Characterization of Zn-Mg-Sr Type Soldering Alloy and Study of Ultrasonic Soldering of SiC Ceramics and Cu-SiC Composite
Abstract
1. Introduction
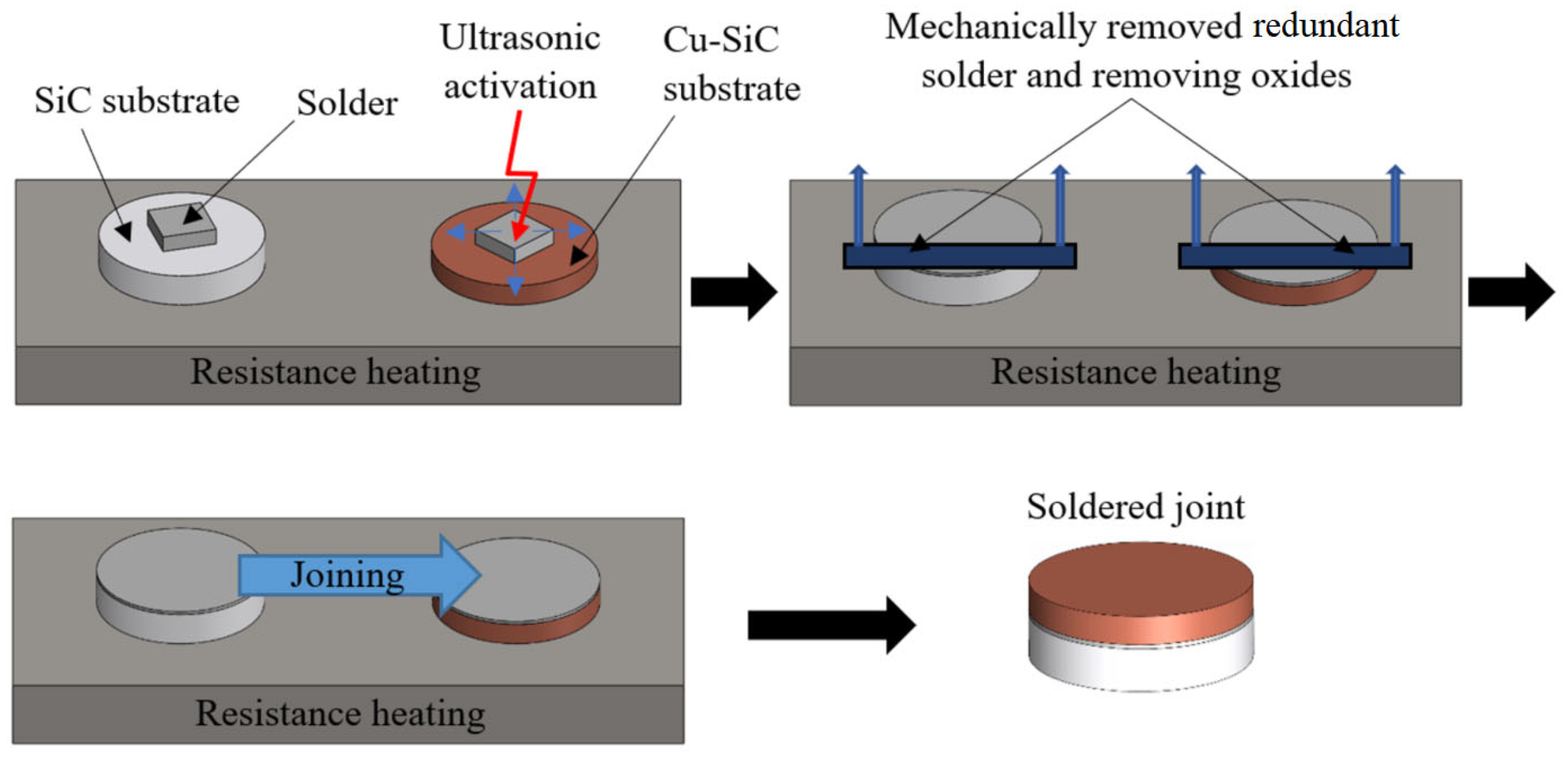
2. Materials and Methods
- ceramic SiC substrates in the form of discs Ø 15 × 3 mm,
- metal-ceramic Cu-SiC substrate with 4N purity and dimensions of Ø 15 × 3 mm and 10 × 10 × 3 mm.
3. Results
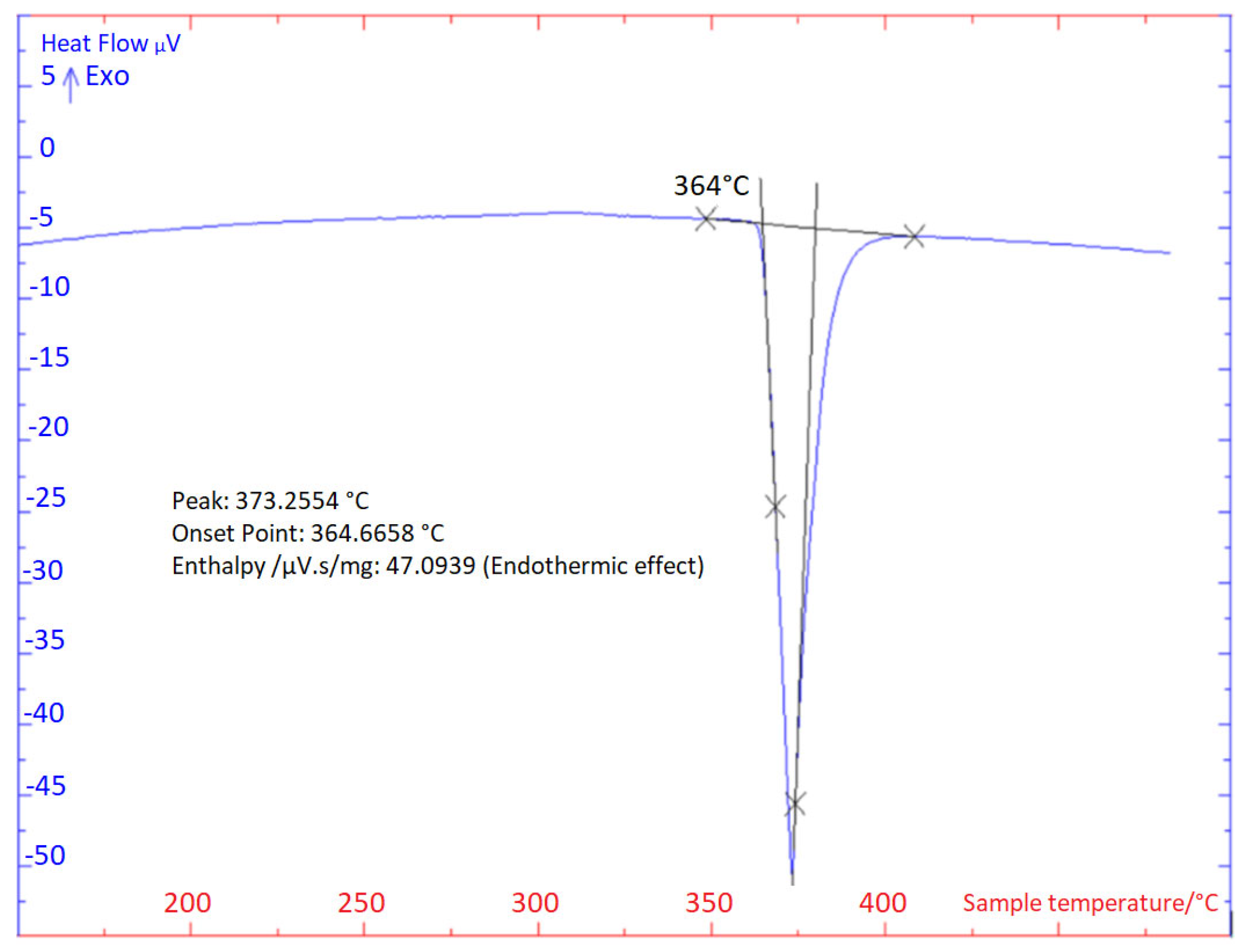
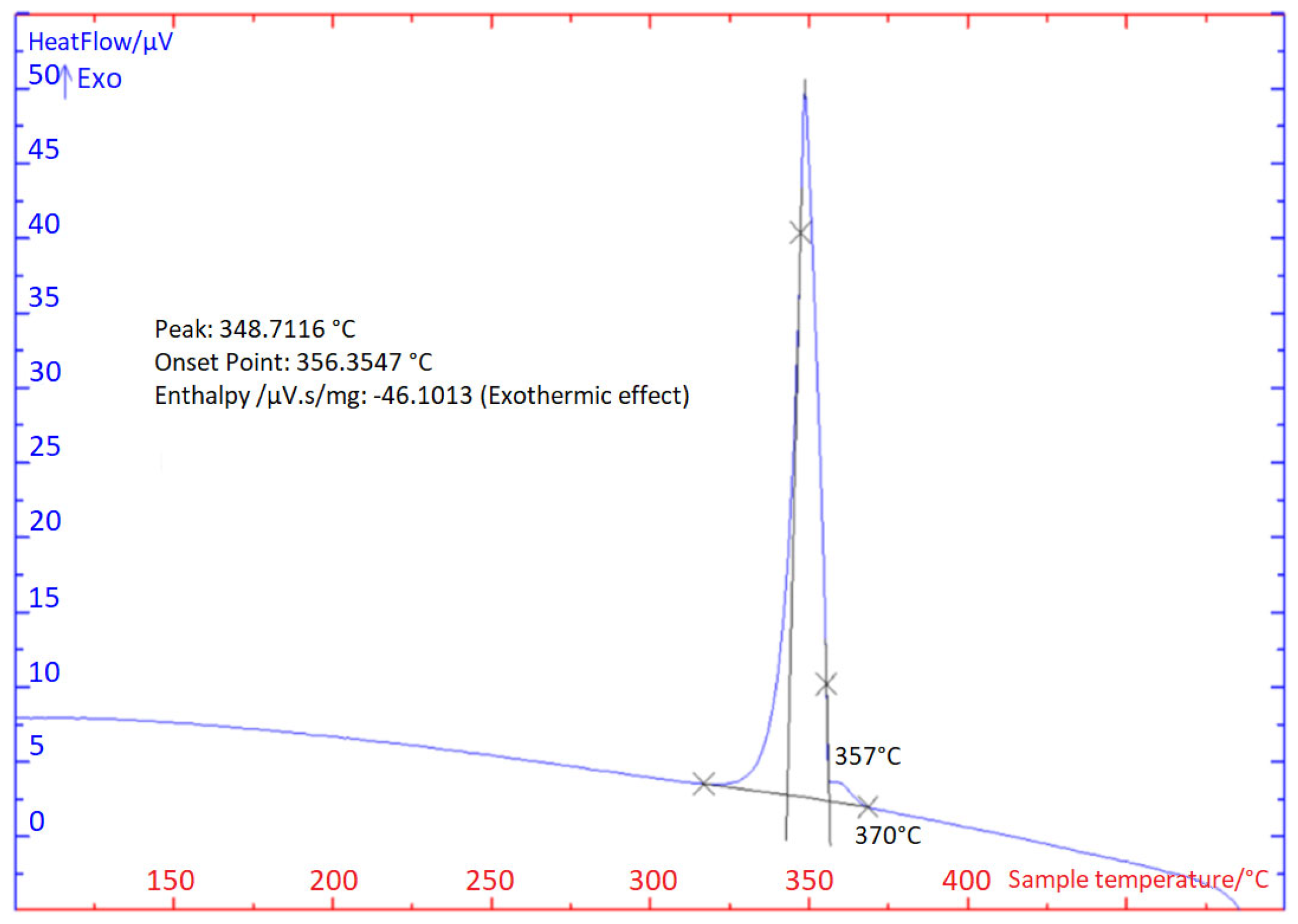
