Finite Element Modelling of a Synthetic Paediatric Spine for Biomechanical Investigation
Abstract
:1. Introduction
2. Methods
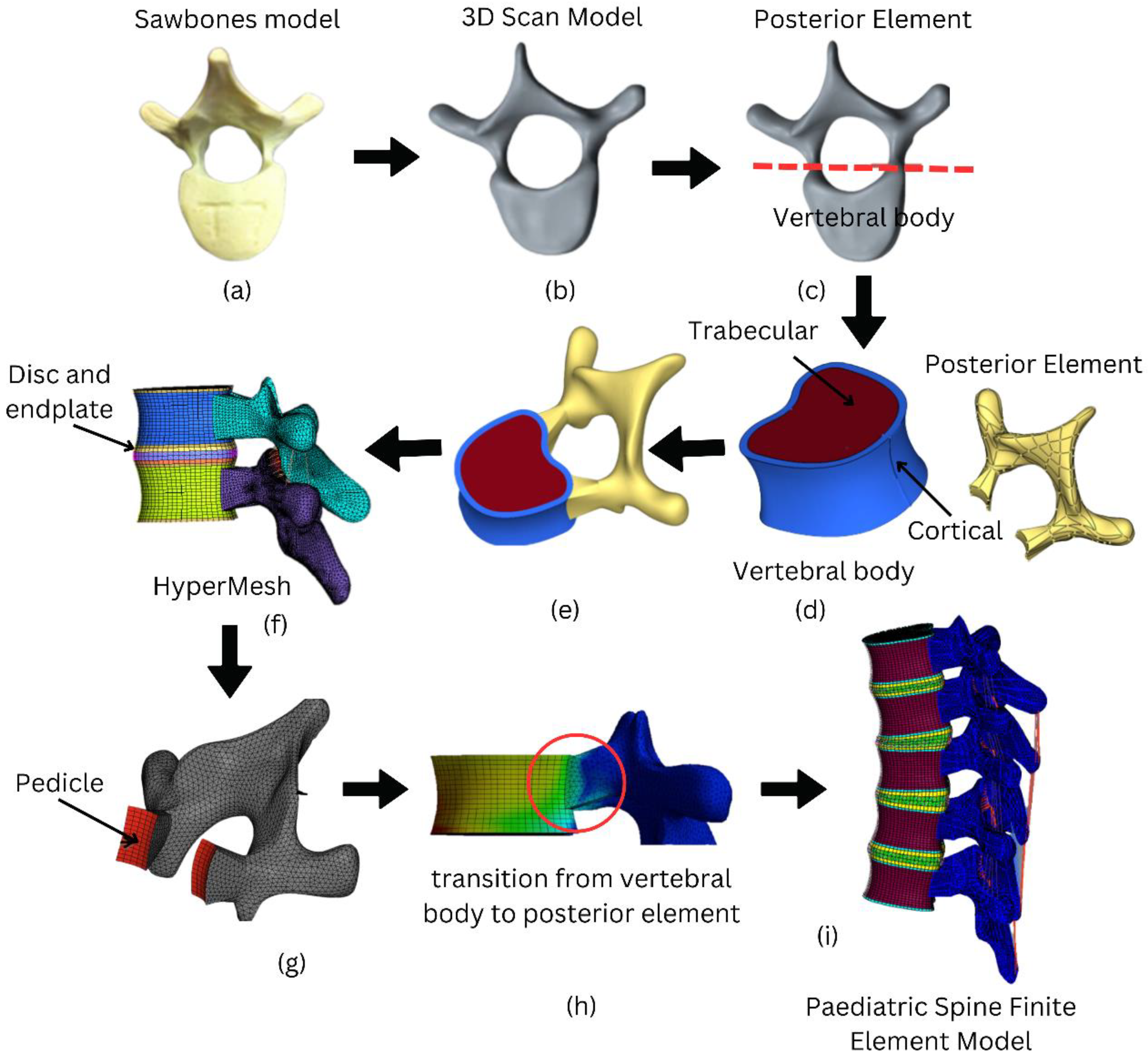
2.1. Geometrical Modelling of Paediatric Spine
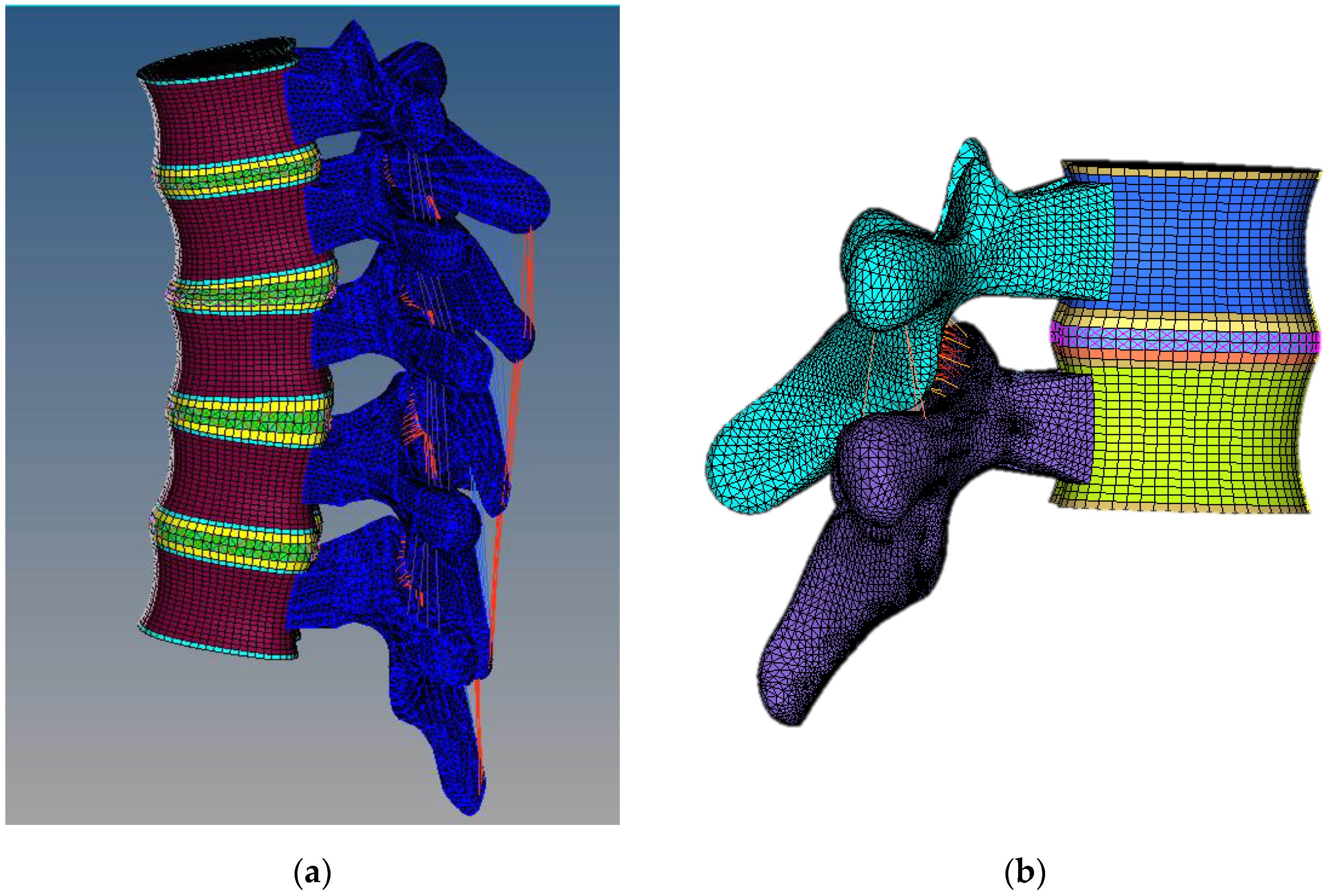
2.2. Meshing
2.3. Material Properties
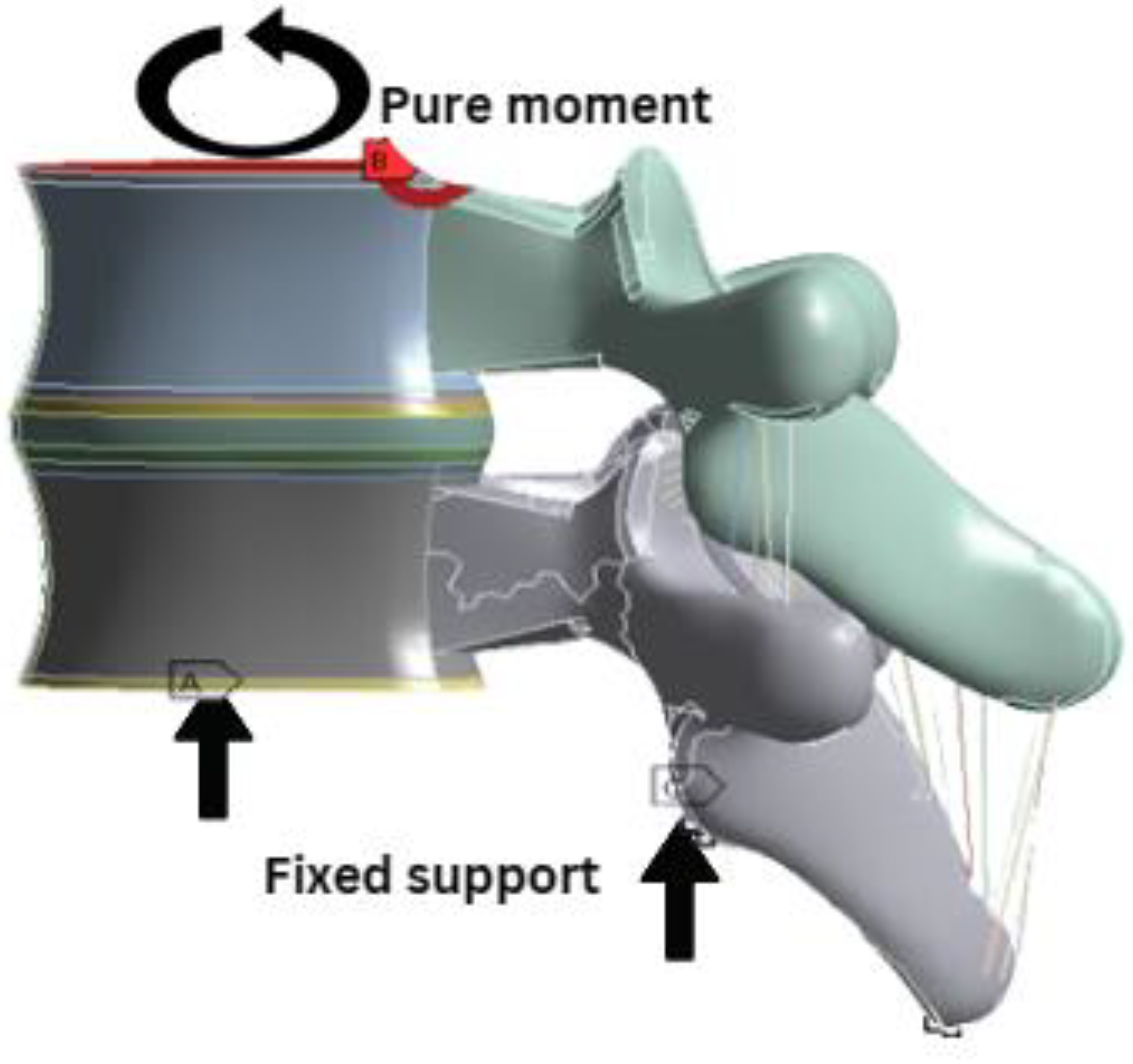
2.4. Boundary Conditions
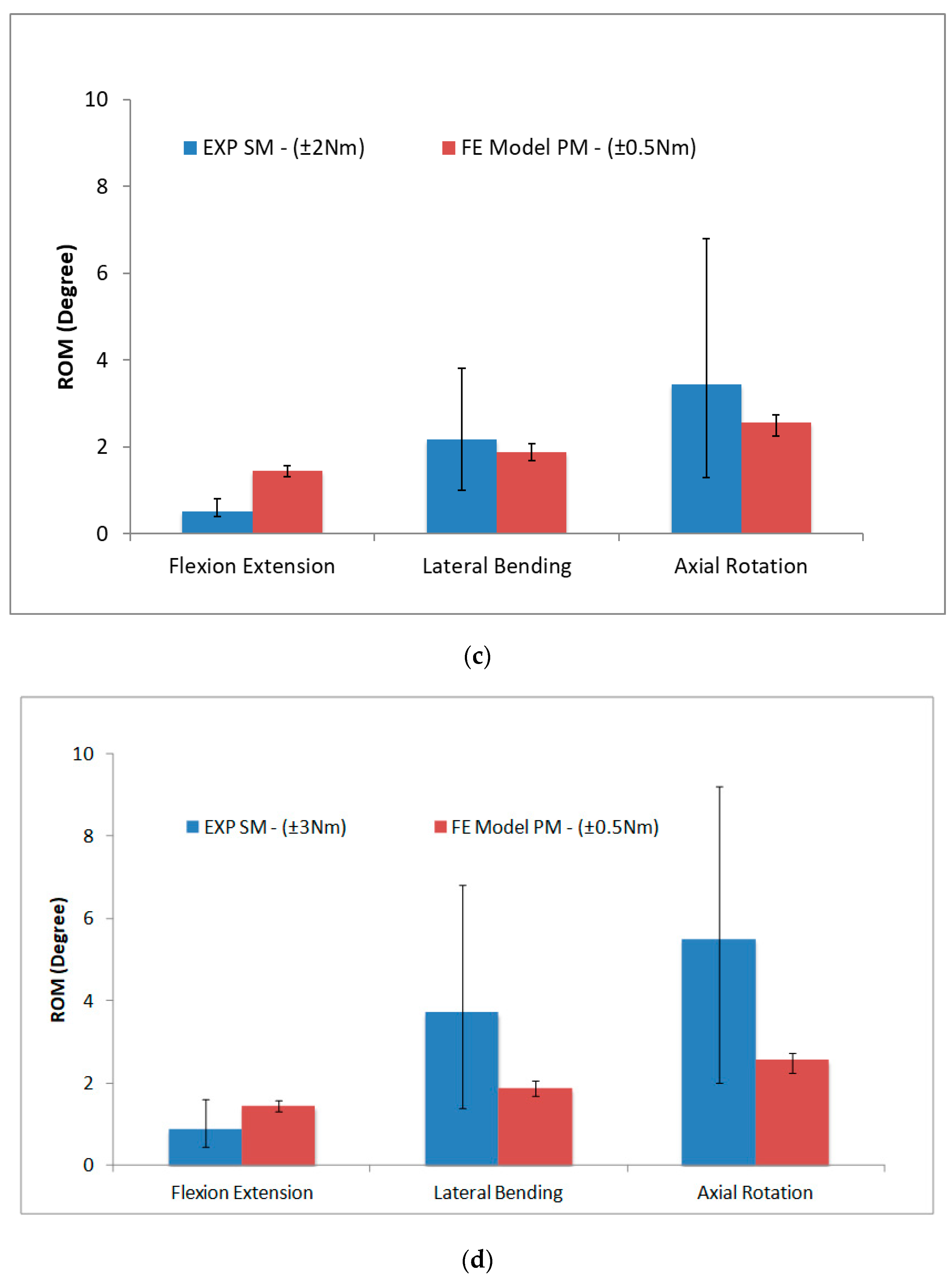
2.5. Validation of FE Model
2.6. Synthetic Spine Model Validation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiba, K.; Taneichi, H.; Namikawa, T.; Inami, S.; Takeuchi, D.; Nohara, Y. Osseointegration improves bone–implant interface of pedicle screws in the growing spine: A biomechanical and histological study using an in vivo immature porcine model. Eur. Spine J. 2017, 26, 2754–2762. [Google Scholar] [CrossRef]
- Lavelle, W.F.; Moldavsky, M.; Cai, Y.; Ordway, N.R.; Bucklen, B.S. An initial biomechanical investigation of fusionless anterior tether constructs for controlled scoliosis correction. Spine J. 2016, 16, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Fekete, T.F.; Kleinstück, F.S.; Mannion, A.F.; Kendik, Z.S.; Jeszenszky, D.J. Prospective study of the effect of pedicle screw placement on development of the immature vertebra in an in vivo porcine model. Eur. Spine J. 2011, 20, 1892–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, B. Experimental study on the loosening of pedicle screws implanted to synthetic bone vertebra models and under non-pull-out mechanical loads. J. Mech. Behav. Biomed. Mater. 2019, 98, 200–204. [Google Scholar] [CrossRef] [PubMed]
