The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Tetranuclear Ti(IV)–Oxo Complex (1) Stabilized by Acetylsalicylate Ligands
2.3. Preparation of the Composite Foils
2.4. Biocompatibility Study of PCL and PCL + (1) Composite Materials
2.4.1. Samples Sterilization and Preparation for Cell Culture
2.4.2. Cell Culture
2.4.3. Cytotoxicity Assays
Extract Assay
Direct Cytotoxicity
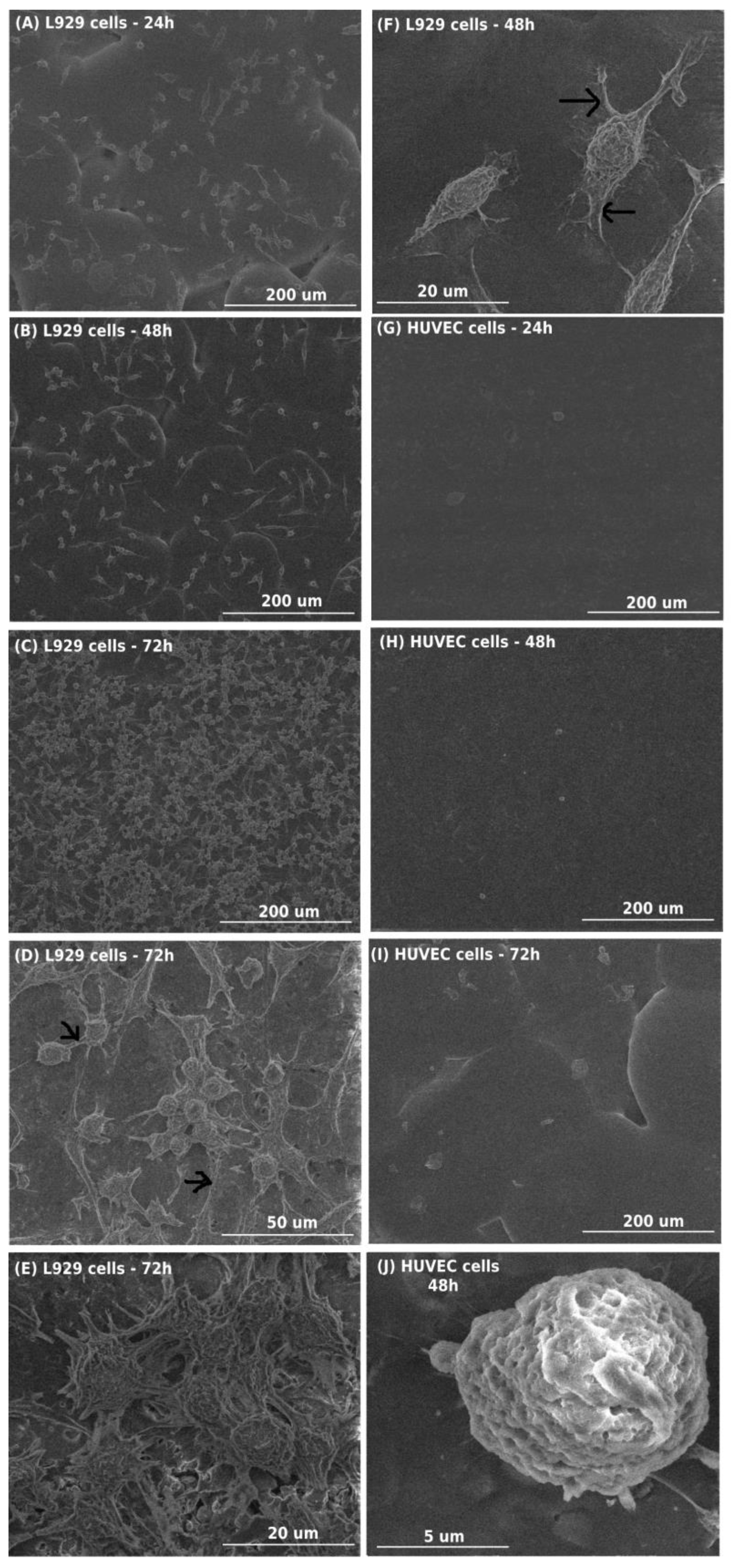
2.4.4. SEM Analysis
2.4.5. Statistical Analysis in the MTT Assay
2.5. Antibacterial and Anticandidal Activity of PCL + (1) Composite Materials
2.6. Electron Paramagnetic Resonance Research
3. Results
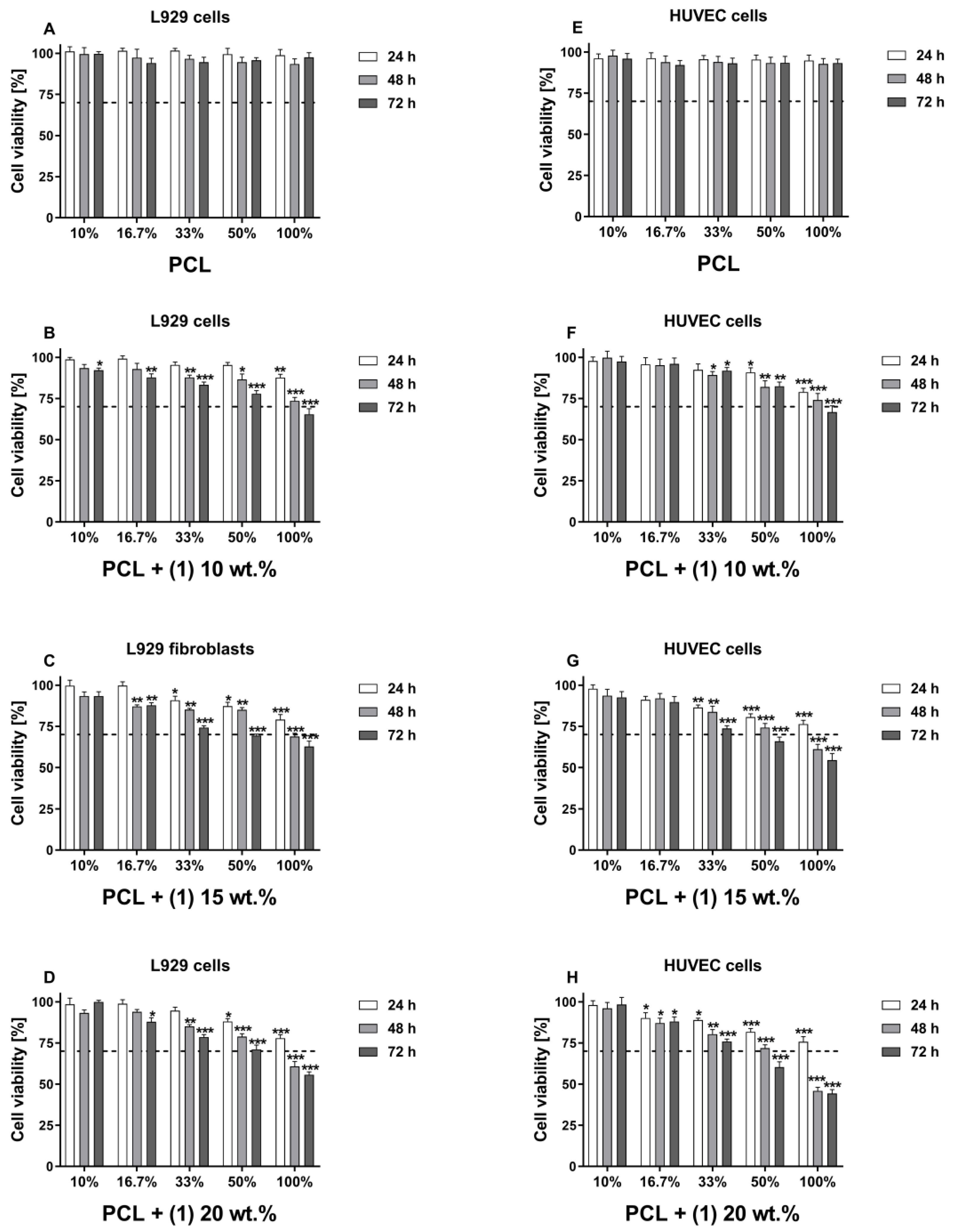
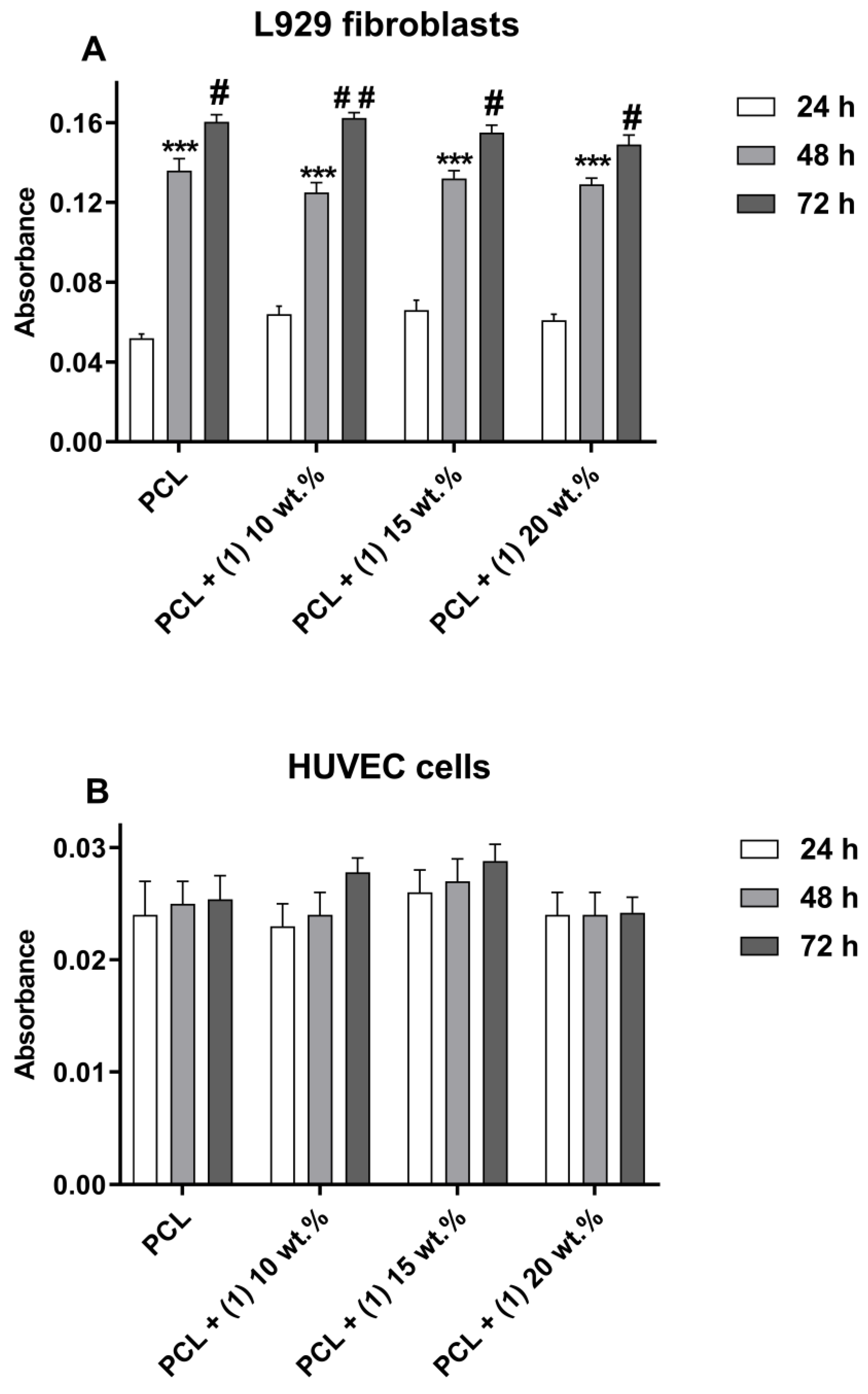
3.1. Cytotoxicity Evaluation of PCL/PCL + (1)
3.2. Antimicrobial Activity of PCL + (1) Composite Materials
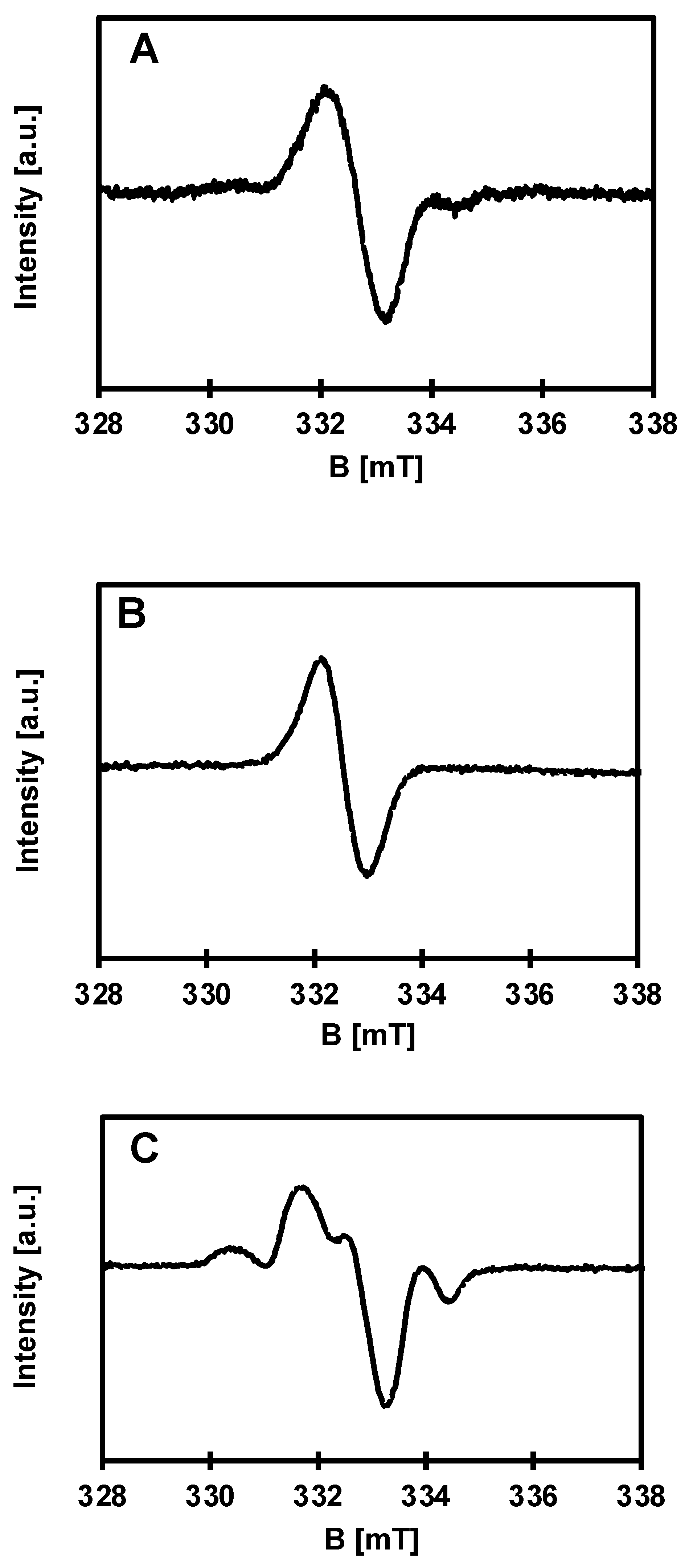
3.3. EPR Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubiak, B.; Radtke, A.; Topolski, A.; Wrzeszcz, G.; Golińska, P.; Kaszkowiak, E.; Sobota, M.; Włodarczyk, J.; Stojko, M.; Piszczek, P. The Composites of PCL and Tetranuclear Titanium(IV)-Oxo Complexes as Materials Exhibiting the Photocatalytic and the Antimicrobial Activity. Int. J. Mol. Sci. 2021, 22, 7021. [Google Scholar] [CrossRef] [PubMed]
- Piszczek, P.; Kubiak, B.; Golińska, P.; Radtke, A. Oxo-Titanium(IV) Complex/Polymer Composites—Synthesis, Spectroscopic Characterization and Antimicrobial Activity Test. Int. J. Mol. Sci. 2020, 21, 9663. [Google Scholar] [CrossRef] [PubMed]
