Role of Plasticizers on PHB/bio-TPE Blends Compatibilized by Reactive Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blend Preparation
2.3. Characterization
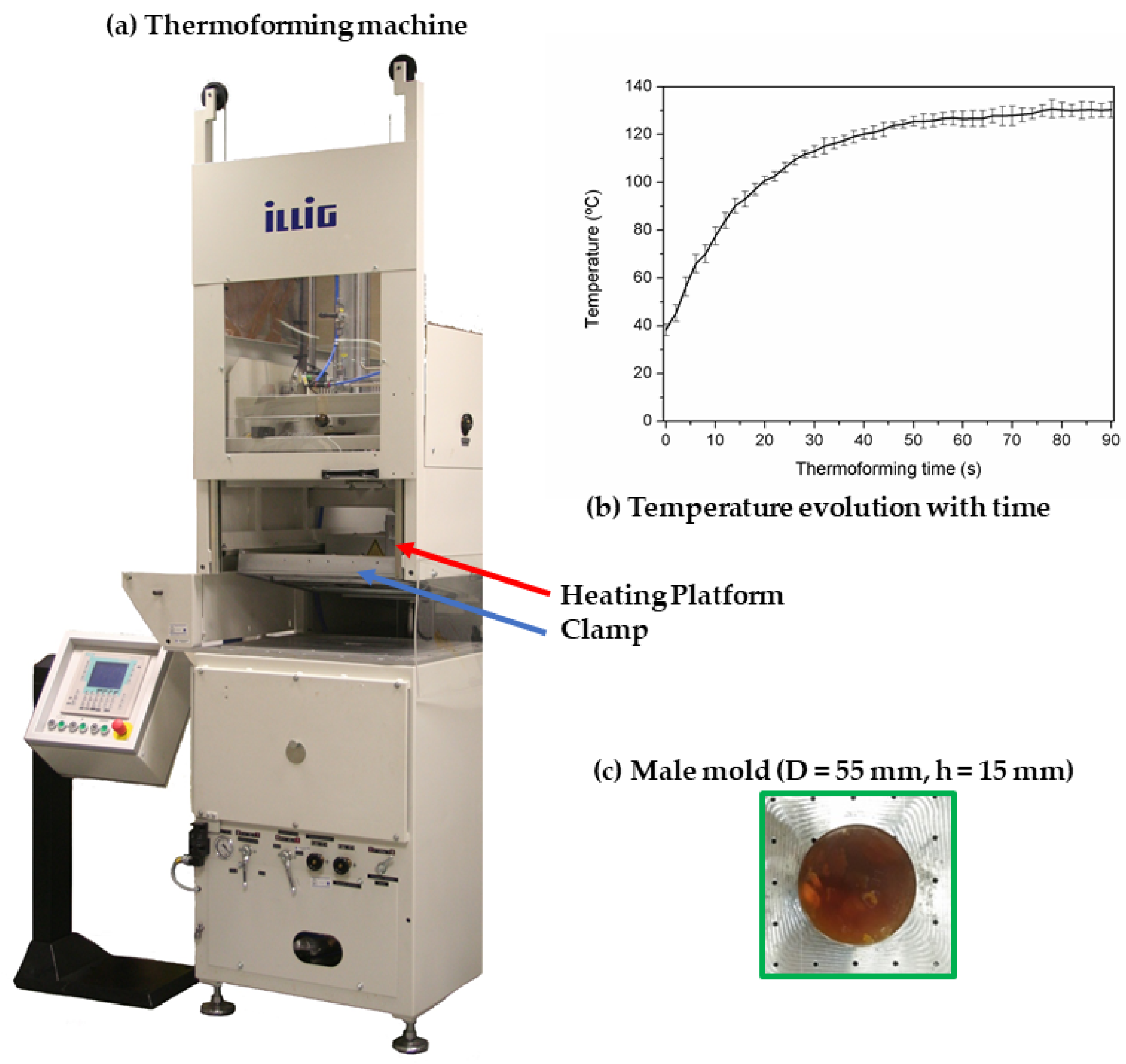
2.3.1. Thermoforming Ability
- (a)
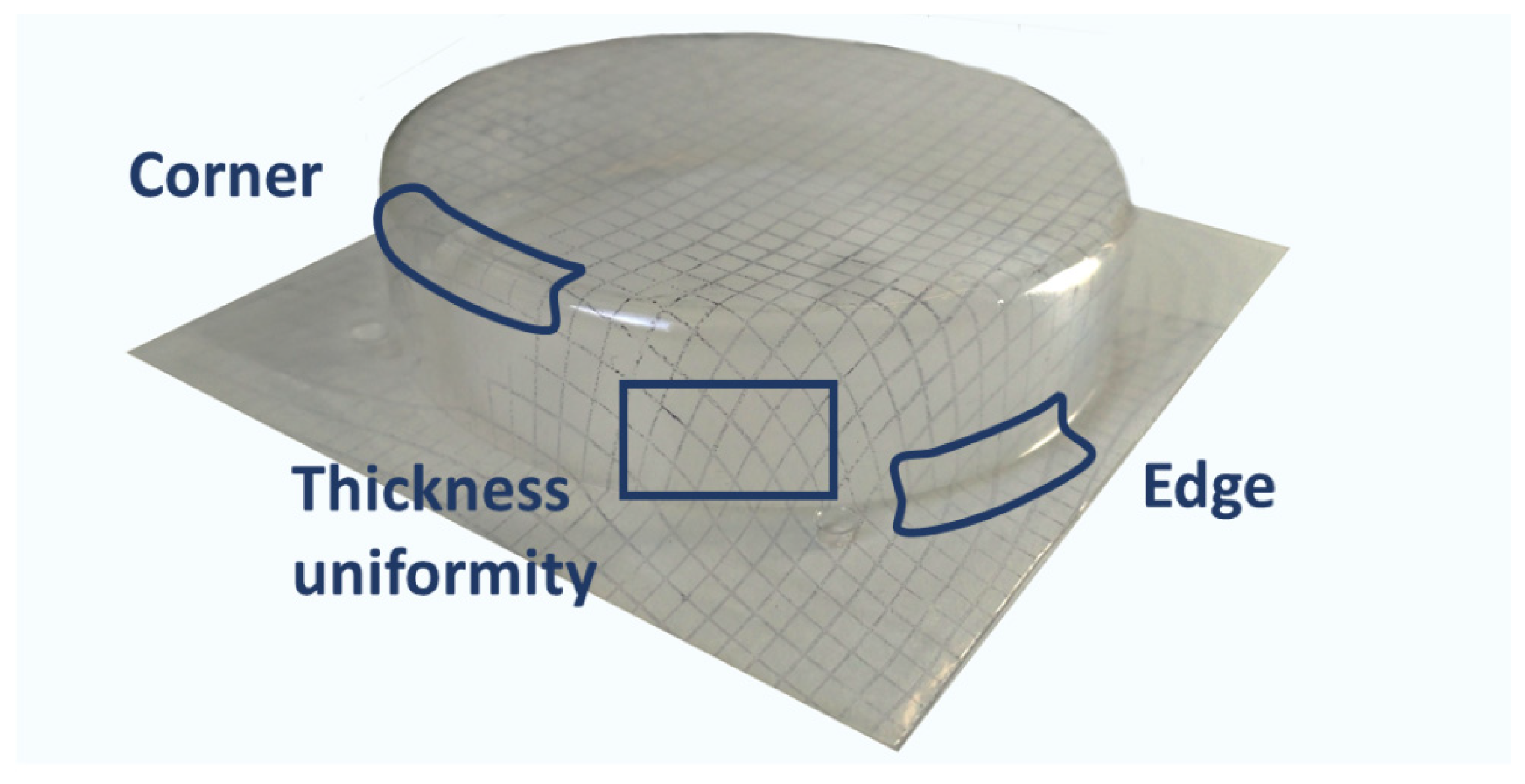
- Edge inspection: Assesses the linearity in the joint section between the flat surface of the original sheet and the onset of the deformation (for the case of a tray-shaped mold, this would be the line defined by the intersecting planes of the original flat sheet and the vertical sides of the tray).