- González, S.G.; Jiménez, J.F.V.; Bastida, G.C.; Vlad, M.D.; López, J.L.; Aguado, E.F. Synthetic open cell foams versus a healthy human vertebra: Anisotropy, fluid flow and μ-CT structural studies. Mater. Sci. Eng. C 2020, 108, 110404. [Google Scholar] [CrossRef] [PubMed]
- Muhayudin, N.A.; Basaruddin, K.S.; Yazid, H.; Salleh, A.F. Development of synthetic spine for biomechanical research: An overview. J. Phys. Conf. Ser. 2021, 2051, 012072. [Google Scholar] [CrossRef]
- Wang, T.; Ball, J.R.; Pelletier, M.H.; Walsh, W.R. Biomechanical evaluation of a biomimetic spinal construct. J. Exp. Orthop. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Bohl, M.A.; Mooney, M.A.; Repp, G.J.; Nakaji, P.; Chang, S.W.; Turner, J.D.; Kakarla, U.K. The barrow biomimetic spine. Spine 2018, 43, E1368–E1375. [Google Scholar] [CrossRef]
- Bohl, M.A.; McBryan, S.; Newcomb, A.G.U.S.; Lehrman, J.N.; Kelly, B.P.; Nakaji, P.; Chang, S.W.; Uribe, J.S.; Turner, J.D.; Kakarla, U.K. Range of Motion Testing of a Novel 3D-Printed Synthetic Spine Model. Glob. Spine J. 2019, 10, 419–424. [Google Scholar] [CrossRef]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.A.; Maiman, D.J. Biomechanical study of pediatric human cervical spine: A finite element approach. J. Biomech. Eng. 2000, 122, 60–71. [Google Scholar] [CrossRef]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.A. Finite element analysis of the cervical spine: A material property sensitivity study. Clin. Biomech. 1999, 14, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Meijer, G.J.M. Development of A Non-Fusion Scoliosis Correction Device. Ph.D. Thesis, Faculty of Engineering Technology, Enschede, The Netherlands, 2011. [Google Scholar] [CrossRef] [Green Version]