- Śmigiel, J.; Muzioł, T.; Piszczek, P.; Radtke, A. Titanium(IV) Oxo-Complex with Acetylsalicylic Acid Ligand and Its Polymer Composites: Synthesis, Structure, Spectroscopic Characterization, and Photocatalytic Activity. Materials 2022, 15, 4408. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Madras, G.; Basu, B. In Vitro/In Vivo Assessment and Mechanisms of Toxicity of Bioceramic Materials and Its Wear Particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The Difference of Fibroblast Behavior on Titanium Substrata with Different Surface Characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial Cell Control of Thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [Green Version]
- Sumpio, B.E.; Timothy Riley, J.; Dardik, A. Cells in Focus: Endothelial Cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512. [Google Scholar] [CrossRef]
- Svensson, F.G.; Seisenbaeva, G.A.; Kessler, V.G. Mixed-Ligand Titanium “Oxo Clusters”: Structural Insights into the Formation and Binding of Organic Molecules and Transformation into Oxide Nanostructures on Hydrolysis and Thermolysis. Eur. J. Inorg. Chem. 2017, 2017, 4117–4122. [Google Scholar] [CrossRef]
- Janek, M.; Radtke, A.; Muzioł, T.; Jerzykiewicz, M.; Piszczek, P. Tetranuclear Oxo-Titanium Clusters with Different Carboxylate Aromatic Ligands: Optical Properties, DFT Calculations, and Photoactivity. Materials 2018, 11, 1661. [Google Scholar] [CrossRef] [Green Version]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, Mechanical Characteristics and in Vitro Degradation, Cytotoxicity, Genotoxicity and Mutagenicity of Novel Biodegradable Zn–Mg Alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting Properties and Cytotoxicity Evaluation of a Premixed Bioceramic Root Canal Sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar-Fayyad, D. Cytocompatibility of New Bioceramic-Based Materials on Human Fibroblast Cells (MRC-5). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.-J.; Chang, S.-W.; Lee, S.-I.; Kum, K.-Y.; Bae, K.-S.; Kim, E.