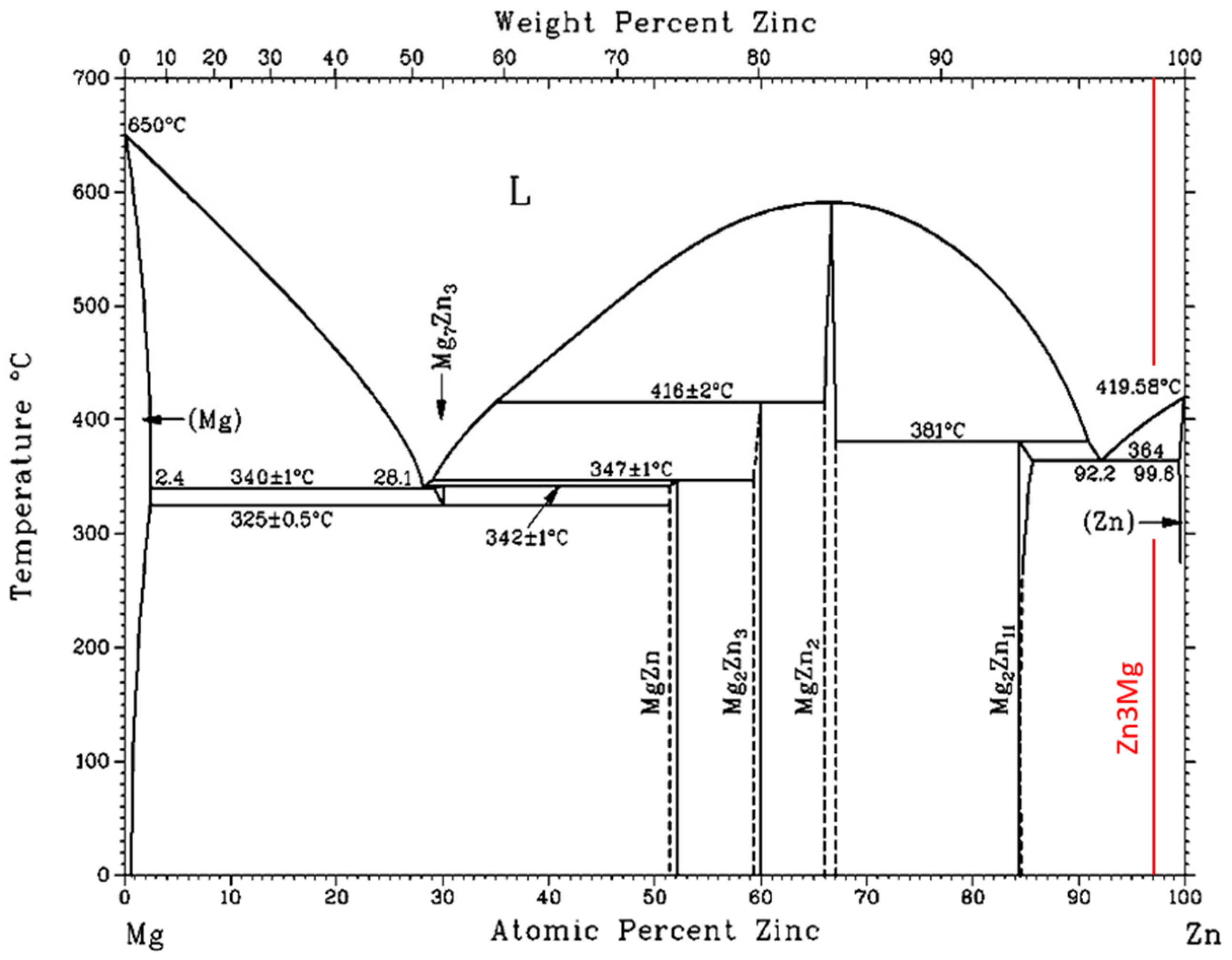
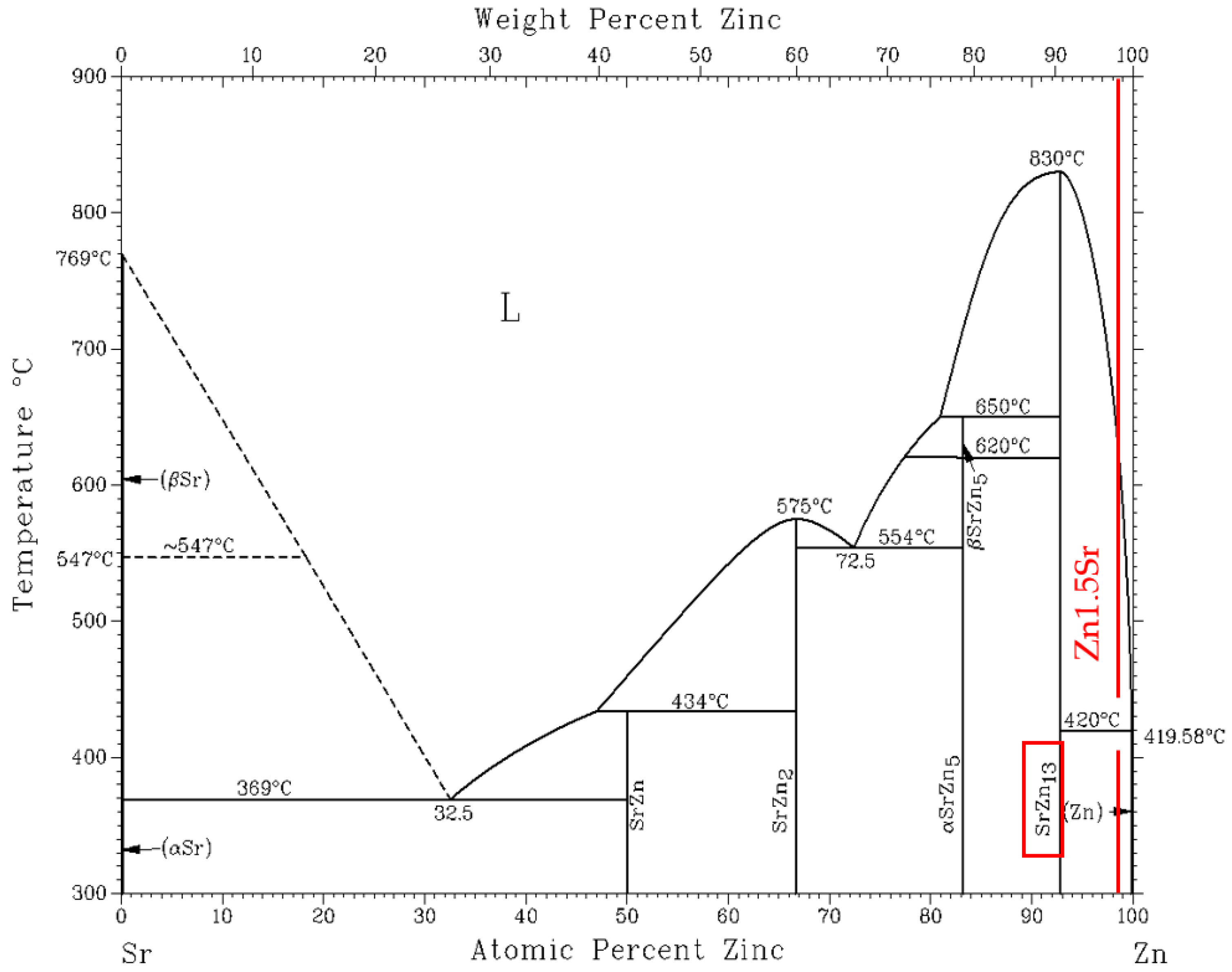
3.1. TG/DTA Analysis
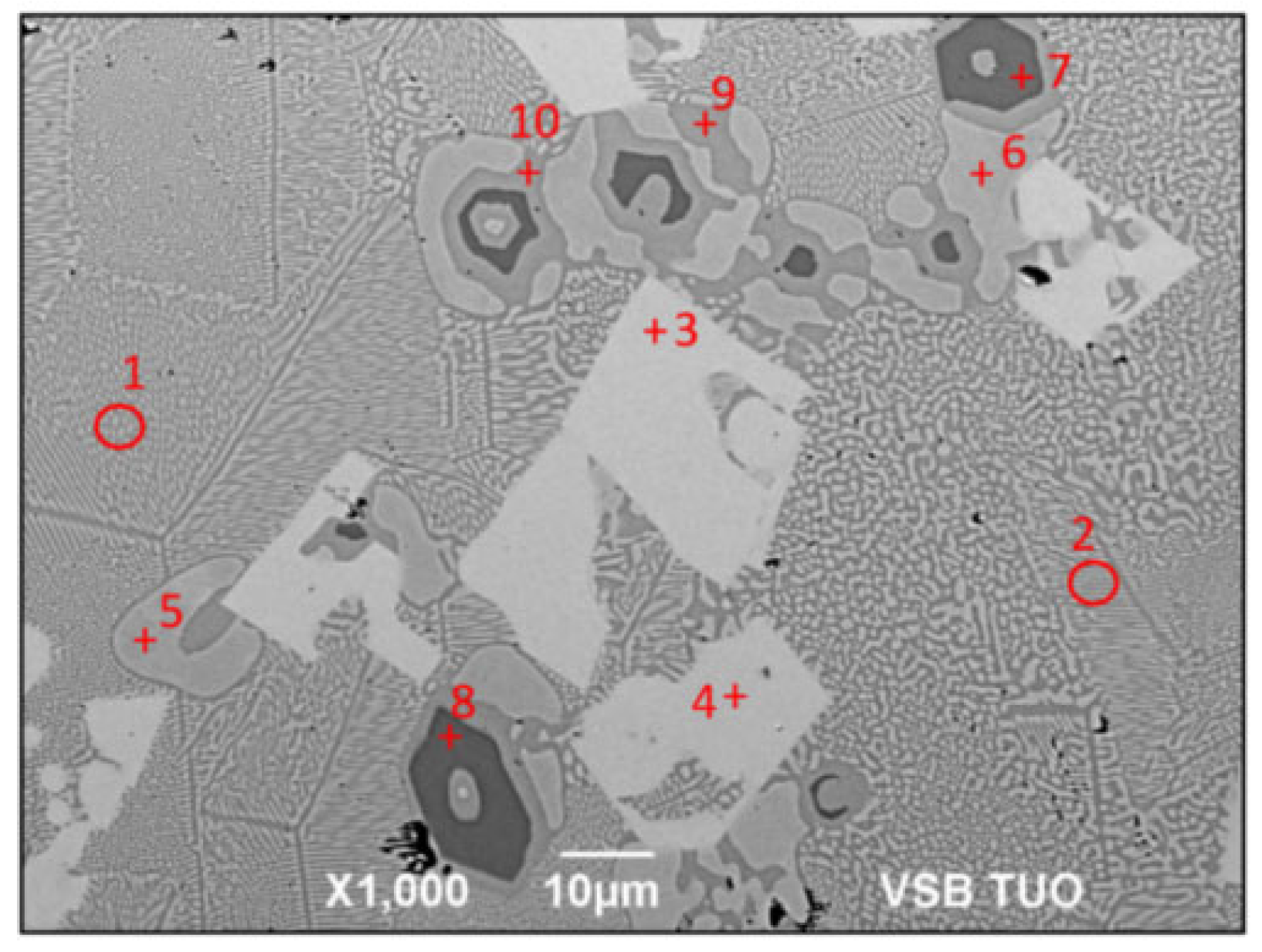
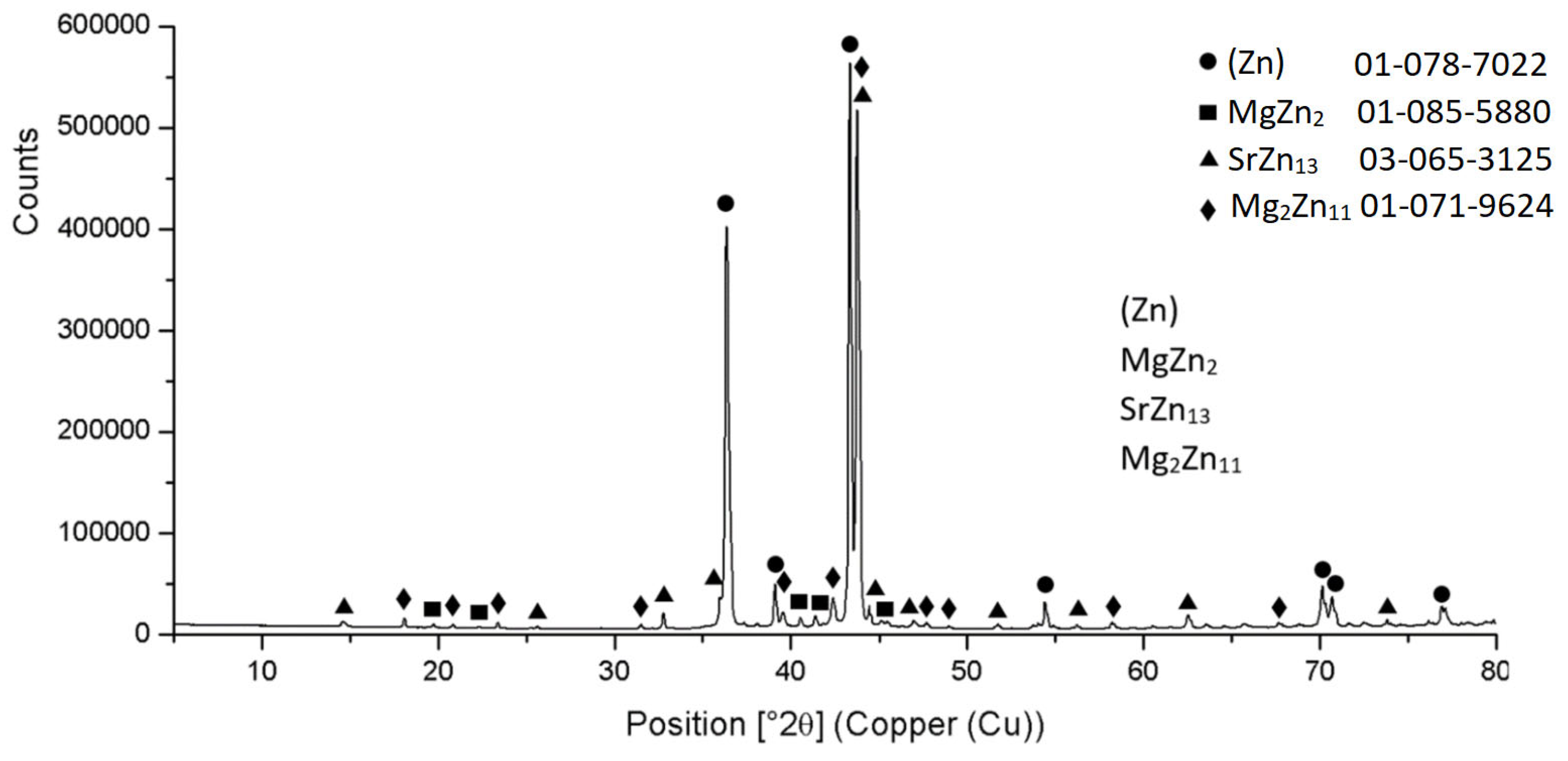
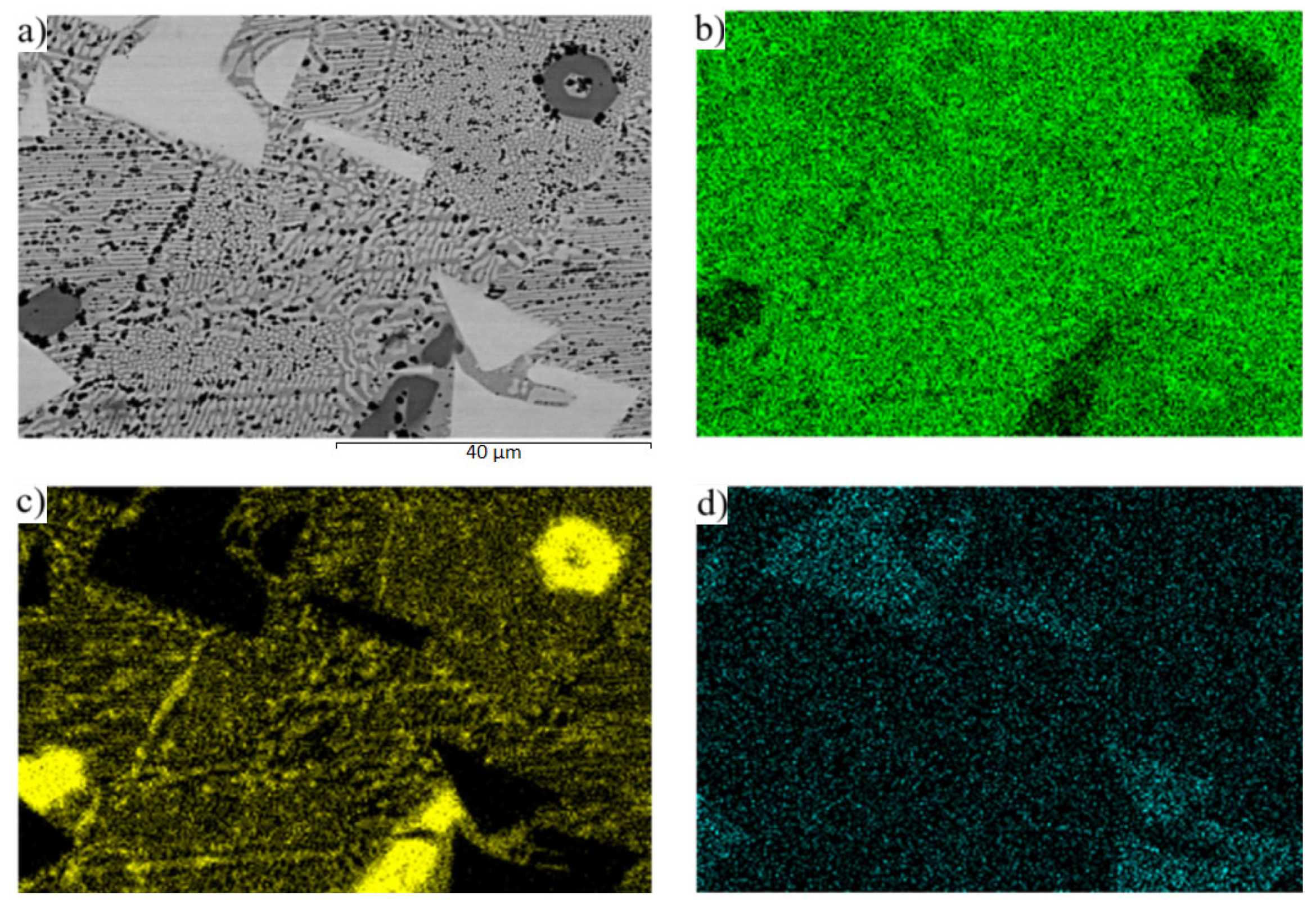
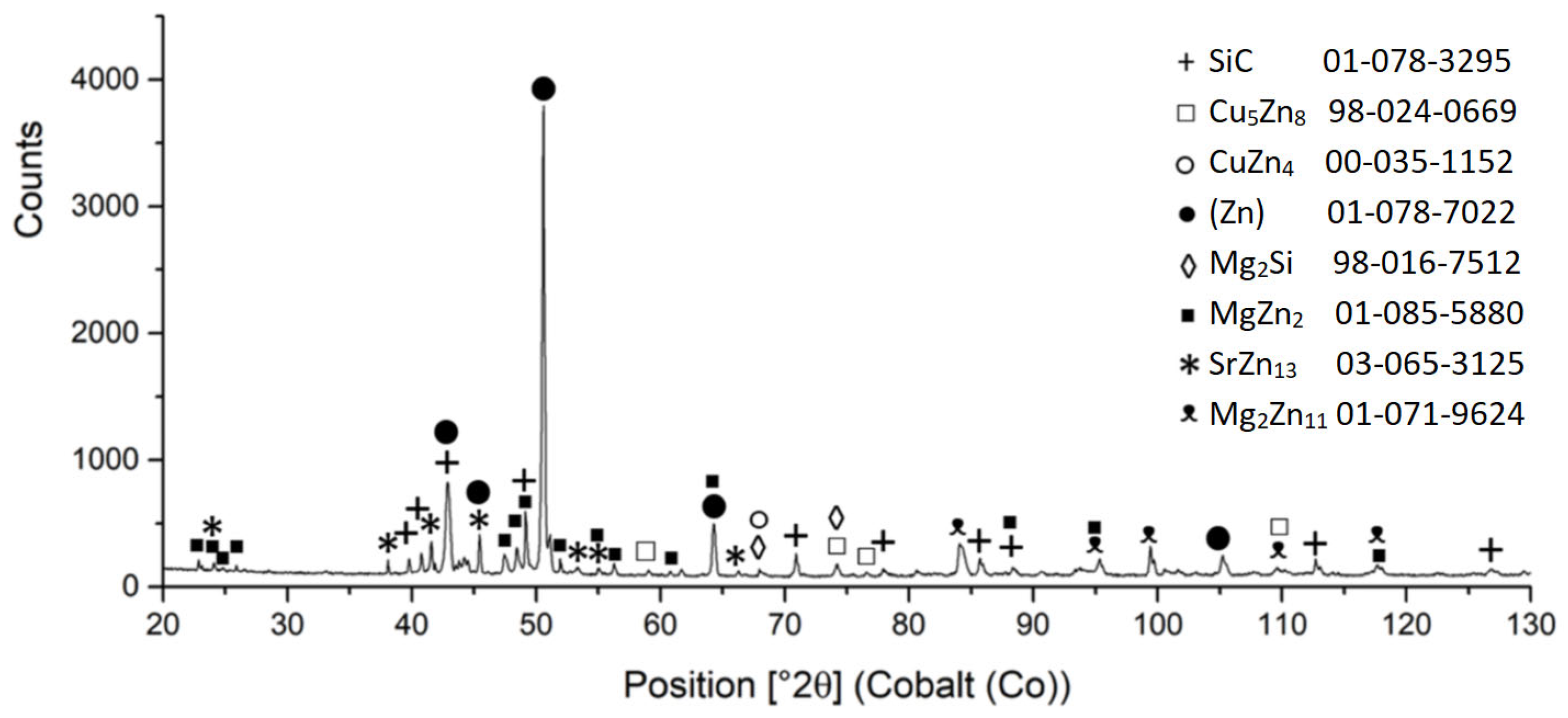
3.2. Microstructure of Zn3Mg1.5Sr Solder
3.3. Tensile Strength Test of Soldering Alloy
3.4. Microstructure of SiC/Zn3Mg1.5Sr/Cu-SiC Joint
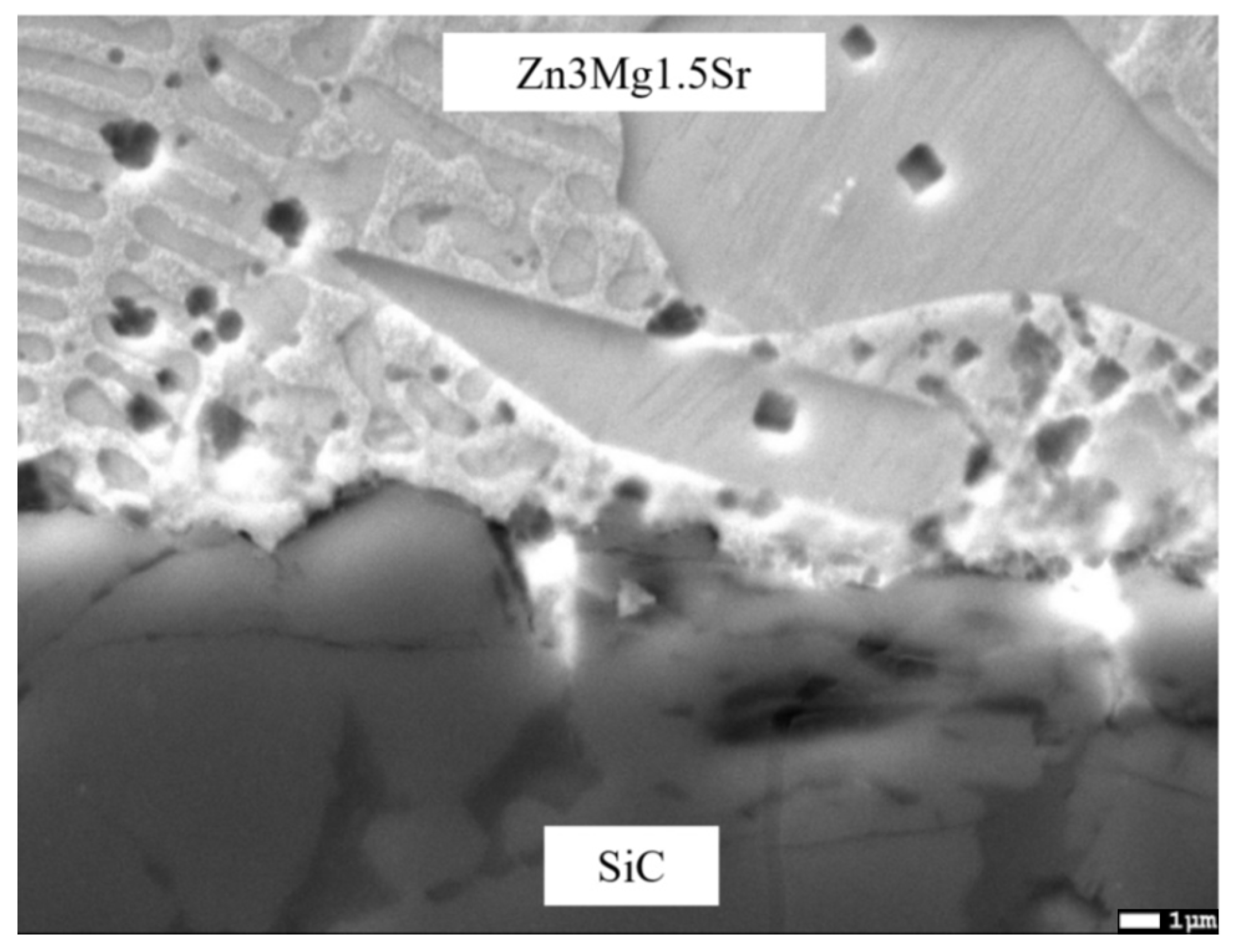