- Jebaseelan, D.D.; Jebaraj, C.; Yoganandan, N.; Rajasekaran, S. Validation efforts and flexibilities of an eight-year-old human juvenile lumbar spine using a three-dimensional finite element model. Med. Biol. Eng. Comput. 2010, 48, 1223–1231. [Google Scholar] [CrossRef]
- Phuntsok, R.; Mazur, M.D.; Ellis, B.J.; Ravindra, V.M.; Brockmeyer, D.L. Development and initial evaluation of a finite element model of the pediatric craniocervical junction. J. Neurosurg. Pediatr. 2016, 17, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Song, G.; Su, Z.; Wang, G. Development, validation, and application of ligamentous cervical spinal segment C6–C7 of a six-year-old child and an adult. Comput. Methods Programs Biomed. 2020, 183, 105080. [Google Scholar] [CrossRef]
- Li, N.; Wei, W.; Wu, S.; Du, X.; Liu, Y.; Rong, P. Development of a 3-y-old Pediatric Cervical Spine Finite Element Model. IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 012035. [Google Scholar] [CrossRef]
- Muhayudin, N.A.; Basaruddin, K.S.; Daud, R.; McEvoy, F.; Tansey. Experimental Analysis of Fabricated Synthetic Midthoracic Paediatric Spine as Compared to the Porcine Spine Based on Range of Motion (ROM). Appl. Bionics Biomech. 2021, 2021, 2799415. [Google Scholar] [CrossRef]
- Ruggiero, A.; D’Amato, R.; Affatato, S. Comparison of Meshing Strategies in THR Finite Element Modelling. Materials 2019, 12, 2332. [Google Scholar] [CrossRef] [Green Version]
