Synthesis Optimization of BaGdF5:x%Tb3+ Nanophosphors for Tunable Particle Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis
2.3.1. Terbium Content Optimization
2.3.2. Variation of Synthesis Conditions
3. Results and Discussion
3.1. Terbium Content Optimization
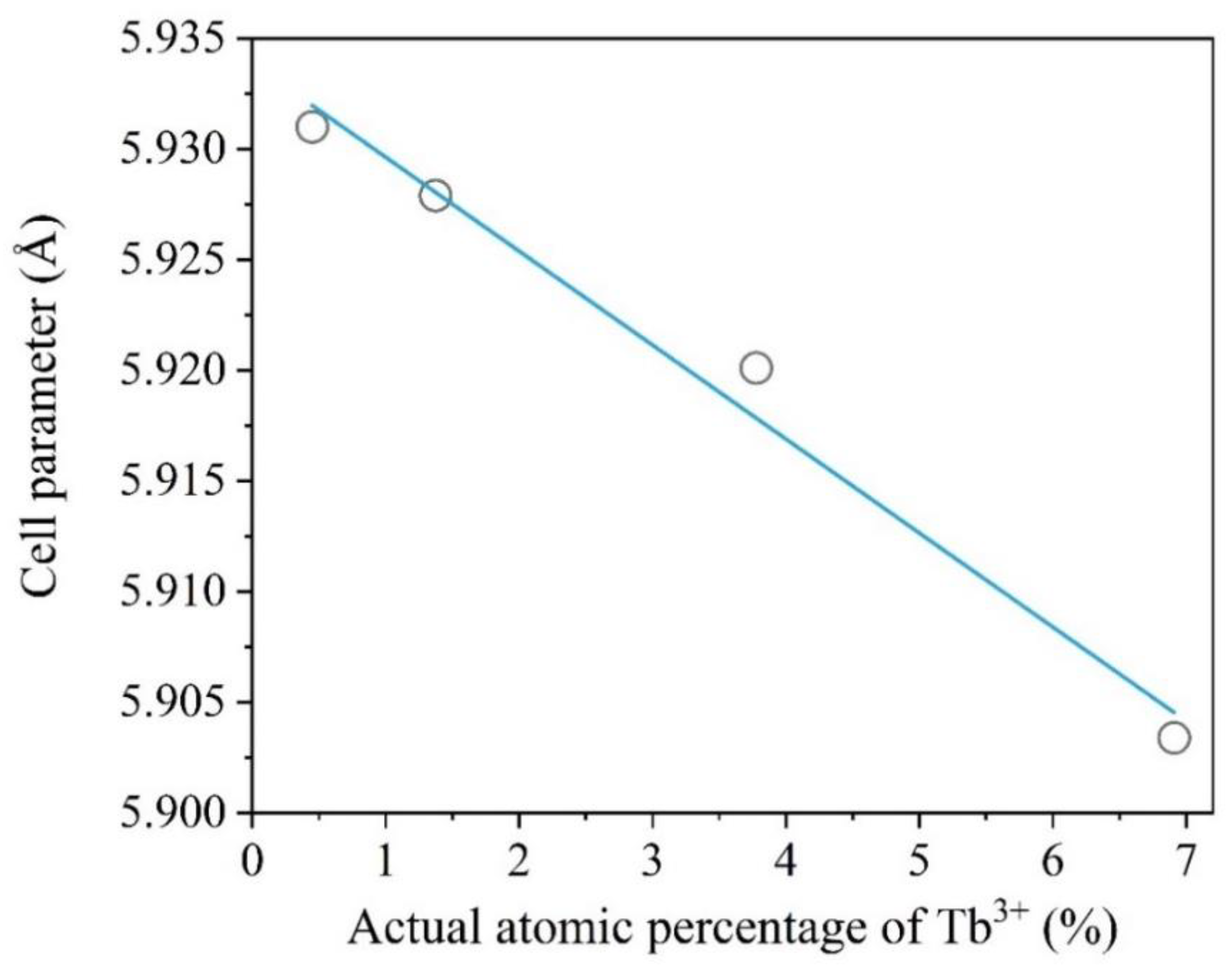
3.1.1. X-Ray Diffraction of BaGdF5: x%Tb3+
3.1.2. X-Ray Fluorescence Measurements of BaGdF5: x%Tb3+
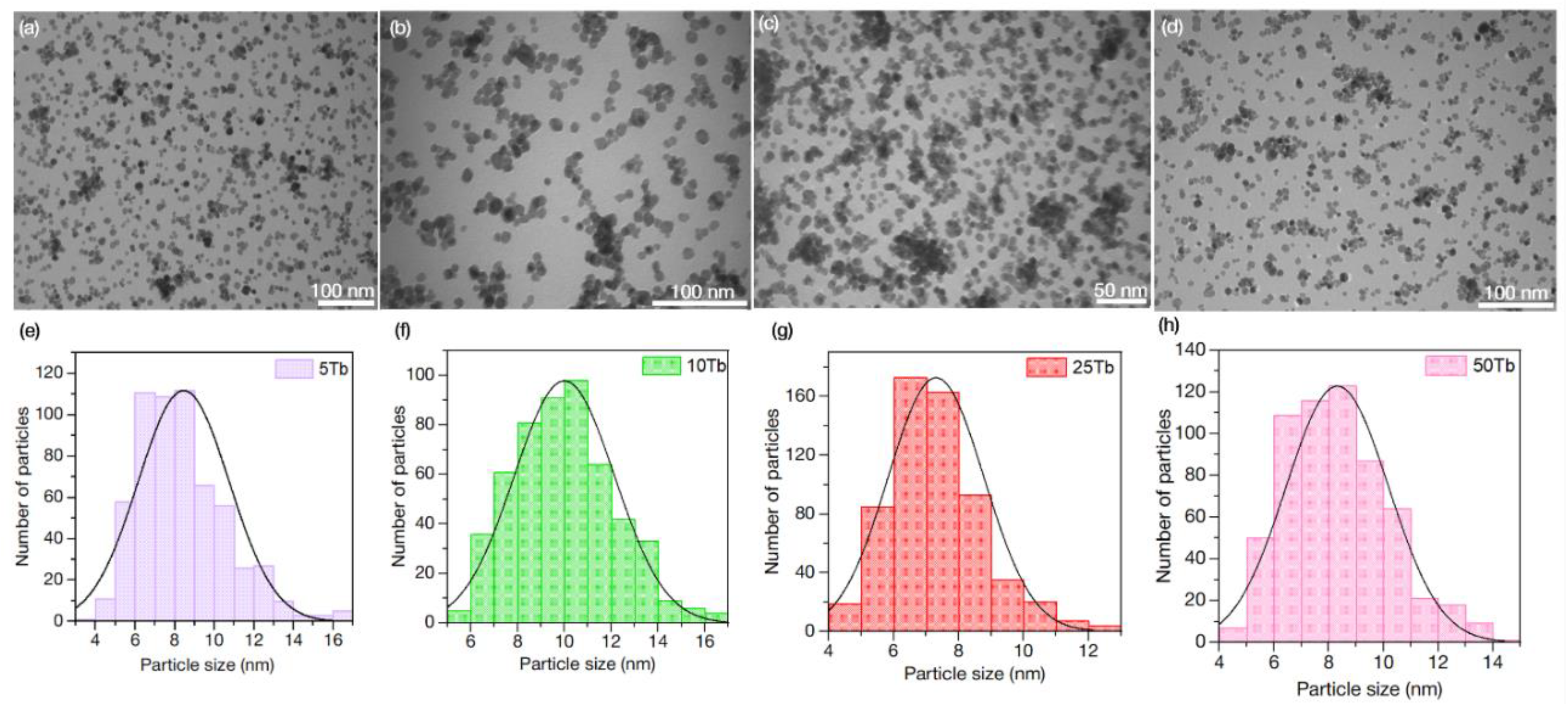
3.1.3. Transmission Electron Microscopy (TEM) of BaGdF5: x%Tb3+
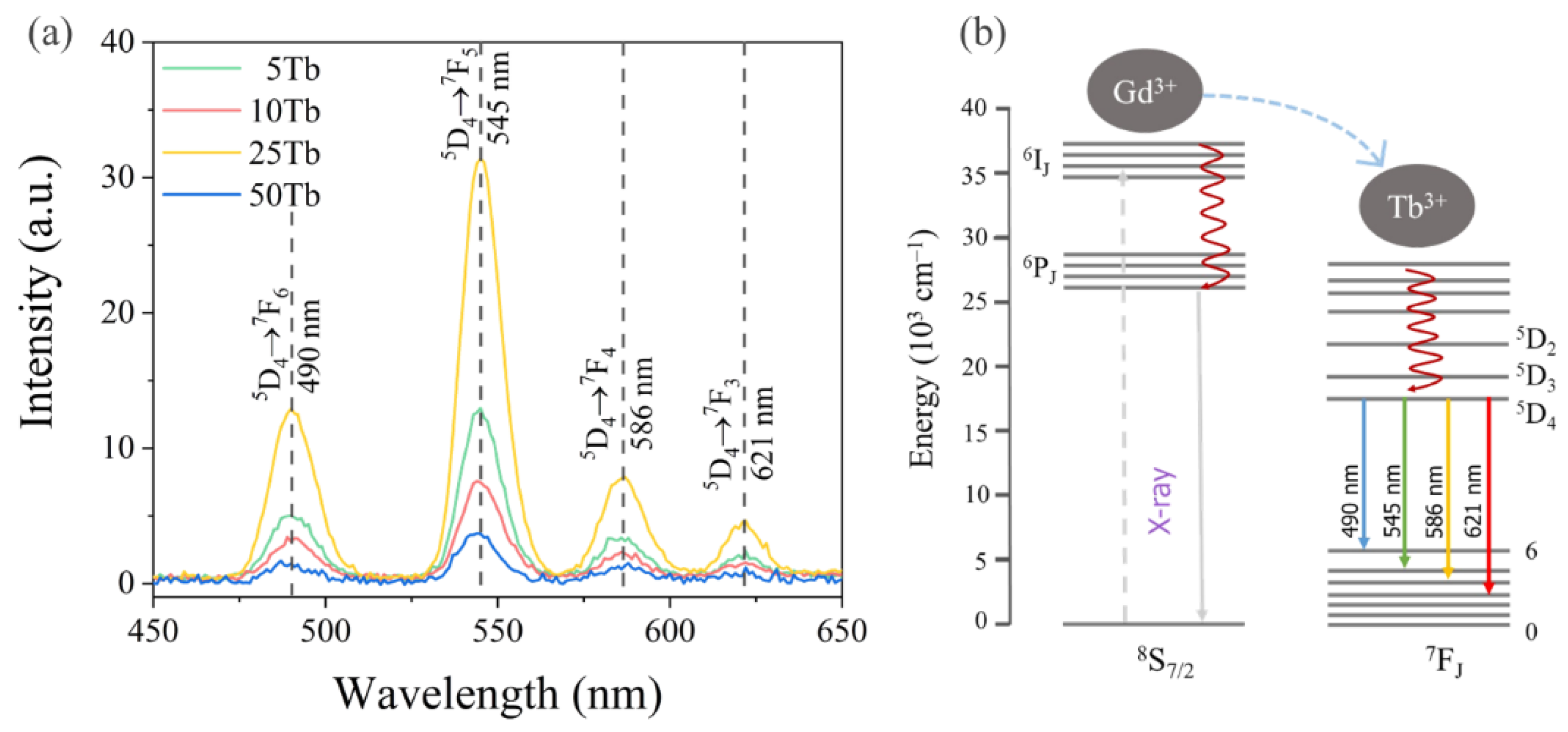
3.1.4. X-Ray Excited Optical Luminescence
3.2. Tuning Size of BaGdF5: 25Tb3+ Nanoparticles by Various Synthesis Conditions
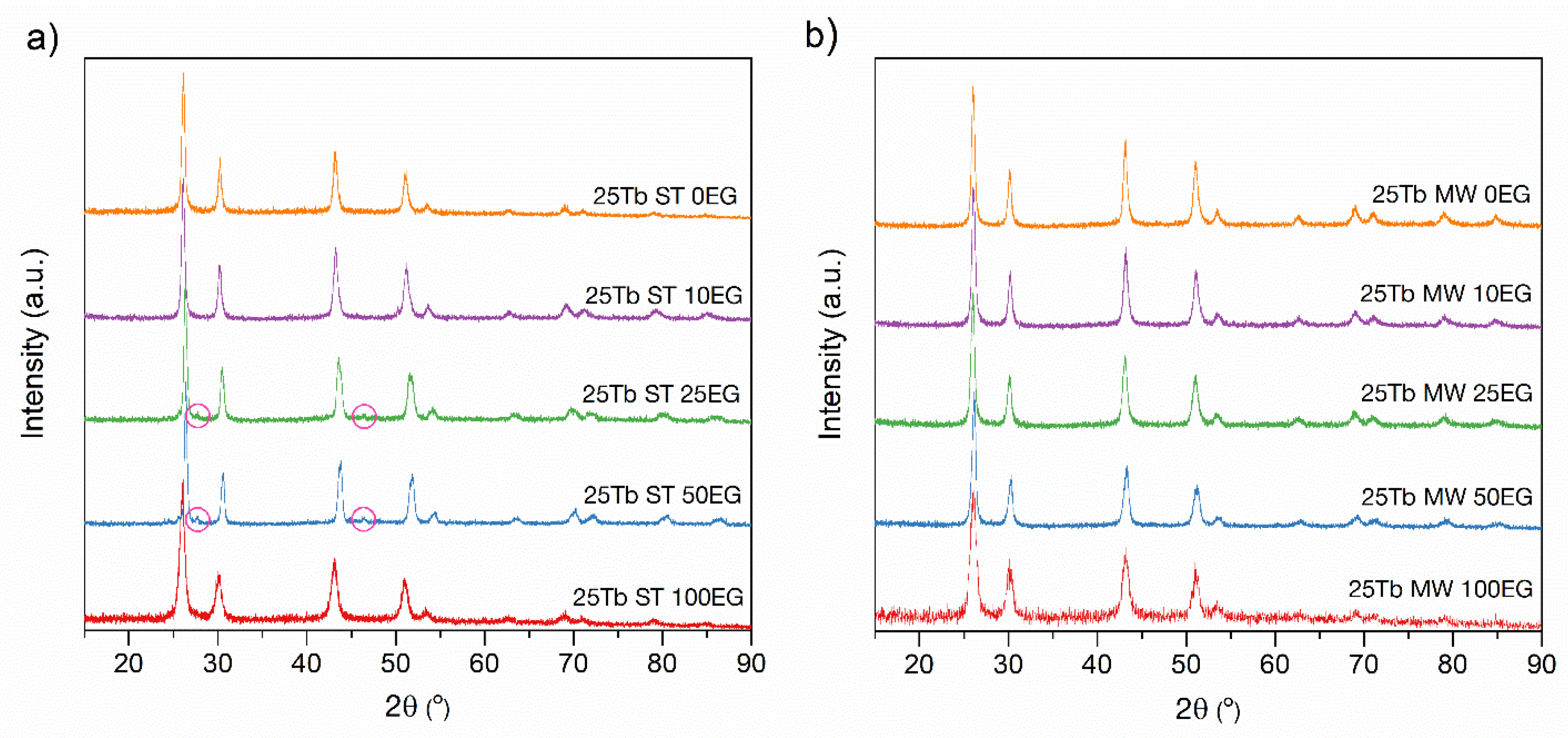
3.2.1. X-Ray Diffraction of BaGdF5: 25Tb3+ Obtained by the MW and ST Techniques
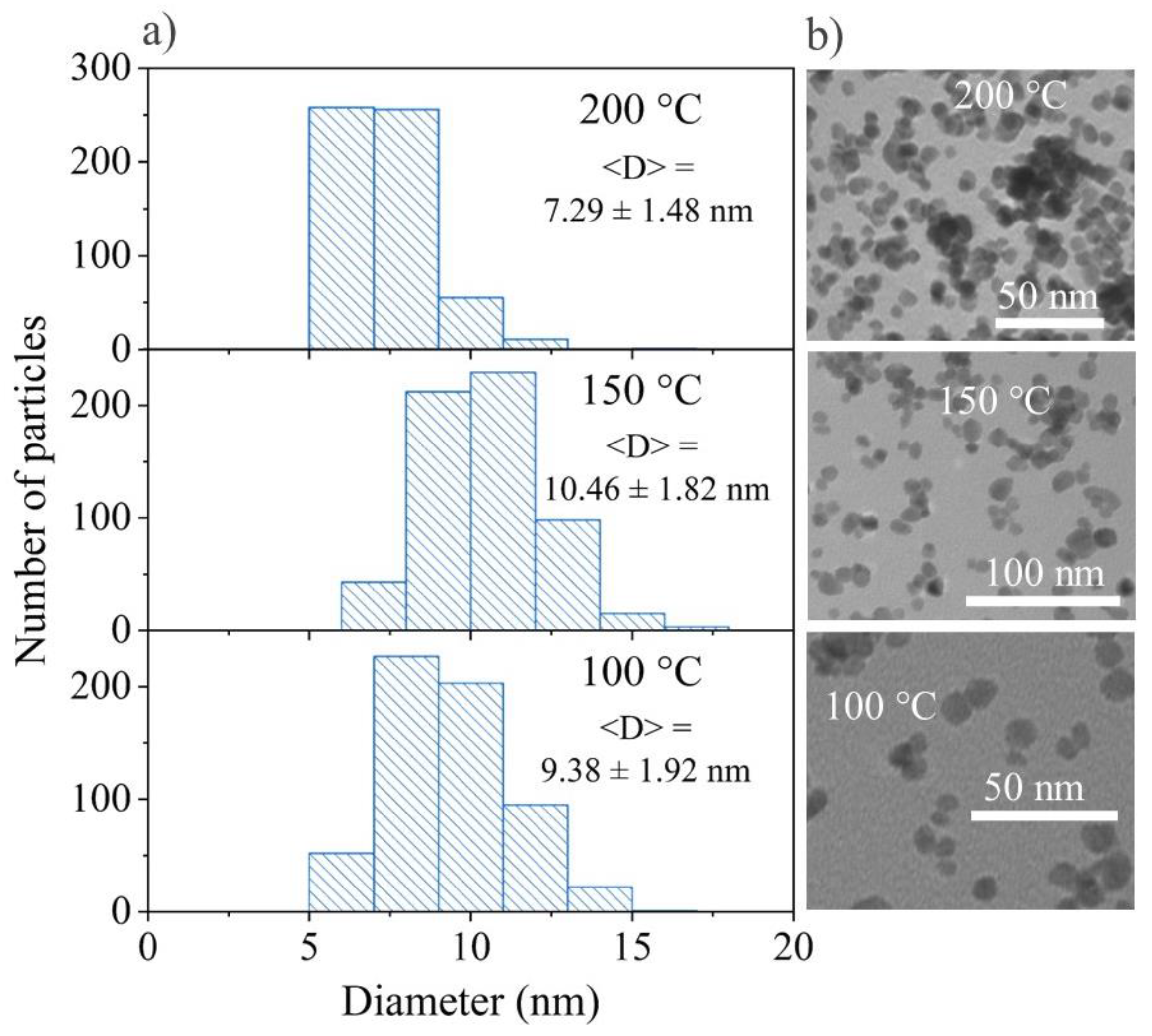
3.2.2. Transmission Electron Microscopy Measurements of BaGdF5:25Tb3+ Obtained by MW and ST Techniques
3.2.3. Microwave Synthesis at Different Temperature and Different Percentage of EG
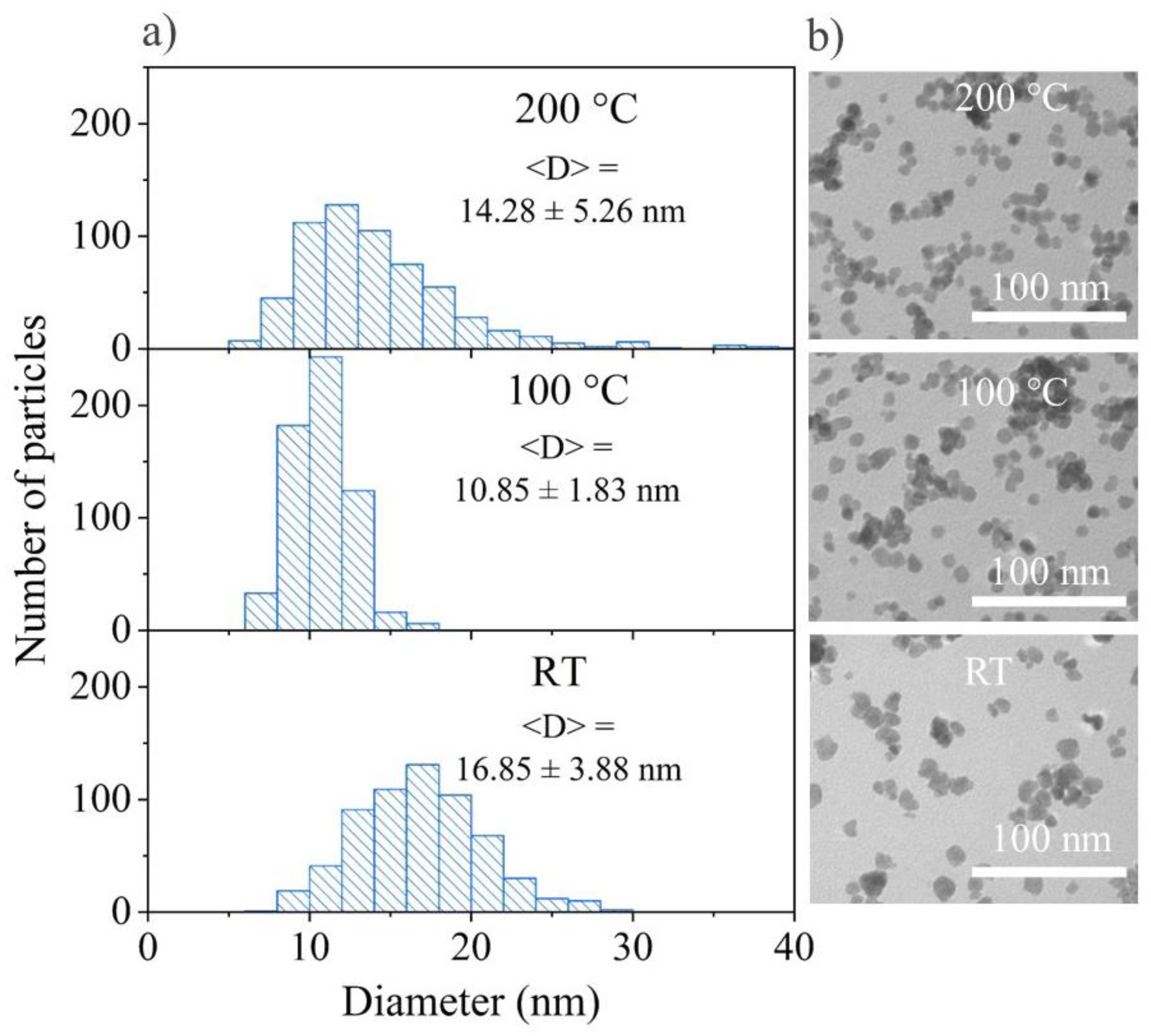
3.2.4. Solvothermal Synthesis at Different Temperature and Different Percentage of EG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann. Oncol. 2019, 30, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Dual-mode, tunable color, enhanced upconversion luminescence and magnetism of multifunctional BaGdF5:Ln3+ (Ln = Yb/Er/Eu) nanophosphors. Phys. Chem. Chem. Phys. 2016, 18, 21518–21526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive Oxygen Species in the Immune System. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive Oxygen Species in Cancer Biology and Anticancer Therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative Strategies for Hypoxic-Tumor Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Bulin, A.