Exploiting Partial Solubility in Partially Fluorinated Thermoplastic Blends to Improve Adhesion during Fused Deposition Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation
2.3. Dog-Bone Production
2.3.1. Fused Deposition Modeling (FDM)
2.3.2. Injection Molding (IM)
2.4. Methods
2.4.1. Apparent Viscosity Measurements
2.4.2. Thermal Analysis
2.4.3. Tensile Testing
2.4.4. Attenuated Total Reflectance Spectroscopy (ATR-FTIR)
2.4.5. Gel Permeation Chromatography (GPC)
3. Results and Discussion
3.1. Apparent Viscosity Calculations
3.2. Thermal Analysis
3.3. Tensile Testing
3.4. Attenuated Total Reflectance Spectroscopy (ATR-FTIR)
3.5. Gel Permeation Chromatography (GPC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babu, S.S.; Love, L.; Dehoff, R.; Peter, W.; Watkins, T.R.; Pannala, S. Additive Manufacturing of Materials: Opportunities and Challenges. MRS Bull. 2015, 40, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Arenas, J.M.; Narbón, J.J.; Alía, C. Optimum Adhesive Thickness in Structural Adhesives Joints Using Statistical Techniques Based on Weibull Distribution. Int. J. Adhes. Adhes. 2010, 30, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Bellehumeur, C.; Li, L.; Sun, Q.; Gu, P. Modeling of Bond Formation between Polymer Filaments in the Fused Deposition Modeling Process. J. Manuf. Process. 2004, 6, 170–178. [Google Scholar] [CrossRef]
- Iragi, M.; Pascual-González, C.; Esnaola, A.; Lopes, C.S.; Aretxabaleta, L. Ply and Interlaminar Behaviours of 3D Printed Continuous Carbon Fibre-Reinforced Thermoplastic Laminates; Effects of Processing Conditions and Microstructure. Addit. Manuf. 2019, 30, 100884. [Google Scholar] [CrossRef] [Green Version]