- (b)
- Corner inspection: Provides information about the mold reproducibility at the corners of the tray.
- (c)
- Thickness inspection: Evaluates the uniformity in the path and span of the squares in the grid (the shape of the grid elements is related to the local draw ratio and to the thickness distribution; high draw ratios result in high span and low thickness. On the other hand, even square-grid deformation is related to a uniform thickness distribution).
2.3.2. Mechanical Characterization
2.3.3. Morphology Characterization
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermogravimetry with Coupled Fourier Transformed Infrared (TGA-FTIR)
3. Results
3.1. Characterization
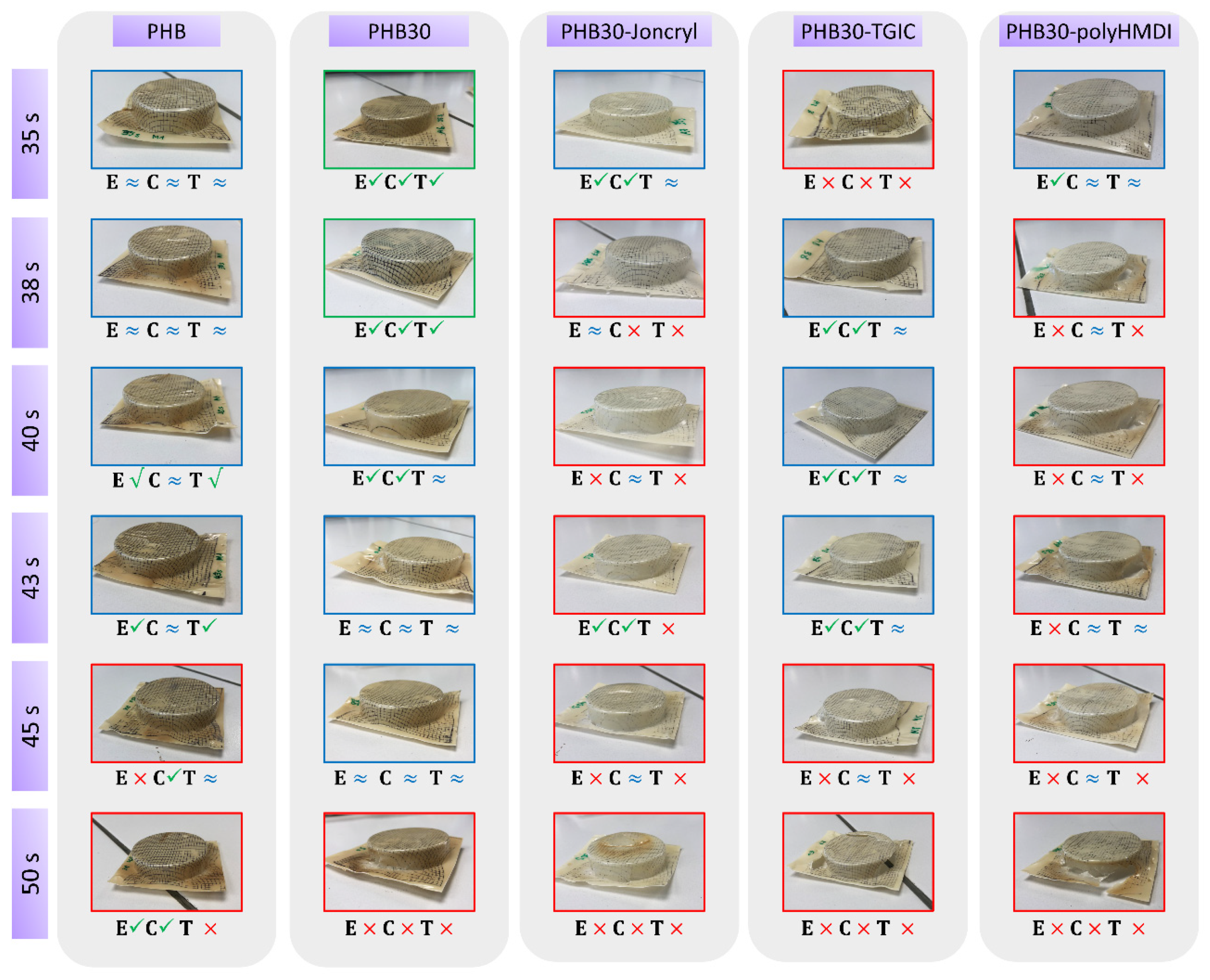
3.1.1. Thermoforming Ability
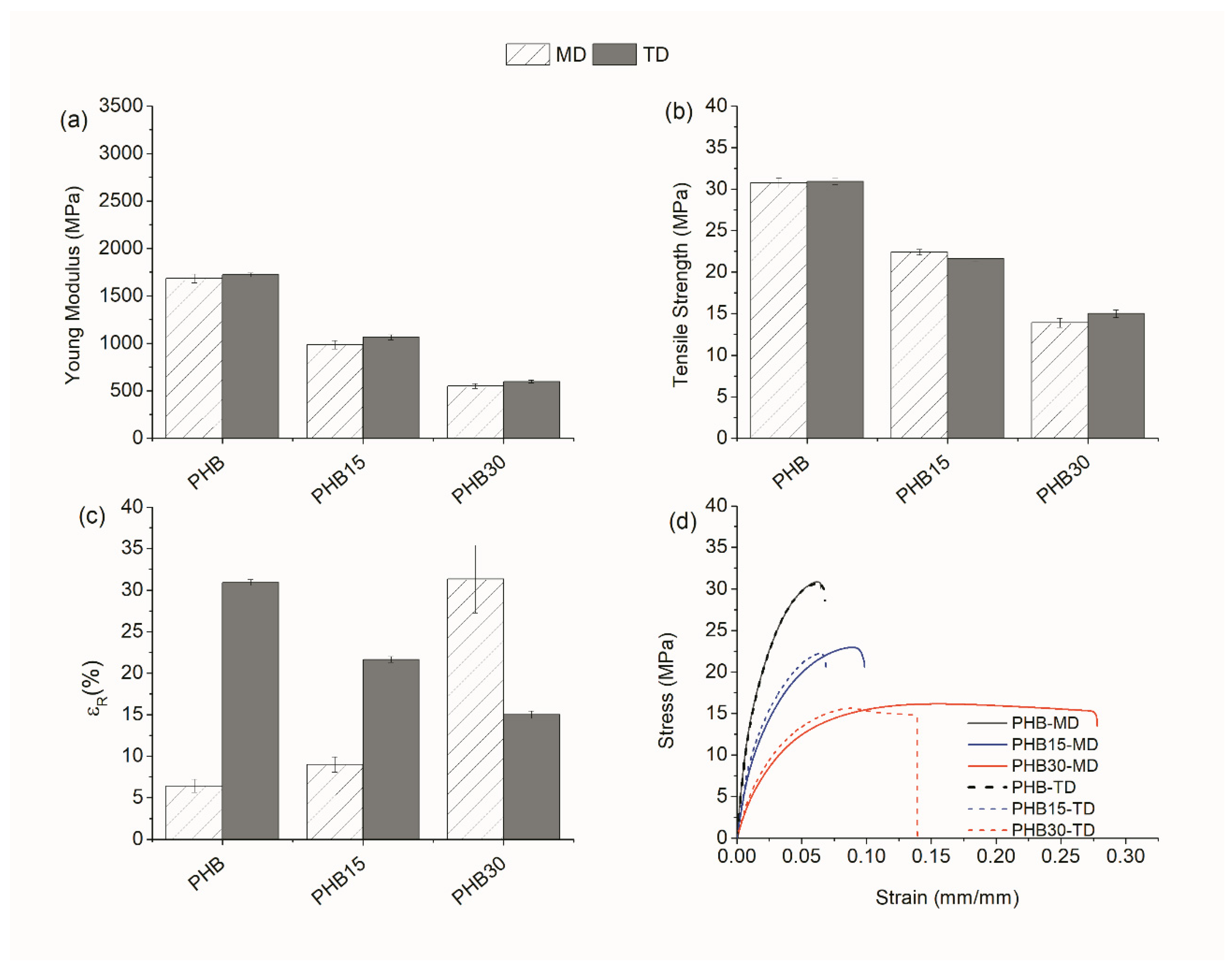
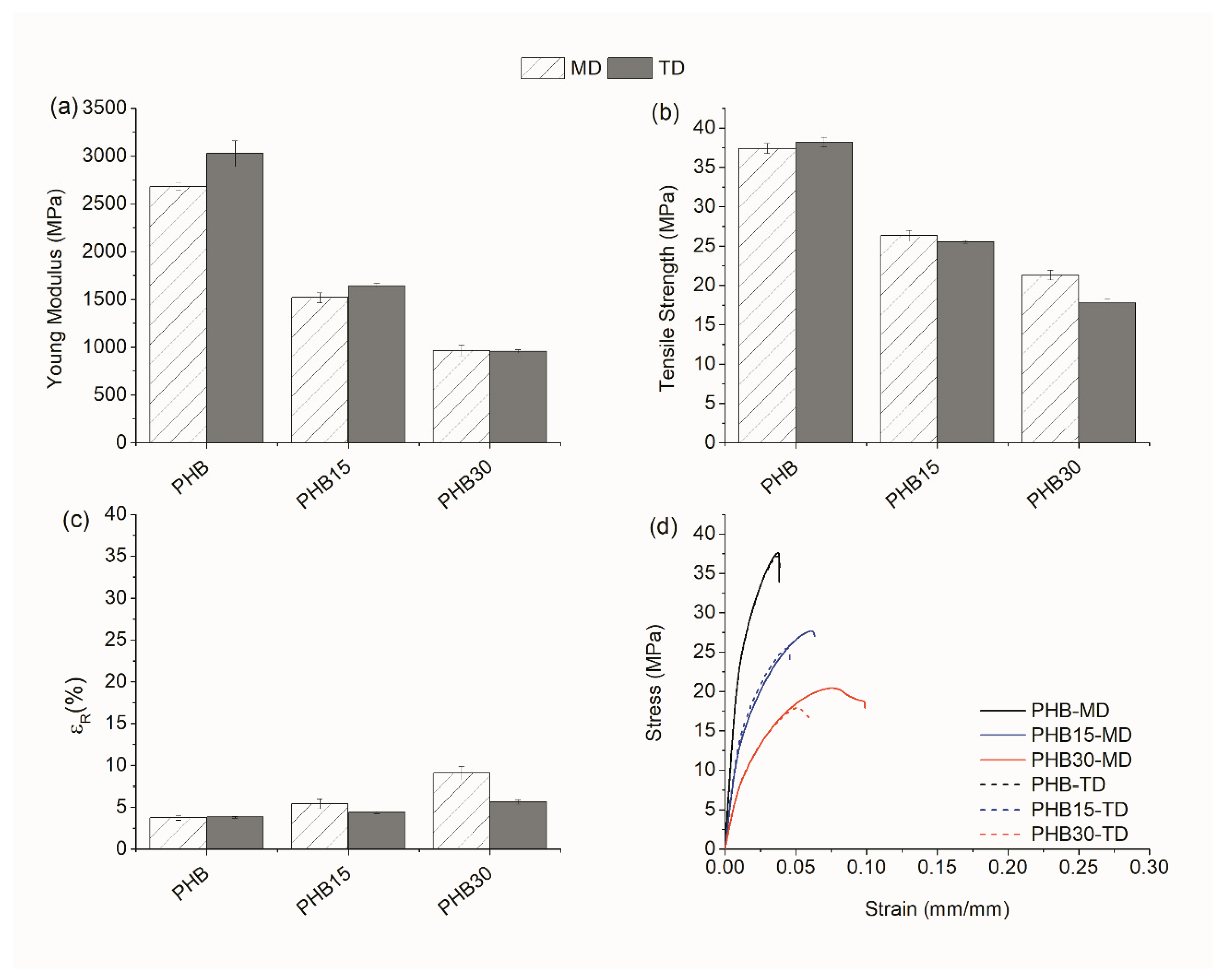