-L.; Truillet, C.; Chouikrat, R.; Lux, F.; Frochot, C.; Amans, D.; Ledoux, G.; Tillement, O.; Perriat, P.; Barberi-Heyob, M.; et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. J. Phys. Chem. C 2013, 117, 21583–21589. [Google Scholar] [CrossRef]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Zolotukhin, P.; Kit, O.; Kirsanova, D.; Soldatov, A. Nanocomposites for X-Ray Photodynamic Therapy. Int. J. Mol. Sci. 2020, 21, 4004. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.-Z.; Liu, J.-H.; Liu, Y.; Yuan, B.-Y.; Gong, X.; Yuan, Q.-H.; Gong, T.-T.; Wang, L. Synthesis of PEGylated BaGdF5 Nanoparticles as Efficient CT/MRI Dual-modal Contrast Agents for Gastrointestinal Tract Imaging. Chin. J. Anal. Chem. 2020, 48, 1004–1011. [Google Scholar] [CrossRef]
- Wang, T.; Jia, G.; Cheng, C.; Wang, Q.; Li, X.; Liu, Y.; He, C.; Chen, L.; Sun, G.; Zuo, C. Active targeted dual-modal CT/MR imaging of VX2 tumors using PEGylated BaGdF5 nanoparticles conjugated with RGD. New J. Chem. 2018, 42, 11565–11572. [Google Scholar] [CrossRef]
- Li, H.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Eu3+ /Tb3+ doped cubic BaGdF 5 multifunctional nanophosphors: Multicolor tunable luminescence, energy transfer and magnetic properties. J. Lumin 2017, 186, 6–15. [Google Scholar] [CrossRef]
- Grzyb, T.; Mrówczyńska, L.; Szczeszak, A.; Śniadecki, Z.; Runowski, M.; Idzikowski, B.; Lis, S. Synthesis, characterization, and cytotoxicity in human erythrocytes of multifunctional, magnetic, and luminescent nanocrystalline rare earth fluorides. J. Nanoparticle Res. 2015, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kirsanova, D.; Polyakov, V.; Butova, V.; Zolotukhin, P.; Belanova, A.; Gadzhimagomedova, Z.; Soldatov, M.; Pankin, I.; Soldatov, A. The Rare-Earth Elements Doping of BaGdF5 Nanophosphors for X-ray Photodynamic Therapy. Nanomaterials 2021, 11, 3212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tu, D.; Zhu, H.; Li, R.; Luo, W.; Chen, X. A Strategy to Achieve Efficient Dual-Mode Luminescence of Eu3+ in Lanthanides Doped Multifunctional NaGdF4 Nanocrystals. Adv. Mater. 