The Damage Performance of Uncarbonated Limestone Cement Pastes Partially Exposed to Na2SO4 Solution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
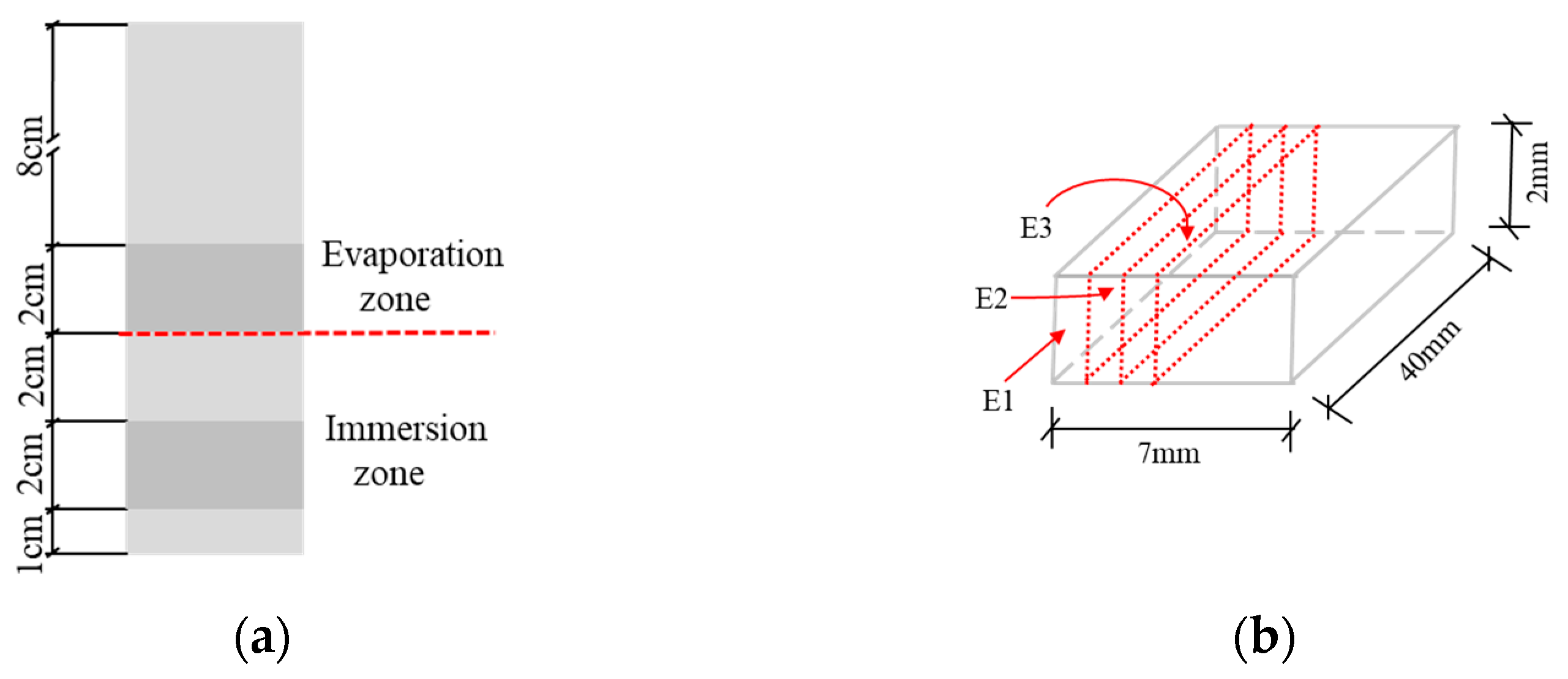
2.2. Specimens Preparation and Exposure Conditions
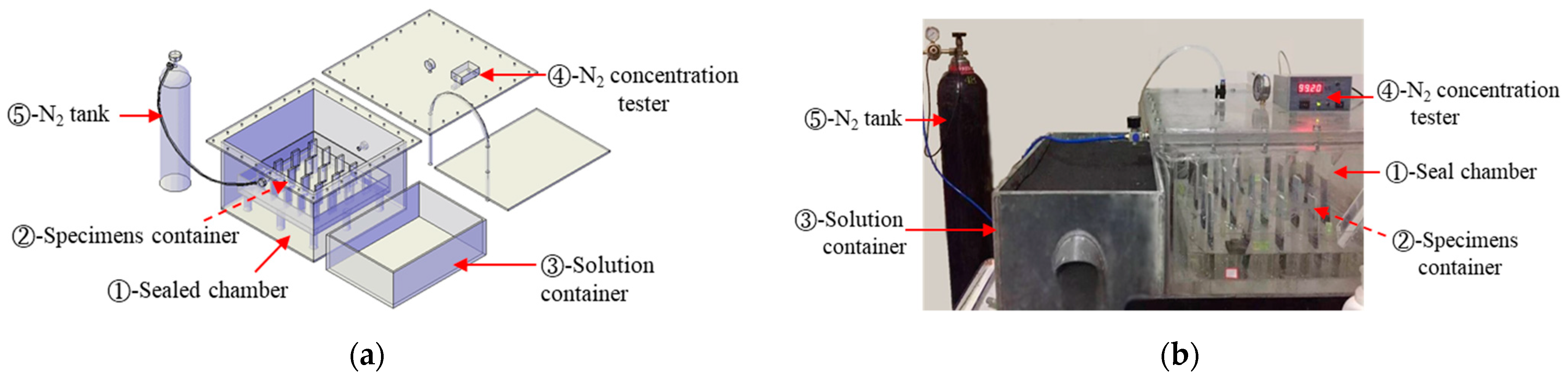
2.3. Test Setup
2.4. 1H NMR, XRD and SEM Analysis