3.1.2. Mechanical Properties
Tensile Test
Tear Strength
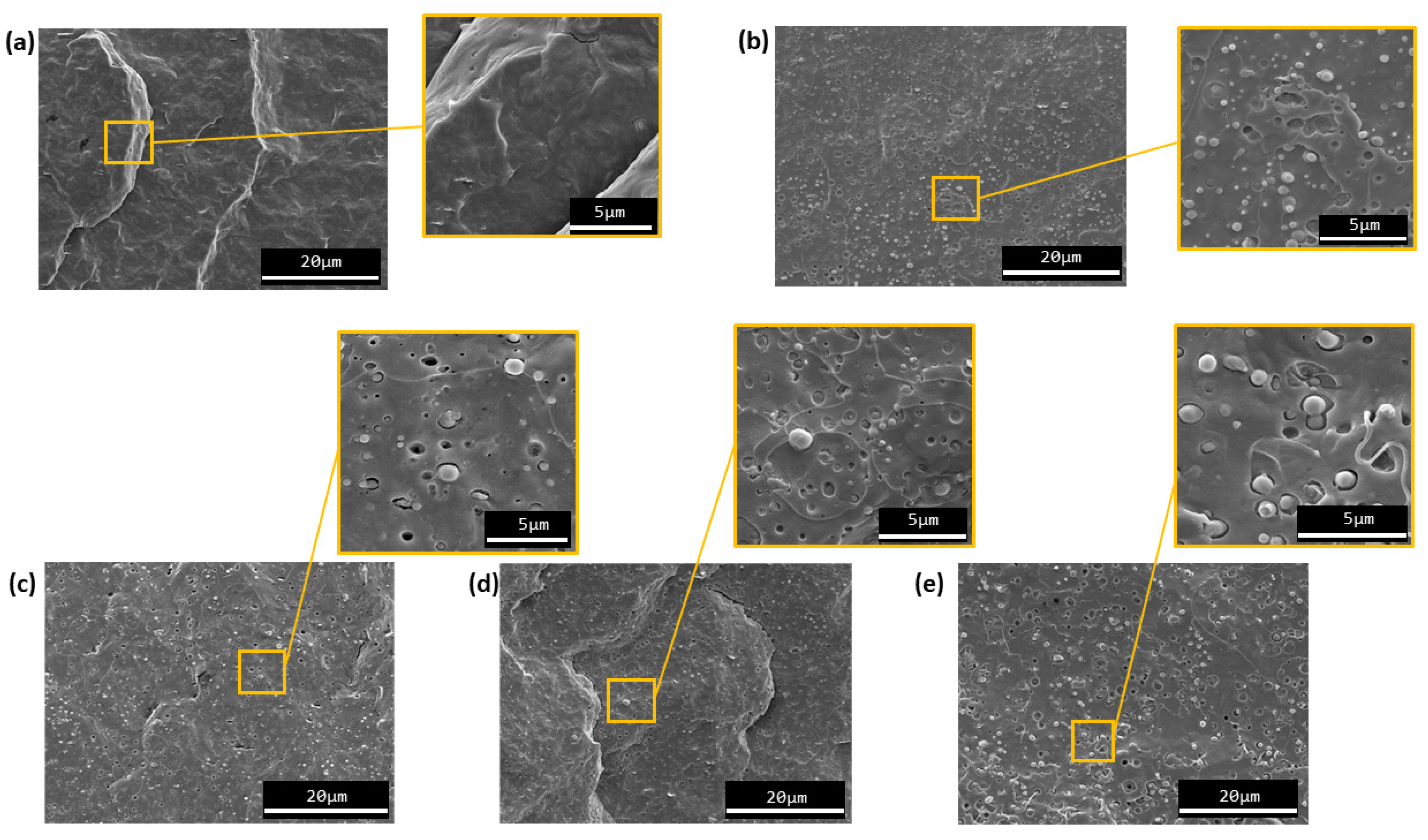
3.1.3. Morphology Characterization
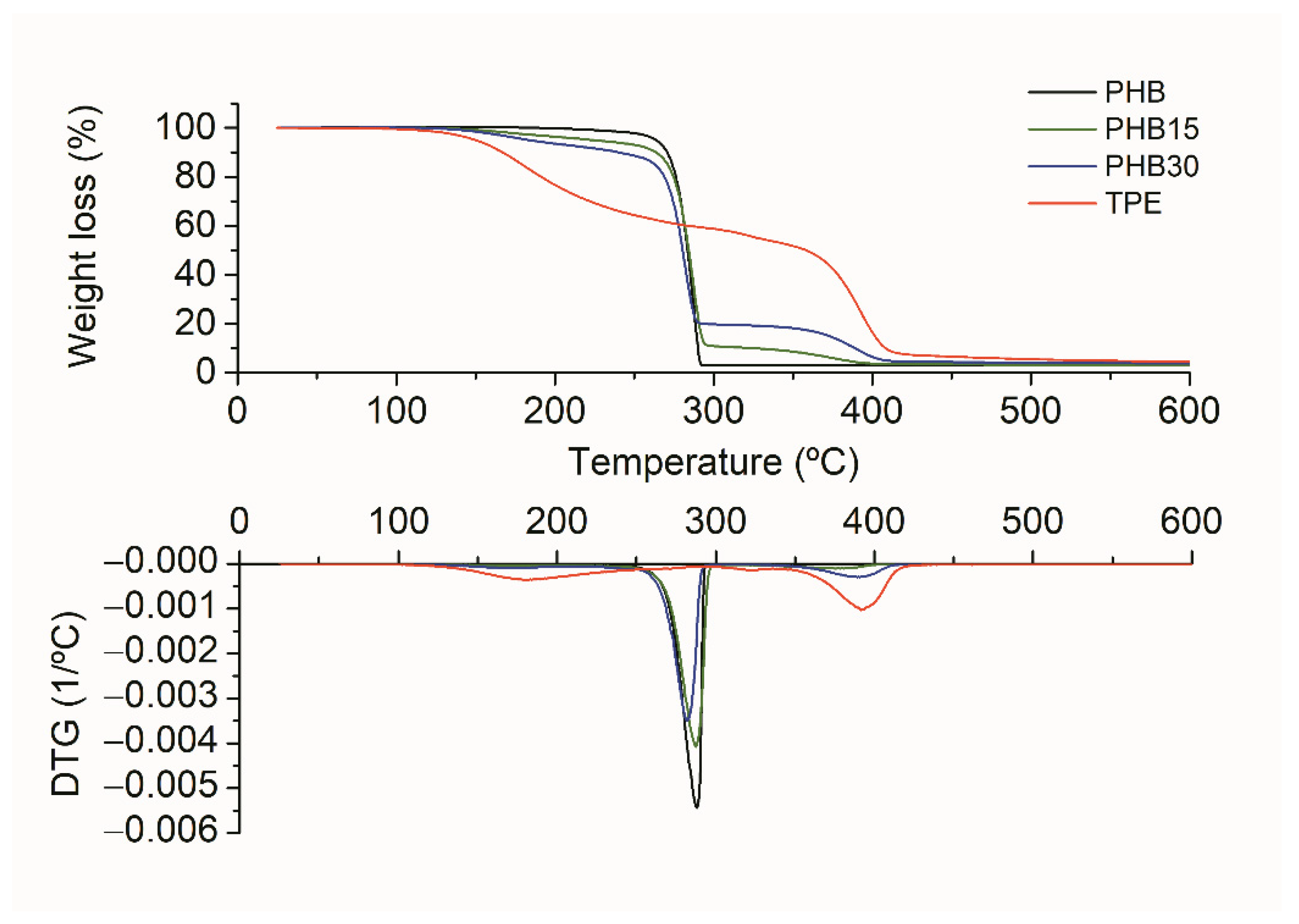
3.1.4. Thermal Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A European Strategy for Plastics in a Circular Economy. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee ofthe Regions; European Commission: Brussels, Belgium, 2018.