-C. Human Periodontal Ligament Cell Response to a Newly Developed Calcium Phosphate–Based Root Canal Sealer. J. Endod. 2010, 36, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Reis, R.L.; Cunha, A.M.; Blitterswijk, C.A.; de Bruijn, J.D. Cytocompatibility and Response of Osteoblastic-like Cells to Starch-Based Polymers: Effect of Several Additives and Processing Conditions. Biomaterials 2001, 22, 1911–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relou, I.A.; Damen, C.A.; van der Schaft, D.W.; Groenewegen, G.; Griffioen, A.W. Effect of culture conditions on endothelial cell growth and responsiveness. Tissue Cell 1998, 30, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.; Tekinay, A.B.; Guler, M.O. Selective adhesion and growth of vascular endothelial cells on bioactive peptide nanofiber functionalized stainless steel surface. Biomaterials 2011, 32, 8797–8805. [Google Scholar] [CrossRef]
- Tiwari, A.; Jung, J.J.; Inamdar, S.M.; Brown, C.O.; Goel, A.; Choudhury, A. Endothelial cell migration on fibronectin is regulated by syntaxin 6-mediated alpha5beta1 integrin recycling. J. Biol. Chem. 2011, 286, 36749–36761. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Pinto, D.J.; Guiza-Arguello, V.R.; Becerra-Bayona, S.M.; Erndt-Marino, J.; Samavedi, S.; Malmut, S.; Russell, B.; Hӧӧk, M.; Hahn, M.S. Collagen-mimetic hydrogels promote human endothelial cell adhesion, migration and phenotypic maturation. J. Mater. Chem. B 2015, 3, 7912–7919. [Google Scholar] [CrossRef] [Green Version]
- Schnaper, H.W.; Kleinman, H.K.; Grant, D.S. Role of laminin in endothelial cell recognition and differentiation. Kidney Int. 1993, 43, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Kook, Y.M.; Kim, H.; Kim, S.; Heo, C.Y.; Park, M.H.; Lee, K.; Koh, W.G. Promotion of Vascular Morphogenesis of Endothelial Cells Co-Cultured with Human Adipose-Derived Mesenchymal Stem Cells Using Polycaprolactone/Gelatin Nanofibrous Scaffolds. Nanomaterials 2018, 8, 117. [Google Scholar] [CrossRef]
