 V/V) | Increase in proton conductivity which results in increase in current and power densities | [191] |
| SPEEK | Polydopamine-modified GRO (DGRO) (2.5, 5, 7.5, and 10 wt%) | Dimethyl formamide (DMF) | Improvement of the proton conductivity, power and current densities | [203] |
| Nafion | Graphite oxide (4 wt%) | N,N-dimethylacetamide (DMAc) | Enhancement of proton conductivity and peak power density | [187] |
| Nafion | Polyoxometalate coupled GRO (1%) | Deionized water | Improvement of the proton conductivity and water retention capacity, decrease in ohmic resistance | [188] |
| Nafion | GRO Pt-GR (0.5–4.5 wt%) | Water and isopropyl alcohol (IPA) | Increase in tensile strength, but the results obtained with Pt-GR were not optimum | [173] |
| Nafion | Pt–GR/SiO2 (0.5–3 wt%) | Deionized water and IPA | Improvement of cell performance up to 1.5 wt% concentration of Pt-GR, increase in water uptake and proton conductivity | [174] |
| Nafion | GRO (2, 3 and 5 wt%) | N,N-dimethylacetamide (DMAc) | Increase in tensile strength, water uptake, proton and electrical conductivities | [185] |
| Nafion/Pt–TiO2 | GRO (1 wt%) | IPA-water mixture | Enhancement of proton and electrical conductivities | [175] |
| Graphene based membranes in DMFCs | ||||
| Nafion | SGRO (0.5 wt%) | N,N-dimethyl formamide | Improvement of proton conductivity, reduction in activation energy and methanol crossover | [204] |
| SPEEK | SGRO (0–10 wt%) | N,N-DMAc | Increase in proton conductivity, water retention capacity and mechanical properties, decrease methanol crossover | [205] |
| SPEEK/PVA | SGRO/Fe3O4 (3–7 wt%) | N,N-DMAc | Decrease in methanol crossover and improvement in proton conductivity and mechanical stability | [206] |
| Nafion 115 | GRO (0–2 wt%) | Deionized water | Decrease in methanol crossover and improvement in proton conductivity | [210] |
| Nafion | GRO (0.1–2%) | Dimethyl formamide (DMF) | Retention of ionic conductivity, increase in thermal and mechanical properties, reduction of methanol crossover | [209] |
| Nafion | PDDA/GRO | - | Improvement of power density and lowering of methanol crossover | [208] |
| Sulfonated Polyimide (SPI) | Sulfonated propyl-9 Silane GRO | N,N-DMAc | Enhancement of thermal, mechanical, and chemical stabilities, increase in proton conductivity and water retention characteristics | [198] |
| SPEEK | Carboxyl-functionalized graphene (G(c)) (0.1–0.25 wt%) | DMF | Increase in proton conductivity and reduction in methanol crossover, improvement in water retention capacity and self-humidifying characteristics | [176] |
| SPEEK | Sodium dodecylbenzene sulfonate (SDBS) adsorbed GRO (5 wt%) | DMF | Improvement of methanol permeability, water retention capacity, proton and electrical conductivities | [202] |
| Nafion | SGRO (0.05–0.5 wt%) | N,N-DMAc | Reduction in methanol uptake and swelling ratio, increase in proton conductivity and water retention capacity | [189] |
| SPEEK | GRO (1–6 wt%) | DMF | Improvement of proton conductivity, selectivity and reduction in methanol crossover | [201] |