- Ning, F.; Cong, W.; Qiu, J.; Wei, J.; Wang, S. Additive Manufacturing of Carbon Fiber Reinforced Thermoplastic Composites Using Fused Deposition Modeling. Compos. Part B Eng. 2015, 80, 369–378. [Google Scholar] [CrossRef]
- Ueda, M.; Kishimoto, S.; Yamawaki, M.; Matsuzaki, R.; Todoroki, A.; Hirano, Y.; Le Duigou, A. 3D Compaction Printing of a Continuous Carbon Fiber Reinforced Thermoplastic. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105985. [Google Scholar] [CrossRef]
- Drzal, L.T.; Raghavendran, V.K. Adhesion of Thermoplastic Matrices to Carbon Fibers: Effect of Polymer Molecular Weight and Fiber Surface Chemistry. J. Thermoplast. Compos. Mater. 2003, 16, 21–30. [Google Scholar] [CrossRef]
- Kim, K.S.; Heo, J.C.; Kim, K.W. Effects of Temperature on the Microscale Adhesion Behavior of Thermoplastic Polymer Film. Tribol. Lett. 2010, 38, 97–106. [Google Scholar] [CrossRef]
- de León, A.S.; Domínguez-Calvo, A.; Molina, S.I. Materials with Enhanced Adhesive Properties Based on Acrylonitrile-Butadiene-Styrene (ABS)/Thermoplastic Polyurethane (TPU) Blends for Fused Filament Fabrication (FFF). Mater. Des. 2019, 182, 108044. [Google Scholar] [CrossRef]
- Sánchez-Adsuar, M.S. Influence of the Composition on the Crystallinity and Adhesion Properties of Thermoplastic Polyurethane Elastomers. Int. J. Adhes. Adhes. 2000, 20, 291–298. [Google Scholar] [CrossRef]