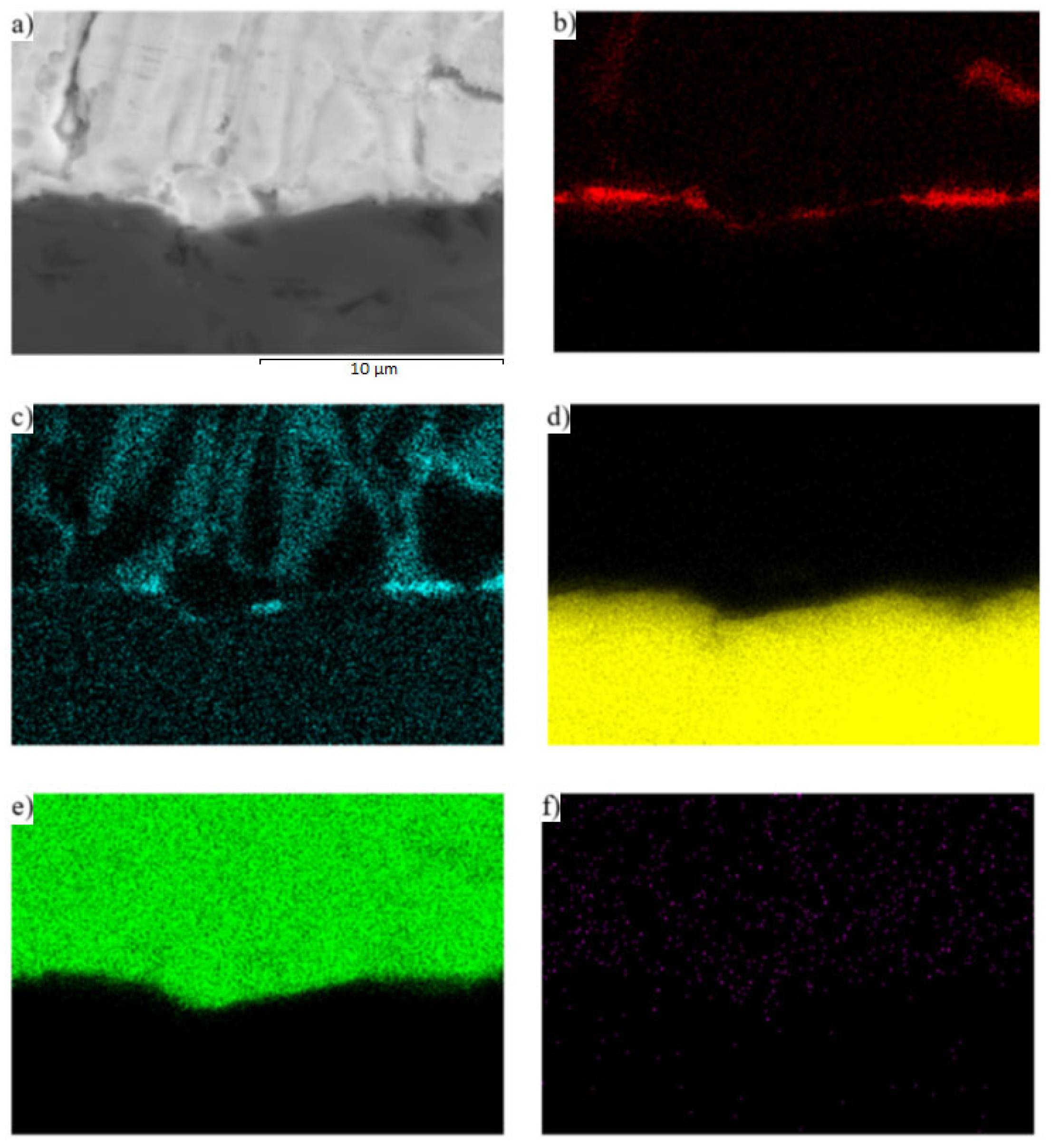
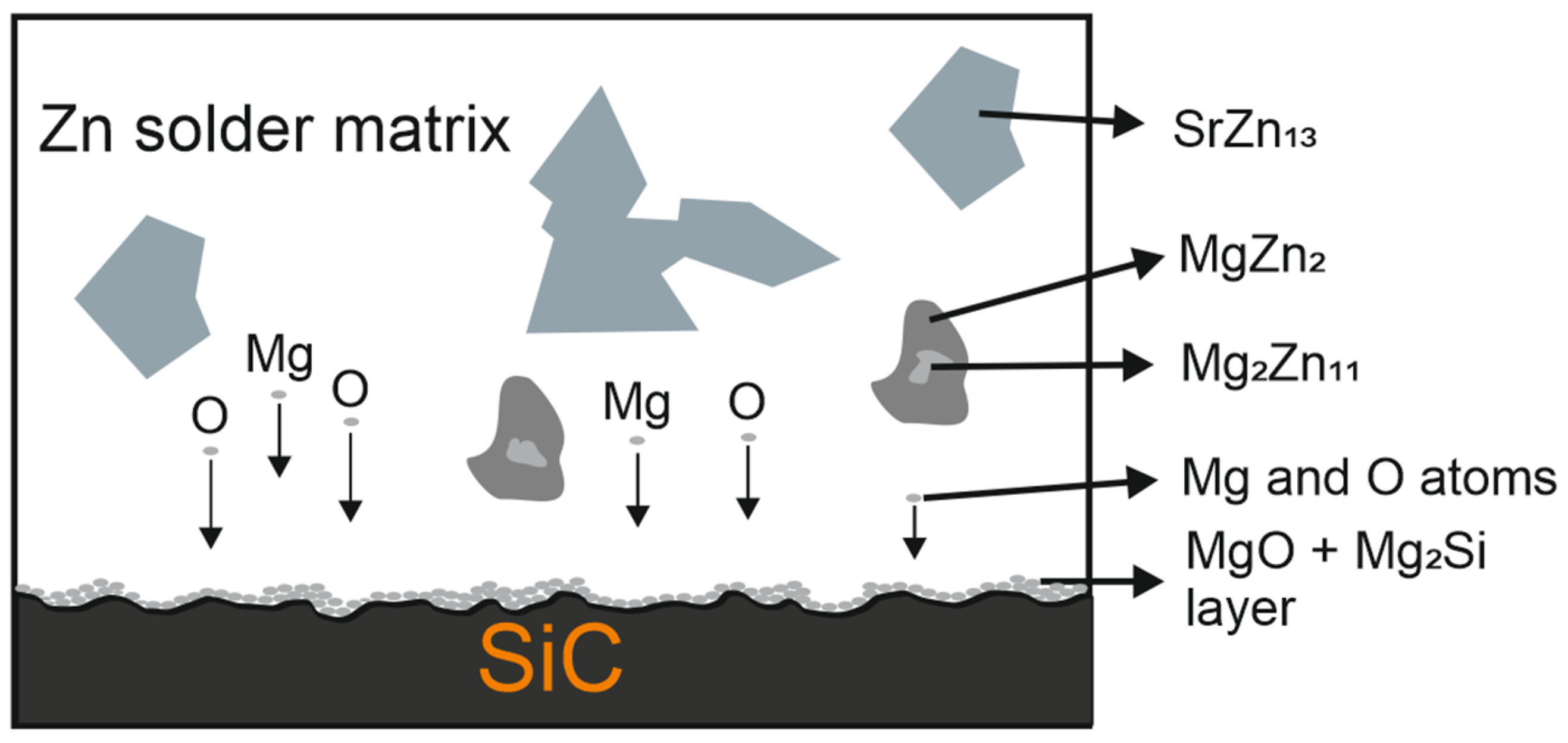
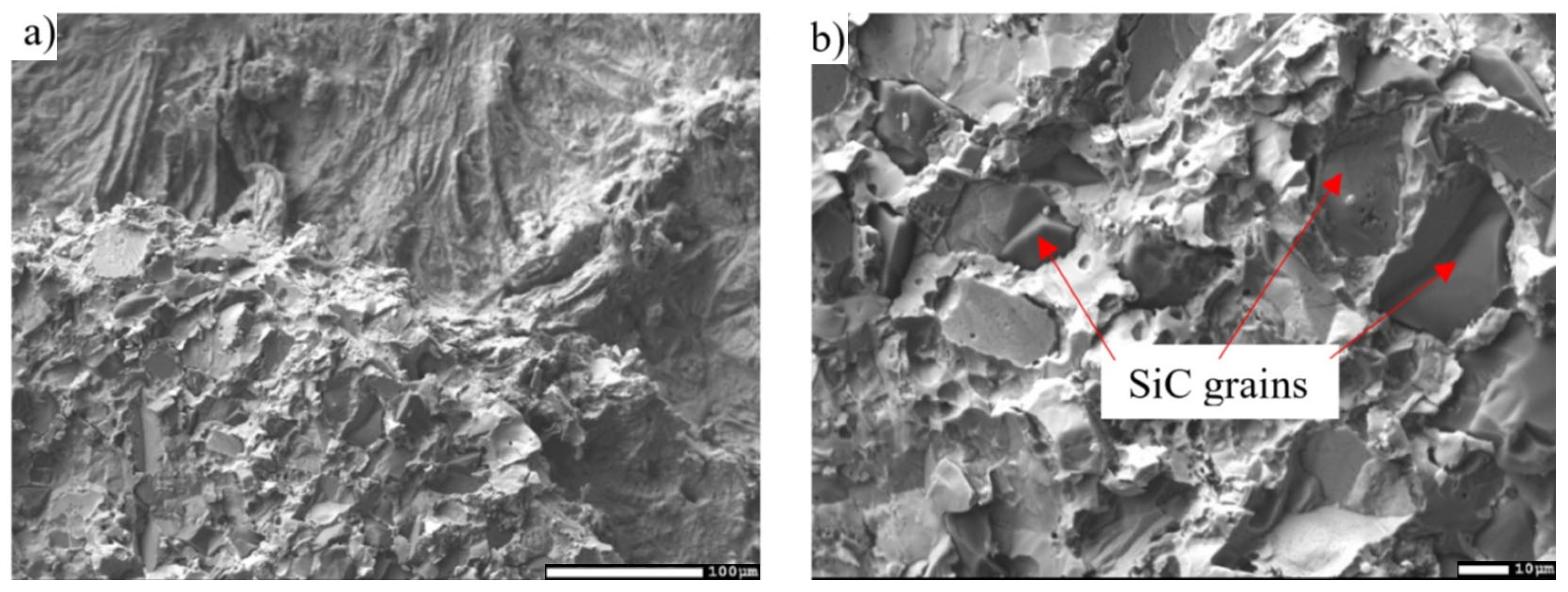
3.5. Analysis of Transition Zone of SiC/Zn3Mg1.5Sr Joint
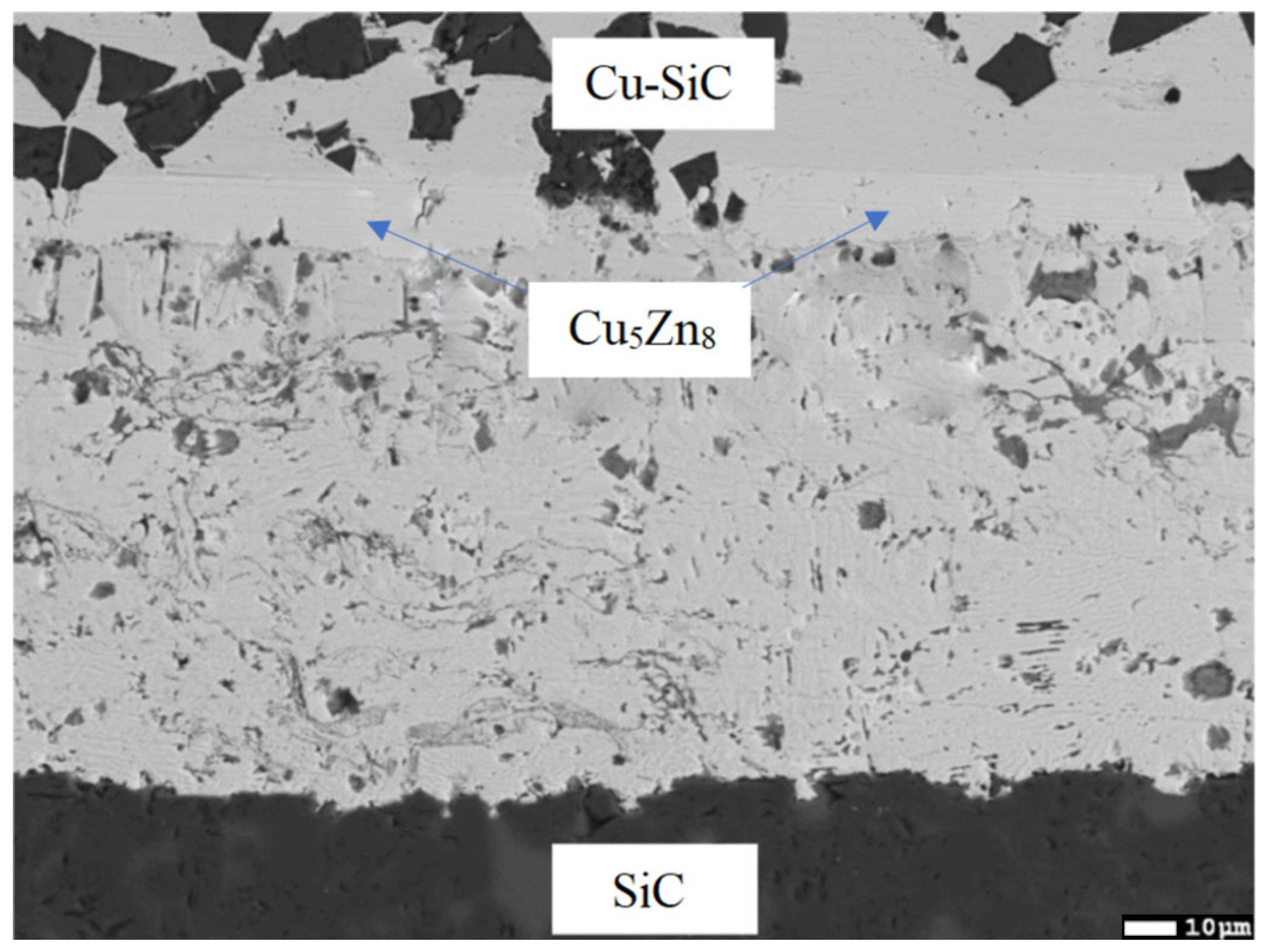
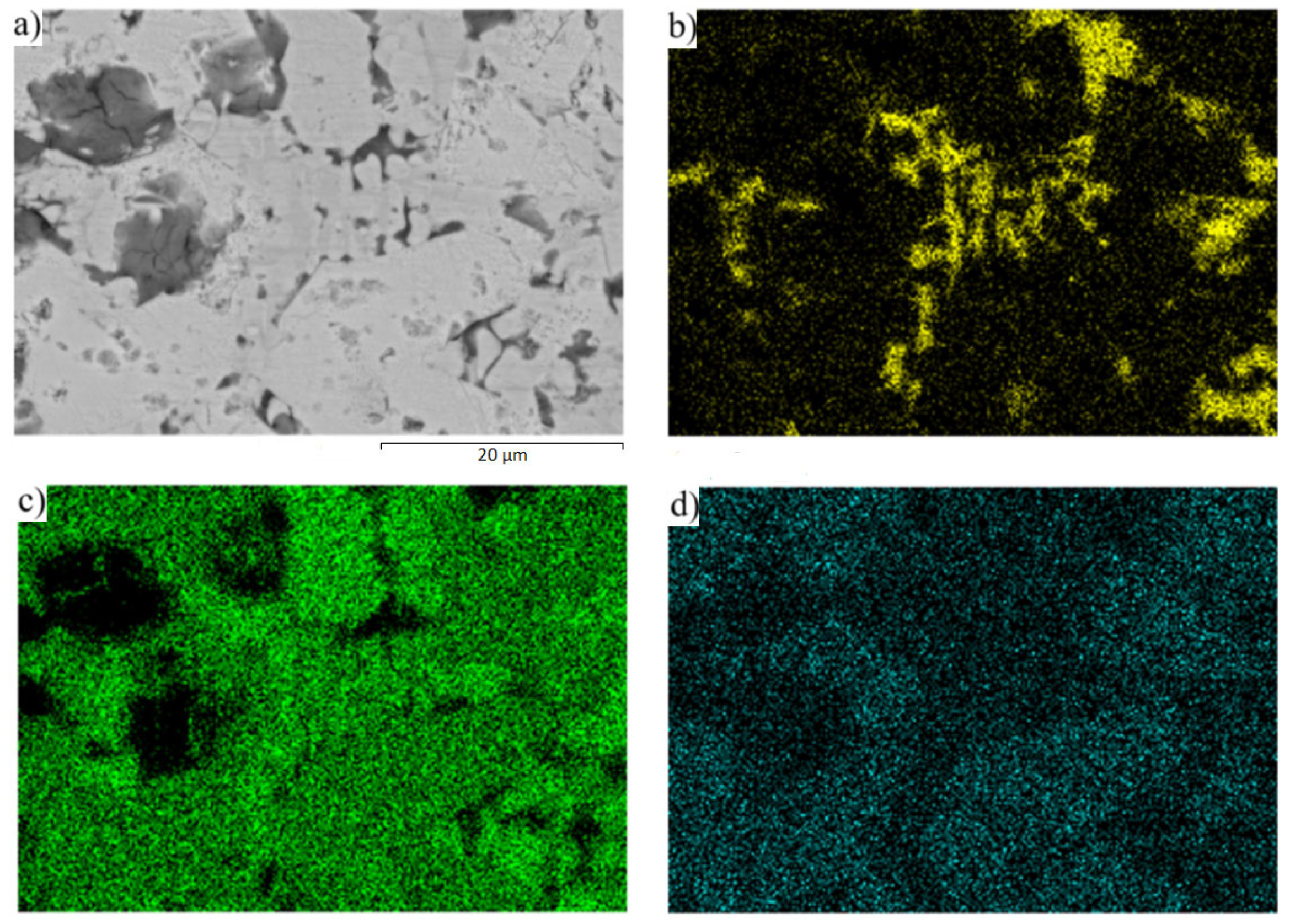
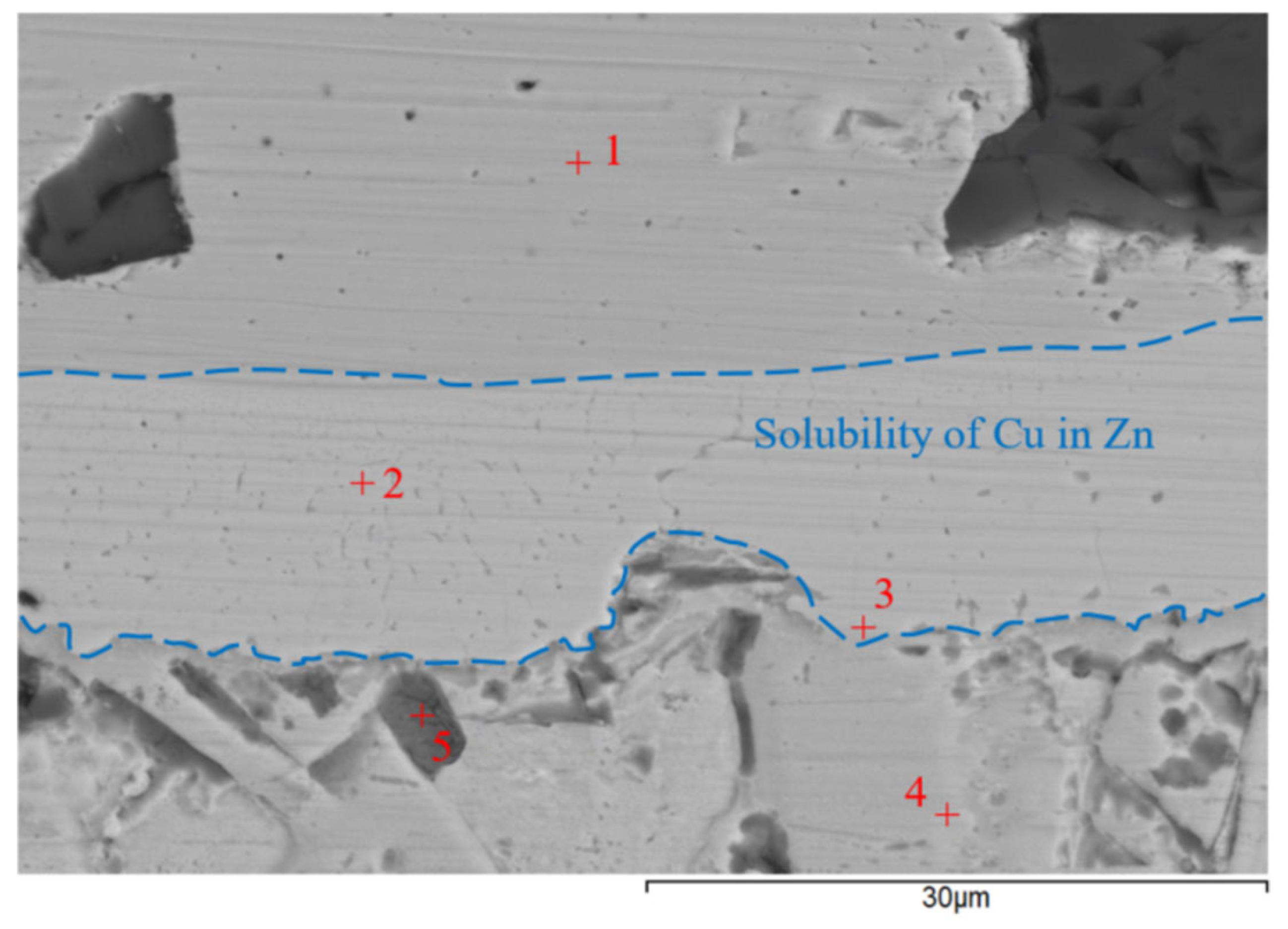
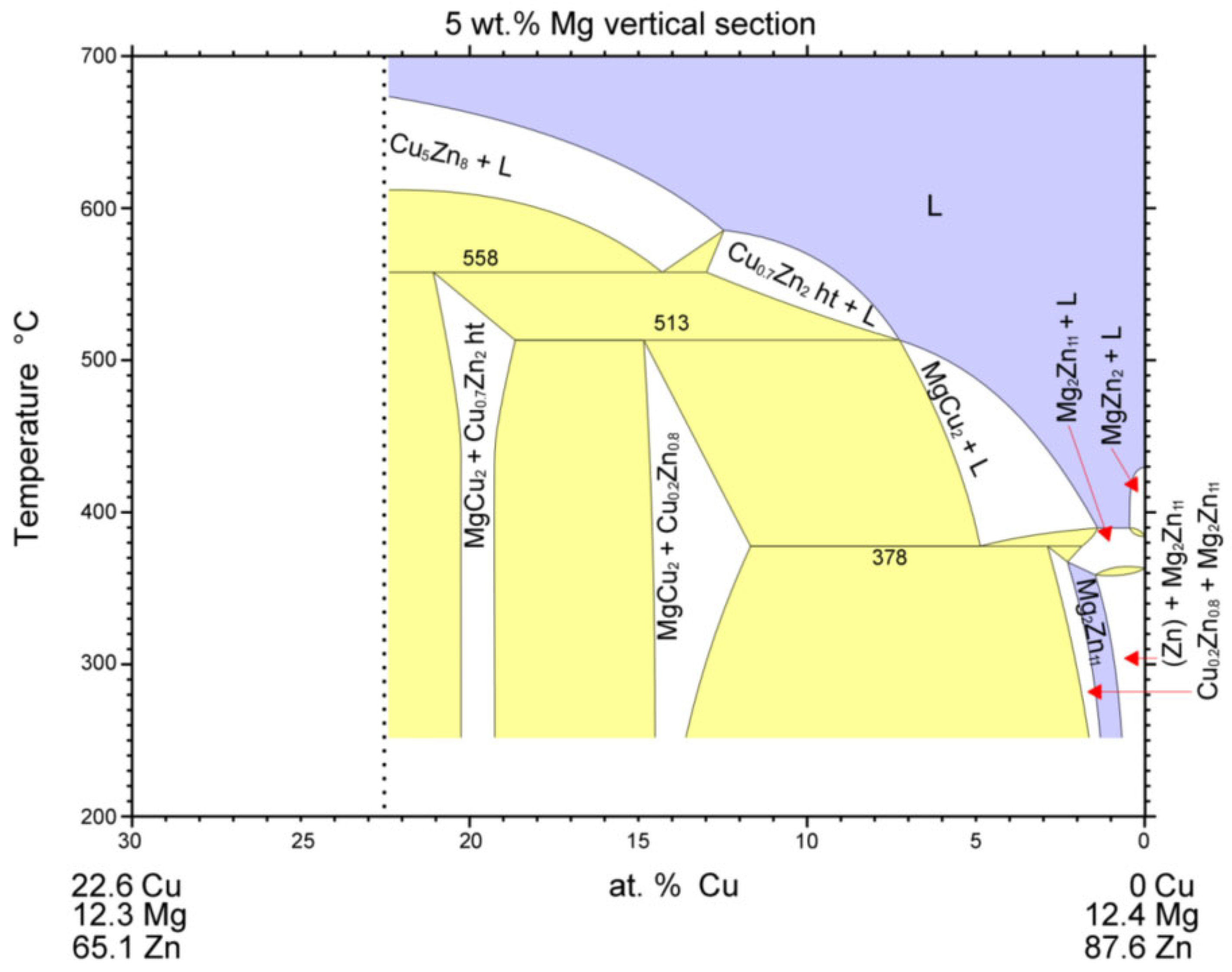
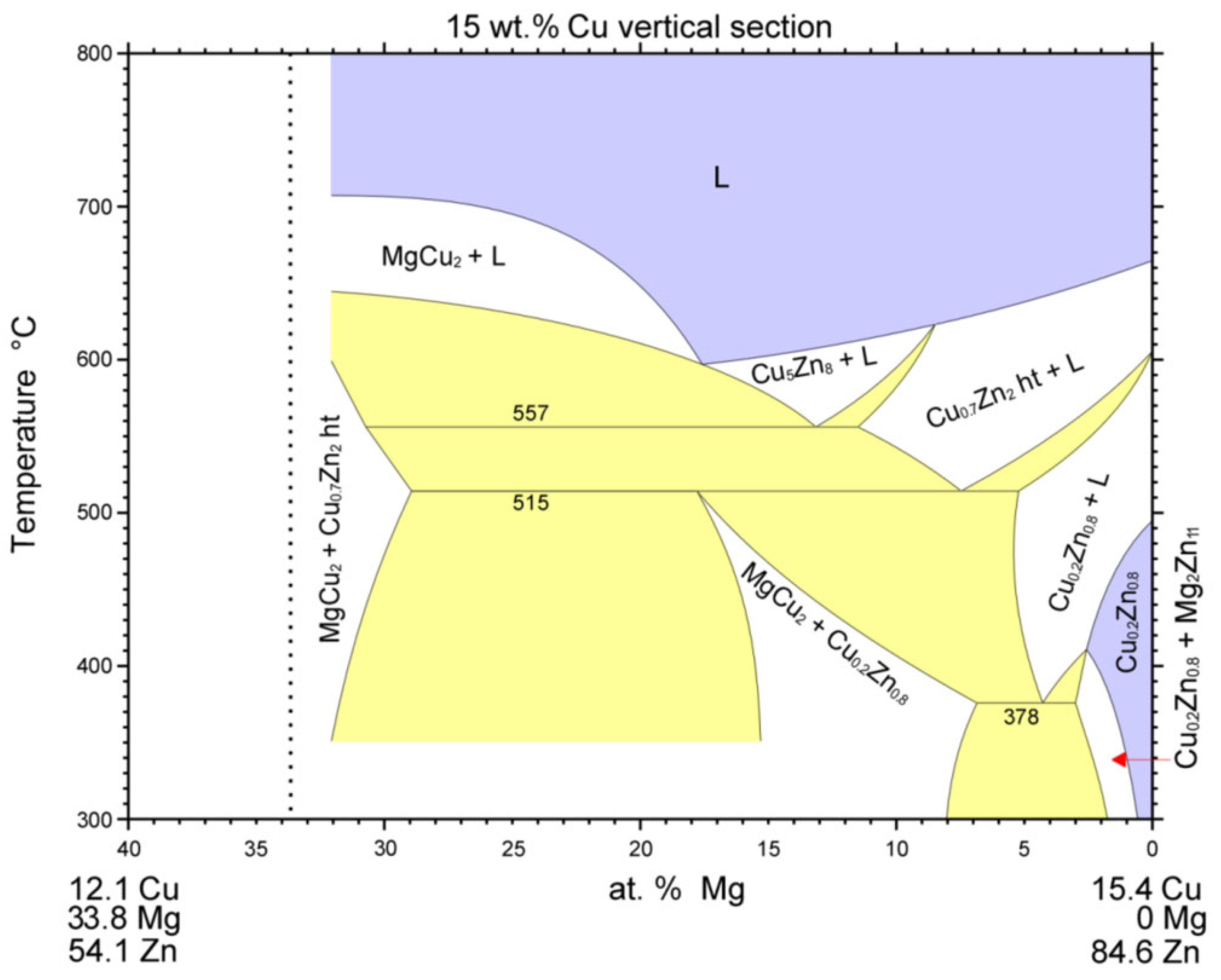
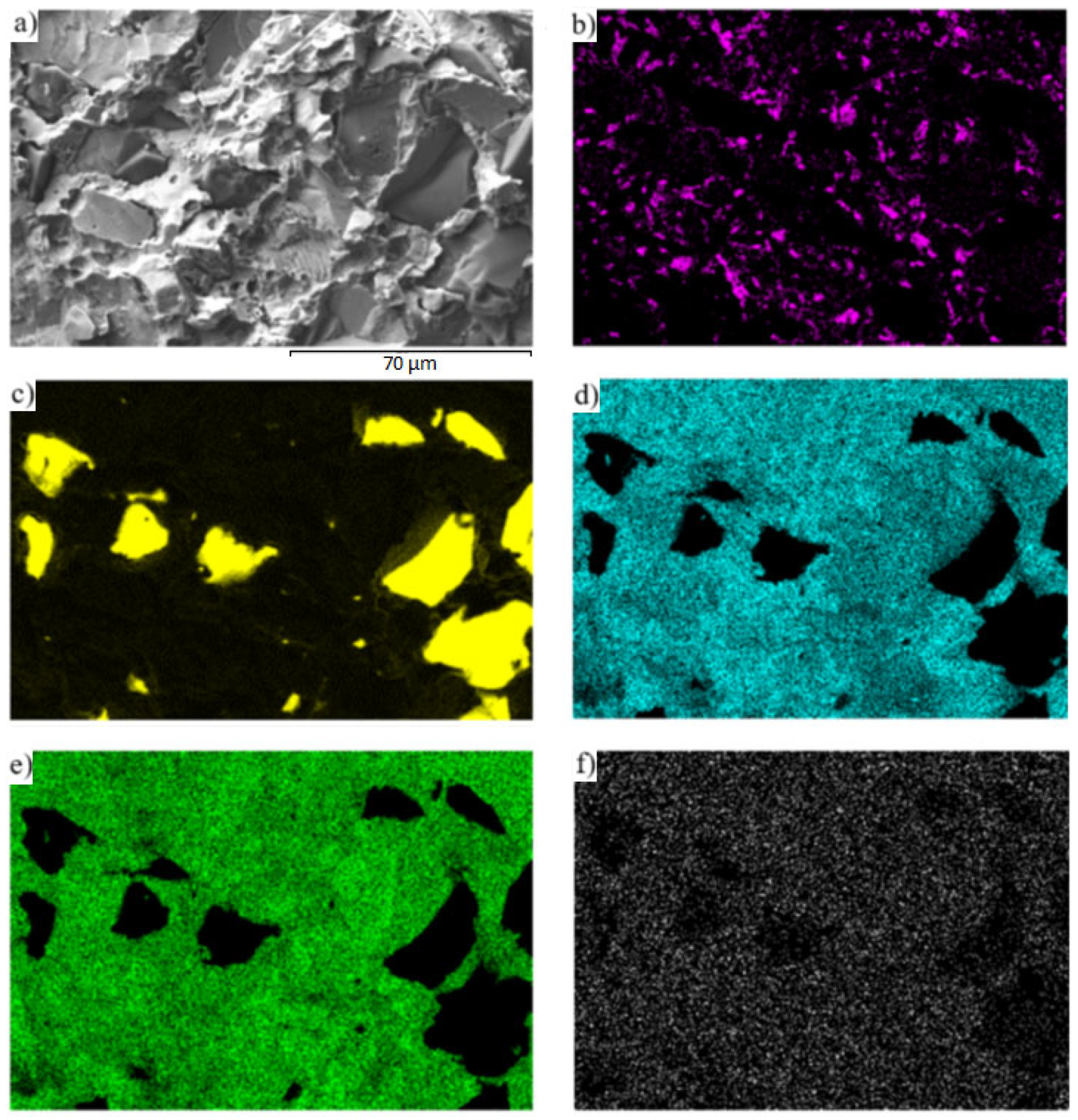