- Plastics Europe’s Market Research and Statistics Group (PMERG) Plastics—The Facts; Plastics Europe: Brussels, Belgium, 2018; p. 38.
- Chinthapalli, R.; Skoczinski, P.; Carus, M.; Baltus, W.; De Guzman, D.; Käb, H.; Raschka, A.; Ravenstijn, J. Bio-based Building Blocks and Polymers-Global Capacities, Production and Trends 2018–2023; Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2019. [Google Scholar]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q. Plastics Completely Synthesized by Bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar] [CrossRef]
- El-malek, F.A.; Khairy, H.; Farag, A.; Omar, S. The sustainability of microbial bioplastics, production and applications. Int. J. Biol. Macromol. 2020, 157, 319–328. [Google Scholar] [CrossRef]
- Castilho, L.R.; Mitchell, D.A.; Freire, D.M.G. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour. Technol. 2009, 100, 5996–6009. [Google Scholar] [CrossRef]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the biological degradation of polymers in various environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Plackett, D.; Siró, I. Polyhydroxyalkanoates (PHAs) for food packaging. Multifunct. Nanoreinforced Polym. Food Packag. 2011, 498–526. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In vivo and Post-synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2020, 8, 619266. [Google Scholar] [CrossRef]
- Albuquerque, P.B.S.; Malafaia, C.B. Perspectives on the production, structural characteristics and potential applications of bioplastics derived from polyhydroxyalkanoates. Int. J. Biol. Macromol. 2018, 107, 615–625. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Miao, L.; Walton, W.C.; Wang, L.; Li, L.; Wang, Y. Characterization of polylactic acids-polyhydroxybutyrate based packaging film with fennel oil, and its application on oysters. Food Packag. Shelf Life 2019, 22. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Clarinval, A.M.; Halleux, J. Classification of biodegradable polymers. In Biodegradable Polymers for Industrial Applications; Woodhead Publishing: Sawston, UK, 2005; pp. 3–31. ISBN 9781855739345. [Google Scholar]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- De Koning, G.J.M.; Lemstra, P.J.; Hill, D.J.T.; Carswell, T.G.; O’Donnell, J.H. Ageing phenomena in bacterial poly[(R)-3-hydroxybutyrate]. 1. A study on the mobility in poly[(R)-3-hydroxybutyrate] powders by monitoring the radical decay with temperature after γ-radiolysis at 77 K. Polymer 1992, 33, 3295–3297. [Google Scholar] [CrossRef][Green Version]
- Biddlestone, F.; Harris, A.; Hay, J.N.; Hammond, T. The Physical Ageing of Amorphous Poly(hydroxybutyrate). Polym. Int. 1996, 39, 221–229. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Cabedo, L.; Gámez-Pérez, J. Study of the compatibilization effect of different reactive agents in phb/natural fiber-based composites. Polymers 2020, 12, 1967. [Google Scholar] [CrossRef]