- Wang, P.; Zou, B.; Xiao, H.; Ding, S.; Huang, C. Effects of Printing Parameters of Fused Deposition Modeling on Mechanical Properties, Surface Quality, and Microstructure of PEEK. J. Mater. Process. Technol. 2019, 271, 62–74. [Google Scholar] [CrossRef]
- Mieth, F.; Tromm, M. Multicomponent Technologies; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780323371216. [Google Scholar]
- McCollum, J.; Morey, A.M.; Iacono, S.T. Morphological and Combustion Study of Interface Effects in Aluminized Poly(Vinylidene Fluoride) Composites. Mater. Des. 2017, 134, 64–70. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Zhuang, Y.; Drioli, E.; Lee, Y.M. Crystalline Polymorphism in Poly(Vinylidenefluoride) Membranes. Prog. Polym. Sci. 2015, 51, 94–126. [Google Scholar] [CrossRef]
- El Mohajir, B.E.; Heymans, N. Changes in Structural and Mechanical Behaviour of PVDF with Processing and Thermomechanical Treatments. 1. Change in Structure. Polymer 2001, 42, 5661–5667. [Google Scholar] [CrossRef]
- Chiu, F.C.; Chen, C.C.; Chen, Y.J. Binary and Ternary Nanocomposites Based on PVDF, PMMA, and PVDF/PMMA Blends: Polymorphism, Thermal, and Rheological Properties. J. Polym. Res. 2014, 21. [Google Scholar] [CrossRef]
- Cauda, V.; Stassi, S.; Bejtka, K.; Canavese, G. Nanoconfinement: An Effective Way to Enhance PVDF Piezoelectric Properties. ACS Appl. Mater. Interfaces 2013, 5, 13. [Google Scholar] [CrossRef]
- Lin, B.; Giurgiutiu, V. Modeling and Testing of PZT and PVDF Piezoelectric Wafer Active Sensors. Smart Mater. Struct. 2006, 15, 1085–1093. [Google Scholar] [CrossRef]