3. Results and Discussion
3.1. Damage of Specimens
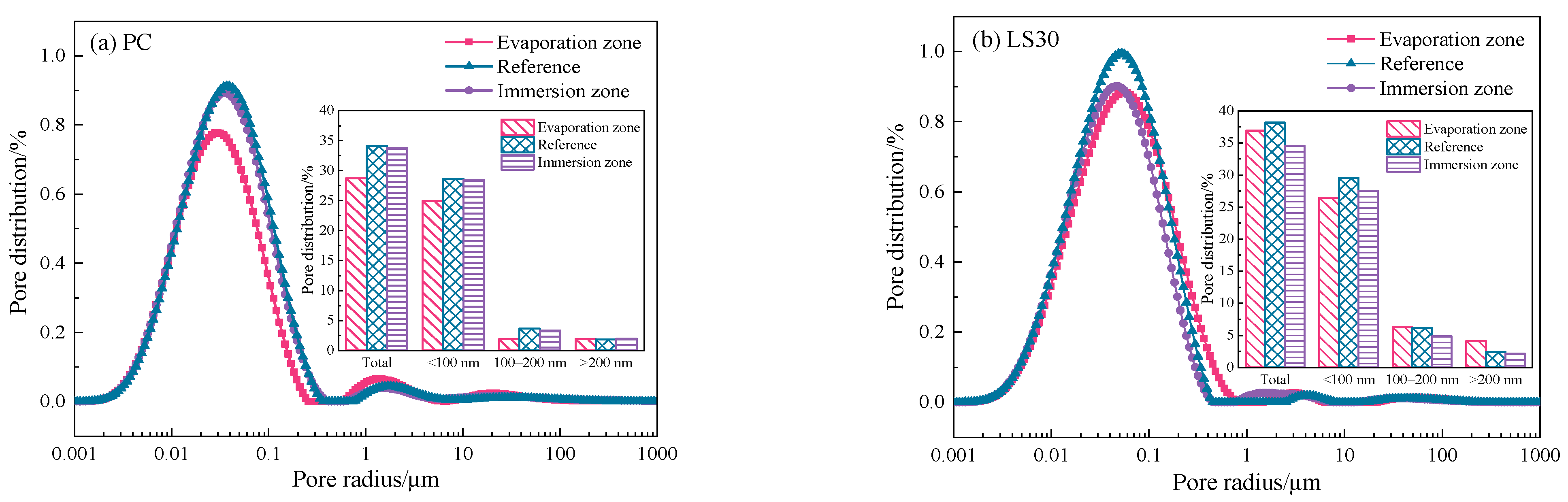
3.2. Pore Structure Analysis
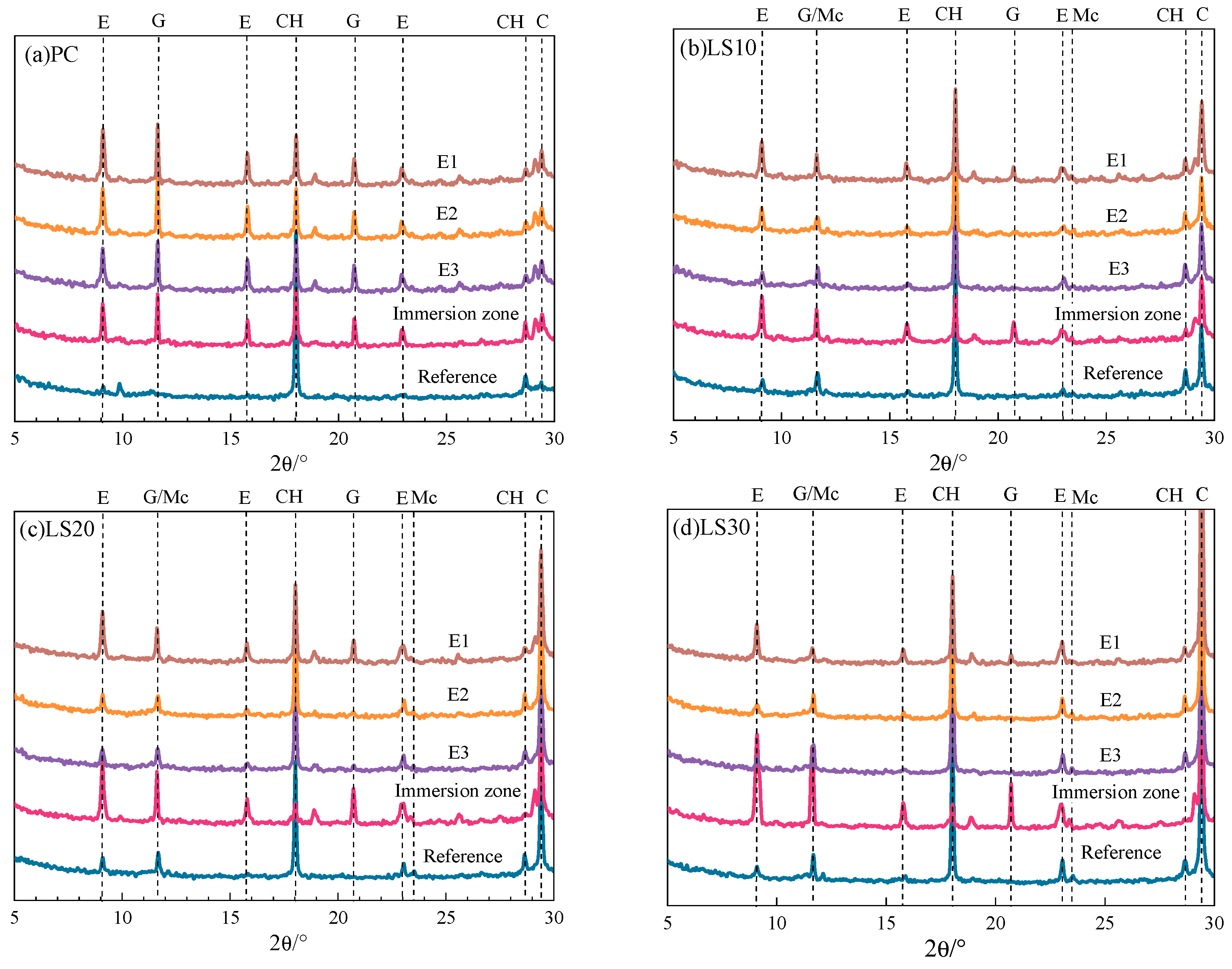
3.3. Compositional Analysis
3.4. Microstructure Analysis
4. Conclusions
- (1)
- The large quantity of small pores (<100 nm) in PC specimens caused strong capillary adsorption that favors the migration of sulfate ions to the evaporation zone. The reactions between these ions and hydrates led to the massive formation of expansive products (ettringite and gypsum) that were filled in the pores and caused cracking of the specimen, whereas the damage in the immersion zone was relatively slight.