- Huang, J.J.; Keskkula, H.; Paul, D.R. Comparison of the toughening behavior of nylon 6 versus an amorphous polyamide using various maleated elastomers. Polymer 2006, 47, 639–651. [Google Scholar] [CrossRef]
- Parulekar, Y.; Mohanty, A.K. Biodegradable toughened polymers from renewable resources: Blends of polyhydroxybutyrate with epoxidized natural rubber and maleated polybutadiene. Green Chem. 2006, 8, 206–213. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Arrillaga, A.; Anakabe, J.; Gamez-Perez, J.; Cabedo, L. PHBV/TPU/cellulose compounds for compostable injection molded parts with improved thermal and mechanical performance. J. Appl. Polym. Sci. 2019, 136, 47257. [Google Scholar] [CrossRef]
- Fortelny, I.; Ujcic, A.; Fambri, L.; Slouf, M. Phase Structure, Compatibility, and Toughness of PLA/PCL Blends: A Review. Front. Mater. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Harada, M.; Iida, K.; Okamoto, K.; Hayashi, H.; Hirano, K. Reactive compatibilization of biodegradable poly(lactic acid)/poly(ɛ-caprolactone) blends with reactive processing agents. Polym. Eng. Sci. 2008, 48, 1359–1368. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Kotaki, M.; Hamada, H. Effect of compounding procedure on mechanical properties and dispersed phase morphology of poly(lactic acid)/polycaprolactone blends containing peroxide. J. Appl. Polym. Sci. 2007, 103, 1066–1074. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; González-Ausejo, J.; Lagarón, J.M.; Cabedo, L. Biodegradable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/thermoplastic polyurethane blends with improved mechanical and barrier performance. Polym. Degrad. Stab. 2016, 132, 52–61. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.; Arrillaga, A.; Anakabe, J.; Cabedo, L.; Gamez-Perez, J. Toughness Enhancement of PHBV/TPU/Cellulose Compounds with Reactive Additives for Compostable Injected Parts in Industrial Applications. Int. J. Mol. Sci. 2018, 19, 2102. [Google Scholar] [CrossRef] [PubMed]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012 97 2012, 9, 676–682. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sanchez-Safont, E.; Lagaron, J.M.; Olsson, R.T.; Gamez-Perez, J.; Cabedo, L. Assessing the thermoformability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(acid lactic) blends compatibilized with diisocyanates. Polym. Test. 2017, 62, 235–245. [Google Scholar] [CrossRef]
- Gunaratne, L.M.W.K.; Shanks, R.A. Isothermal crystallisation kinetics of poly(3-hydroxybutyrate) using step-scan DSC. J. Therm. Anal. Calorim. 2006, 83, 313–319. [Google Scholar] [CrossRef]
- Gunaratne, L.M.W.K.; Shanks, R.A.; Amarasinghe, G. Thermal history effects on crystallisation and melting of poly(3-hydroxybutyrate). Thermochim. Acta 2004, 423, 127–135. [Google Scholar] [CrossRef]
- Farris, G.; Cinelli, P.; Anguillesi, I.; Salvadori, S.; Coltelli, M.B.; Lazzeri, A. Effect of ageing time on mechanical properties of plasticized poly(hydroxybutyrate) (PHB). AIP Conf. Proc. 2014, 1599, 294–297. [Google Scholar] [CrossRef]