| Self-supporting membrane | GRO laminates (3 mg/l) | Deionized water | Better power density in comparison to Nafion membrane without any decrease in open circuit potential | [207] |
| Others | ||||
| SPEEK in Microbial FC | Single layer GRO (0.25 wt%) | N-Methyl−2-pyrrolidone (NMP) | Increase in water retention capability, selectivity, proton conductivity and oxygen diffusion coefficient | [220] |
| Polybenzimidazole (PBI) in Alkaline Anion Exchange Membrane FCs | Ionic Liquid-GRO (ILGRO) | DMSO | Increase in water uptake, thermal stability, tensile strength, swelling ratio and conductivities | [221] |
| PVA in Direct Methanol Alkaline FC | GR nanosheets (0.1–1.4 wt%) | Deionized water | Decrease in methanol crossover, improvement in tensile strength and ionic conductivity | [214] |
| Chloromethylated polysulfone (CMPSU) in alkaline FC | Quaternized graphenes (QGs) (0.25–1 wt%) | DMF | Enhancement of mechanical properties and bicarbonate conductivity | [222] |
| KOH in alkaline FC | GRO (5 mg/mL) | - | Reduction in hydrogen permeability, improvement in ionic conductivity and peak power density | [223] |
| GR Based Material | Electrode/Function | Sheet Resistance | Transmittance (%) | Category and Configuration of Solar Cells | Power Conversion Efficiency | Ref. |
|---|---|---|---|---|---|---|
| r-GRO | TA | 1.8 k Ω/sq | 70 | Solid-state DSSC: r-GRO/glass/dye/TiO2 spiro-OMeTAD/Au | 0.26% | [266] |
| r-GRO | TA | 3.2 k Ω/sq | 65% | OPV: r-GRO/PET/PEDOT:PSS P3HT: TiO2/PCBM/Al | 0.78% | [241] |
| CVD-GR | TA | 0.25 k Ω/sq | 95% | OPV: GR/quartz/PEDOT:PSS/ CuPc: BCP/C60/Ag | 0.85% | [253] |
| r-GRO-CNT | TA | 0.6 k Ω/sq | 87% | OPV: r-GRO-CNT/glass/PEDOT:PSS P3HT: Ca:Al/PCBM | 0.85% | [93] |
| CVD-GR | TA | 0.08 k Ω/sq | 90% | OPV: GR/quartz/MoO3+ PEDOT:PSS/P3HT:PCBM/Al/LiF | 2.5% | [256] |
| Au-doped GR | TA | 0.293 k Ω/sq | 90% | OPV: Au-GR/PEDOT:PSS/ P3HT:PCBM/ITO/ZnO | 3.04% | [267] |
| r-GRO | TC | 0.42 k Ω/sq | 61% | Hybrid solar cell: r-GRO/quartz/ZnO/ P3HT/Au/PEDOT:PSS | 0.31% | [259] |
| CVD-GR | TC | 0.22 k Ω/sq | 84 | Thin film solar cell: GR/glass/CdTe/ CdS/graphite paste/ZnO | 4.17% | [232] |
| Al-TiO2 modified GR | TC | 1.2 k Ω/sq | 96 | OPV: GR/Al-TiO2/P3HT:PCBM/MoO3/Ag/Au | 2.58% | [243] |
| fr-GRO | CCE | - | - | Liquid DSSC: r-GRO/FTO/dye/I3−/I1−/TiO2/mediated electrolyte | 4.99% | [269] |
| CNT-r-GRO paper | CCE | - | - | Liquid DSSC: CNT-r-GRO/TiO2/FTO/I3−/I1− mediated electrolyte/dye/ | 6.05% | [267] |
| GR platelets | CCE | - | - | Liquid DSSC: GR/FTO/Co(III)/(II) mediated electrolyte TiO2/dye | 9.3% | [270] |
| GR QDs | Sensitizer of dye | - | - | Liquid DSSC: GR QD/dye/FTO//I3−/I1– mediated electrolyte/Pt/TiO2 | <0.1% | [264] |
| GR/n-Si | SJL | Schottky junction solar cell: Ag/GR/Ti/n-Si/Pb/Au | 1.65% | [226] | ||
| GR-TiO2 | SJL | Liquid DSSC: TiO2 -GR/FTO/Pt dye/I3−/I1- mediated electrolyte | 6.06% | [271] | ||
| GRO | HTL | OPV: GRO/ITO/Al/P3HT:PCBM | 3.5 | [257] | ||
| MoO3-GR | Interfacial layer | Series tandem solar cell: GR-MoO3/PEDOT:PSS/ITO/P3HT:PCBM/ZnPc:C60/Al/LiF | 2.3% | [254] | ||