2010, 22, 3266–3271. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Law, W.-C.; Wu, F.; Swihart, M.T.; Ågren, H.; Prasad, P.N. Core/Shell NaGdF4:Nd3þ/NaGdF4 Nanocrystals with Efficient Near-Infrared to Near-Infrared Downconversion Photoluminescence for Bioimaging Applications. ACS Nano 2012, 6, 2969–2977. [Google Scholar] [CrossRef]
- Chouryal, Y.N.; Sharma, R.K.; Ivanovskikh, K.V.; Ishchenko, A.V.; Shi, Q.; Ivanov, V.Y.; Nigam, S.; Pandey, A.; Ghosh, P. Temperature dependent quantum cutting in cubic BaGdF5:Eu3+ nanophosphors. New J. Chem. 2020, 45, 1463–1473. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Q.; Zou, H.; Zhang, X.; Xiao, X.; Shi, Z.; Song, Y. Ionic liquid/H2O two-phase synthesis and luminescence properties of BaGdF5:RE3+ (RE = Ce/Dy/Eu/Yb/Er) octahedra. New J. Chem. 2020, 45, 742–750. [Google Scholar] [CrossRef]
- Sengar, M.; Narula, A.K. Luminescence sensitization of Ln3+ impurity ions in BaGdF5 host matrix: Structural investigation, color tunable luminescence and energy transfer. Opt. Mater. 2020, 110. [Google Scholar] [CrossRef]
- Lee, G.; Struebing, C.; Wagner, B.; Summers, C.; Ding, Y.; Bryant, A.; Thadhani, N.; Shedlock, D.; Star-Lack, J.; Kang, Z. Synthesis and characterization of a BaGdF5:Tb glass ceramic as a nanocomposite scintillator for x-ray imaging. Nanotechnology 2016, 27, 205203. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Sheng, Y.; Song, Y.; Zheng, K.; Xu, C.; Xie, X.; Dai, Y.; Zou, H. White light-emitting, tunable color luminescence, energy transfer and paramagnetic properties of terbium and samarium doped BaGdF5 multifunctional nanomaterials. RSC Adv. 2016, 6, 73160–73169. [Google Scholar] [CrossRef]