- Aid, S.; Eddhahak, A.; Khelladi, S.; Ortega, Z.; Chaabani, S.; Tcharkhtchi, A. On the Miscibility of PVDF/PMMA Polymer Blends: Thermodynamics, Experimental and Numerical Investigations. Polym. Test. 2019, 73, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Bencomo, J.A.J.A.; McCollum, J.M.J.; Iacono, S.T.S.T.; McCollum, J.M.J. 3D Printing Multifunctional Fluorinated Nanocomposites: Tuning Electroactivity, Rheology and Chemical Reactivity. J. Mater. Chem. A 2018, 6, 12308–12315. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, Y.J.; Bae, I.; Kim, K.J.; Kim, H.C.; Bauer, S.; Thomas, E.L.; Park, C. Printable Ferroelectric PVDF/PMMA Blend Films with Ultralow Roughness for Low Voltage Non-Volatile Polymer Memory. Adv. Funct. Mater. 2009, 19, 2812–2818. [Google Scholar] [CrossRef]
- Ma, T.; Cui, Z.; Wu, Y.; Qin, S.; Wang, H.; Yan, F.; Han, N.; Li, J. Preparation of PVDF Based Blend Microporous Membranes for Lithium Ion Batteries by Thermally Induced Phase Separation: I. Effect of PMMA on the Membrane Formation Process and the Properties. J. Membr. Sci. 2013, 444, 213–222. [Google Scholar] [CrossRef]
- Xiong, X.; Zhi, R.; Zhou, Q.; Yan, W.; Zhu, Y.; Chen, Y.; Fu, L.; Yu, N.; Wu, Y. A Binary PMMA/PVDF Blend Film Modified Substrate Enables a Superior Lithium Metal Anode for Lithium Batteries. Mater. Adv. 2021, 2, 4240–4245. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Buraidah, M.H.; Madhavan, J.; Arof, A.K. Effect of Tetrabutylammonium Iodide Content on PVDF-PMMA Polymer Blend Electrolytes for Dye-Sensitized Solar Cells. Ionics 2015, 21, 2889–2896. [Google Scholar] [CrossRef]