| ZnO-GRO-PEDOT:PSS | Interfacial layer | Series tandem solar cell: ZnO-GRO-PEDOT:PSS/PEDOT:PSS/ITO/P3HT:PCBM/Ca/P3HT:PCBM/Al | 4.14% | [255] |
| Material | Medium | Composition | Reduction in COF (%) | Reduction in Wear (%) | Ref. |
|---|---|---|---|---|---|
| GR | Synthetic oil | 78 | 90 | [286] | |
| reduced f-GRO | Oil | 16 | 30 | [310] | |
| GRO | 10W-40 oil | 37.5 | 36.4 | [289] | |
| GRO | Oil-in-water emulsion | 21.8 | 27.9 | [292] | |
| Exfoliated GR | Oleic acid | 0.02 to 0.06 wt% | 17 | 14 | [301] |
| GRO + Zinc borate | 500 SN oil | 2 wt% | 48.2 | 40 | [314] |
| GR | Grease in semi-solid state | - | 40 to 60 | 50 | [297] |
| Thermally converted graphite oxide | H2SO4 | 30 | 75 | [318] | |
| GR | Deionized H2O | 23.8 to 110 μg/ml | 81.3 | 61.8 | [300] |
| GRO | H2O | 0.2 wt% | 57 | [284] | |
| SiO2/GRO composite | C2H6O2 | 0.125 wt% | 38 | 31 | [320] |
| GRO nanosheets | SN150 | 0.1 wt% | 30 | - | [282] |
| ZrO2 nanoparticles/r-GRO nanosheets | Paraffin oil | 0.06 wt% | 56 | 6.4 | [307] |
| Modified GO | Multi alkylated cyclo pentanes | 27 | 74 | [309] | |
| GR nanosheets | Grease | 3 wt% | 61 | 45 | [283] |
| MoS2/GR nanocomposite | PFPE | 1 wt% | 57.1 | 97 | [311] |
| Multi-layered GR | PAO2 | 0.05 wt% | 78 | 16 | [281] |
| Ag/GR nanocomposites | 10W40 oil | 0.06 to 0.10 wt% | 30.4 | 27.4 | [302] |
| GR nanosheet | Vegetable based oil | 50 ppm | 13.5 | 9.7 | [290] |
| Nanographite platelets | Mineral oil | 0.25 | 17 | 24.1 | [299] |
| GR | PAO4 | 0.04 wt% | 78 | 90 | [295] |
| Modified GR platelets | 350 SN oil | 0.075 wt% | 37 | [285] | |
| Single layer GR | C2H5OH | 1 mg/mL | 48 | [287] | |
| GRO | C2H5OH + SAE20W-50 oil | - | - | 60 to 80 | [298] |
| GR | Engine oil | 0.025 wt% | ∼80 | ∼33 | [291] |
| Polyacrylamide-grafted-f-GRO | Water | 0.2 to 1.0 wt% | 46 to 55 | 13 to 37 | [313] |
| Octadecylamine fGR | C16H34 | 0.06 wt% | 26 | 9 | [317] |
| r-GRO nanosheets | PEG 200 | 0.03 mg/mL | 38 | 55 | [304] |
| GR nanoplatelets | Palm-oil TMP ester + PAO | 0.05 wt% | 5 | 15 | [296] |
| Urea-modified fluorinated GR | Water | 1 mg/mL | 64.4 | [322] | |
| Cu nanoparticles/PDA f-GRO nanosheets | Soyabean oil | 0.1 wt% | 57 | 27 | [319] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, D.; Dang, R.K.; Goyal, T.; Saxena, K.K.; Mohammed, K.A.; Dixit, S. Graphene: A Path-Breaking Discovery for Energy Storage and Sustainability. Materials 2022, 15, 6241. https://doi.org/10.3390/ma15186241
Goyal D, Dang RK, Goyal T, Saxena KK, Mohammed KA, Dixit S. Graphene: A Path-Breaking Discovery for Energy Storage and Sustainability. Materials. 2022; 15(18):6241. https://doi.org/10.3390/ma15186241
Chicago/Turabian StyleGoyal, Deepam, Rajeev Kumar Dang, Tarun Goyal, Kuldeep K. Saxena, Kahtan A. Mohammed, and Saurav Dixit. 2022. "Graphene: A Path-Breaking Discovery for Energy Storage and Sustainability" Materials 15, no. 18: 6241. https://doi.org/10.3390/ma15186241
APA StyleGoyal, D., Dang, R. K., Goyal, T., Saxena, K. K., Mohammed, K. A., & Dixit, S. (2022). Graphene: A Path-Breaking Discovery for Energy Storage and Sustainability. Materials, 15(18), 6241. https://doi.org/10.3390/ma15186241