- Cao, C.-Y.; Xie, A.; Yu, X.-G. Controlled synthesis and optical properties of Ce3+/Tb3+ co-doped BaGdF5 nanocrystals. Rare Met. 2014, 34, 673–678. [Google Scholar] [CrossRef]
- Yanes, A.; Del-Castillo, J.; Ortiz, E. Energy transfer and tunable emission in BaGdF5: RE3+ (RE= Ce, Tb, Eu) nano-glass-ceramics. J. Alloy. Compd. 2019, 773, 1099–1107. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, L.; Li, X.; He, A. Manipulating upconversion emission of cubic BaGdF5:Ce3+/Er3+/Yb3+ nanocrystals through controlling Ce3+ doping. J. Alloy. Compd. 2017, 721, 374–382. [Google Scholar] [CrossRef]
- He, F.; Li, C.; Zhang, X.; Chen, Y.; Deng, X.; Liu, B.; Hou, Z.; Huang, S.; Jin, D.; Lin, J. Optimization of upconversion luminescence of Nd3+-sensitized BaGdF5-based nanostructures and their application in dual-modality imaging and drug delivery. Dalton Trans. 2015, 45, 1708–1716. [Google Scholar] [CrossRef]
- Guan, H.; Song, Y.; Zheng, K.; Sheng, Y.; Zou, H. BaGdF5:Dy3+,Tb3+,Eu3+ multifunctional nanospheres: Paramagnetic, luminescence, energy transfer, and tunable color. Phys. Chem. Chem. Phys. 2016, 18, 13861–13873. [Google Scholar] [CrossRef]
- Huignard, A.; Buissette, V.; Franville, A.-C.; Gacoin, T.; Boilot, J.-P. Emission Processes in YVO4:Eu Nanoparticles. J. Phys. Chem. B 2003, 107, 6754–6759. [Google Scholar] [CrossRef]
- Jorma, H.; Eija, K. Crystal Fields in REOF:Eu3+ (RE=La, Gd and Y). J. Chem. Soc. Faraday Trans. 1995, 91, 1503–1509. [Google Scholar]
- King, A.; Singh, R.; Nayak, B.B. Phase and photoluminescence analysis of dual-color emissive Eu3+-doped ZrO2 nanoparticles for advanced security features in anti-counterfeiting. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 631, 127715. [Google Scholar] [CrossRef]