- Cicco, S.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira Weissflogii Silica Shells. Bioengineering 2016, 3, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, F.; Janzen, D.M.; Knecht, D.A. Contribution of Filopodia to Cell Migration: A Mechanical Link between Protrusion and Contraction. Int. J. Cell Biol. 2010, 2010, 507821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearce, H.; Kalia, N.; Bardhan, K.; Brown, N. Effects of Aspirin and Indomethacin on Endothelial Cell Proliferation In Vitro. J. Gastroenterol. Hepatol. 2003, 18, 1180–1187. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Wojciechowska, M.; Bilska, A.; Stangret, A.; Szewczyk, G.; Mittal, T.K.; Watroba, M.; Kochanowski, J. Aspirin Action in Endothelial Cells: Different Patterns of Response Between Chemokine CX3CL1/CX3CR1 and TNF-α/TNFR1 Signaling Pathways. Cardiovasc. Drugs 2015, 29, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Borthwick, G.M.; Sarah Johnson, A.; Partington, M.; Burn, J.; Wilson, R.; Arthur, H.M. Therapeutic Levels of Aspirin and Salicylate Directly Inhibit a Model of Angiogenesis through a Cox- Independent Mechanism. FASEB J. 2006, 20, 2009–2016. [Google Scholar] [CrossRef]
- Nordin, M.; Wieslander, A.; Martinson, E.; Kjellstrand, P. Effects of Exposure Period of Acetylsalicylic Acid, Paracetamol and Isopropanol on L929 Cytotoxicity. Toxicol. Vitr. 1991, 5, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Raza, H.; John, A.; Benedict, S. Acetylsalicylic Acid-Induced Oxidative Stress, Cell Cycle Arrest, Apoptosis and Mitochondrial Dysfunction in Human Hepatoma HepG2 Cells. Eur. J. Pharmacol. 