- Sim, O.; Ryu, D.; Lee, J.; Lee, C. Stress distribution on spinal cord according to type of laminectomy for large focal cervical ossification of posterior longitudinal ligament based on finite element method. Bioengineering 2022, 9, 519. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.; Xie, H.; Wen, W.; Xu, H.; Du, J.; Miao, J. Biomechanical behaviour of tension-band-reconstruction titanium plate in open-door laminoplasty: A study based on finite element analysis. BMC Musculoskelet. Disord. 2022, 23, 851. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Brand, R.A.; White, A.A. Mechanical properties of the human thoracic spine as shown by three-dimensional load-displacement curves. J. Bone Jt. Surg. 1976, 58, 642–652. [Google Scholar] [CrossRef]
- Muhayudin, N.A.; Basaruddin, K.S.; McEvoy, F.; Tansey, A. Development and evaluation of a paediatric mid-thoracic spine finite element model. AIP Conf. Proc. 2021, 2339, 020250. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, Q.; Zhao, W.; Xu, Y.; Chen, W.; Zhong, S. Biomechanical assessment of the pediatric cervical spine under bending and tensile loading. Spine 2005, 30, E716–E723. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, S.; Yoganandan, N.; Pintar, F.A. Finite element modeling approaches of human cervical spine facet joint capsule. J. Biomech. 1998, 31, 371–376. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Panjabi, M. Clinical Biomechanics of the Spine, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1990. [Google Scholar]