- Rezende, M.; Montes, P.J.; Valerio, M.; Jackson, R.A. The optical properties of Eu3+ doped BaAl2O4: A computational and spectroscopic study. Opt. Mater. 2012, 34, 1434–1439. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Teleanu, R.I. Neuronanomedicine: An Up-to-Date Overview. Pharmaceutics 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Elmenoufy, A.H.; Tang, Y.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Lin, S.-L.; Chang, C.A. Lanthanide-Doped Core–Shell–Shell Nanocomposite for Dual Photodynamic Therapy and Luminescence Imaging by a Single X-ray Excitation Source. ACS Appl. Mater. Interfaces 2018, 10, 7859–7870. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.D.; Chuang, Y.-J.; Zhen, Z.; Chen, X.; Biddinger, P.; Hao, Z.; Liu, F.; Shen, B.; Pan, Z.; et al. Nanoscintillator-Mediated X-ray Inducible Photodynamic Therapy for In Vivo Cancer Treatment. Nano Lett. 2015, 15, 2249–2256. [Google Scholar] [CrossRef]
- Gadzhimagomedova, Z.; Polyakov, V.; Pankin, I.; Butova, V.; Kirsanova, D.; Soldatov, M.; Khodakova, D.; Goncharova, A.; Mukhanova, E.; Belanova, A.; et al. BaGdF5 Nanophosphors Doped with Different Concentrations of Eu3+ for Application in X-ray Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 13040. [Google Scholar] [CrossRef]
- Becerro, A.I.; González-Mancebo, D.; Cantelar, E.; Cussó, F.; Stepien, G.; de la Fuente, J.M.; Ocaña, M. Ligand-Free Synthesis of Tunable Size Ln:BaGdF5 (Ln = Eu3+ and Nd3+) Nanoparticles: Luminescence, Magnetic Properties, and Biocompatibility. Langmuir 2016, 32, 411–420. [Google Scholar] [CrossRef]
- Kvítek, L.; Prucek, R.; Panáček, A.; Novotný, R.; Hrbáč, J.; Zbořil, R. The influence of complexing agent concentration on particle size in the process of SERS active silver colloid synthesis. J. Mater. Chem. 2005, 15, 1099–1105. [Google Scholar] [CrossRef]