- Jost, V.; Miesbauer, O. Effect of different biopolymers and polymers on the mechanical and permeation properties of extruded PHBV cast films. J. Appl. Polym. Sci. 2018, 135, 46153. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, H.; Wang, R.; Peng, C.; Zhou, Z.; Zhu, M. Morphology and properties of renewable poly(3-hydroxybutyrate- co -3-hydroxyvalerate) blends with thermoplastic polyurethane. Polym. Eng. Sci. 2013, 54, 1113–1119. [Google Scholar] [CrossRef]
- Abate, R.; Ballistreri, A.; Montando, G.; Impallomeni, G. Thermal Degradation of Microbial Poly(4-hydroxybutyrate). Macromolecules 1994, 27, 332–336. [Google Scholar] [CrossRef]
- Cervantes-Uc, J.M.; Espinosa, J.I.M.; Cauich-Rodríguez, J.V.; Ávila-Ortega, A.; Vázquez-Torres, H.; Marcos-Fernández, A.; Román, J.S. TGA/FTIR studies of segmented aliphatic polyurethanes and their nanocomposites prepared with commercial montmorillonites. Polym. Degrad. Stab. 2009, 94, 1666–1677. [Google Scholar] [CrossRef]
- Scatto, M.; Salmini, E.; Castiello, S.; Coltelli, M.B.; Conzatti, L.; Stagnaro, P.; Andreotti, L.; Bronco, S. Plasticized and nanofilled poly(lactic acid)-based cast films: Effect of plasticizer and organoclay on processability and final properties. J. Appl. Polym. Sci. 2013, 127, 4947–4956. [Google Scholar] [CrossRef]
- Tanrattanakul, V.; Bunkaew, P. Effect of different plasticizers on the properties of bio-based thermoplastic elastomer containing poly(lactic acid) and natural rubber. Express Polym. Lett. 2014, 8, 387–396. [Google Scholar] [CrossRef]
- Park, H.M.; Misra, M.; Drzal, L.T.; Mohanty, A.K. “Green” nanocomposites from cellulose acetate bioplastic and clay: Effect of eco-friendly triethyl citrate plasticizer. Biomacromolecules 2004, 5, 2281–2288. [Google Scholar] [CrossRef]
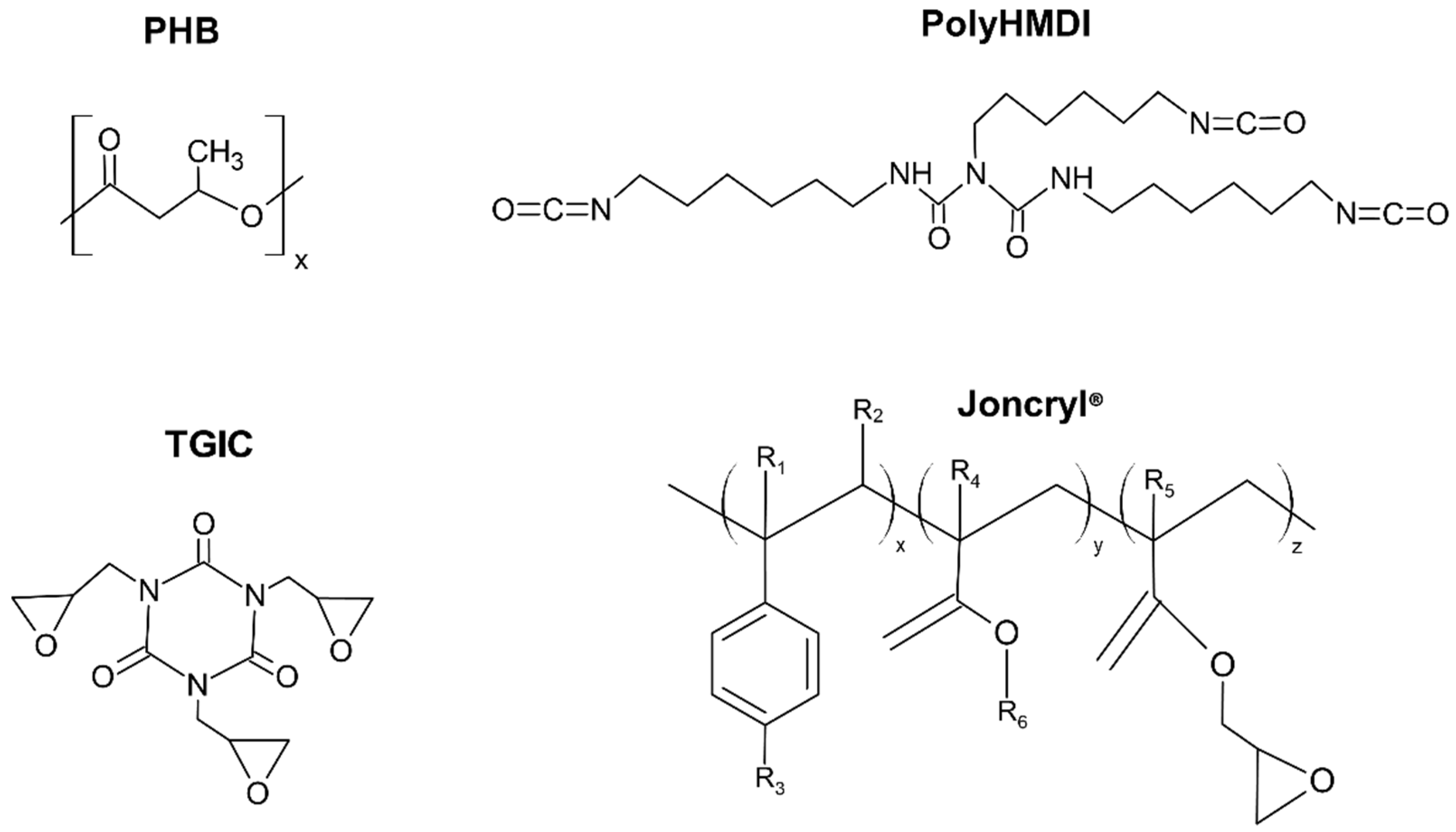



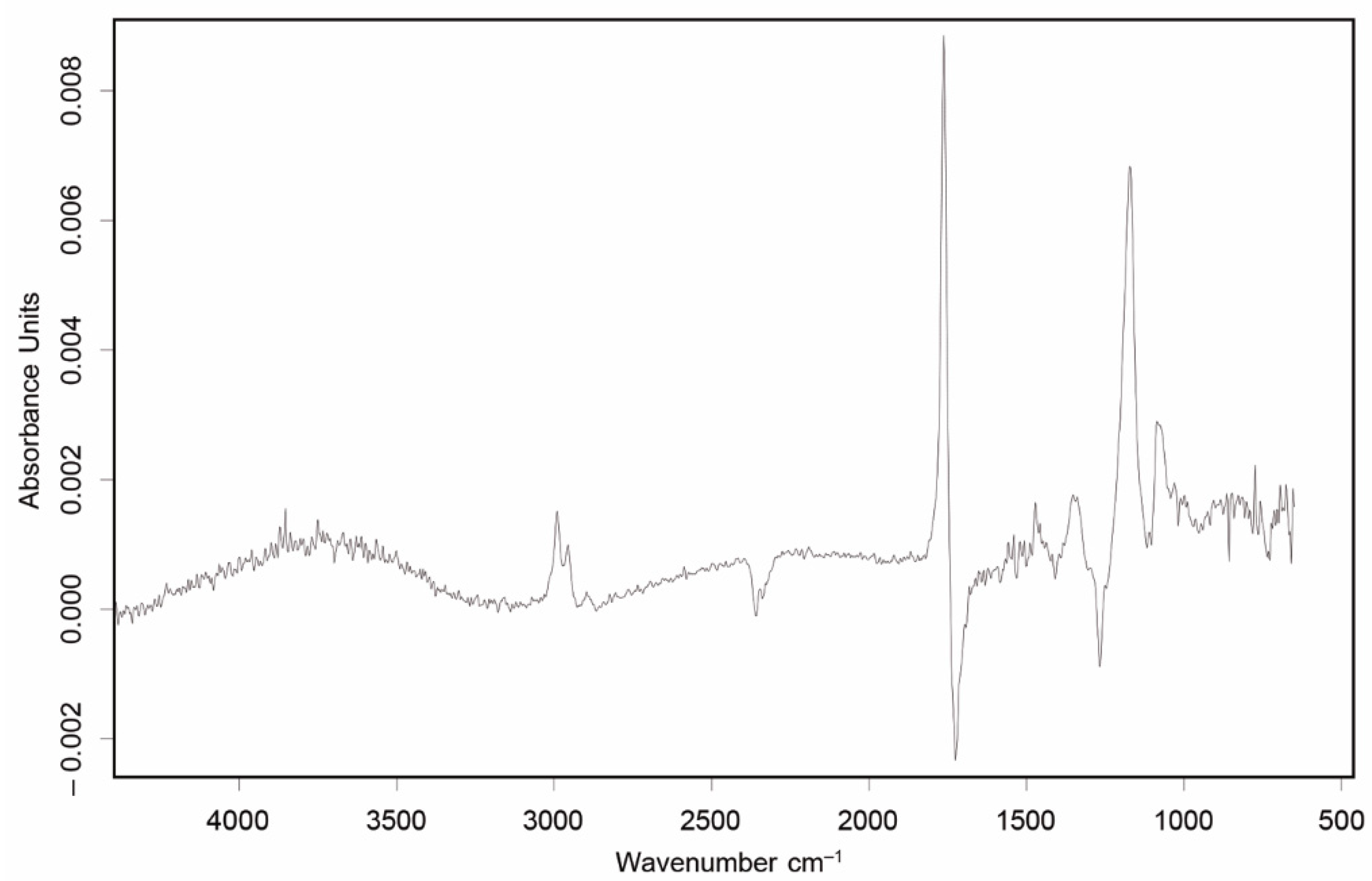
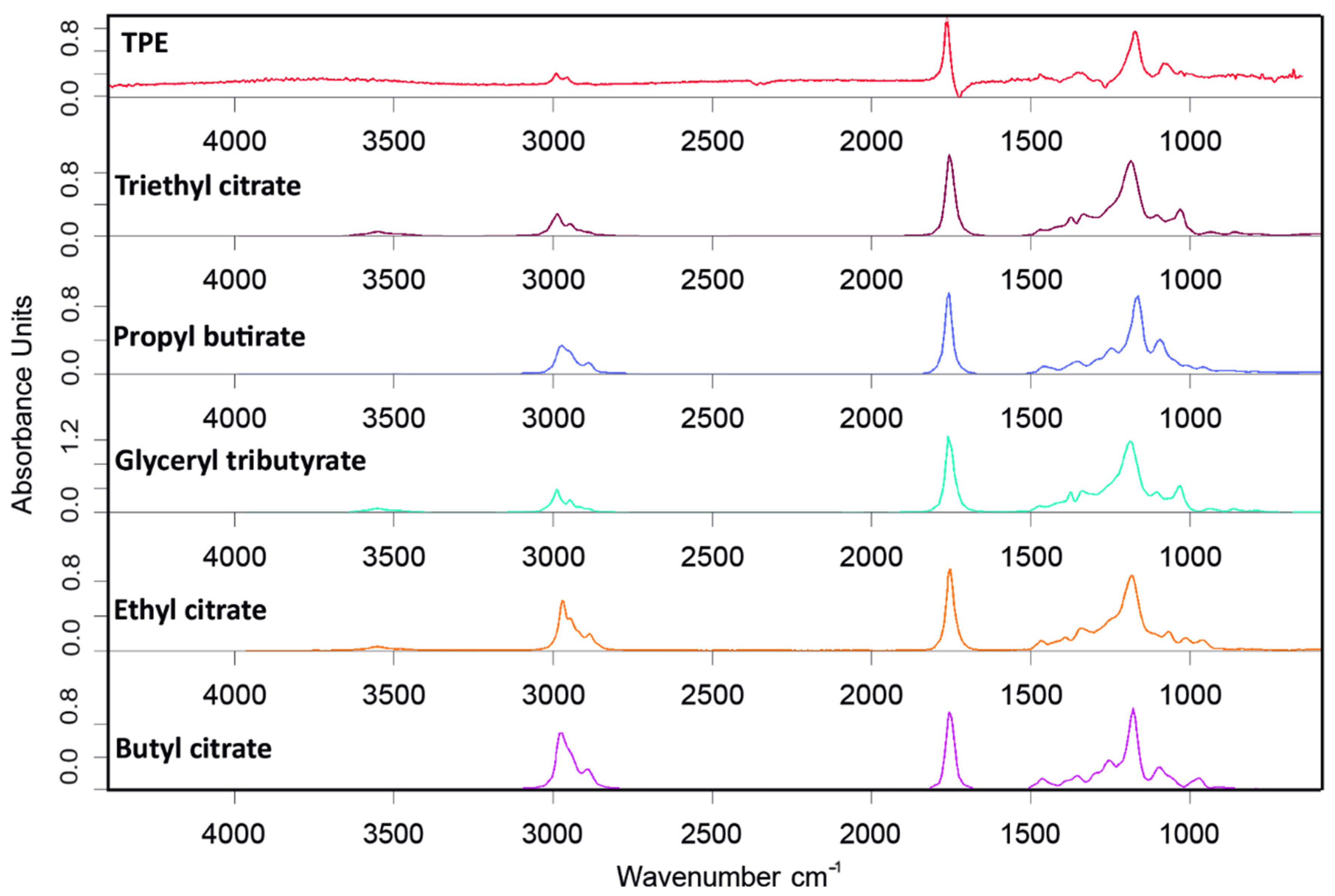
| Sample | Neat PHB (wt%) | TPE (wt%) | TGIC (phr) 1 | PolyHMDI | Joncryl® |
|---|---|---|---|---|---|
| PHB | 100 | - | - | - | - |
| PHB15 | 85 | 15 | - | - | - |
| PHB15-TGIC | 85 | 15 | 1 | - | - |
| PHB15-Joncry® | 85 | 15 | - | - | 1 |
| PHB15-PolyHMDI | 85 | 15 | - | 1 | - |
| PHB30 | 70 | 30 | - | - | - |
| PHB30-TGIC | 70 | 30 | 1 | - | - |
| PHB30-Joncry® | 70 | 30 | - | - | 1 |
| PHB30-PolyHMDI | 70 | 30 | - | 1 | - |
| 0 Days | 15 Days | ||||||
|---|---|---|---|---|---|---|---|
| Samples | E (MPa) | σmax (MPa) | εR (%) | E (MPa) | σmax (MPa) | εR (%) | |
| PHB | MD | 1680 | 30.8 | 6.4 | 2680 | 37.4 | 3.8 |
| TD | 1720 | 30.9 | 6.7 | 3030 | 38.2 | 3.8 | |
| PHB15 | MD | 990 | 22.4 | 9.0 | 1520 | 26.3 | 5.4 |
| TD | 1070 | 21.6 | 6.6 | 1640 | 25.5 | 4.4 | |
| PHB15-TGIC | MD | 820 | 21.1 | 11.1 | 1290 | 25.4 | 6.8 |
| TD | 760 | 18.1 | 10.0 | 1260 | 22.0 | 5.4 | |
| PHB15-Joncryl® | MD | 920 | 20.5 | 7.6 | 1450 | 26.1 | 5.1 |
| TD | 1050 | 20.2 | 6.0 | 1630 | 25.0 | 4.1 | |
| PHB15-polyHMDI | MD | 960 | 20.4 | 8.1 | 1430 | 24.8 | 5.3 |
| TD | 1070 | 20.4 | 5.1 | 1570 | 23.3 | 3.9 | |