- (2)
- Limestone addition significantly increased the larger pores (>100 nm) while decreased the finer pores (<100 nm) in specimens, which resulted in a gradual coarsening of the pore structure and an increase in porosity. As such, the capillary absorption effect in limestone-cements was weakened, and, therefore, sulfate attack in the evaporation zone was alleviated. However, the larger pores promoted diffusion of sulfate ions and thus serious damage chemical attack was found in the immersion zone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Mu, S.; Liu, J. Influence of chlorides and salt concentration on salt crystallization damage of cement-based materials. J. Build. Eng. 2022, 61, 105260. [Google Scholar] [CrossRef]
- Yoshida, N.; Matsunami, Y.; Nagayama, M.; Sakai, E. Salt Weathering in Residential Concrete Foundations Exposed to Sulfate-bearing Ground. J. Adv. Concr. Technol. 2010, 8, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Bassuoni, M.T.; Rahman, M.M. Response of concrete to accelerated physical salt attack exposure. Cem. Concr. Res. 2016, 79, 395–408. [Google Scholar] [CrossRef]
- Al-Amoudi, O.S.B. Attack on plain and blended cements exposed to aggressive sulfate environments. Cem. Concr. Compos. 2002, 24, 305–316. [Google Scholar] [CrossRef]
- Sousa, L.; Siegesmund, S.; Wedekind, W. Salt weathering in granitoids: An overview on the controlling factors. Environ. Earth Sci. 2018, 77, 502. [Google Scholar] [CrossRef]
- Chen, F.; Gao, J.; Qi, B.; Shen, D. Deterioration mechanism of plain and blended cement mortars partially exposed to sulfate attack. Constr. Build. Mater. 2017, 154, 849–856. [Google Scholar] [CrossRef]
- Xiong, C.; Jiang, L.; Xu, Y.; Song, Z.; Chu, H.; Guo, Q. Influences of exposure condition and sulfate salt type on deterioration of paste with and without fly ash. Constr. Build. Mater. 2016, 113, 951–963. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, X.; Zhao, L.; Zhao, M.; Liu, Y.; Zeng, X. Effect of nanosilica on sulfate resistance of cement mortar under partial immersion. Constr. Build. Mater. 2020, 231, 117180. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Thaulow, N.; Sahu, S. Mechanism of concrete deterioration due to salt crystallization. Mater. Charact. 2004, 53, 123–127. [Google Scholar] [CrossRef]
- Bellmann, F.; Möser, B.; Stark, J. Influence of sulfate solution concentration on the formation of gypsum in sulfate resistance test specimen. Cem. Concr. Res. 2006, 36, 358–363. [Google Scholar] [CrossRef]
- Ogawa, S.; Nozaki, T.; Yamada, K.; Hirao, H.; Hooton, R. Improvement on sulfate resistance of blended cement with high alumina slag. Cem. Concr. Res. 2012, 42, 244–251. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, D.; De Schutter, G. Does concrete suffer sulfate salt weathering? Constr. Build. Mater. 2014, 66, 692–701. [Google Scholar] [CrossRef]
- Suleiman, A.R.; Nehdi, M.L. Exploring effects of supplementary cementitious materials in concrete exposed to physical salt attack. Mag. Concr. Res. 2017, 69, 576–585. [Google Scholar] [CrossRef]
- Guan, B. Transport Behavior of Sulfate Ions in Concrete with Attack Damage. Cem. Concr. Res. 2020, 39, 3169–3174+3183. [Google Scholar]
- Sakr, M.; Bassuoni, M. Performance of concrete under accelerated physical salt attack and carbonation. Cem. Concr. Res. 2021, 141, 106324. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]
- Liu, J.; Yao, S.; Ba, M.; He, Z.; Li, Y. Effects of carbonation on micro structures of hardened cement paste. J. Wuhan Univ. Technol. Sci. Ed. 2016, 31, 146–150. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, W.; Pei, M.; Deng, D. The role of carbonation in the occurrence of MgSO4 crystallization distress on concrete. Constr. Build. Mater. 2018, 192, 167–178. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, L.; Deng, D.; Zhang, F.; Hu, W. Crystallization damage of carbonated concrete with sulfate attack. J. Chin. Ceram. Soc. 2017, 45, 1621–1628. [Google Scholar]