2011, 668, 15–24. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Shafarin, J. NAC Attenuates LPS-Induced Toxicity in Aspirin-Sensitized Mouse Macrophages via Suppression of Oxidative Stress and Mitochondrial Dysfunction. PLoS ONE 2014, 9, e103379. [Google Scholar] [CrossRef] [Green Version]
- Fukui, A.; Naito, Y.; Handa, O.; Kugai, M.; Tsuji, T.; Yoriki, H.; Qin, Y.; Adachi, S.; Higashimura, Y.; Mizushima, K.; et al. Acetyl Salicylic Acid Induces Damage to Intestinal Epithelial Cells by Oxidation-Related Modifications of ZO-1. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G927–G936. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, J.; Zhang, S.; Wu, W.; Qi, R. Aspirin Inhibits the Production of Reactive Oxygen Species by Downregulating Nox4 and Inducible Nitric Oxide Synthase in Human Endothelial Cells Exposed to Oxidized Low-Density Lipoprotein. J. Cardiovasc. Pharmacol. 2012, 59, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Grosser, N.; Schröder, H. Aspirin Protects Endothelial Cells from Oxidant Damage Via the Nitric Oxide-CGMP Pathway. ATVB 2003, 23, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Jorda, A.; Aldasoro, M.; Aldasoro, C.; Guerra-Ojeda, S.; Iradi, A.; Vila, J.M.; Campos-Campos, J.; Valles, S.L. Action of Low Doses of Aspirin in Inflammation and Oxidative Stress Induced by Aβ 1-42 on Astrocytes in Primary Culture. Int. J. Med. Sci. 2020, 17, 834–843. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron Spin Resonance Spectroscopy for the Study of Nanomaterial-Mediated Generation of Reactive Oxygen Species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti 3 + in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2012, 2012, 831524. [Google Scholar] [CrossRef] [Green Version]