- Verma, H.; Mishra, K.; Rai, D.K. Microporous PVDF–PMMA Blend-Based Gel Polymer Electrolyte for Electrochemical Applications: Effect of PMMA on Electrochemical and Structural Properties. J. Electron. Mater. 2021. [Google Scholar] [CrossRef]
- Hu, Y.H.; Chen, C.Y.; Wang, C.C. Viscoelastic Properties and Thermal Degradation Kinetics of Silica/PMMA Nanocomposites. Polym. Degrad. Stab. 2004, 84, 545–553. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S. Crystallization Behaviors of Poly(Vinylidene Fluoride) and Poly(Methyl Methacrylate)-Block-Poly(2-Vinyl Pyridine) Block Copolymer Blends. J. Therm. Anal. Calorim. 2016, 125, 215–230. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, J.; Wang, X. Crystallizaion and Surface Morphology of Poly(Vinylidene Fluoride)/Poly(Methylmethacrylate) Films by Solution Casting on Different Substrates. Appl. Surf. Sci. 2008, 254, 2947–2954. [Google Scholar] [CrossRef]
- Elashmawi, I.S.; Hakeem, N.A. Effect of PMMA Addition on Characterization and Morphology of PVDF. Polym. Eng. Sci. 2008, 48, 895–901. [Google Scholar] [CrossRef]
- Lee, M.; Koo, T.; Lee, S.; Byong Hun Min, J.H.K. Morphology and Physical Properties of Nanocomposites Based on Poly(Methyl Methacrylate)/Poly(Vinylidene Fluoride) Blends and Multiwalled Carbon Nanotubes Minho. Polym. Compos. 2015, 16, 101–113. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, Y.M. Preparation and Investigation of PVDF/PMMA/TiO2 Composite Film. J. Mater. Sci. 2009, 44, 2977–2984. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Zhang, J.; Zhang, W.; Wang, X. Crystallization of PVDF in the PVDF/PMMA Blends Precipitated from Their Non-Solvents: Special Orientation Behavior, Morphology, and Thermal Properties. J. Cryst. Growth 2011, 328, 74–80. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Gregorio, R.; Cestari, M. Effect of Crystallization Temperature on the Crystalline Phase Content and Morphology of Poly(Vinylidene Fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
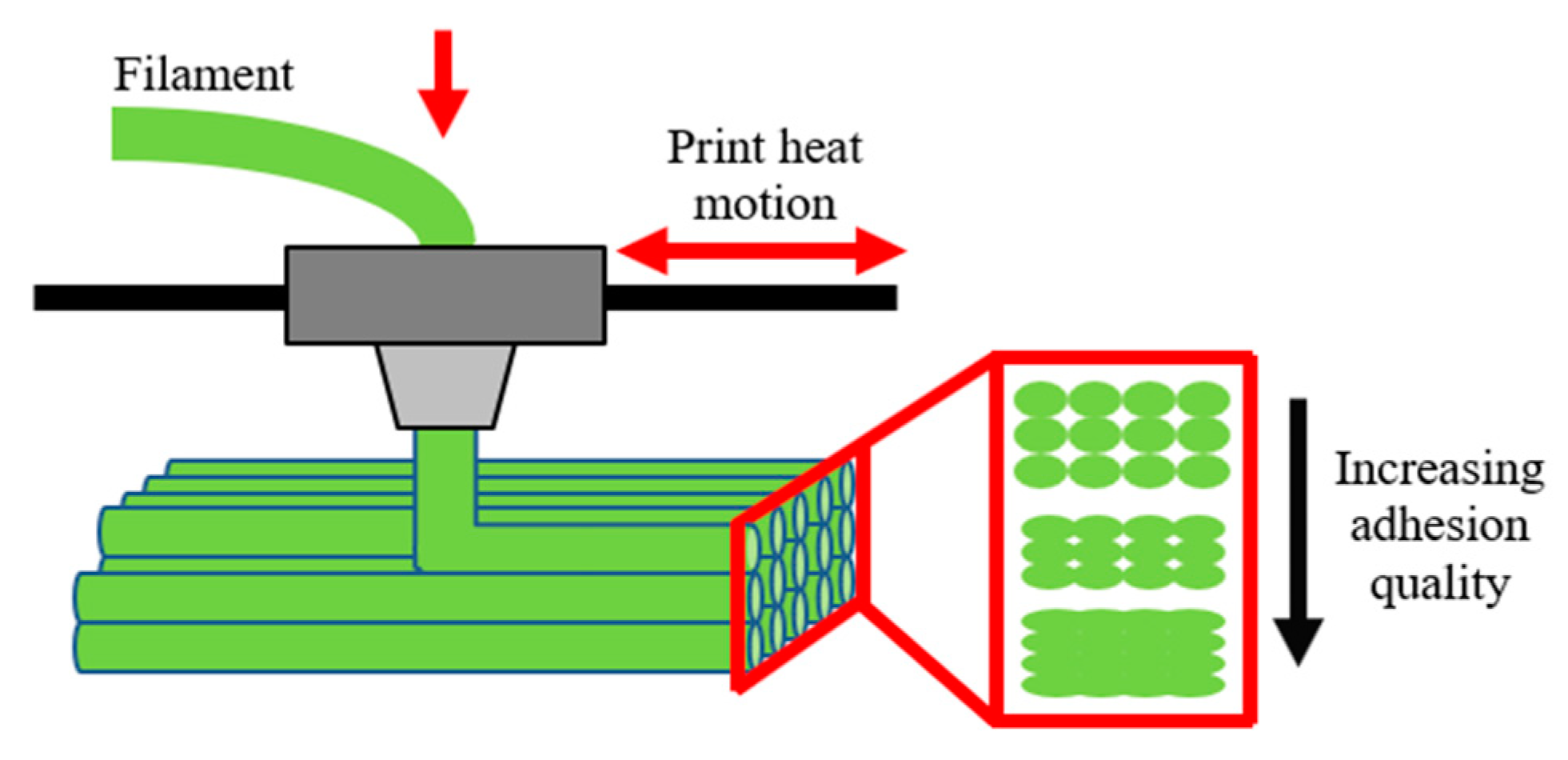
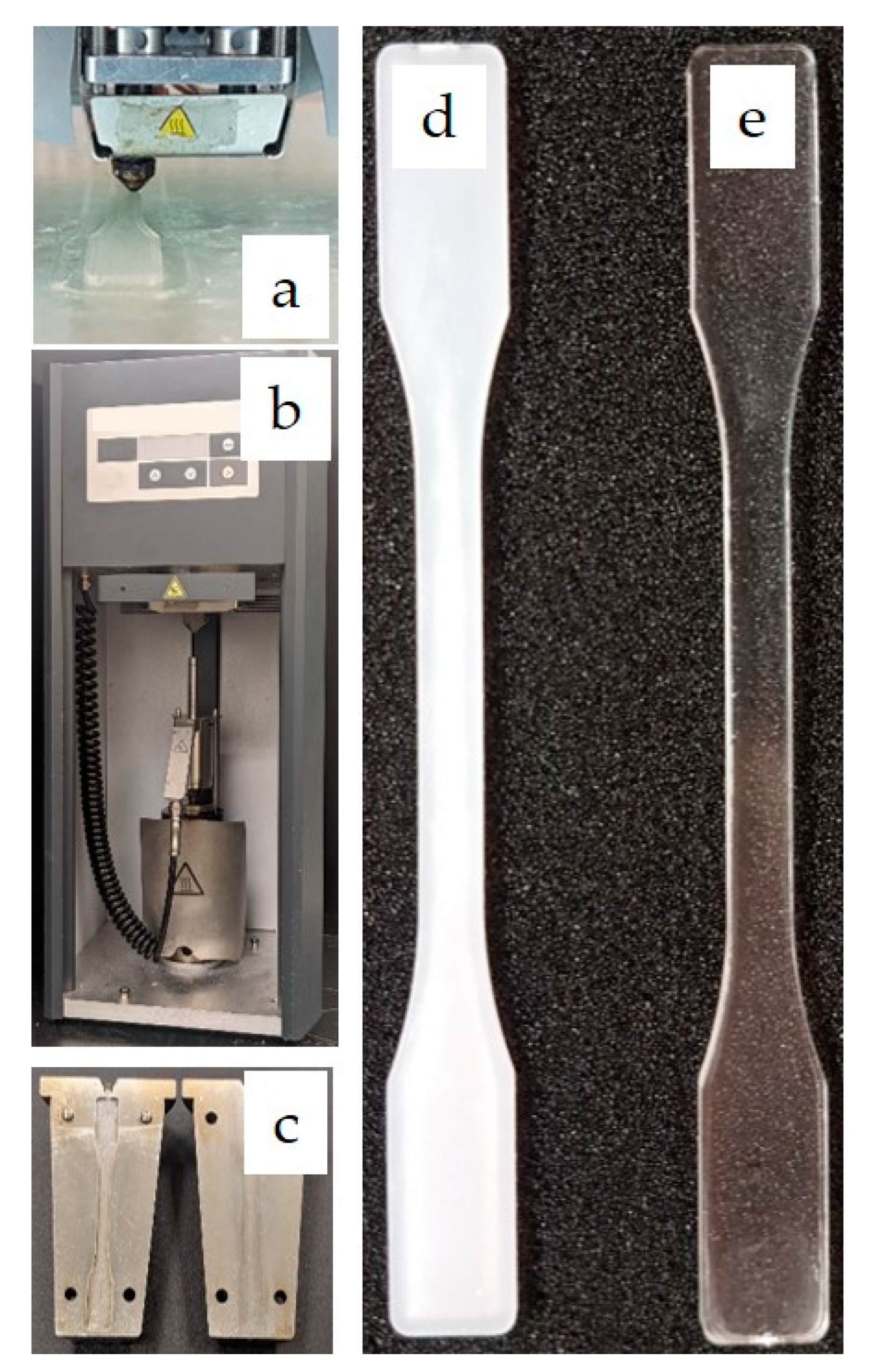
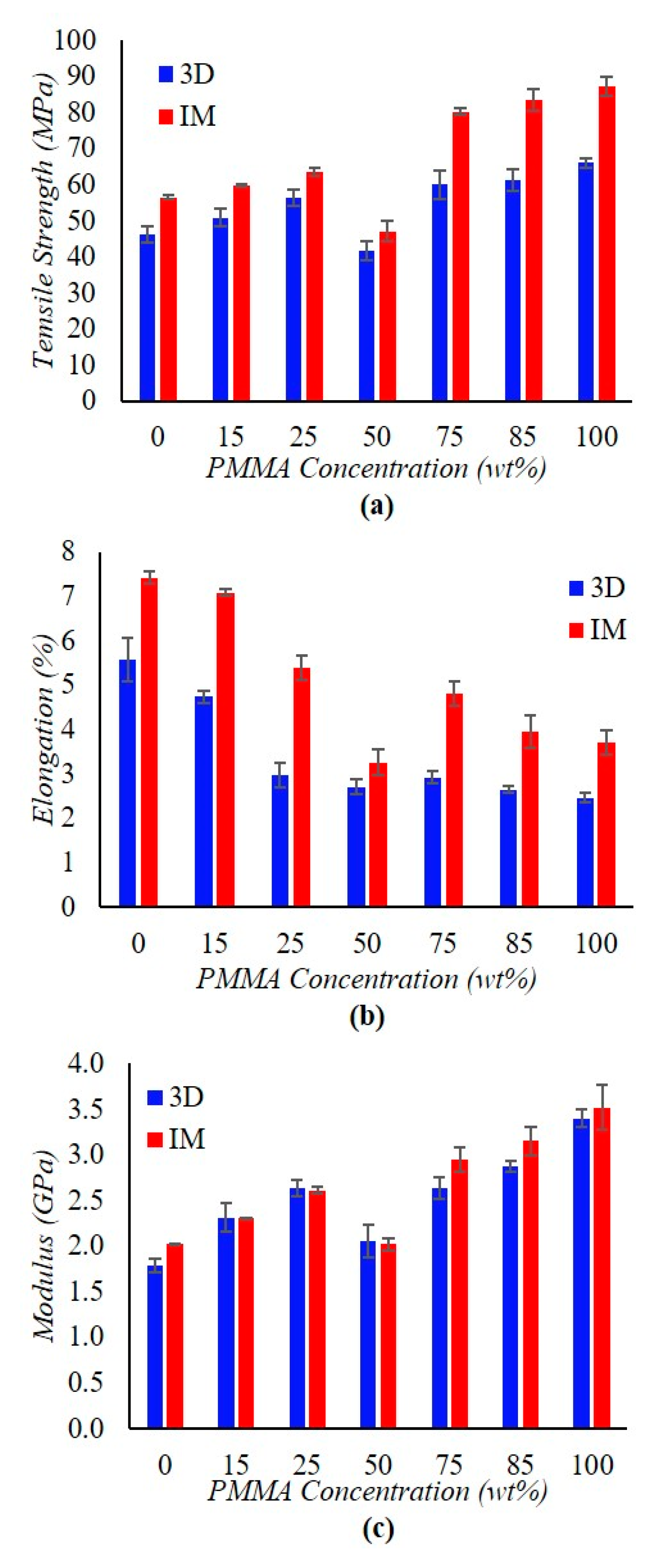
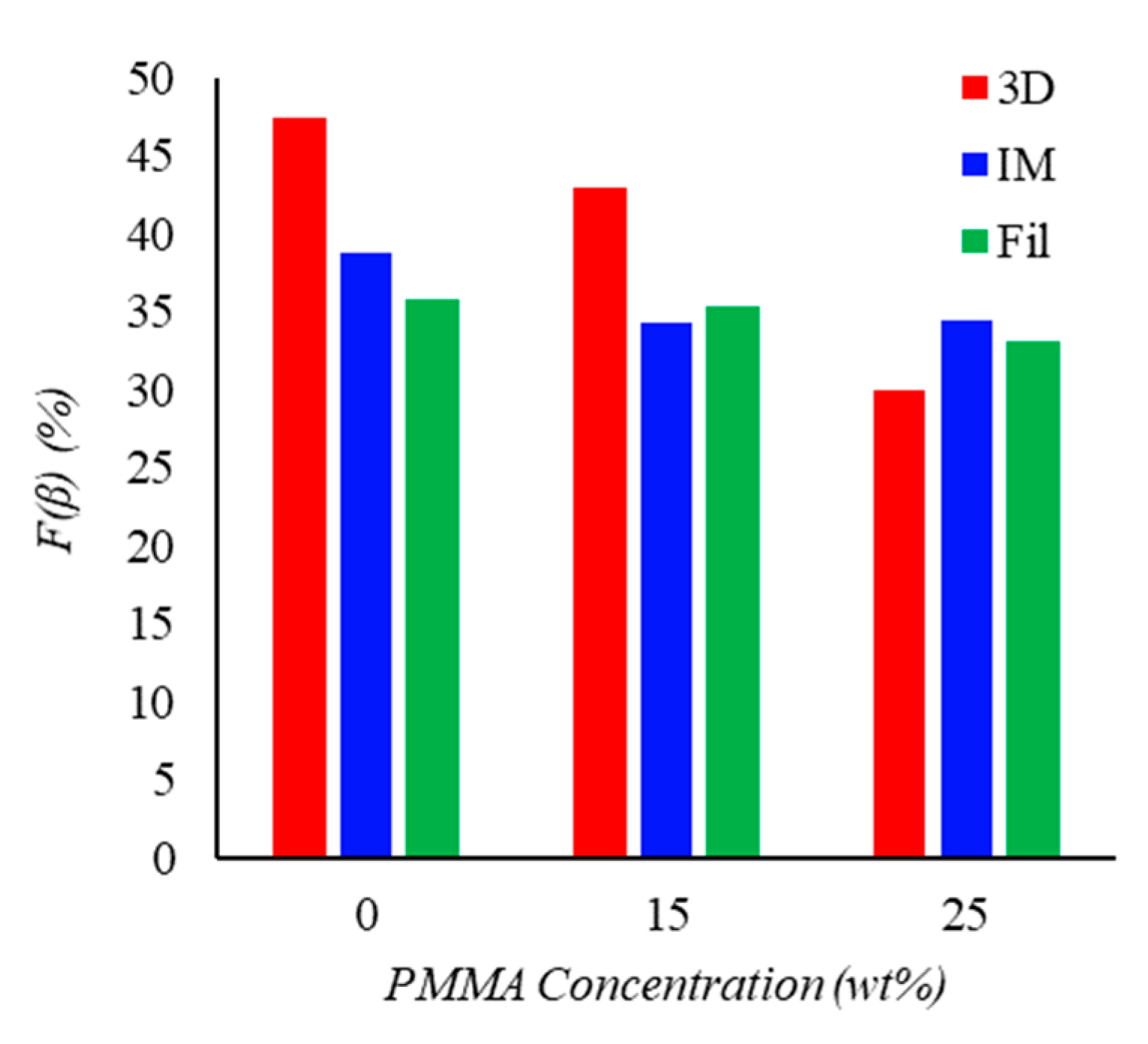
| Sample | Apparent Viscosity [kPa·s] |
|---|---|
| PMMA0 | 60.37 ± 0.51 |
| PMMA15 | 65.05 ± 0.38 |
| PMMA25 | 74.75 ± 0.44 |
| PMMA50 | 147.08 ± 6.97 |
| PMMA75 | 223.47 ± 2.22 |
| PMMA 85 | 235.49 ± 3.75 |
| PMMA 100 | 247.16 ± 5.35 |
| Filament | 3D Printed | IM | Filament | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | Hm (J/g) | Xc | Tg (°C) | Tm (°C) | Hm (J/g) | Xc | Tg (°C) | Tm (°C) | Hm (J/g) | Xc | |
| PMMA0 | -- | 164 | 45.0 | 43% | -- | 166 | 37.8 | 36% | -- | 161 | 38.4 | 37% |
| PMMA15 | 54 | 157 | 35.5 | 40% | 58 | 162 | 30.6 | 34% | 57 | 160 | 30.2 | 34% |
| PMMA25 | 57 | 160 | 28.8 | 37% | 60 | 164 | 32.1 | 41% | 61 | 157 | 21.0 | 27% |
| PMMA50 | 61 | 146 | 16.5 | 32% | 62 | 151 | 11.9 | 23% | 63 | 156 | 0.6 | 1% |
| PMMA75 | 88 | -- | -- | 0% | 86 | -- | -- | 0% | 99 | -- | -- | 0% |
| PMMA85 | 98 | -- | -- | 0% | 101 | -- | -- | 0% | 110 | -- | -- | 0% |
| PMMA100 | 111 | -- | -- | 0% | 106 | -- | -- | 0% | 112 | -- | -- | 0% |
| Sample | (g/mol) | (g/mol) | Dispersity |