3.6. Analysis of Transition Zone of Cu-SiC/Zn3Mg1.5Sr Joint
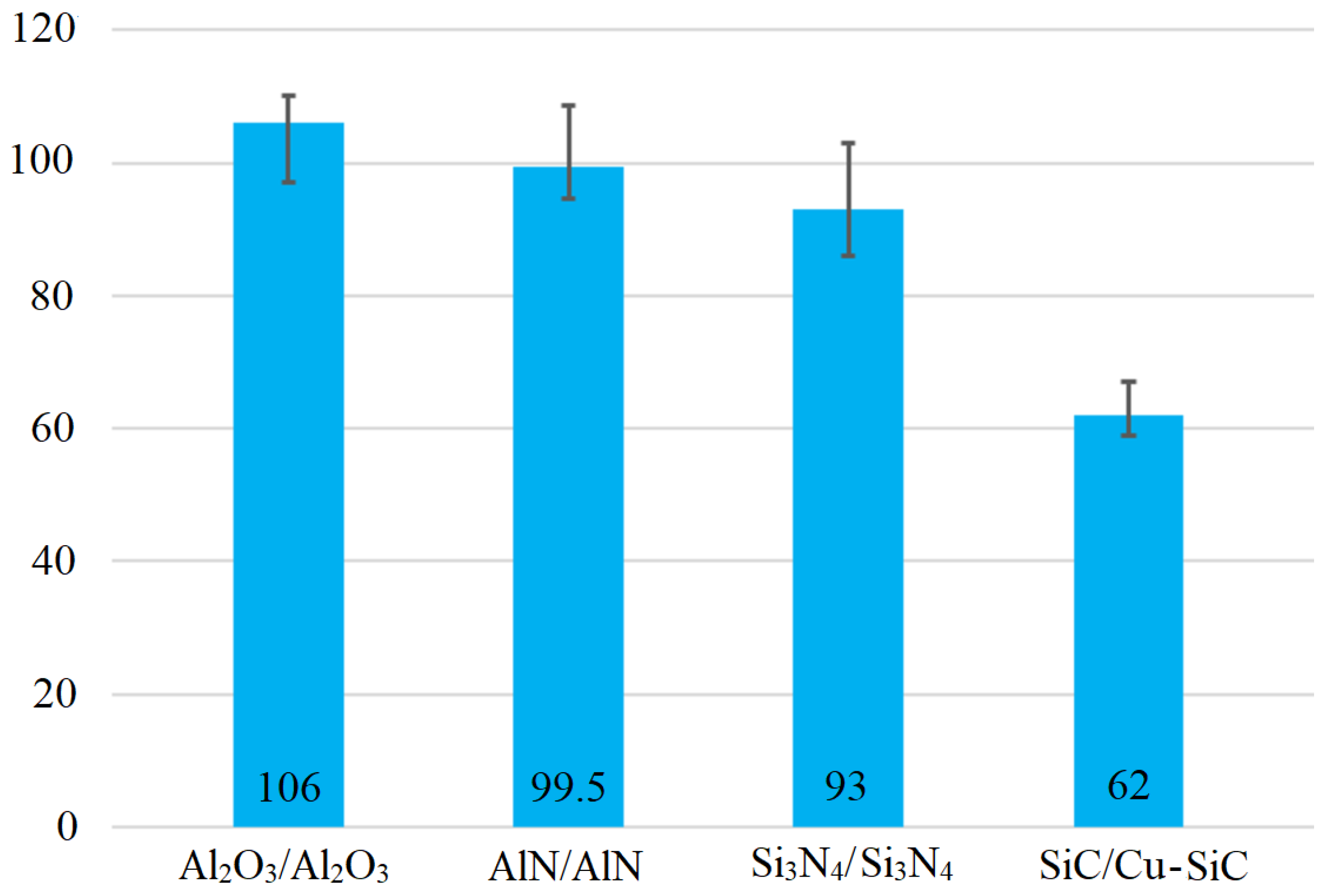
3.7. Shear Strength of Soldered Joints
4. Conclusions
- For the determination of the solder melting point, TG/DTA analysis was applied. The record may show a significant peak, which corresponds to a eutectic reaction. The Zn– Mg system is thus of a eutectic type, with a reaction temperature of 364 °C. The effect of strontium on phase transformations was minimal, owing to its lower content.
- The microstructure of the soldering alloy type Zn3Mg1.5Sr is formed of a very fine eutectic matrix, where the strontium phases (light-grey constituents) and magnesium phases (grey constituents) are segregated. The XRD analysis of the solder revealed the solid solution of zinc (Zn), intermetallic phases of magnesium—MgZn2, Mg2Zn11, and the presence of an intermetallic phase of strontium—SrZn13.
- The solder type Zn3Mg1.5Sr has an average tensile strength of 98.6 MPa. The tensile strength is partially increased by solder alloying with magnesium and strontium.
- The SiC/solder bond is formed due to the distribution of magnesium from the solder to the boundary with SiC ceramics at the formation of the Mg2Si phase. Due to the effect of soldering in air, the metals oxidize during soldering and the oxides formed are combined with the silicon oxides that occur on the surface of SiC ceramics. An interaction based on oxygen as a medium takes place, thus ensuring the wettability of SiC ceramics.
- A massive interaction on the boundary of the Cu-SiC/solder joint took place between the molten zinc solder and copper matrix of the composite substrate at the formation of a wide transition zone, formed mainly of the new phase of γCu (Cu5Zn8). The bond formation is thus affected mainly by the Zn from the solder.