- Ryan, G.; Pandit, A.; Apatsidis, D. Stress distribution in the intervertebral disc correlates with strength distribution in subdiscal trabecular bone in the porcine lumbar spine. Clin. Biomech. 2008, 23, 859–869. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Kim, H.Y.; Hosoda, H.; Miyazaki, S. Superelastic properties of biomedical (Ti-Zr)-Mo-Sn alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 11–20. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Laille, D.; Heraud, L.; Gordin, D.; Castany, P.; Gloriant, T. Design of novel superelastic Ti-23Hf-3Mo-4Sn biomedical alloy combining low modulus, high strength and large recovery strain. Mater. Lett. 2016, 177, 39–41. [Google Scholar] [CrossRef]
- Ijaz, M.F.; Alharbi, H.F.; Bahri, Y.A.; Sherif, E.S.M. Alloy design and fabrication of duplex titanium-based alloys by spark plasma sintering for biomedical implant applications. Materials 2022, 15, 8562. [Google Scholar] [CrossRef]
- Héraud, L.; Castany, P.; Ijaz, M.F.; Gordin, D.M.; Gloriant, T. Large-strain functional fatigue properties of superelastic metastable β titanium and NiTi alloys: A comparative study. J. Alloys Compd. 2023, 953, 170170. [Google Scholar] [CrossRef]



| Component | Element | |
|---|---|---|
| Type | Number | |
| Bony endplate | 8-noded brick | 594 |
| Cortical shell | 8-noded brick | 1024 |
| Trabecular bone | 8-noded brick | 8480 |
| Posterior element | 4-noded tetra | 24,960–30,559 |
| Cartilage endplate | 8-noded brick | 594 |
| Nucleus Pulposus | 8-noded brick | 676 |
| Annulus Fibrosus matrix | 8-noded brick | 512 |
| Disc Fibres | 2-noded truss | 336 |
| ALL | Nonlinear spring | 200 |
| PLL | Nonlinear spring | 120 |
| ISL | 2-noded truss | 5 |
| SSL | 2-noded truss | 5 |
| TL | 2-noded truss | 4 |
| CL | 2-noded truss | 30 |
| LF | Nonlinear spring | 6 |
| Synthetic Materials | Paediatric Spine | |||