- Guo, X.; Yan, H.; Zhao, S.; Li, Z.; Li, Y.; Liang, X. Effect of calcining temperature on particle size of hydroxyapatite synthesized by solid-state reaction at room temperature. Adv. Powder Technol. 2013, 24, 1034–1038. [Google Scholar] [CrossRef]
- Shi, A.-M.; Li, D.; Wang, L.-J.; Li, B.-Z.; Adhikari, B. Preparation of starch-based nanoparticles through high-pressure homogenization and miniemulsion cross-linking: Influence of various process parameters on particle size and stability. Carbohydr. Polym. 2011, 83, 1604–1610. [Google Scholar] [CrossRef]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents Synthesis, Formation, Assembly and Application; Springer: London, UK, 2009. [Google Scholar]
- Lai, J.; Niu, W.; Luque, R.; Xu, G. Solvothermal synthesis of metal nanocrystals and their applications. Nano Today 2015, 10, 240–267. [Google Scholar] [CrossRef]
- Wang, H.; Xie, C.; Zeng, D. Controlled growth of ZnO by adding H2O. J. Cryst. Growth 2005, 277, 372–377. [Google Scholar] [CrossRef]
- Lian, J.; Liang, Y.; Kwong, F.-L.; Ding, Z.; Ng, D.H. Template-free solvothermal synthesis of ZnO nanoparticles with controllable size and their size-dependent optical properties. Mater. Lett. 2012, 66, 318–320. [Google Scholar] [CrossRef]
- Kirsanova, D.Y.; Butova, V.V.; Polyakov, V.A.; Zolotukhin, P.V.; Belanova, A.A.; Legostaev, V.M.; Kuchma, E.A.; Gadzhimagomedova, Z.M.; Soldatov, A.V. X-Ray nanophosphors based on BaGdF5 for x-ray photodynamic therapy in oncology. Nanotechnologies Russ. 2020, 15, 105–111. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Für Krist.