| PHB30 | MD | 550 | 13.9 | 31.3 | 970 | 21.3 | 9.1 |
| TD | 600 | 15.0 | 11.7 | 960 | 17.8 | 5.7 | |
| PHB30-TGIC | MD | 610 | 17.9 | 31.8 | 910 | 20.7 | 9.3 |
| TD | 560 | 15.1 | 14.7 | 860 | 16.9 | 6.6 | |
| PHB30-Joncryl® | MD | 660 | 16.6 | 13.9 | 880 | 19.8 | 9.3 |
| TD | 600 | 13.3 | 7.5 | 750 | 14.3 | 5.5 | |
| PHB30-polyHMDI | MD | 630 | 17.6 | 17.4 | 1010 | 21.5 | 7.4 |
| TD | 510 | 14.4 | 11.0 | 1080 | 20.0 | 5.8 | |
| 0 Days | 15 Days | ||
|---|---|---|---|
| Samples | Tear Strength (N/mm) | Tear Strength (N/mm) | |
| PHB | MD | 10.1 | 8.8 |
| TD | 10.8 | 9.5 * | |
| PHB15 | MD | 7.2 | 4.8 |
| TD | 8.6 | 6.3 | |
| PHB15-TGIC | MD | 6.6 | 5 |
| TD | 10.9 * | 9.5 * | |
| PHB15-Joncryl® | MD | 5.5 | 3.7 |
| TD | 8.9 | 7.5 * | |
| PHB15-polyHMDI | MD | 6.9 | 3.9 |
| TD | 7.2 | 5.9 * | |
| PHB30TPE | MD | 7.5 | 4.4 |
| TD | 17.7 * | 14.6 * | |
| PHB30-TGIC | MD | 7.7 | 5.5 |
| TD | 29.2 * | 13 * | |
| PHB30-Joncryl® | MD | 2.6 | 1.9 |
| TD | 8.7 * | 4.9 * | |
| PHB30-polyHMDI | MD | 8.8 | 3.9 |
| TD | 26.5 * | 8.7 * | |
| Composition | Tc (°C) | ΔHc (J/g) | Tm(°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|
| PHB | 122 | 90 | 172 | 93 | 64 |
| TPE | 67 | - | 19/99 1 | - | - |
| PHB/15TPE | 108 | 64 | 168 | 66 | 53 |
| PHB/15TPE-TGIC | 108 | 59 | 167 | 64 | 52 |
| PHB/15TPE-Joncryl® | 109 | 72 | 169 | 73 | 59 |
| PHB/15TPE-polyHMDI | 108 | 70 | 169 | 69 | 56 |
| PHB/30TPE | 104 | 53 | 165 | 57 | 55 |
| PHB/30TPE-TGIC | 106 | 61 | 167 | 62 | 61 |
| PHB/30TPE-Joncryl® | 106 | 59 | 166 | 62 | 61 |
| PHB/30TPE-polyHMDI | 107 | 61 | 167 | 61 | 60 |
| Composition | T5% (°C) | Tdmax (°C) |
|---|---|---|
| PHB | 264 | 288 |
| PHB/15TPE | 224 | 287 |
| PHB/15TPE-TGIC | 214 | 283 |
| PHB/15TPE-Joncryl® | 230 | 286 |
| PHB/15TPE-polyHMDI | 220 | 265 |
| PHB/30TPE | 183 | 281 |
| PHB/30TPE-TGIC | 193 | 282 |
| PHB/30TPE-Joncryl® | 191 | 285 |
| PHB/30TPE-polyHMDI | 185 | 274 |
| TPE | 150 | 392 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego, K.; Matos, A.; Sánchez-Safont, E.; Candal, M.V.; Lagaron, J.M.; Cabedo, L.; Gamez-Perez, J. Role of Plasticizers on PHB/bio-TPE Blends Compatibilized by Reactive Extrusion. Materials 2022, 15, 1226. https://doi.org/10.3390/ma15031226
Samaniego K, Matos A, Sánchez-Safont E, Candal MV, Lagaron JM, Cabedo L, Gamez-Perez J. Role of Plasticizers on PHB/bio-TPE Blends Compatibilized by Reactive Extrusion. Materials. 2022; 15(3):1226. https://doi.org/10.3390/ma15031226
Chicago/Turabian StyleSamaniego, Kerly, Armando Matos, Estefanía Sánchez-Safont, María V. Candal, Jose M. Lagaron, Luis Cabedo, and Jose Gamez-Perez. 2022. "Role of Plasticizers on PHB/bio-TPE Blends Compatibilized by Reactive Extrusion" Materials 15, no. 3: 1226. https://doi.org/10.3390/ma15031226
APA StyleSamaniego, K., Matos, A., Sánchez-Safont, E., Candal, M. V., Lagaron, J. M., Cabedo, L., & Gamez-Perez, J. (2022). Role of Plasticizers on PHB/bio-TPE Blends Compatibilized by Reactive Extrusion. Materials, 15(3), 1226. https://doi.org/10.3390/ma15031226