|---|---|---|---|
| Pellet | 8.95 × 104 (±2.21%) | 1.41 × 105 (±1.33%) | 1.57 (±2.58%) |
| Filament | 9.08 × 104 (±1.37%) | 1.46 × 105 (±1.08%) | 1.61 (±1.74%) |
| Injection Molded | 8.29 × 104 (±4.76%) | 1.33 × 105 (±2.12%) | 1.59 (±5.21%) |
| 3D Printed | 8.62 × 104 (±3.71%) | 1.37 × 105 (±1.73%) | 1.62 (±4.09%) |
| Sample | (g/mol) | (g/mol) | Dispersity |
|---|---|---|---|
| Pellet | 4.57 × 104 (±7.11%) | 8.27 × 104 (±1.19%) | 1.81 (±7.21%) |
| Filament | 4.41 × 104 (±9.72%) | 8.24 × 104 (±1.77%) | 1.87 (±9.88%) |
| Injection Molded | 4.13 × 104 (±8.38%) | 8.25 × 104 (±1.31%) | 1.99 (±8.49%) |
| 3D Printed | 4.09 × 104 (±8.66%) | 8.02 × 104 (±1.27%) | 1.96 (±8.75%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldaña-Baqué, P.; Strutton, J.W.; Shankar, R.; Morgan, S.E.; McCollum, J.M. Exploiting Partial Solubility in Partially Fluorinated Thermoplastic Blends to Improve Adhesion during Fused Deposition Modeling. Materials 2022, 15, 8062. https://doi.org/10.3390/ma15228062
Saldaña-Baqué P, Strutton JW, Shankar R, Morgan SE, McCollum JM. Exploiting Partial Solubility in Partially Fluorinated Thermoplastic Blends to Improve Adhesion during Fused Deposition Modeling. Materials. 2022; 15(22):8062. https://doi.org/10.3390/ma15228062
Chicago/Turabian StyleSaldaña-Baqué, Pau, Jared W. Strutton, Rahul Shankar, Sarah E. Morgan, and Jena M. McCollum. 2022. "Exploiting Partial Solubility in Partially Fluorinated Thermoplastic Blends to Improve Adhesion during Fused Deposition Modeling" Materials 15, no. 22: 8062. https://doi.org/10.3390/ma15228062