- The shear strength was measured using several ceramic materials. The average shear strength of the combined joint of SiC/Cu-SiC fabricated by using Zn3Mg1.5Sr solder was 62 MPa. When soldering similar ceramic materials mutually, a shear strength of as much as around 100 MPa was observed. The limit value of shear strength for the application of power electronic modules is 40 MPa. The application of Zn3Mg1.5Sr solder greatly exceeded this value and it is therefore an excellent candidate for such applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, K.N.; Gusak, A.M.; Li, M. Physics and materials challenges for lead-free solders. J. Appl. Phys. 2003, 93, 1335–1353. [Google Scholar] [CrossRef]
- Menon, S.; George, E.; Osterman, M.; Pecht, M. High lead solder (over 85%) solder in the electronics industry: RoHS exemptions and alternatives. J. Mater. Sci. Mater. Electron. 2015, 26, 4021–4030. [Google Scholar] [CrossRef]
- European Commission. Recast of the WEEE and RoHS Directives Proposed; European Commission: Brussels, Belgium, 13 August 2012. [Google Scholar]
- Rashid, M.H. Power Electronics Handbook, 4th ed.; Butterworth-Heinemann: Portsmouth, NH, USA, 2018; ISBN 978-0-12-8111407-0. [Google Scholar]
- Chidambaram, V.; Hald, J.; Hattel, J. Development of Au–Ge based candidate alloys as an alternative to high-lead content solders. J. Alloys Compd. 2010, 490, 170–179. [Google Scholar] [CrossRef]
- Chidambaram, V.; Hattel, J.; Hald, J. Design of lead-free candidate alloys for high-temperature soldering based on the Au–Sn system. Mater. Des. 2010, 31, 4638–4645. [Google Scholar] [CrossRef]
- Shi, Y.; Fang, W.; Xia, Z.; Lei, Y.; Guo, F.; Li, X. Investigation of rare earth-doped BiAg high-temperature solders. J. Mater. Sci. Mater. Electron. 2010, 21, 875–881. [Google Scholar] [CrossRef]
- Song, J.M.; Chuang, H.Y.; Wen, T.X. Thermal and tensile properties of Bi–Ag alloys. Metall. Mater. Trans. A 2007, 38, 1371–1375. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Li, X.; Mei, Y.H.; Lu, G.Q. Study on high temperature bonding reliability of sintered nano-silver joint on bare copper plate. Microelectron. Reliab. 2015, 55, 2524–2531. [Google Scholar] [CrossRef]
- Sharif, A.; Gan, C.L.; Chen, Z. Transient liquid phase Ag-based solder technology for high-temperature packaging applications. J. Alloys Compd. 2014, 587, 365–368. [Google Scholar] [CrossRef]
- Kang, N.; Na, H.S.; Kim, S.J.; Kang, C.Y. Alloy design of Zn–Al–Cu solder for ultra high temperatures. J. Alloys Compd. 2009, 467, 246–250. [Google Scholar] [CrossRef]
- Rettenmayr, M.; Lambracht, P.; Kempf, B.; Tschudin, C. Zn–Al based alloys as Pb-free solders for die attach. J. Electron. Mater. 2002, 31, 278–285. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, Z.G.; Liu, Y.; Jiang, H.F. Effect of zirconium on the microstructure and mechanical properties of Zn–4% Al hypoeutectic alloy. J. Alloys Compd. 2014, 592, 127–134. [Google Scholar] [CrossRef]
- Panaghie, C.; Cimpoeșu, R.; Zegan, G.; Roman, A.-M.; Ivanescu, M.C.; Aelenei, A.A.; Benchea, M.; Cimpoeșu, N.; Ioanid, N. In Vitro Corrosion Behavior of Zn3Mg0.7Y Biodegradable Alloy in Simulated Body Fluid (SBF). Appl. Sci. 2022, 12, 2727. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Antoniac, I.-V.; Lupescu, Ș.-C. Current Research Studies of Mg–Ca–Zn Biodegradable Alloys Used as Orthopedic Implants—Review. Crystals 2022, 12, 1468. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolný, I.; Sahul, M. Direct bonding of silicon with solders type Sn-Ag-Ti. Solder. Surf. Mt. Technol. 2016, 28, 149–158. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolný, I.; Drápala, J.; Sahul, M.; Urminský, J. Characterizing the Soldering Alloy Type In–Ag–Ti and the Study of Direct Soldering of SiC Ceramics and Copper. Metals 2018, 8, 274. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolný, I.; Drápala, J.; Babincová, P.; Zacková, P.; Gogola, P. Characterization of Soldering Alloy Type Bi-Ag-Ti and the Study of Ultrasonic Soldering of Silicon and Copper. Metals 2021, 11, 624. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.Y.; Wang, L.; Huang, S.Y.; Du, X.M.; Liu, Z.Q. Interfacial reaction behavior and mechanical properties of ultrasonically brazed Cu/Zn–Al/Cu joints. Mater. Des. 2015, 73, 42–49. [Google Scholar] [CrossRef]
- Xiao, Y.; Ji, H.J.; Li, M.Y.; Kim, J. Ultrasound-assisted brazing of Cu/Al dissimilar metals using a Zn–3Al filler metal. Mater. Des. 2013, 52, 740–747. [Google Scholar] [CrossRef]
- Guo, W.B.; Luan, T.M.; He, J.S.; Yan, J.C. Ultrasonic-assisted soldering of fine-grained 7034 aluminum alloys using ZnAl filler metals. Mater. Des. 2017, 125, 85–93. [Google Scholar] [CrossRef]
- Chen, X.; Yan, J.; Ren, S.; Wang, Q.; Wei, J.; Fan, G. Microstructure, mechanical properties, and bonding mechanism of ultrasonic-assisted brazed joints of SiC ceramics with ZnAlMg filler metals in air. Ceram. Int. 2014, 40, 683–689. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Peng, L.; Yan, J. Ultrafast ultrasonic-assisted transient liquid bonding Al/Mg in air. Mat. Char. 2022, 190, 111987. [Google Scholar] [CrossRef]
- Kolenak, R.; Kostolny, I.; Drapala, J.; Drienovsky, M.; Sahul, M. Research on joining metal-ceramics composite Al/Al2O3 with Cu substrate using solder type Zn–In–Mg. J. Compos. Mater. 2019, 53, 1411–1422. [Google Scholar] [CrossRef]
- Kolenak, R.; Melus, T.; Drapala, J.; Gogola, P.; Pasak, M. Study of Bond Formation in Ceramic and Composite Materials Ultrasonically Soldered with Bi-Ag-Mg-Type Solder. Materials 2023, 16, 2991. [Google Scholar] [CrossRef] [PubMed]
- Massalski, T.B. Binary Alloy Phase Diagrams; ASM: Metals Park, OH, USA, 1996. [Google Scholar]
- Liang, P.; Seifert, H.J.; Lukas, H.L.; Ghosh, G.; Effenberg, G.; Aldinger, F. Thermodynamic modelling of the Cu-Mg-Zn ternary system. Calphad 1998, 22, 527–544. [Google Scholar] [CrossRef]








| Specimen | Charge [wt. %] | ICP-AES [wt. %] | ||||
|---|---|---|---|---|---|---|
| Zn | Mg | Sr | Zn | Mg | Sr | |
| Zn3Mg1.5Sr | 95.5 | 3 | 1.5 | 96.04 | 2.84 | 1.12 |
| Ultrasound power | 400 | [W] |
| Working frequency | 40 | [kHz] |
| Amplitude | 2 | [μm] |
| Soldering temperature | 150 | [°C] |
| Time of ultrasound activation | 5 | [s] |
| DTA Analysis | TL (°C) | TE (°C) | |
|---|---|---|---|
| Heating | 1st | 368 | 365 |
| 2nd | 368 | 364 | |
| Cooling | 1st | 370 | 356 |
| 2nd | 370 | 357 | |
| Spectrum | Mg [at.%] | Zn [at.%] | Sr [at.%] | Solder Component |
|---|---|---|---|---|
| Spectrum 1 | 8.41 | 91.59 | - | Eutectic (Zn) + Mg2Zn11 |
| Spectrum 2 | 9.13 | 90.87 | - | Eutectic (Zn) + Mg2Zn11 |
| Spectrum 3 | - | 92.81 | 7.19 | SrZn13 phase |
| Spectrum 4 | - | 92.81 | 7.19 | SrZn13 phase |
| Spectrum 5 | - | 100.00 | - | Zn matrix |
| Spectrum 6 | - | 100.00 | - | Zn matrix |
| Spectrum 7 | 34.05 | 65.95 | - | MgZn2 phase |
| Spectrum 8 | 33.71 | 66.29 | - | MgZn2 phase |
| Spectrum 9 | 16.61 | 83.39 | - | Mg2Zn11 phase |
| Spectrum 10 | 16.19 | 83.81 | - | Mg2Zn11 phase |
| Spectrum | Zn [at.%] | Mg [at.%] | Si [at.%] | O [at.%] |
|---|---|---|---|---|
| Spectrum 1 | 97.62 | 2.38 | 0 | 0 |
| Spectrum 2 | 91.78 | 8.22 | 0 | 0 |
| Spectrum 3 | 51.75 | 1.00 | 41.28 | 5.97 |
| Spectrum 4 | 21.83 | 11.16 | 21.36 | 45.65 |
| Spectrum 5 | 42.42 | 15.33 | 21.13 | 21.12 |
| Spectrum | Zn [at.%] | Mg [at.%] | Si [at.%] | O [at.%] | Cu [at.%] |
|---|---|---|---|---|---|
| Spectrum 1 | 0 | 0 | 1.68 | 0 | 98.32 |
| Spectrum 2 | 65.61 | 0 | 1.06 | 0 | 33.33 |
| Spectrum 3 | 73.45 | 14.98 | 0 | 0 | 11.57 |
| Spectrum 4 | 78.35 | 18.73 | 0 | 0 | 2.92 |
| Spectrum 5 | 45.60 | 0.95 | 0 | 52.09 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolenak, R.; Pluhar, A.; Drapala, J.; Babincova, P.; Pasak, M. Characterization of Zn-Mg-Sr Type Soldering Alloy and Study of Ultrasonic Soldering of SiC Ceramics and Cu-SiC Composite. Materials 2023, 16, 3795. https://doi.org/10.3390/ma16103795
Kolenak R, Pluhar A, Drapala J, Babincova P, Pasak M. Characterization of Zn-Mg-Sr Type Soldering Alloy and Study of Ultrasonic Soldering of SiC Ceramics and Cu-SiC Composite. Materials. 2023; 16(10):3795. https://doi.org/10.3390/ma16103795
Chicago/Turabian StyleKolenak, Roman, Alexej Pluhar, Jaromir Drapala, Paulina Babincova, and Matej Pasak. 2023. "Characterization of Zn-Mg-Sr Type Soldering Alloy and Study of Ultrasonic Soldering of SiC Ceramics and Cu-SiC Composite" Materials 16, no. 10: 3795. https://doi.org/10.3390/ma16103795
APA StyleKolenak, R., Pluhar, A., Drapala, J., Babincova, P., & Pasak, M. (2023). Characterization of Zn-Mg-Sr Type Soldering Alloy and Study of Ultrasonic Soldering of SiC Ceramics and Cu-SiC Composite. Materials, 16(10), 3795. https://doi.org/10.3390/ma16103795