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Yang, D.; Kang, X.; Shang, M.; Li, G.; Peng, C.; Li, C.; Lin, J. Size and shape controllable synthesis and luminescent properties of BaGdF5:Ce3+/Ln3+ (Ln = Sm, Dy, Eu, Tb) nano/submicrocrystals by a facile hydrothermal process. Nanoscale 2011, 3, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, P.; Wang, Z.; Li, T.; Bai, Q.; Sun, J.; Yang, Z. Luminescence and energy transfer of Eu2+/Tb3+/Eu3+ in LiBaBO3 phosphors with tunable-color emission. J. Mater. Chem. C 2015, 3, 9112–9121. [Google Scholar] [CrossRef]
- Lee, T.; Luo, L.-Y.; Diau, E.W.-G.; Chen, T.-M.; Cheng, B.M.; Tung, C.-Y. Visible quantum cutting through downconversion in green-emitting K2GdF5:Tb3+ phosphors. Appl. Phys. Lett. 2006, 89, 131121. [Google Scholar] [CrossRef]
- Cheng, S.D.; Kam, C.H.; Buddhudu, S. Enhancement of green emission from Tb3+:GdOBr phosphors with Ce3+ ion co-doping. Mater. Res. Bull. 2001, 31, 1131–1137. [Google Scholar] [CrossRef]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhao, Q.; Lü, W.; Guo, N.; Jia, Y.; Lv, W.; Shao, B.; Jiao, M.; You, H. Inorganic-salt-induced morphological transformation and luminescent performance of GdF3 nanostructures. Dalton Trans. 2013, 42, 6902–6908. [Google Scholar] [CrossRef]
- Bohne, D.; Fischer, S.; Obermeier, E. Thermal, Conductivity, Density, Viscosity, and Prandtl-Numbers of Ethylene Glycol-Water Mixtures. Ber. Der Bunsenges. Für Phys. Chem. 1984, 88, 739–742. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalińska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]



| Sample | BaCl2 ∙ 2H2O | GdCl3 | TbCl3 ∙ 6H2O | NH4F | ||||
|---|---|---|---|---|---|---|---|---|
| mg | mmol | mg | mmol | mg | mmol | mg | mmol | |
| 5Tb MW | 244.3 | 1 | 250.4 | 0.95 | 13.3 | 0.05 | 203.7 | 5.5 |
| 10Tb MW | 210.9 | 0.9 | 26.5 | 0.1 | ||||
| 25Tb MW | 197.7 | 0.75 | 66.3 | 0.25 | ||||
| 50Tb MW | 131.8 | 0.5 | 132.6 | 0.5 | ||||
| Type of Synthesis | Sample | % EG | Temperature, °C |
|---|---|---|---|
| Microwave (MW) | 25Tb MW 100EG 200T | 100 | 200 |