- Hawkins, P.; Tennis, P.D.; Detwiler, R.J. The Use of Limestone in Portland Cement: A State-of-the-Art Review; Portland Cement Association: Skokie, IL, USA, 1996. [Google Scholar]
- Li, C.; Jiang, L.; Xu, N.; Jiang, S. Pore structure and permeability of concrete with high volume of limestone powder addition. Powder Technol. 2018, 338, 416–424. [Google Scholar] [CrossRef]
- Zhu, P.; Yang, G.; Jiang, L.; Shi, Y.; Xu, N.; Jin, M.; Gu, Y. Influence of high-volume limestone powder on hydration and microstructural development of cement. Adv. Cem. Res. 2021, 33, 197–209. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Yang, J.; Zou, Y.; Wang, Z. Understanding of the deterioration characteristic of concrete exposed to external sulfate attack: Insight into mesoscopic pore structures. Constr. Build. Mater. 2020, 260, 119932. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Z.; He, F.; Huang, J.; Zhou, J. Sulfate diffusion in calcium sulphoaluminate mortar. Constr. Build. Mater. 2020, 234, 117312. [Google Scholar] [CrossRef]
- Han, F.; Wang, Q.; Liu, M.; Mei, Y. Early hydration properties of composite binder containing limestone powder with different finenesses. J. Therm. Anal. Calorim. 2016, 123, 1141–1151. [Google Scholar]
- Nadelman, E.; Kurtis, K. Durability of Portland-limestone cement-based materials to physical salt attack. Cem. Concr. Res. 2019, 125, 105859. [Google Scholar] [CrossRef]
- Ikumi, T.; Sergio, H.C.; Ignacio, S. The role of porosity in external sulphate attack. Cem. Concr. Compos. 2019, 97, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Flatt, R.J. Salt damage in porous materials: How high supersaturations are generated. J. Cryst. Growth 2020, 242, 435–454. [Google Scholar] [CrossRef]
- Liu, K.; Sun, D.; Wang, A.; Zhang, G.; Tang, J. Long-Term Performance of Blended Cement Paste Containing Fly Ash against Sodium Sulfate Attack. J. Mater. Civ. Eng. 2018, 30, 04018309. [Google Scholar] [CrossRef]
- Deng, D.; Xiao, J.; Yuan, Q.; Liu, Z.; Zhang, W. Effect of limestone powder on the sulfate-resistance of materials based on cement and its mechanism. J. Chin. Ceram. Soc. 2006, 34, 81–86. [Google Scholar]
- Shi, Z.; Ferreiro, S.; Lothenbach, B.; Geiker, M.R.; Kunther, W.; Kaufmann, J.; Herfort, D.; Skibsted, J. Sulfate resistance of calcined clay–Limestone–Portland cements. Cem. Concr. Res. 2019, 116, 238–251. [Google Scholar] [CrossRef]
- Müllauer, W.; Robin, E.B.; Detlef, H. Sulfate attack expansion mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]





| CaO | SiO2 | Fe2O3 | MgO | Al2O3 | SO3 | TiO2 | K2O | |
|---|---|---|---|---|---|---|---|---|
| Cement | 62.68 | 19.62 | 2.96 | 1.89 | 4.37 | 2.06 | 0.24 | 0.71 |
| Limestone | 54.99 | 0.21 | 0.07 | 0.55 | 0.24 | 0.63 | / | 0.01 |
| Number | Cement/% | Limestone/% | W/B |
|---|---|---|---|
| PC | 100 | 0 | 0.55 |
| LS10 | 90 | 10 | |
| LS20 | 80 | 20 | |
| LS30 | 70 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Pei, M.; Huang, J.; Hou, W.; Liu, Z. The Damage Performance of Uncarbonated Limestone Cement Pastes Partially Exposed to Na2SO4 Solution. Materials 2022, 15, 8351. https://doi.org/10.3390/ma15238351
Cui Y, Pei M, Huang J, Hou W, Liu Z. The Damage Performance of Uncarbonated Limestone Cement Pastes Partially Exposed to Na2SO4 Solution. Materials. 2022; 15(23):8351. https://doi.org/10.3390/ma15238351
Chicago/Turabian StyleCui, Yu, Min Pei, Ju Huang, Wei Hou, and Zanqun Liu. 2022. "The Damage Performance of Uncarbonated Limestone Cement Pastes Partially Exposed to Na2SO4 Solution" Materials 15, no. 23: 8351. https://doi.org/10.3390/ma15238351