- Suriye, K.; Lobo-Lapidus, R.J.; Yeagle, G.J.; Praserthdam, P.; Britt, R.D.; Gates, B.C. Probing Defect Sites on TiO2 with [Re3(CO)12H3]: Spectroscopic Characterization of the Surface Species. Chem. Eur. J. 2008, 14, 1402–1414. [Google Scholar] [CrossRef]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef]
- Richards, E.; Murphy, D.M.; Che, M. An EPR Characterisation of Stable and Transient Reactive Oxygen Species Formed under Radiative and Non-Radiative Conditions. Res. Chem. Intermed. 2019, 45, 5763–5779. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of Ultraviolet Light Assisted Titanium Dioxide Photocatalysis for Food Safety: A Review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Wang, S.; Wang, X.; Fu, X.; Weng, J. Plasma Pre-Treatment and TiO2 Coating of PMMA for the Improvement of Antibacterial Properties. Surf. Coat. Technol. 2010, 205, 465–469. [Google Scholar] [CrossRef]
- Jesline, A.; John, N.P.; Narayanan, P.M.; Vani, C.; Murugan, S. Antimicrobial Activity of Zinc and Titanium Dioxide Nanoparticles against Biofilm-Producing Methicillin-Resistant Staphylococcus Aureus. Appl. Nanosci. 2015, 5, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, M.J.; Sharifian, G.M.; Wu, T.; Li, Y.; Chang, C.-M.; Ma, J.; Dai, H.-L. Determination of Bacterial Surface Charge Density via Saturation of Adsorbed Ions. Biophys. J. 2021, 120, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Victoria Elorza, M.; ValentÃn, E.; Sentandreu, R. Molecular Organization of the Cell Wall of Candida Albicans and Its Relation to Pathogenicity. FEMS Yeast Res. 2006, 6, 14–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]

| Microorganisms | |||||