| 25Tb MW 50EG | 50 | ||
| 25Tb MW 25EG | 25 | ||
| 25Tb MW 10EG | 10 | ||
| 25Tb MW 0EG | 0 | ||
| 25Tb MW 100EG 150T | 100 | 150 | |
| 25Tb MW 100EG 100T | 100 | 100 | |
| Solvothermal (ST) | 25Tb ST 100EG 200T | 100 | 200 |
| 25Tb ST 50EG | 50 | ||
| 25Tb ST 25EG | 25 | ||
| 25Tb ST 10EG | 10 | ||
| 25Tb ST 0EG | 0 | ||
| 25Tb ST 100EG 100T | 100 | 100 | |
| 25Tb ST 100EG RT | 100 | RT |
| Sample | Expected Tb Content, at. % | Actual Tb Content, at. % | Cell Parameters. Å | Cell Volume. Å3 | Goodness of Fit (GOF) | R-Factor | Crystal Size. nm |
|---|---|---|---|---|---|---|---|
| 5Tb MW | 0.71 | 0.45 | 5.9310(12) | 208.64(7) | 1.03 | 0.1282 | 8.53 |
| 10Tb MW | 1.43 | 1.37 | 5.9279(14) | 208.30(8) | 1.12 | 0.1120 | 8.87 |
| 25Tb MW | 3.57 | 3.78 | 5.9201(16) | 207.49(3) | 1.04 | 0.1546 | 7.67 |
| 50Tb MW | 7.14 | 6.91 | 5.9034(9) | 205.73(5) | 0.99 | 0.1141 | 10.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyakov, V.; Gadzhimagomedova, Z.; Kirsanova, D.; Soldatov, A. Synthesis Optimization of BaGdF5:x%Tb3+ Nanophosphors for Tunable Particle Size. Materials 2022, 15, 8559. https://doi.org/10.3390/ma15238559
Polyakov V, Gadzhimagomedova Z, Kirsanova D, Soldatov A. Synthesis Optimization of BaGdF5:x%Tb3+ Nanophosphors for Tunable Particle Size. Materials. 2022; 15(23):8559. https://doi.org/10.3390/ma15238559
Chicago/Turabian StylePolyakov, Vladimir, Zaira Gadzhimagomedova, Daria Kirsanova, and Alexander Soldatov. 2022. "Synthesis Optimization of BaGdF5:x%Tb3+ Nanophosphors for Tunable Particle Size" Materials 15, no. 23: 8559. https://doi.org/10.3390/ma15238559
APA StylePolyakov, V., Gadzhimagomedova, Z., Kirsanova, D., & Soldatov, A. (2022). Synthesis Optimization of BaGdF5:x%Tb3+ Nanophosphors for Tunable Particle Size. Materials, 15(23), 8559. https://doi.org/10.3390/ma15238559