|---|---|---|---|---|
| Component | Young’s Modulus (MPa) | Poisson’s Ratio | Young’s Modulus (MPa) | Poisson’s Ratio |
| Bone | ||||
| Cortical bone | 3036 | 0.25 | Exx = 645.7 Eyy = 645.7 Ezz = 1257 | νxy = 0.484 νxz = 0.203 νxz = 0.203 |
| Trabecular bone | 90 | 0.2 | Exx = 105.3 Eyy = 105.3 Ezz = 150 | νxy = 0.450 νxz = 0.315 νxz = 0.315 |
| Cartilage Endplate/Growth Plate | 7.5 | 0.45 | 23.8 | 0.4 |
| Posterior bone | 90 | 0.2 | 200 | 0.25 |
| Intervertebral disc | ||||
| Nucleus pulposus (fluid) | 1.6 | 0.4999 | 0.2 | 0.4999 |
| Annulus Fibrosus matrix | 15.63 | 0.45 | Hyperelastic, Mooney Rivlin, C1-0.12, C2-0.03 | |
| Spinal Ligaments | ||||
| ALL | Force-deflection curve of synthetic ligaments. | 90% of adult ligament stiffness value | ||
| PLL | ||||
| LF | ||||
| TL | 0.62 | 0.3 | 52.83 | 0.3 |
| ISL | 10.44 | 0.3 | ||
| SSL | 13.5 | 0.3 | ||
| CL | 29.61 | 0.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhayudin, N.A.; Basaruddin, K.S.; Ijaz, M.F.; Daud, R. Finite Element Modelling of a Synthetic Paediatric Spine for Biomechanical Investigation. Materials 2023, 16, 4514. https://doi.org/10.3390/ma16134514
Muhayudin NA, Basaruddin KS, Ijaz MF, Daud R. Finite Element Modelling of a Synthetic Paediatric Spine for Biomechanical Investigation. Materials. 2023; 16(13):4514. https://doi.org/10.3390/ma16134514
Chicago/Turabian StyleMuhayudin, Nor Amalina, Khairul Salleh Basaruddin, Muhammad Farzik Ijaz, and Ruslizam Daud. 2023. "Finite Element Modelling of a Synthetic Paediatric Spine for Biomechanical Investigation" Materials 16, no. 13: 4514. https://doi.org/10.3390/ma16134514