|---|---|---|---|---|---|
| Sample | Escherichia coli ATCC 8739 | Escherichia coli ATCC 25922 | Staphylococcus aureus ATCC 6538 | Staphylococcus aureus ATCC 25923 | Candida albicans ATCC 10231 |
| R | R | R | R | R | |
| PCL | +0.15 * | 0.2 | +0.3 * | +0.4 * | +1.0 * |
| PCL + (1) 10 wt.% | 1.0 | 1.8 | 4.5 | 4.6 | +0.1 * |
| PCL + (1) 15 wt.% | 2.1 | 2.8 | 4.5 | 4.6 | 1.1 |
| PCL + (1) 20 wt.% | 2.0 | 3.7 | 4.5 | 4.6 | 0.6 |
| Sample | g-Factors | Species |
|---|---|---|
| (1) | 2.0036 | Radical |
| PCL + (1) 10 wt.% | 2.017; 2.0036; 1.993 | O− + Radical + Ti(III) |
| PCL + (1) 15 wt.% | 2.0036 | Radical |
| PCL + (1) 20 wt.% | 2.017; 2.009; 2.002; 1.992 | O− + Radical + Ti(III) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmigiel, J.; Piszczek, P.; Wrzeszcz, G.; Jędrzejewski, T.; Golińska, P.; Radtke, A. The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy. Materials 2023, 16, 297. https://doi.org/10.3390/ma16010297
Śmigiel J, Piszczek P, Wrzeszcz G, Jędrzejewski T, Golińska P, Radtke A. The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy. Materials. 2023; 16(1):297. https://doi.org/10.3390/ma16010297
Chicago/Turabian StyleŚmigiel, Julia, Piotr Piszczek, Grzegorz Wrzeszcz, Tomasz Jędrzejewski, Patrycja Golińska, and Aleksandra Radtke. 2023. "The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy" Materials 16, no. 1: 297. https://doi.org/10.3390/ma16010297
APA StyleŚmigiel, J., Piszczek, P., Wrzeszcz, G., Jędrzejewski, T., Golińska, P., & Radtke, A. (2023). The Composites of PCL and Tetranuclear Titanium(IV)–Oxo Complex with Acetylsalicylate Ligands—Assessment of Their Biocompatibility and Antimicrobial Activity with the Correlation to EPR Spectroscopy. Materials, 16(1), 297. https://doi.org/10.3390/ma16010297